Abstract
Background
Statins reportedly have anti-inflammatory effects aside from their lipid-lowering impact. We investigated the effects of statin therapy on the level of C-reactive protein (CRP) or highly sensitive CRP (hs-CRP), a liver-derived marker of systemic inflammation, among stroke patients.
Methods
An online search was performed in Scopus, PubMed/MEDLINE, ISI Web of Science, and Google Scholar up to November 2020 to recognize clinical trials investigating the effects of statins on the CRP level in stroke patients.
Results
Overall, nine studies (11 treatment arms) with 1659 participants met the inclusion criteria. Six out of 9 studies (8 out of 11 arms) were categorized as studies with a high-quality methodological approach using the Cochrane Collaboration's tool. Data from 5 treatment arms indicated a significant decrease in CRP concentration, and in one treatment arm, CRP concentration did not suggest any considerable alteration following statin therapy. Moreover, two treatment arms showed a significant reduction in hs-CRP concentration and three treatment arms revealed no significant alteration in hs-CRP concentration following statin therapy. Generally, results were heterogeneous and independent of the type of statin, statin dose, treatment duration, and changes in plasma low-density lipoprotein cholesterol concentration.
Conclusion
The results suggest that statin therapy could reduce and, therefore, could be considered in these patients as potential anti-inflammatory agents.
1. Introduction
Stroke is a leading cause of severe and long-term disability and is considered the third common cause of human mortality. According to the estimates, annually, 15 million people suffer stroke worldwide, with an annual mortality rate of about 5 million [1]. High blood pressure and atrial fibrillation are the most important risk factors for stroke [2].
Ischemic stroke also referred to as brain ischemia or cerebral ischemia is the most common type of stroke, accounting for 80% of all cases [3]. The leading cause of ischemic stroke is the narrowing of the arteries due to atherosclerosis. However, there are many other causes of cerebral ischemic pathogenesis, including endothelial dysfunction, thrombogenesis, inflammatory and oxidative stress damages, and defects in angiogenesis [4].
Inflammatory damage plays a pivotal role in the pathogenesis of ischemic stroke. The collective contribution of the inflammatory cells in the ischemic tissue usually results in longstanding vascular inflammation and ischemic brain injury [5, 6]. Among various proinflammatory cytokines and mediators, serum C-reactive protein (CRP) is of particular importance. According to several studies, this protein is a neuroinflammation marker and an indicator of treatment efficacy [7]. Furthermore, studies have revealed that CRP could also be potentially used to predict impending atherosclerotic-related diseases, including ischemic stroke and cardiovascular disorders [8].
Statins are inhibitors of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, which have been proved to improve endothelial function, modulate thrombogenesis, and significantly diminish cardiovascular disorders [9–11]. In addition, the preventive and ameliorative effects of statins on myocardial infarctions and stroke have been thought to lower serum cholesterol levels [12]. However, it has been demonstrated that the inhibitory effects of statins on HMG-CoA reductase could result in pleiotropic effects beyond reducing the serum low-density lipoprotein (LDL) and cholesterol [13–20]. In this regard, it has been proved that statins could prevent ischemic stroke through attenuating inflammatory damage [21]. Moreover, various preclinical and clinical studies have reported the beneficial effect of statins on CRP reduction [22–24].
Despite the published clinical trials reporting the effects of statins on the CRP level in stroke patients, the findings of these studies have not been systematically reviewed. Therefore, we aimed to perform a systematic review of published clinical studies assessing the effects of statins on CRP levels in patients with stroke.
2. Material and Methods
This systematic review was designed and reported using the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [25].
2.1. Search Strategy
We performed a conclusive systematic search on medical databases including Scopus, ISI Web of Science, PubMed, and Google Scholar databases from inception up to 12 November 2020 using the following keywords: (“statin therapy” OR “statin” OR “atorvastatin” OR “fluvastatin” OR “lovastatin” OR “pitavastatin” OR “pravastatin” OR “rosuvastatin” OR “simvastatin”) AND (“stroke” OR “Brain attack” or “Cerebrovascular accident” OR “CVA” OR “Hemorrhagic stroke” OR “Ischemic stroke”) AND (“CRP” OR “hs-CRP” OR “high sensitivity C-reactive protein” OR “C-reactive protein” OR “C-reactive protein”) AND (“Intervention Study” OR “Intervention Studies” OR “Controlled trial” OR “Randomized controlled trial” OR “Randomized clinical trial” OR “Non-Randomized Controlled Trials” OR “Clinical Trial” OR “Non-Randomized Controlled Trials” OR “Cross-Over study” OR “Cross-Over trial” OR “Cross Over trial” OR “Cross Over study” OR “Double-Blind Method” OR “Double-Blind” OR “Double-Blind trial” OR “Double-Blind study”). In addition, whenever possible, Medical Subject Headings (MESH) terms were used.
2.2. Study Selection
The title and abstract of all papers, which were found in early search, were independently reviewed by two authors (M.B. and G.A.). Articles that did not meet the inclusion criteria were excluded using a screen form with a hierarchical approach based on the study design, population, exposure, and outcome. To explore additional studies, reference lists of relevant review articles were reviewed. The full text of the eligible citation was reviewed. Any disagreements were discussed and agreed.
2.3. Inclusion Criteria
The search was conducted to identify articles examining the effects of statin therapy on CRP or hs-CRP in stroke patients. In the present systematic review, only original articles following these criteria were included: (1) using clinical trial design, (2) using statins as a drug, (3) conducted on patients with stroke disease as a primary disease, (4) assessing CRP or hs-CRP, and (5) using the English language.
2.4. Exclusion Criteria
Studies were excluded if they (1) were a nonhuman experimental disease, (2) reported duplicate data, and (3) were reviews, letters, editorial articles, study protocol, or case reports.
2.5. Data Extraction
Relevant articles were selected after screening records in the initial search. The following information was extracted from eligible and included articles and reported in Table 1: publication information including first author's last name, publication date, and study location, details of the clinical trial including target population, sample size, gender, the mean of age (years), study design, intervention (treatment), dose, control, duration of treatment, and main results of the studies.
Table 1.
Summarize the studies included in the systematic review (arranged alphabetically by first author's last name).
| ID | First author, country, year | Target population | Sample size (intervention, control) | Gender (male/female) | Age (mean ± SD) | Study design | Intervention | Dose | Control | Duration | Main results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Antonino Tuttolomondo, Italy, 2016 [28] | Acute ischemic stroke | 42 (22/20) | 23/19 | 66.27 ± 19.34 | Randomized parallel trial | Atorvastatin | 80 mg/day | No treatment | 72 h | CRP ↓ |
| 2 | Antonino Tuttolomondo, Italy, 2016 [28] | Acute ischemic stroke | 42 (22/20) | 23/19 | 66.27 ± 19.34 | Randomized parallel trial | Atorvastatin | 80 mg/day | No treatment | 7 days | CRP ↓ |
| 3 | Christopher Beer, Australia, 2012 [33] | Acute ischemic stroke | 40 (20/20) | NM | 68.6 ± 13.8 | Randomized parallel trial | Atorvastatin | 80 mg/day | Placebo | 3 days | hs-CRP ↔ |
| 4 | Christopher Beer, Australia, 2012 [33] | Acute ischemic stroke | 38 (17/21) | NM | 68.6 ± 13.8 | Randomized parallel trial | Atorvastatin | 80 mg/day | Placebo | 30 days | hs-CRP ↔ |
| 5 | Antonio Muscari, Italy, 2011 [29] | Ischemic stroke | 62 (31/31) | 20/42 | 75.3 ± 11.9 | Double-blind, placebo-controlled, parallel group study | Atorvastatin | 80 mg/day | Placebo | 7 days | CRP ↔ |
| 6 | Xingyu Chen, China, 2018 [31] | Acute ischemic stroke | 117 (60/57) | 73/43 | 61.67 ± 11.67 | Preliminary, randomized controlled | Atorvastatin | 60 mg/day | Atorvastatin 20 mg/day | 7 days | hs-CRP ↓ |
| 7 | Kazuo Kitagawa, Japan, 2017 [27] | Non-cardiogenic ischemic stroke | 1095 (545/550) | 755/340 | 66.2 ± 8.5 | Randomized open-label trial | Pravastatin | 10 mg/day | No treatment | 2 months | hs-CRP ↓ |
| 8 | Jae-Kwan Cha, South Korea, 2004 [35] | Atherosclerotic ischemic stroke | 32 (32/0) | 28/4 | 68.5 | Trial | Simvastatin | 20 mg/day | No control group | 12 weeks | CRP ↓ |
| 9 | Joan Montaner, Spain, 2008 [32] | Cortical stroke | 56 (28/28) | 29/27 | 72.7 ± 12.6 | Pilot, double-blind, randomized, multicenter clinical trial | Simvastatin + aspirin or simvastatin + triflusal | Simvastatin 40 mg/day firs week 20 mg/day until day 90 aspirin 300 mg/day or triflusal 900 mg/day and followed with aspirin 300 mg/day or triflusal 600 mg/day until day 90 | Placebo + aspirin or placebo + triflusal | 90 days | hs-CRP ↔ |
| 10 | A. Vijaya Anand, India, 2009 [34] | Stroke | 95 (35/60) | 64/31 | 60.1 ± 7.4 | Clinical controlled trial | Atorvastatin | 10 mg/day | No treatment | 3 months | CRP ↓ |
| 11 | Guo-jun Cao, China2017 [30] | Cerebral infarction | 120 (60/60) | 65/55 | 46.29 ± 7.48 | Randomized parallel trial | Clopidogrel (75 mg) + rosuvastatin 10 mg/day | 10 mg/day | Clopidogrel (75 mg) + atorvastatin 20 mg/day | 6 months | CRP ↓ |
2.6. Quality Assessment
The quality of the included studies was assessed by two independent researchers (M.B.) and (G.A.) using the Cochrane Collaboration's tool [26]. The following vital parts are included in this tool: random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting. Each item was categorized as low/unclear/high risk of bias. Consequently, if a study had more than two items of low risk, it was classified as a study with good quality. If a study had two items of low risk, it was considered a study with acceptable quality, and if a study had less than two items of low risk of bias, it was considered a study with weak quality [26].
3. Results
3.1. Search Results and Study Selection
A total of 5308 studies were acknowledged during the initial search, 5119 references in Scopus, 115 in PubMed, and 74 studies in the Web of Sciences, of which 32 records were duplicated. After reading the title and abstracts, 5222 irrelevant records were omitted. Full texts of these 54 remained articles were reviewed, and according to our inclusion and exclusion criteria, 45 articles were omitted due to the following reasons: review papers (n = 14), Chinese language (n = 9), working on cardiovascular diseases (n = 8), did not report CRP as an outcome (n = 5), duplicated data (n = 4), retrospective cohort (n = 3), and conference report (n = 2). The results of the search are shown in Figure 1. Thus, data extraction was done on nine articles with 11 arms. The characteristics of each selected paper are shown in Table 1.
Figure 1.
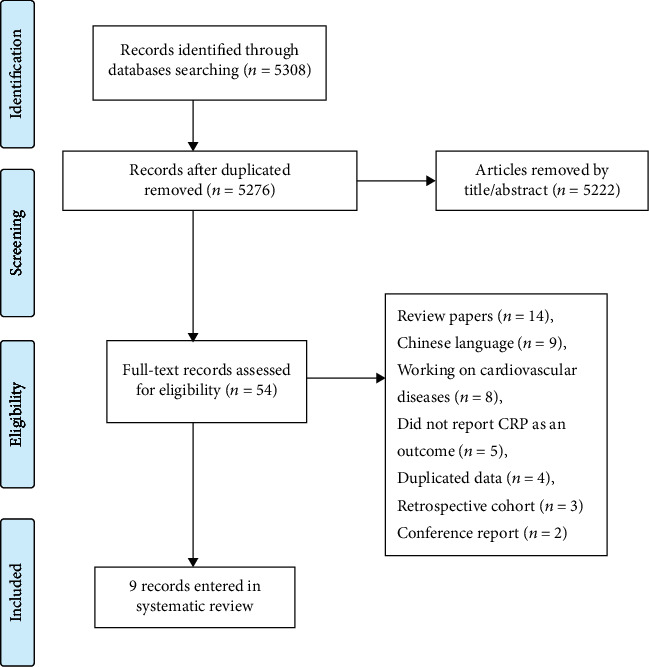
Flow chart of the process of the study selection.
3.2. Characteristics of the Included Studies
Included studies were published between 2004 and 2018 and were conducted in Japan (n = 1) [27], Italy (n = 2) [28, 29], China (n = 2) [30, 31], and Spain (n = 1) [32], and one each from Australia [33], India [34], and South Korea [35]. In total, 1659 participants with a mean age of 46–75 years were allocated to these studies and sample sizes ranged from 32 [35] to 1095 [27]. Eight studies included both men and women, and one study did not report the gender status of participants [33]. The duration of statin intervention ranged between 72 h [28] and six months [30]. Five studies used atorvastatin alone, three at the dose of 80 mg/day [28, 29, 33], one at the dose of 60 mg/day [31], and one at the dose of 10 mg/day [34]. One study used 10 mg/day of pravastatin alone [27], and one study used 20 mg/day of simvastatin alone [35]. Combined therapy was administered in 2 trials. One study used rosuvastatin 10 mg/day plus clopidogrel [30], and in one study, simvastatin, 20 and 40 mg/day, was used in combination with aspirin or triflusal [32]. Finally, five trials measured CRP [28–30, 34, 35] and four trials measured hs-CRP [27, 31–33].
3.3. Main Results
Changes in plasma CRP/hs-CRP concentrations following statin therapy were reported in 11 treatment arms. Data of 5 treatment arms indicated a significant decrease in CRP concentration [28, 30, 34, 35], and one treatment arm CRP concentration did not reveal any significant alteration following statin therapy [29]. Moreover, two treatment arms showed a significant reduction in hs-CRP concentration [27, 31] and the three treatment arms revealed no significant alteration in hs-CRP concentration following statin therapy [32, 33].
When the included studies were arranged according to the type of statin used, CRP concentration in 3 treatment arms was decreased [28, 34], and in one arm, it did not alter after atorvastatin therapy [29]. On the other hand, hs-CRP concentration in one study arm decreased after atorvastatin monotherapy [31]. In 2 treatment arms, alteration in hs-CRP concentration was not significant when single doses of atorvastatin were used [33]. In a different arm, hs-CRP concentration significantly decreased after monotherapy of pravastatin [27]. A significant reduction in CRP concentration was observed after monotherapy of simvastatin in one treatment arm [35], but combination therapy of simvastatin with aspirin or triflusal did not alter the hs-CRP concentration in another study arm [32]. Finally, CRP concentration significantly decreased in one study arm after using rosuvastatin combined with clopidogrel [30].
Overall, statin therapy with lower doses was more effective than higher doses. A significant reduction in CRP/hs-CRP concentrations was observed upon administration of low-dose statin treatments. There was no association between the duration of statin therapy and changes in plasma CRP/hs-CRP concentrations.
3.4. Quality of the Included Studies
As shown in Table 2, 6 out of 9 studies had a high-quality methodological approach [28–33], one study was categorized as a study with acceptable quality [27], and two studies had a weak methodological design [34, 35].
Table 2.
Risk of bias assessment for included clinical trials.
| First author (publication year) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other risks of bias |
|---|---|---|---|---|---|---|---|
| Antonino Tuttolomondo (2016) | L | L | H | H | L | L | U |
| Christopher Beer (2012) | L | L | L | L | U | L | H |
| Antonio Muscari,(2011) | L | L | L | L | L | L | U |
| Xingyu Chen (2018) | L | L | L | L | L | L | L |
| Kazuo Kitagawa (2017) | L | L | H | H | U | U | H |
| Jae-Kwan Cha (2004) | H | H | H | H | H | L | U |
| J. Montaner (2007) | L | L | L | L | L | L | U |
| Vijaya Anand (2009) | H | H | U | U | U | U | U |
| Guo-jun Cao (2017) | L | L | U | U | L | L | U |
L: low risk of bias; H: high risk of bias; U: unclear risk of bias.
4. Discussion
This review is aimed at systematically and comprehensively evaluating the evidence regarding the effect of statin on CRP in patients with stroke to provide the groundwork for future studies. To our knowledge, this systematic review is the first to assess the association between statin therapy and plasma CRP concentrations among patients with stroke. As described in the results, since the population of statin-treated patients and follow-up durations were heterogeneous across included studies, we did not conduct a meta-analysis. However, the main finding of the current qualitative study is that statin therapy in stroke patients is associated with reducing CRP as an acute-phase reactant and sensitive marker of systemic inflammation. Furthermore, the role of inflammation as a triggering factor for increasing blood viscosity, promoting plaque formation, and accelerating atherosclerosis, through CRP, tumor necrosis factor-alpha (TNF-α), and interleukin 6 (IL-6), as nonspecific markers of the acute stage of the systemic inflammatory response, is well established. Thus, there is a complementary relationship between the level of inflammation and atherosclerotic plaque formation [30, 34, 36].
Although the beneficial effects of statins are mediated predominantly by their lipid-lowering effects, recent evidence from clinical trials suggests that statins have anti-inflammatory effects and their benefits may extend beyond their cholesterol-lowering effects that are important for prognosis and treatment in cardiovascular and stroke events [34, 37, 38]. Statin therapy has suggested protecting vascular events through anti-inflammatory activities reflected by CRP reductions [32, 34]. Growing evidence of CRP-reducing effects of statins indicates that this class of medications reduces the risk of cardiovascular events in patients with coronary artery disease [37, 39–41]. In this regard, Ridker et al. demonstrated that statin therapy resulted in a more significant clinical benefit when CRP levels were high and that statins decreased CRP levels in a manner essentially independent of LDL-C levels [42].
It is well documented that the induction of an inflammatory response plays an essential role in the pathogenesis of brain damage. For example, elevated serum levels of CRP in the inflammatory process of atherosclerosis have been widely considered to increase intimal thickness and plaque rupture, resulting in acute cerebral infarction [30, 31]. This phenomenon implies that the onset and development of atherosclerotic lesions could be modulated by reducing inflammation. In this regard, anti-inflammatory therapies are neuroprotective and preventing neuroinflammation may add a better clinical outcome to ischemic stroke [30, 43].
In addition to their effects in reducing cardiovascular risk, there is increasing evidence that prior or early use of statins may reduce the severity of an acute ischemic stroke and improve its outcome [22, 32, 43–46]. It has been shown that statins stabilize atherosclerotic plaque and increase cerebral blood flow [30, 44]. Data from several systematic reviews and meta-analyses support statins' benefit in stroke patients [47–52]. In several trials, statin therapy has been shown to significantly reduce the plasma level of CRP and patients with any stroke history who have low CRP levels after statin therapy have better clinical outcomes than those with higher CRP levels [27, 34, 43, 53, 54].
In the present review, only two studies were designed to directly assess the effects of statins on CRP levels in stroke patients due to their pleiotropic activities [27, 34]. However, the results of these two studies showed a significant reduction in CRP concentrations after statin therapy, confirming the potential anti-inflammatory effects of statins aside from their cholesterol-lowering impact. Moreover, in noncardiogenic ischemic stroke patients, pravastatin decreased the hs-CRP levels and elevated hs-CRP levels were suggested to increase the risk of recurrent stroke and vascular events [27].
In two studies, the effect of statins on the inflammatory markers in stroke patients was evaluated. In a randomized parallel trial, atorvastatin 80 mg/day acutely administered immediately after an atherosclerotic ischemic stroke was found to reduce serum levels of inflammatory markers including CRP, confirming the neuroinflammatory protection of statins [28]. In another trial, atorvastatin and rosuvastatin revealed lipid-lowering and anti-inflammatory effects in stroke patients and reduced CRP levels, although rosuvastatin showed better therapeutic benefits [30]. In addition, in other trials, CRP was also measured after statin usage in stroke patients. These studies showed a decrease in plasma CRP concentration in stroke patients after using statins [31, 35].
In contrast to the above studies, there have also been studies in which statin therapy did not affect the concentrations of CRP in stroke patients. In the study of Montaner and coworkers, assessment of inflammatory markers, including CRP, after using simvastatin did not show any difference in their levels regarding treatment allocation and authors report a nonsignificant increase in mortality and a more significant proportion of infections in the simvastatin group as the primary safety concerns [32]. Moreover, two independent studies demonstrated that administration of atorvastatin 80 mg/day did not affect the concentration of CRP in stroke patients. Muscari and coworkers observed a lack of increase in the CRP level in the atorvastatin group, compared with the significant increase in the placebo group. They conclude that this difference was likely due to the anti-inflammatory effect of atorvastatin [29]. In the second study, atorvastatin prescription for 3 and 30 days did not reduce CRP in stroke patients and did not appear to modify infarct growth substantially [33].
Although some studies did not meet our inclusion criteria, their results concerning statins' effect on CRP levels were interesting. Notably, in two studies, reducing CRP levels following statin therapy was significantly associated with favorable 3-month outcomes and improved patients' survival and readmission rates after acute ischemic stroke [43, 53]. Moreover, in one retrospective study, patients with a history of stroke after prescribing pitavastatin showed a reduced CRP level and potentially limited atherosclerosis in high-risk stroke patients [54]. Furthermore, in the Justification for the Use of Statins in Primary Prevention (JUPITER) trial, a study conducted on healthy subjects without hyperlipidemia and with elevated CRP levels; rosuvastatin 20 mg was administered daily and was shown to decrease both LDL cholesterol and CRP levels, reducing the occurrence of ischemic stroke [55]. In our systematic review, the association between statin therapy and CRP levels did not vary upon the statin type and dose.
The mechanisms by which statins reduce CRP levels in stroke patients are not precisely known. CRP is synthesized mainly by the liver in response to proinflammatory cytokines, particularly IL-6 derived from activated leukocytes, adipose tissue, and in part from the liver [56]. After releasing in the bloodstream, CRP induces upregulation of the vasoconstrictor endothelin-1 and IL-6 by endothelial cells and increases vascular cell adhesion molecule-1, intercellular adhesion molecule-1, E-selectin, and monocyte chemoattractant protein-1 (MCP-1) and thus enhances leukocyte recruitment in an inflammatory process [38]. As depicted in Figure 2, statins possibly reduce IL-6-induced CRP expression in human hepatocytes. At the transcriptional level, statins act on the geranylgeranyl pathway and decrease activation of the transcription factor STAT3, leading to the attenuation of inflammation and neuroprotection effects after stroke.
Figure 2.
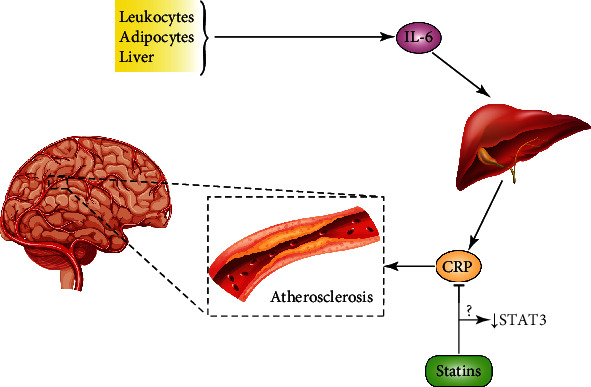
Potential mechanisms leading to statin-induced reduction of CRP release from hepatocytes in stroke. Statins reduce activation of transcription factor STAT3, leading to a decrease of CRP release by hepatocytes. Reduction of the CRP level attenuates inflammatory response and eventually leads to the neuroprotective effects during the stroke.
Several potential limitations to this study should be noted. First, the total number of studies was limited and the trial population size was relatively small. Second, because studies with almost high heterogeneity were included in this study, estimates from these studies could not be reliably combined to conduct a meta-analysis. Finally, this heterogeneity made us interpret our results cautiously. Differences in the duration of treatment, statin dose, control group treatment, and design of studies were some reasons for potential heterogeneity across studies.
Furthermore, various changes in plasma CRP concentrations are dependent on different pharmacokinetic profiles of statins used. Third, we included only studies published in English and might have missed relevant articles in languages other than English. Finally, if the literature search fails to find all relevant reports, the present study is at risk of bias.
5. Conclusion
Our research showed the beneficial impact of statins in patients after a stroke by reducing CRP levels confirming available evidence regarding the potential benefits of statins as anti-inflammatory agents. These findings could propose that statin treatment should be started in patients with stroke, irrespective of their levels of cholesterol; however, well-designed trials in patients with stroke are needed to precisely examine the CRP-reducing benefits of statin therapy in the future, considering potential differences by dosage, duration of use, study population, and other factors.
Acknowledgments
Isfahan University approved this study of Medical Sciences with the grant number 199467 and ethical code IR.MUI.MED.REC.1399.841. There is no financial support for this study.
Data Availability
There is no raw data associated with this systematic review.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
All authors contributed to the conceptualization, drafting, and final editing of the manuscript. All authors approved the final version for submission.
References
- 1.Donkor E. S. Stroke in the 21(st) century: a snapshot of the burden, epidemiology, and quality of life. Stroke Research and Treatment. 2018;2018, article 3238165:1–10. doi: 10.1155/2018/3238165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R. C., Xu W. D., Lei Y. L., et al. The risk of stroke and associated risk factors in a health examination population: a cross-sectional study. Medicine. 2019;98(40, article e17218) doi: 10.1097/MD.0000000000017218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehme A. K., Esenwa C., Elkind M. S. V. Stroke risk factors, genetics, and prevention. Circulation Research. 2017;120(3):472–495. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musuka T. D., Wilton S. B., Traboulsi M., Hill M. D. Diagnosis and management of acute ischemic stroke: speed is critical. CMAJ. 2015;187(12):887–893. doi: 10.1503/cmaj.140355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. Journal of Leukocyte Biology. 2010;87(5):779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anrather J., Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13(4):661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidari B. C-reactive protein and other markers of inflammation in hemodialysis patients. Caspian Journal of Internal Medicine. 2013;4(1):611–616. [PMC free article] [PubMed] [Google Scholar]
- 8.Moon A. R., Choi D. H., Jahng S. Y., et al. High-sensitivity C-reactive protein and mean platelet volume as predictive values after percutaneous coronary intervention for long-term clinical outcomes: a comparable and additive study. Blood Coagulation & Fibrinolysis: An International Journal in Haemostasis and Thrombosis. 2016;27(1):70–76. doi: 10.1097/MBC.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 9.Kapur N. K., Musunuru K. Clinical efficacy and safety of statins in managing cardiovascular risk. Vascular Health and Risk Management. 2008;Volume 4(2):341–353. doi: 10.2147/VHRM.S1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han X., Zhang Y., Yin L., et al. Statin in the treatment of patients with myocardial infarction: a meta-analysis. Medicine. 2018;97(12, article e0167e) doi: 10.1097/MD.0000000000010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toso A., Leoncini M., De Servi S. Statins and myocardial infarction: from secondary ‘prevention’ to early ‘treatment’. Journal of Cardiovascular Medicine. 2019;20(4):220–222. doi: 10.2459/JCM.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 12.Byrne P., Cullinan J., Smith A., Smith S. M. Statins for the primary prevention of cardiovascular disease: an overview of systematic reviews. BMJ Open. 2019;9(4, article e023085) doi: 10.1136/bmjopen-2018-023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afshari A. R., Mollazadeh H., Henney N. C., Jamialahmad T., Sahebkar A. Effects of statins on brain tumors: a review. Seminars in Cancer Biology. 2021;73:116–133. doi: 10.1016/j.semcancer.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Bahrami A., Bo S., Jamialahmadi T., Sahebkar A. Effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors on ageing: molecular mechanisms. Ageing Research Reviews. 2020;58:p. 101024. doi: 10.1016/j.arr.2020.101024. [DOI] [PubMed] [Google Scholar]
- 15.Ferretti G., Bacchetti T., Sahebkar A. Effect of statin therapy on paraoxonase-1 status: a systematic review and meta-analysis of 25 clinical trials. Progress in Lipid Research. 2015;60:50–73. doi: 10.1016/j.plipres.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Mollazadeh H., Tavana E., Fanni G., et al. Effects of statins on mitochondrial pathways. Journal of Cachexia, Sarcopenia and Muscle. 2021;12(2):237–251. doi: 10.1002/jcsm.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiner Ž., Hatamipour M., Banach M., et al. Statins and the Covid-19 main protease: in silico evidence on direct interaction. Archives of Medical Science. 2020;16(3):490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahebkar A., Serban C., Mikhailidis D. P., et al. Association between statin use and plasma d-dimer levels. a systematic review and meta-analysis of randomised controlled trials. Thrombosis and Haemostasis. 2015;114(3):546–557. doi: 10.1160/TH14-11-0937. [DOI] [PubMed] [Google Scholar]
- 19.Sahebkar A., Serban C., Ursoniu S., et al. The impact of statin therapy on plasma levels of von Willebrand factor antigen. Thrombosis and Haemostasis. 2016;115(3):520–532. doi: 10.1160/th15-08-0620. [DOI] [PubMed] [Google Scholar]
- 20.Serban C., Sahebkar A., Ursoniu S., et al. A systematic review and meta-analysis of the effect of statins on plasma asymmetric dimethylarginine concentrations. Scientific Reports. 2015;5(1) doi: 10.1038/srep09902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J., Zhang X., Dong L., Wen Y., Cui L. The many roles of statins in ischemic stroke. Current Neuropharmacology. 2014;12(6):564–574. doi: 10.2174/1570159X12666140923210929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura M., Fukukawa T., Kitagawa K., et al. Ten-year standardization of lipids and high-sensitivity C-reactive protein in a randomized controlled trial to assess the effects of statins on secondary stroke prevention: Japan statin treatment against recurrent stroke. Annals of Clinical Biochemistry. 2018;55(1):128–135. doi: 10.1177/0004563217693651. [DOI] [PubMed] [Google Scholar]
- 23.Joshi P. H., Jacobson T. A. Therapeutic options to further lower C-reactive protein for patients on statin treatment. Current Atherosclerosis Reports. 2010;12(1):34–42. doi: 10.1007/s11883-009-0075-x. [DOI] [PubMed] [Google Scholar]
- 24.Albert M. A., Danielson E., Rifai N., Ridker P. M., Investigators P., Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. Journal of the American Medical Association. 2001;286(1):64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 25.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J. P. Cochrane handbook for systematic reviews of interventions version 5.0.1. The Cochrane Collaboration. 2008. http://www.cochrane-handbook.org.
- 27.Kitagawa K., Hosomi N., Nagai Y., et al. Reduction in high-sensitivity C-reactive protein levels in patients with ischemic stroke by statin treatment: Hs-CRP sub-study in J-STARS. Journal of Atherosclerosis and Thrombosis. 2017;24(10):1039–1047. doi: 10.5551/jat.39354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuttolomondo A., di Raimondo D., Pecoraro R., et al. Early high-dosage atorvastatin treatment improved serum immune-inflammatory markers and functional outcome in acute ischemic strokes classified as large artery atherosclerotic stroke: a randomized trial. Medicine. 2016;95(13):p. e3186. doi: 10.1097/MD.0000000000003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muscari A., Puddu G. M., Santoro N., et al. The atorvastatin during ischemic stroke study: a pilot randomized controlled trial. Clinical Neuropharmacology. 2011;34(4):141–147. doi: 10.1097/WNF.0b013e3182206c2f. [DOI] [PubMed] [Google Scholar]
- 30.Cao G. J., Zhang X. F., Zheng K. D. Effects of atorvastatin and rosuvastatin on blood lipids, platelet aggregation rate and inflammatory factors in patients with cerebral infarction. Tropical Journal of Pharmaceutical Research. 2017;16(10):2507–2513. doi: 10.4314/tjpr.v16i10.26. [DOI] [Google Scholar]
- 31.Chen X., Zhuang X., Peng Z., Yang H., Chen L., Yang Q. Intensive statin therapy for acute ischemic stroke to reduce the number of microemboli: a preliminary, randomized controlled study. European Neurology. 2019;80(3-4):163–170. doi: 10.1159/000494989. [DOI] [PubMed] [Google Scholar]
- 32.Montaner J., Chacón P., Krupinski J., et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. European Journal of Neurology. 2008;15(1):82–90. doi: 10.1111/j.1468-1331.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- 33.Beer C., Blacker D., Bynevelt M., Hankey G. J., Puddey I. B. A randomized placebo controlled trial of early treatment of acute ischemic stroke with atorvastatin and irbesartan. International Journal of Stroke. 2012;7(2):104–111. doi: 10.1111/j.1747-4949.2011.00653.x. [DOI] [PubMed] [Google Scholar]
- 34.Anand A. V., Chandrasek M., Kalavathy S., et al. The influencing aspects of atorvastatin on C-reactive protein and lipid profile in patients with stroke. International Journal of Biological Chemistry. 2008;3(1):30–34. doi: 10.3923/ijbc.2009.30.34. [DOI] [Google Scholar]
- 35.Cha J.-K., Jeong M.-H., Kim J. W. Statin reduce the platelet p-selectin expression in atherosclerotic ischemic stroke. Journal of Thrombosis and Thrombolysis. 2004;18(1):39–42. doi: 10.1007/s11239-004-0172-1. [DOI] [PubMed] [Google Scholar]
- 36.Li J.-J., Chen X.-J. Simvastatin inhibits interleukin-6 release in human monocytes stimulated by C-reactive protein and lipopolysaccharide. Coronary Artery Disease. 2003;14(4):329–334. doi: 10.1097/01.mca.0000078062.22445.60. [DOI] [PubMed] [Google Scholar]
- 37.Hyun M. H., Lee Y., Choi B. G., et al. Roles of achieved levels of low-density lipoprotein cholesterol and high- sensitivity C-reactive protein on cardiovascular outcome in statin therapy. Cardiovascular Therapeutics. 2019;2019:1–10. doi: 10.1155/2019/3824823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnaud C., Burger F., Steffens S., et al. Statins reduce interleukin-6–induced C-reactive protein in human hepatocytes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(6):1231–1236. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- 39.Kang D. O., Park Y., Seo J. H., et al. Time-dependent prognostic effect of high sensitivity C-reactive protein with statin therapy in acute myocardial infarction. Journal of Cardiology. 2019;74(1):74–83. doi: 10.1016/j.jjcc.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Horne B. D., Muhlestein J. B., Carlquist J. F., et al. Statin therapy, lipid levels, C-reactive protein and the survival of patients with angiographically severe coronary artery disease. Journal of the American College of Cardiology. 2000;36(6):1774–1780. doi: 10.1016/S0735-1097(00)00950-5. [DOI] [PubMed] [Google Scholar]
- 41.Ridker P. M., Cannon C. P., Morrow D., et al. C-reactive protein levels and outcomes after statin therapy. New England Journal of Medicine. 2005;352(1):20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 42.Ridker P. M., Rifai N., Clearfield M., et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. New England Journal of Medicine. 2001;344(26):1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 43.Tsai N.-W., Lee L.-H., Huang C.-R., et al. The association of statin therapy and high-sensitivity C‐reactive protein level for predicting clinical outcome in acute non-cardioembolic ischemic stroke. Clinica Chimica Acta. 2012;413(23-24):1861–1865. doi: 10.1016/j.cca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 44.Yaghi S., Elkind M. S. Lipid control and beyond: current and future indications for statin therapy in stroke. Current Treatment Options in Cardiovascular Medicine. 2016;18(4) doi: 10.1007/s11936-016-0448-8. [DOI] [PubMed] [Google Scholar]
- 45.Colivicchi F., Bassi A., Santini M., Caltagirone C. Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke. 2007;38(10):2652–2657. doi: 10.1161/STROKEAHA.107.487017. [DOI] [PubMed] [Google Scholar]
- 46.Chen P. S., Cheng C. L., Kao Yang Y. H., Li Y. H. Statin adherence after ischemic stroke or transient ischemic attack is associated with clinical outcome. Circulation Journal. 2016;80(3):731–737. doi: 10.1253/circj.CJ-15-0753. [DOI] [PubMed] [Google Scholar]
- 47.Hong K.-S., Lee J. S. Statins in acute ischemic stroke: a systematic review. Journal of Stroke. 2015;17(3):282–301. doi: 10.5853/jos.2015.17.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milionis H., Ntaios G., Korompoki E., Vemmos K., Michel P. Statin-based therapy for primary and secondary prevention of ischemic stroke: a meta-analysis and critical overview. International Journal of Stroke. 2020;15(4):377–384. doi: 10.1177/1747493019873594. [DOI] [PubMed] [Google Scholar]
- 49.Christophe B., Karatela M., Sanchez J., Pucci J., Connolly E. S. Statin therapy in ischemic stroke models: a meta-analysis. Translational Stroke Research. 2020;11(4):590–600. doi: 10.1007/s12975-019-00750-7. [DOI] [PubMed] [Google Scholar]
- 50.Eun M.-Y., Jung J.-M., Choi K.-H., Seo W.-K. Statin effects in atrial fibrillation-related stroke: a systematic review and meta-analysis. Frontiers in Neurology. 2020;11 doi: 10.3389/fneur.2020.589684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ní Chróinín D., Asplund K., Åsberg S., et al. Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44(2):448–456. doi: 10.1161/STROKEAHA.112.668277. [DOI] [PubMed] [Google Scholar]
- 52.Squizzato A., Romualdi E., Dentali F., Ageno W. Statins for acute ischemic stroke. The Cochrane database of systematic reviews. 2011;8, article Cd007551 doi: 10.1002/14651858.CD007551.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Arévalo-Lorido J. C., Carretero-Gómez J., Fernández-Recio J. M., et al. Lowering C-reactive protein with statins after an ischemic stroke avoids mortality and readmissions. A prospective cohort study. Annals of Medicine. 2015;47(3):226–232. doi: 10.3109/07853890.2015.1010227. [DOI] [PubMed] [Google Scholar]
- 54.Sugimoto H., Konno S., Nomoto N., et al. The long-term effects of pitavastatin on blood lipids and platelet activation markers in stroke patients: impact of the homocysteine level. PLoS One. 2014;9(11, article e113766) doi: 10.1371/journal.pone.0113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Everett B. M., Glynn R. J., MacFadyen J. G., Ridker P. M. Rosuvastatin in the prevention of stroke among men and women with elevated levels of C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER) Circulation. 2010;121(1):143–150. doi: 10.1161/CIRCULATIONAHA.109.874834. [DOI] [PubMed] [Google Scholar]
- 56.Sucajtys-Szulc E., Debska-Slizien A., Rutkowski B., et al. Hepatocyte nuclear factors as possible C-reactive protein transcriptional inducer in the liver and white adipose tissue of rats with experimental chronic renal failure. Molecular and Cellular Biochemistry. 2018;446(1-2):11–23. doi: 10.1007/s11010-018-3268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no raw data associated with this systematic review.


