Abstract
Background
Lung cancer is one of the most common malignancies, and there is a trend of increasing incidence in young patients. The preoperative diagnosis of pulmonary nodules is mainly based on the combination of imaging and tumor markers. There is no relevant report on the diagnostic value of tumor markers in young pulmonary nodules. Our study was designed to explore the value of five tumor markers in young patients with pulmonary nodules.
Methods
We reviewed the medical records of 390 young patients (age ≤45 years) with pulmonary nodules treated at two separate centers from January 1, 2015, to January 1, 2021. Malignant pulmonary nodules were confirmed in 318 patients, and the other 72 patients were diagnosed with benign pulmonary nodules. The gold standard for diagnosis of pulmonary nodules was surgical biopsy. The conventional serum biomarkers included cytokeratin 19 (CYFRA21‐1), pro‐gastrin‐releasing‐peptide (ProGRP), carcinoembryonic antigen (CEA), neuron‐specific enolase (NSE), and squamous cell carcinoma‐associated antigen (SCCA). The diagnostic values of five tumor markers were analyzed by receiver operating characteristic (ROC) curves.
Results
There were no significant differences in the expression of five tumor markers between the groups (p > 0.05). Single tumor marker (CYFRA21‐1, ProGRP, CEA, NSE, and SCCA) showed a limited value in the diagnosis of malignant pulmonary nodules, with the AUC of 0.506, 0.503 0.532, 0.548, and 0.562, respectively. The AUC of the combined examination was only 0.502~0.596, which did not improve the diagnostic value.
Conclusions
Five conventional tumor markers had a limited diagnostic value in young patients with pulmonary nodules.
Keywords: pulmonary nodules, tumor markers, young patients
Preoperative diagnosis of pulmonary nodules is mainly based on the combination of imaging and tumor markers. Our study found the expression of the five tumor markers (CYFRA21‐1, ProGRP, CEA, NSE, and SCCA) did not differ significantly in young patients with pulmonary nodules. The AUC of tumor markers did not exceed 0.600, whether it was a single tumor marker or a combination of tumor markers.

1. INTRODUCTION
Study1 had shown that there were about 228,150 new cases in 2019 and about 142,670 deaths in 2019, accounting for approximately 25 percent of all cancer deaths in the United States. Lung cancer was still the leading cause of death from cancer. In addition, there was a trend of increasing incidence in young patients.2, 3 Another study4 had reported that the pathological characteristics and prognosis of young patients with lung cancer were different from the elderly, suggesting that young patients with lung cancer should be regarded as a special subtype of lung cancer. Many studies defined young lung cancer as an age inferior than 45 years.2, 5, 6, 7, 8 Research from the American National Cancer Data Base8 showed that young patients can achieve better survival benefits than the elderly at lower stages. Therefore, early diagnosis of young lung cancer was crucial. A trial demonstrated that a person's cumulative probability of 1 or more false‐positive low‐dose CT examinations was 21%.9 To improve the diagnostic accuracy and avoid unnecessary invasive procedures, it was necessary to combine tumor markers with imaging. The conventional serum biomarkers for lung cancer included cytokeratin 19 (CYFRA21‐1), pro‐gastrin‐releasing‐peptide (ProGRP), carcinoembryonic antigen (CEA) neuron‐specific enolase (NSE), and squamous cell carcinoma‐associated antigen (SCCA).10, 11 Study12 indicated that for solitary pulmonary lesions, using the tumor markers alone, the highest sensitivity (27.2%) and accuracy (40.4%) were found with CEA, the highest specificity (100%) with CYFRA 21‐1, and with NSE. Rafael Molina13 reported that the sensitivities of CYFRA21‐1, ProGRP, CEA, NSE, and SCCA for the diagnosis of lung cancer were 56.1%, 17.1%, 56.5%, 19.1%, and 20.7%, respectively, while the specificities were 96.1%, 95.2%, 93.5%, 99.5%, and 97.8%, respectively. His study also indicated that the specificity of CYFRA21‐1, ProGRP, CEA, NSE, and SCCA for the diagnosis of lung cancer could be over 90%, even if nodule size was less than 30 mm. However, many studies about tumor markers in pulmonary nodules did not distinguish the young patients from the old. There was no relevant report on the diagnostic value of conventional tumor markers in young patients with pulmonary nodules.
Therefore, this retrospective study was designed to explore the value of conventional tumor markers in young patients with pulmonary nodules.
2. PATIENTS AND METHODS
In this double‐center retrospective study, we reviewed the medical records of young patients hospitalized for pulmonary nodules at two separate centers (Fujian Medical University Union Hospital and Fujian Provincial Hospital) from January 1, 2015, to January 1, 2021. Malignant pulmonary nodules were pathologically defined as primary lung cancer including adenocarcinoma, carcinoma in situ, squamous cell carcinoma, and carcinoid. Benign pulmonary nodules included pneumonic benign nodules, pulmonary cryptococcus, tuberculosis, and pulmonary hamartoma. Inclusion criteria: (1) Patients were defined as age younger than 45 years, and pulmonary nodule's diameter less than 30 mm, including solitary and multiple pulmonary nodules; (2) there were definite pathological reports of pulmonary nodules; (3) complete case's data. Exclusion criteria: Pulmonary nodules were pathologically diagnosed as lung metastases from other tumors. Finally, a total of 390 patients were identified as eligible for enrollment in the study. We divided them into malignant and benign pulmonary nodules groups according to pathology. The malignant pulmonary nodules group was confirmed in 318 patients, and the other 72 patients were diagnosed with benign pulmonary nodules.
The medical records of these patients were documented and reviewed, including demographic information, diagnosis, laboratory testing results, and histopathology findings. Serum samples of tumor markers (CYFRA21‐1, ProGRP, CEA, NSE, and SCCA) were collected within three days before surgery and sent to the clinical laboratory at the center for testing within 120 min.
3. STATISTICAL ANALYSIS
Normally distributed data were expressed as mean ± standard derivation; otherwise, they were expressed as medians. Differences of normally distributed data between groups were analyzed by the independent Student's t test, and non‐normally distributed data between groups were compared using the Mann‐Whitney U test. Enumeration data were expressed as n (%) and were compared using the Chi‐square test. ROC curves were constructed for assessing diagnostic potentials, with sensitivity (%) as the Y‐axis and 100‐specificity (%) as the X‐axis. The area under curve (AUC), sensitivity [sensitivity = true‐positive rate/(true‐positive rate + false‐negative rate) × 100%], and specificity [specificity = true‐negative rate/(true‐negative rate + false‐positive rate) × 100%] were calculated. SPSS25.0 and MedCalc_v12.3 were used for statistical analyses. p < 0.05 was considered statistically significant.
4. RESULTS
The characteristics of the enrolled patients were shown in Table 1. The malignant and benign pulmonary nodules group did not differ significantly in terms of age, gender, smoke, personal or family cancer history, or nodule size (p > 0.05).
TABLE 1.
The characteristics of the enrolled patients
| Malignant (n = 318) | Benign (n = 72) | p value | |
|---|---|---|---|
| Ageb | 39.21(6.70) | 38.49(5.32) | 0.392 |
| Sexa | — | — | 0.155 |
| Male | 88 (27.67%) | 26 (36.11%) | — |
| Female | 230 (72.33%) | 46 (63.89%) | — |
| Personal cancer historya | 23 (7.23%) | 2 (2.78%) | 0.260 |
| Family cancer historya | 27 (8.49%) | 8 (11.11%) | 0.482 |
| Smokea | 38 (11.95%) | 12 (16.67%) | 0.280 |
| Nodule sizea | — | — | 0.235 |
| ≤10 mm | 166 (52.20%) | 32 (44.44%) | — |
| 11−30 mm | 152 (47.80%) | 40 (55.56%) | — |
Data were expressed as the number of cases (percentage).
Data were expressed as mean ± standard derivation.
Our study found that the expression of five tumor markers (CYFRA21‐1, ProGRP, CEA, NSE, and SCCA) did not differ significantly in the malignant and benign pulmonary nodules group (p > 0.05; Table 2).
TABLE 2.
Tumor markers values in the two groups
| Malignant (n = 318) | Benign (n = 72) | p value | |
|---|---|---|---|
| CYFRA21‐1 (ng/ml)a | 2.19 (1.68–2.84) | 2.38 (1.67–2.88) | 0.875 |
| ProGRP (Pg/ml)a | 38.19 (32.18–47.71) | 38.92 (30.88–47.28) | 0.940 |
| CEA (ng/ml)a | 1.40 (0.90–2.10) | 1.40 (0.81–1.98) | 0.399 |
| NSE (ng/ml)a | 11.75 (10.26–13.59) | 11.45 (10.16–12.49) | 0.201 |
| SCCA (ng/ml)a | 0.90 (0.60–1.10) | 0.80 (0.50–1.05) | 0.097 |
Data were expressed as medians (quartile).
As reported by some studies,13 the nodule size may affect the expression of tumor markers. Therefore, according to the nodule size, we did a subgroup analysis and found that the expression of tumor markers was still not significantly different in the two groups (p > 0.05; Table 3).
TABLE 3.
Tumor marker values stratified by nodule size
| Nodule Size ≤10 mm | Nodule Size 11–30 mm | |||||
|---|---|---|---|---|---|---|
| Malignant (n = 166) | Benign (n = 32) | p value | Malignant (n = 152) | Benign (n = 40) | p value | |
| CYFRA21‐1 (ng/ml)a | 2.16 (1.62–2.84) | 2.55 (1.67–3.17) | 0.315 | 2.21 (1.77–2.83) | 2.20 (1.66–2.53) | 0.217 |
| ProGRP (Pg/ml)a | 38.25 (32.40–46.90) | 40.63 (31.22–48.30) | 0.423 | 38.12 (32.11–50.07) | 37.80 (30.88–46.41) | 0.568 |
| CEA (ng/ml)a | 1.35 (0.90–1.97) | 1.41 (1.03–1.96) | 0.467 | 1.51 (0.96–2.30) | 1.30 (0.70–1.99) | 0.069 |
| NSE (ng/ml)a | 11.71 (10.25–13.77) | 11.60 (10.00–12.99) | 0.522 | 11.95 (10.25–13.52) | 11.37 (10.33–12.43) | 0.251 |
| SCCA (ng/ml)a | 0.90 (0.70–1.10) | 0.85 (0.50–1.10) | 0.205 | 0.80 (0.60–1.10) | 0.80 (0.53–1.00) | 0.329 |
Data were expressed as medians (quartile).
Further analysis of tumor markers showed that the diagnostic value was very limited in young patients with pulmonary nodules. The area under the ROC curve of five conventional tumor markers was less than 0.600 (the AUC of CYFRA21‐1, ProGRP, CEA, NSE, and SCCA was 0.506, 0.503 0.532, 0.548, and 0.562, respectively), which could not provide a good diagnostic value (Table 4, Figure 1).
TABLE 4.
Individual TM ROC results for lung cancer
| CYFRA21‐1 | ProGRP | CEA | NSE | SCCA | |
|---|---|---|---|---|---|
| The threshold | 2.35 | 35.49 | 0.75 | 12.56 | 0.5 |
| Sensitivity (%) | 44.65 (39.10–50.30) | 40.25 (34.80–45.90) | 88.36 (84.30–91.70) | 39.94 (34.50–45.60) | 87.74 (83.60–91.10) |
| Specificity (%) | 47.22 (35.30–59.30) | 66.67 (54.60–77.30) | 22.22 (13.30–33.60) | 79.17 (68.00–87.80) | 26.39 (16.70–38.10) |
| PPV (%) | 78.90 (74.40–82.80) | 84.20 (78.90–88.40) | 83.40 (81.50–85.10) | 89.40 (84.10–93.10) | 84.00 (82.00–85.90) |
| NPV (%) | 16.20 (12.90–20.10) | 20.20 (17.30–23.30) | 30.20 (20.30–42.30) | 23.00 (20.50–25.70) | 32.80 (23.10–44.20) |
| Positive likelihood ratio | 0.85 (0.70–1.10) | 1.21 (0.80–1.70) | 1.14 (1.00–1.30) | 1.92 (1.20–3.10) | 1.19 (1.00–1.40) |
| Negative likelihood ratio | 1.17 (0.90–1.50) | 0.90 (0.70–1.10) | 0.52 (0.30–0.90) | 0.76 (0.70–0.90) | 0.46 (0.30–0.80) |
| Youden index | 0.081 | 0.069 | 0.106 | 0.191 | 0.141 |
| AUC | 0.506 (0.455–0.557) | 0.503 (0.452–0.554) | 0.532 (0.481–0.582) | 0.548 (0.497–0.598) | 0.562 (0.512–0.612) |
FIGURE 1.
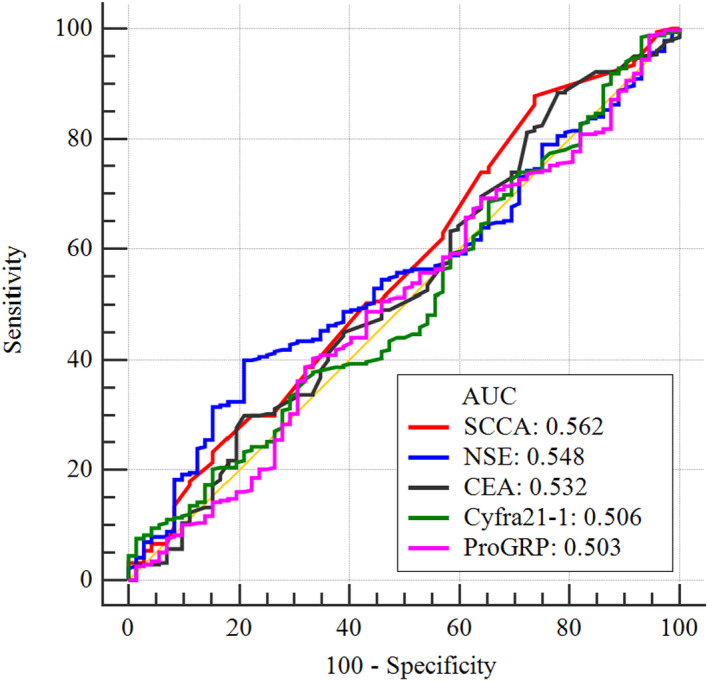
ROC curves for the diagnosis of young patients with pulmonary nodules
In addition, we conducted a combined analysis of the five tumor markers respectively. The area under the ROC curve of the combined tumor markers’ assessment was 0.502~0.596, and its maximum value could not exceed 0.600. The highest AUC was found when the four tumor markers were combined (ProGRP, CEA, NSE, and SCCA), but the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) for lung cancer were 36.16%, 81.94%, 89.80%, and 22.50%, respectively (Table 5).
TABLE 5.
AUC sensitivity, specificity, PPV, and NPV of tumor maker's combined assessment
| AUC | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|
| CYFRA21‐1+ProGRP | 0.505 (0.454–0.555) | 44.03 (38.50–49.70) | 48.61 (36.70–60.70) |
| CYFRA21‐1+CEA | 0.502 (0.451–0.553) | 31.76 (26.70–37.20) | 58.33 (46.10–69.80) |
| CYFRA21‐1+NSE | 0.552 (0.501–0.602) | 24.53 (19.90–29.60) | 87.50 (77.60–94.10) |
| CYFRA21‐1+SCCA | 0.562 (0.511–0.611) | 27.36 (22.50–32.60) | 84.72 (74.30–92.10) |
| ProGRP+CEA | 0.529 (0.478–0.579) | 85.53 (81.20–89.20) | 27.78 (17.9–39.6) |
| ProGRP+NSE | 0.550 (0.499–0.600) | 38.36 (33.00–44.00) | 79.17 (68.00–87.80) |
| ProGRP+SCCA | 0.574 (0.523–0.623) | 76.10 (71.00–80.70) | 40.28 (28.90–52.50) |
| CEA+NSE | 0.547 (0.496–0.597) | 28.93 (24.00–34.30) | 86.11 (75.90–93.10) |
| CEA+SCCA | 0.565 (0.514–0.615) | 79.25 (74.40–83.60) | 33.33 (22.70–45.40) |
| NSE+SCCA | 0.589 (0.538–0.638) | 60.06 (54.40–65.50) | 55.56 (43.40–67.30) |
| CYFRA21‐1+ProGRP+CEA | 0.503 (0.452–0.553) | 33.02 (27.90–38.50) | 55.56 (43.40–67.30) |
| CYFRA21‐1+ProGRP+NSE | 0.548 (0.497–0.598) | 20.75 (16.40–25.60) | 91.67 (82.70–96.90) |
| CYFRA21‐1+ProGRP+SCCA | 0.571 (0.520–0.620) | 72.64 (67.40–77.50) | 41.67 (30.20–53.90) |
| CYFRA21‐1+NSE+CEA | 0.552 (0.501–0.602) | 20.13 (15.90–25.00) | 93.06 (84.50–97.70) |
| CYFRA21‐1+CEA+SCCA | 0.564 (0.513–0.613) | 57.86 (52.20–63.40) | 54.17 (42.00–66.00) |
| CYFRA21‐1+NSE+SCCA | 0.584 (0.534–0.634) | 58.49 (52.90–64.00) | 56.94 (44.70–68.60) |
| ProGRP+CEA+NSE | 0.548 (0.498–0.599) | 38.99 (33.60–44.60) | 76.39 (64.90–85.60) |
| ProGRP+CEA++SCCA | 0.575 (0.525–0.625) | 70.13 (64.80–75.10) | 45.83 (34.00–58.00) |
| ProGRP+NSE+SCCA | 0.594 (0.543–0.643) | 60.38 (54.80– 65.80) | 56.94 (44.70–68.60) |
| CEA+NSE+SCCA | 0.591 (0.540–0.640) | 30.82 (25.80–36.20) | 86.11 (75.90–93.10) |
| CYFRA21‐1+ProGRP+CEA+NSE | 0.545 (0.494–0.595) | 18.87 (14.70–23.60) | 94.44 (86.40–98.50) |
| CYFRA21‐1+ProGRP+CEA+SCCA | 0.571 (0.520–0.621) | 75.47 (70.40–80.10) | 37.50 (26.40–49.70) |
| CYFRA21‐1+ProGRP+NSE+SCCA | 0.590 (0.540–0.640) | 69.50 (64.10–74.50) | 50.00 (38.00–62.00) |
| CYFRA21‐1+CEA+NSE+SCCA | 0.587 (0.536–0.636) | 74.84 (69.70–79.50) | 40.28 (28.90–52.50) |
| ProGRP+CEA+NSE+SCCA | 0.596 (0.546–0.645) | 36.16 (30.90–41.70) | 81.94 (71.10–90.00) |
| CYFRA21‐1+ProGRP+CEA+NSE+SCCA | 0.593 (0.542–0.642) | 62.58 (57.00–67.90) | 54.17 (42.00–66.00) |
5. DISCUSSION
We found, among young patients, female patient accounted for up to 72.33%, and adenocarcinoma was the most common pathological type (95.91%). In addition, smoking was only accounted for 11.95%, while tumor history (including personal and family) was 15.09%, which was consistent with the previous reports.8, 14, 15, 16, 17 Young age at onset and lack of established environmental risk factors suggested genetic predisposition.18
Conventional tumor markers for lung cancer included CYFRA21‐1, ProGRP, CEA, NSE, and SCCA, which served as important auxiliary indicators for the diagnosis of pulmonary nodules. Many studies showed a statistical difference in tumor markers between benign and malignant pulmonary nodules.13, 19, 20 However, most of the existing studies on pulmonary nodules did not distinguish young patients from old, and the diagnostic significance of tumor markers was not clear in young pulmonary nodules. There was a trend of increasing incidence in young patients, and it was necessary to identify the diagnostic value of tumor markers in young patients. Our study found the expression of the five tumor markers (CYFRA21‐1, ProGRP, CEA, NSE, and SCCA) did not differ significantly in young patients with pulmonary nodules (p > 0.05). The tumor markers showed limited diagnostic value with the AUC of CYFRA21‐1, ProGRP, CEA, NSE, and SCCA was 0.506, 0.503 0.532, 0.548, and 0.562, respectively. A previous study has reported that chronic kidney disease may cause an increase in ProGRP.21 In addition, CYFRA 21‐1, CEA, and NSE also were reported to be possibly increased in non‐neoplastic conditions.10 In summary, for young patients with pulmonary nodules, negative tumor markers should not be relaxed, while with elevated tumor markers, a comprehensive judgment should be made based on clinical, imaging, and other indicators to exclude benign lesions and avoid unnecessary surgery.
A study showed when nodule size was less than 10 mm, only CEA showed significant differences, meanwhile; nodule size was 10–30 mm; the five tumor markers were higher in malignant groups than benign groups.13 Therefore, the author inferred that nodule size might affect the expression of tumor markers. However, by subgroup analysis, we found that the difference in nodule size did not lead to the differential results. When nodules size was less than 30 mm, CYFRA21‐1, ProGRP, CEA, NSE, and SCCA showed no significant differences in young patients with different natures of pulmonary nodules (p > 0.05). The results may be related to the patient's young age, fewer underlying diseases, and short smoking history, etc., which is not clear at present and needs further study.
According to the previous research,11, 13, 22, 23 the five tumor markers for lung cancer had high specificity and low sensitivity in the diagnosis, and a combination of tumor markers can improve the diagnostic value. Through the combination of the tumor markers, we found that their diagnostic value was limited in young patients, and the combination could not improve the diagnostic value (the combined AUC was 0.502–0.596). The results may be due to the limited diagnostic value of single tumor markers. Our study indicated that the five tumor markers were not helpful for the young pulmonary nodules in clinical diagnosis. Therefore, other molecular biomarkers should be explored to improve the diagnostic accuracy for young patients, such as DNA methylation,24, 25 miRNA,26 circulating tumor cells,27 and tumor‐associated antigens and autoantibody.28
Previous studies did not identify young lung cancer as a specific subtype. The difference in the diagnostic value of tumor markers may be related to the different proportion of young patients. It was necessary to carry out tumor marker studies in elderly patients with pulmonary nodules to assess the diagnostic value of tumor markers.
The limitations of the present study were the unavoidable selection bias and the limited tumor markers. Further investigation into the diagnostic value of biomarkers in young pulmonary nodules was required.
6. CONCLUSION
Conventional tumor markers (CYFRA21‐1, ProGRP, CEA, NSE, and SCCA) showed limited value to differentiate the nature of young pulmonary nodules.
CONFLICT OF INTERESTS
The authors declared that they had no conflict of interests.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the Clinical Key Specialty Construction Project of Fujian Province (No. 2017YZ0001‐2).
Xu L, Su Z, Xie B. Diagnostic value of conventional tumor markers in young patients with pulmonary nodules. J Clin Lab Anal. 2021;35:e23912. 10.1002/jcla.23912
DATA AVAILABILITY STATEMENT
Although the data that support the findings of this study were not publicly available due to privacy, the data were available from the corresponding author upon reasonable request.
REFERENCES
- 1.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69(3):184‐210. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Chen S‐F, Zhen Y, et al. Multicenter analysis of lung cancer patients younger than 45 years in Shanghai. Cancer. 2010;116(15):3656‐3662. [DOI] [PubMed] [Google Scholar]
- 3.Strand TE, Malayeri C, Eskonsipo PK, Grimsrud TK, Norstein J, Grotmol T. Adolescent smoking and trends in lung cancer incidence among young adults in Norway 1954‐1998. Cancer Causes Control. 2004;15(1):27‐33. [DOI] [PubMed] [Google Scholar]
- 4.Sacher AG, Dahlberg SE, Heng J, Mach S, Jänne PA, Oxnard GR. Association between younger age and targetable genomic alterations and prognosis in non‐small‐cell lung cancer. JAMA Oncol. 2016;2(3):313‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou H, Zhang C, Qi X, et al. Distinctive targetable genotypes of younger patients with lung adenocarcinoma: a cBioPortal for cancer genomics data base analysis. Cancer Biol Ther. 2020;21(1):26‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou H, Zhu H, Zhao H, et al. Comprehensive molecular characterization of young Chinese patients with lung adenocarcinoma identified a distinctive genetic profile. Oncologist. 2018;23(9):1008‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi J, Li D, Liang D, He Y. Epidemiology and prognosis in young lung cancer patients aged under 45 years old in northern China. Sci Rep. 2021;11(1):6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold BN, Thomas DC, Rosen JE, et al. Lung cancer in the very young: treatment and survival in the national cancer data base. J Thorac Oncol. 2016;11(7):1121‐1131. [DOI] [PubMed] [Google Scholar]
- 9.Croswell JM, Baker SG, Marcus PM, Clapp JD, Kramer BS. Cumulative incidence of false‐positive test results in lung cancer screening: a randomized trial. Ann Intern Med. 2010;152(8):505‐512, w176‐w580. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura H, Nishimura T. History, molecular features, and clinical importance of conventional serum biomarkers in lung cancer. Surg Today. 2017;47(9):1037‐1059. [DOI] [PubMed] [Google Scholar]
- 11.Qu T, Zhang J, Xu N, et al. Diagnostic value analysis of combined detection of Trx, CYFRA21‐1 and SCCA in lung cancer. Oncol Lett. 2019;17(5):4293‐4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seemann MD, Beinert T, Fürst H, Fink U. An evaluation of the tumour markers, carcinoembryonic antigen (CEA), cytokeratin marker (CYFRA 21‐1) and neuron‐specific enolase (NSE) in the differentiation of malignant from benign solitary pulmonary lesions. Lung Cancer. 1999;26(3):149‐155. [DOI] [PubMed] [Google Scholar]
- 13.Molina R, Marrades RM, Augé JM, et al. Assessment of a combined panel of six serum tumor markers for lung cancer. Am J Respir Crit Care Med. 2016;193(4):427‐437. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non‐small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol. 2010;5(1):23‐28. [DOI] [PubMed] [Google Scholar]
- 15.Thomas A, Chen Y, Yu T, Jakopovic M, Giaccone G. Trends and characteristics of young non‐small cell lung cancer patients in the United States. Front Oncol. 2015;5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolwin Y, Gillis R, Peled N. Gender and lung cancer‐SEER‐based analysis. Ann Epidemiol. 2020;46:14‐19. [DOI] [PubMed] [Google Scholar]
- 17.Xia J, Li H, Ji Y, et al. Clinicopathologic characteristics and EGFR mutations in lung cancer patients aged below 45 years. Curr Probl Cancer. 2019;43(4):363‐370. [DOI] [PubMed] [Google Scholar]
- 18.Donner I, Katainen R, Sipilä LJ, Aavikko M, Pukkala E, Aaltonen LA. Germline mutations in young non‐smoking women with lung adenocarcinoma. Lung Cancer. 2018;122:76‐82. [DOI] [PubMed] [Google Scholar]
- 19.Fang R, Han H, Yang Y, et al. Clinical characteristics on low‐dose high‐resolution computed tomography and serum tumor markers of malignant pulmonary solid small nodules and postoperative survival analysis. J BUON. 2019;24(3):918‐928. [PubMed] [Google Scholar]
- 20.Chen F, Li J, Qi X, Qi J. Diagnostic value of CYFRA 21‐1 and carcinoembryonic antigen in diagnosis of operable lung cancer from benign lung disease. J Cancer Res Ther. 2018;14(suppl):S400‐S404. [DOI] [PubMed] [Google Scholar]
- 21.Dai Z, Zhu J, Huang H, et al. Expression and clinical value of gastrin‐releasing peptide precursor in nephropathy and chronic kidney disease. Nephrology (Carlton). 2020;25(5):398‐405. [DOI] [PubMed] [Google Scholar]
- 22.Wu H, Wang Q, Liu Q, Zhang Q, Huang Q, Yu Z. The serum tumor markers in combination for clinical diagnosis of lung cancer. Clin Lab. 2020;66(3). 10.7754/Clin.Lab.2019.190533 [DOI] [PubMed] [Google Scholar]
- 23.Li X, Zhang Q, Jin X, Cao L. Combining serum miRNAs, CEA, and CYFRA21‐1 with imaging and clinical features to distinguish benign and malignant pulmonary nodules: a pilot study: Xianfeng Li, et al.: combining biomarker, imaging, and clinical features to distinguish pulmonary nodules. World J Surg Oncol. 2017;15(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang W, Chen Z, Li C, et al. Accurate diagnosis of pulmonary nodules using a non‐invasive DNA methylation test. J Clin Investig. 2021;131. 10.1172/JCI145973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schotten LM, Darwiche K, Seweryn M, et al. DNA methylation of PTGER4 in peripheral blood plasma helps to distinguish between lung cancer, benign pulmonary nodules and chronic obstructive pulmonary disease patients. Eur J Cancer. 2021;147:142‐150. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J‐T, Qin H, Man Cheung FK, et al. Plasma extracellular vesicle microRNAs for pulmonary ground‐glass nodules. J Extracell Vesicles. 2019;8(1):1663666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alix‐Panabières C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med. 2012;63:199‐215. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang R, Wu S, Zhang B, Wang T, Yin B, Huang J, Wei W, Huang M, Zhang M, Wang Y, et al. Clinical value of tumor‐associated antigens and autoantibody panel combination detection in the early diagnostic of lung cancer. Cancer Biomark. 2021. 10.3233/CBM-210099 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Although the data that support the findings of this study were not publicly available due to privacy, the data were available from the corresponding author upon reasonable request.


