Abstract
Cerebral ischemia is a common global disease that is characterized by a loss of neurological function and a poor prognosis in many patients. However, only a limited number of treatments are available for this condition at present. Given that the efficacies of these treatments tend to be poor, cerebral ischemia can create a significant burden on patients, families, and society. Mesenchymal stem cell (MSC) transplantation treatment has shown significant potential in animal models of ischemic stroke; however, the specific mechanisms underlying this effect have yet to be elucidated. Furthermore, clinical trials have yet to yield promising results. Consequently, there is an urgent need to identify new methods to improve the efficiency of MSC transplantation as an optimal treatment for ischemic stroke. In this review, we provide an overview of recent scientific reports concerning novel strategies that promote MSC transplantation as an effective therapeutic approach, including physical approaches, chemical agents, traditional Chinese medicines and extracts, and genetic modification. Our analyses showed that two key factors need to be considered if we are to improve the efficacy of MSC transplantation treatments: survival ability and homing ability. We also highlight the importance of other significant mechanisms, including the enhanced activation of MSCs to promote neurogenesis and angiogenesis, and the regulation of permeability in the blood-brain barrier. Further in-depth investigations of the specific mechanisms underlying MSC transplantation treatment will help us to identify effective methods that improve the efficiency of MSC transplantation for ischemic stroke. The development of safer and more effective methods will facilitate the application of MSC transplantation as a promising adjuvant therapy for the treatment of poststroke brain damage.
1. Introduction
Ischemic stroke is a major global disease that is associated with high incidence and mortality rates. The most common cause of ischemic stroke is a blockage of the cerebral vessels; this often leads to disability, thus placing a huge socioeconomic burden on patients, families, and society [1]. Thrombolytic therapy with recombinant tissue plasminogen activator (rt-PA) is the only treatment approved by the United States-food and drug administration (FDA) for acute ischemic stroke; however, this drug is not widely used clinically due to its restricted time window of efficacy (4.5 hours) and unpredictable complications [2, 3]. Therefore, there is an urgent need to identify novel treatment options to address the limitations associated with current treatment strategies for ischemic stroke [4, 5].
Cell transplantation treatments have shown great potential in animal models to improve the sequelae of neurological diseases. In particular, stem cell transplantation therapy has become a major area of research over recent years. Studies have found that stem cells are immortal and can proliferate into neurons and glial cells when used to treat diseases of the central nervous system, thereby replacing necrotic cells and exerting neuroprotective effects [6]. Mesenchymal stem cells (MSCs) have become the most widely used form of stem cells in biological medical research, largely because of their neuroprotective effects on ischemic stroke in animal models [7–9].
MSCs are multipotent adult stem cells that can self-renew and can be found in a variety of tissues, including bone marrow, adipose tissue, and the umbilical cord [10, 11]. The ease with which MSCs can be isolated from a diverse range of tissues is one of the main advantages of MSC-based therapies. Compared with other stem cell-based therapies, MSCs have several unique advantages. MSCs possess the ability to migrate to an area of damage and can be transplanted before differentiating into mature cells [12, 13]. Furthermore, MSCs with “plastic” immune properties (immunostimulatory and immunosuppressive) can be used in a wider range of applications [14]. Given their ability to cross allogeneic barriers, MSCs may become an “off-the-shelf” stem cell that could be used in certain emergency clinical situations [14]. Furthermore, a considerable body of evidence now supports the fact that MSCs can improve recovery by enhancing angiogenesis and neurogenesis and by inducing immunomodulatory and anti-inflammatory effects [15]. In addition, the paracrine actions of MSCs are known to promote functional recovery via both direct and indirect effects [15, 16].
However, stem cell therapy for transplantation is associated with poor efficacy. Recent studies have shown that after the transplantation of stem cells, only a small number of stem cells reach the damaged area; furthermore, these cells tend to disappear quickly. This indicates that the efficacy of this method is compromised by the poor survival rates of the donor cells [17–19]. In this review, we summarize research progress in in the neuroprotective approaches that can be used to improve the efficiency of MSC transplantation for ischemic stroke.
1.1. The Immunomodulatory Effects of MSCs
Inflammatory signaling is involved in all stages of cerebral ischemia, from the early damaging events triggered by vascular occlusion to the late regenerative processes underlying postischemic tissue repair [20]. Ischemic stroke induces a strong inflammatory response, prompting a large number of leukocytes to accumulate in the ischemic infarct area [20]. In contrast to other types of stem cells, MSCs possess the capability to mediate the immune response. When MSCs enter an inflamed brain, they can be become anti-inflammatory cells by reducing the secretion of tumor necrosis factor alpha (TNF-α) and by increasing the secretion of interleukin-10 (IL-10) [21]. The transplantation of MSCs was found to reduce infarct size and improve functional deficits in a mouse model of middle cerebral artery occlusion (MCAO) by immunomodulating the expression of the IL-23/IL-17 axis [22]. Moreover, the transplantation of MSCs has also been shown to suppress inflammatory responses (by reducing the levels of IL-1β, IL-6, and TNF-α) and neuronal apoptosis during the early stages of focal cerebral ischemia in rabbits [23]. Li et al. confirmed that MSCs restrain astroglial activity in the peri-ischemic area and increase the expression levels of IL-10 to inhibit ischemic injury and improve neurological function [24]. The implantation of MSCs into the injured brain enhances neuroprotection by activating the activity of NF-kappaB (NF-κB) in resident stem cells, thus leading to an increase in IL-6 production and a decrease in apoptosis [25]. In addition to regulating the expression of cytokines to induce immunosuppression, MSCs can also modulate the immune response by inhibiting B cells [26, 27]. Moreover, inflammatory cell (T cell) proliferation was found to be reduced after coculture with MSCs in vitro, thus suggesting that the nitric oxide (NO) produced by MSCs is one of the major mediators of T cell suppression [28].
1.2. The Secretion of Paracrine Factors by MSCs
MSCs have the potential to differentiate into adipocytes, osteoblasts, chondrocytes, and neurons [29]. Furthermore, transplanted MSCs have been shown to migrate to the infarct zone and differentiate into neuronal, glial, and endothelial cells to enhance neuroplasticity [30]. Moreover, the paracrine action of MSCs has been shown to induce regenerative processes by increasing the level of growth factors or receptors [31]. MSC treatment also increases the expression of stromal cell-derived factor-1 (SDF-1), vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), and growth-associated protein-43 (GAP-43), in the peri-infarct region [32].
Furthermore, intravenously transplanted MSCs are known to induce functional improvement and reduce infarct volume in ischemic rats, possibly by providing insulin-like growth factor 1 (IGF-1), inducing vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and basic fibroblast growth factor (bFGF), neurotrophic factors in the host brain [33]. IGF-1 plays an important role in neurological recovery by promoting neurogenesis; MSC transplantation has also been shown to increase the expression levels of IGF-1 and IGF-1 receptors in ischemic brain tissue [33, 34]. These bioactive factors promote functional recovery after stroke in synergistic manner. In addition, the administration of MSCs increases endogenous brain bone morphogenetic protein 2/4 and connexin-43 expression in astrocytes and promotes the expression of synaptophysin; these factors facilitate functional recovery in rats following stroke [35]. Collectively, these data may indicate that the endocrine effects of MSCs may mitigate difficulties in sending sufficient numbers of MSCs to the ischemic infarct area. This may suggest that the endocrine effects of MSCs mitigate the typical difficulties faced in enabling sufficient numbers of MSCs to reach the ischemic infarct area.
2. Physical Approaches to Improve the Efficiency of MSC Transplantation
Oxygen is an essential element for cellular homeostasis; hypoxia has been shown to be involved in the pathological processes of many diseases associated with high morbidity and mortality rates [36]. Bone marrow, one of the most common sources of MSCs, is a hypoxic environment with an oxygen tension of approximately 1% to 7% [37]. Hypoxic pretreatment is a simpler and easier approach than other methods such as genetic modification. Many studies have found that moderate hypoxia preconditioning can improve the cellular activities of MSCs [38]. In a rat model of ischemic stroke, hypoxic preconditioning of bone marrow MSCs (BMSCs) improved their ability to promote cell homing, neuronal differentiation and regeneration, and the recovery of neuronal function via CXCL12/CXC chemokine receptor type 4 (CXCR4) signaling [39–41]. Moreover, experiments involving hypoxic-preconditioned BMSCs in a rat model of MCAO demonstrated the upregulation of hypoxia-inducible factor-1α (HIF-1α) and a number of trophic/growth factors (e.g., BDNF, VEGF, and glial cell-derived neurotrophic factor [GDNF]) and the downregulation of proinflammatory cytokines/chemokines [40]. Further research confirmed that ischemic rat cortical neurons could be rescued by coculturing them with hypoxic-preconditioned BMSCs [42]. In addition, hypoxic preconditioning also reduced the extent of apoptosis in BMSCs during ischemia–reperfusion (I/R) injury by inhibiting caspase-3 activation and increasing the expression of HIF-1α [41]. In a rat model of cardiac arrest-induced global cerebral ischemia, hypoxia was shown to promote the migration and integration of MSCs, rescued a greater number of neurons, and suppressed inflammation in the ischemic brain by activating the phosphoinositide-3 kinase (PI3K)/AKT and HIF-1α/CXCR4 pathways [43]. Furthermore, granulocyte colony-stimulating factor (G-CSF) improved the efficiency of hypoxic preconditioning in promoting the survival and migration of canine BMSCs, thus indicating that a combination of the two interventions (G-CSF and hypoxic preconditioning) may offer a novel strategy for improving the efficiency of MSC transplantation [44]. Collectively, this evidence indicates that hypoxic preconditioning improves the homing ability (via CXCL12/CXCR4 signaling) and survival ability (antiapoptotic and anti-inflammatory properties) of transplanted MSCs, thus helping MSCs to fulfill their therapeutic function in a more efficient manner.
Other physical strategies that improve the homing ability of MSCs have also been reported. Ultrasound-targeted microbubble destruction can disrupt biological barriers and increase vascular permeability, including the blood-brain barrier (BBB) [45]; this technique may help more transplanted cells to migrate to the injured brain area. Qian et al. found that ultrasound-targeted microbubbles enhanced the homing effect of BMSCs during the application of transplantation to treat ischemic stroke [46]. This improved level of homing led to the amelioration of cerebral edema, a reduction in infarct size, and an improved neurological function score in a rat model of MCAO. Moreover, the application of ultrasound microvesicles to assist MSC infusion facilitated the homing of BMSCs to the cerebral infarct and reduced the infarct volume by upregulating VEGF expression and by modulating apoptosis [47].
Improving MSCs so that they can adapt and survive better in the harsh environment of the ischemic brain would increase the efficiency of MSC transplantation treatment. A previous study showed that the short-term preconditioning of human mesenchymal stem cells (hMSCs) via three-dimensional (3D) aggregation promoted resistance to oxidative stress while also restoring energy homeostasis and innate cellular properties [48]. These effects were partly mediated via regulation of the PI3K/AKT pathway and demonstrated that preconditioning improves the therapeutic efficacy of hMSCs in the treatment of ischemic stroke. In another study, Paudyal et al. reported that p5, a 24-residue peptide derived from p35, offers protection to neurons and endothelial cells in vitro [49]. These authors administered human adipose-derived mesenchymal stem cell- (hADMSC-) loaded p5 peptide in a rat model of focal cerebral ischemia; they found that this promoted the survival of transplanted hADMSCs and enhanced neurological recovery. The main mechanisms of action underlying the physical approaches used to improve the efficiency of MSC transplantation are summarized in Table 1 and Figure 1.
Table 1.
Recent studies reporting physical approaches that can improve the efficiency of MSC transplantation in ischemic stroke models.
| Physical approaches | Possible mechanisms | Animal model | Source of MSC | Research time | References |
|---|---|---|---|---|---|
| Hypoxic pretreatment | Promoting cell homing by CXCL12/CXCR4 signaling pathway. | Rat cerebral infarction model | Bone marrow (rat) | 2019 | Hu et al. [39] |
| Hypoxic pretreatment | Promoting the migration and integration of MSCs by PI3K/AKT and HIF-1α/CXCR4 pathways | Rat cardiac arrest-induced global cerebral ischemia model | Bone marrow (rat) | 2017 | Wang et al. [43] |
| Hypoxic pretreatment | Improving survival and inhibiting apoptosis by inhibiting of caspase-3 activation and increasing expression of HIF-1α | Rat cerebral infarction model | Bone marrow (rat) | 2017 | Chen et al. [41] |
| Hypoxia/reoxygenation pretreatment | Promoting cell migration and increasing their ability to rescue ischemic cortical neurons. | Cell model of cerebral ischemia | Bone marrow | 2015 | Kim et al. [42] |
| Hypoxic pretreatment | Promoting the expression of trophic/growth factors and improving regenerative responses. | Rat cerebral infarction model | Bone marrow (rat) | 2012 | Wei et al. [40] |
| p5 peptide loaded | Promoting survival of transplanted MSCs | Rat cerebral infarction model | Adipose (human) | 2020 | Paudyal et al. [49] |
| Three-dimensional aggregation | Promoting the migration and resistance to oxidative stress by PI3K/AKT pathway | Rat cerebral infarction model | Bone marrow (human) | 2019 | Yuan et al. [48] |
| Ultrasound-targeted microbubble | Promoting the migration of MSCs | Rat cerebral infarction model | Bone marrow (rat) | 2019 | Qian et al. [46] |
| Ultrasound microvesicle | Promoting the migration of MSCs | Rat cerebral infarction model | Bone marrow (rat) | 2017 | Chang et al. [47] |
Figure 1.
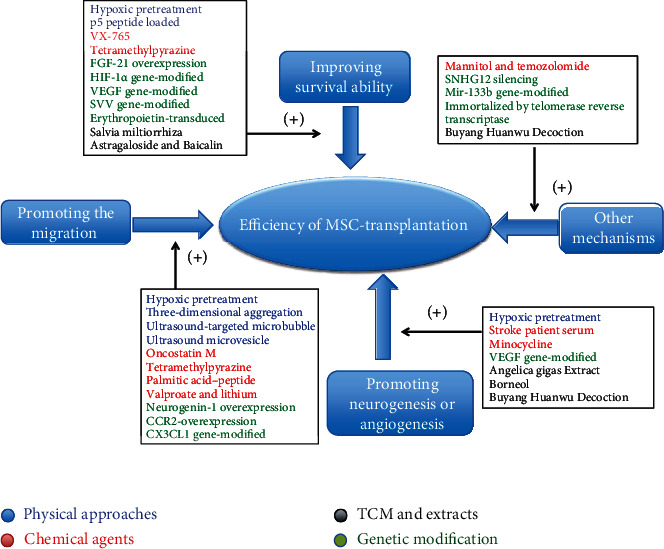
Possible main mechanisms of strategies to improve efficiency of MSC transplantation in ischemic stroke models.
3. The Use of Chemical Agents to Improve the Efficiency of MSC Transplantation
The use of chemical agents to increase the transplantation efficiency of MSCs has also become a significant research topic. Tetramethylpyrazine (TMP) is a biologically active alkaloid that is extracted from the rhizome of the Chinese herb Rhizoma Chuanxiong [50]. Owing to its antioxidant, antiapoptotic, and anti-inflammatory properties, TMP has become a widely accepted therapeutic agent for cardiovascular and cerebrovascular diseases in China [50, 51]. In a series of in vitro experiments, TMP was shown to improve the survival of BMSCs against H2O2-induced apoptosis by regulating the PI3K/AKT and extracellular regulated protein kinases1/2 (ERK1/2) signaling pathways [52]. Furthermore, in a rat model of ischemic stroke, BMSCs combined with TMP promoted the homing of BMSCs towards ischemic areas by upregulating the expression of SDF-1 and CXCR4. In addition, this combined treatment increased the secretion of nerve growth factors (BDNF and VEGF) and promoted angiogenesis and neurogenesis, thus leading to improved functional recovery after cerebral ischemia [53].
Increased transplantation treatment efficiency requires more transplanted cells to survive and function in a harsh environment. Previous studies have shown that VX-765, a novel small molecule caspase-1 inhibitor, is able to suppress inflammation by passing the BBB [54]. In addition, this drug has been used clinically in a phase IIa randomized, double-blind, multicenter, placebo-controlled trial to treat epilepsy [55]. Furthermore, an in-depth study by Sun et al. demonstrated that the pretreatment of human umbilical cord mesenchymal stem cells (HUMSCs) with VX-765 improved cell survival during transplantation treatment by upregulating autophagy via the AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signaling pathway, thus ameliorating inflammatory responses, inhibiting apoptosis, and reducing infarct volume in HUMSC-transplanted models of stroke [56]. These researchers suggested that pretreatment with VX-765 may become a novel method for improving the efficacy of MSC-based regenerative therapies for cerebral ischemia. Minocycline, a second-generation tetracycline, is known to exert a neuroprotective effect on ischemic stroke by inhibiting the activity of matrix metalloproteinase [57]. Recent experiments confirmed that a therapeutic treatment that combined human bone marrow-derived MSCs (hBMSCs) and minocycline promoted neurological recovery and reduced infarct volume, possibly by upregulating neuronal nuclear antigen (NeuN) and VEGF at the boundary of the ischemic area [58]. These results suggested that minocycline may become an adjuvant therapy for improving the efficiency of transplantation treatment.
Improving the ability of MSCs to migrate is still the key approach for improving their transplantation efficiency. The suppression of matrix metalloproteinase-9 (MMP-9) was reported to attenuate the ability of stem cells to migrate to the infarcted brain [59]. In contrast, priming with valproate and/or lithium was shown to improve the migration ability of MSCs by activating CXCR4 and MMP-9 [60]. In turn, this improved the efficiency of transplantation treatment by reducing infarct volume, enhancing angiogenesis, and by improving functional recovery in a rat model of cerebral ischemia. Both valproate and lithium have been used to treat bipolar disorder [61, 62]; additional supporting evidence will help to further confirm the importance of these factors to MSC transplantation treatment for ischemic stroke. Oncostatin M (OSM), a member of the IL-6 cytokine family [63], has been shown to bind to specific receptor complexes and activate particular signaling pathways to regulate downstream events [63]. In addition, OSM preconditioning has been shown to improve MSC migration, possibly by enhancing the secretion of growth factors and cytokines [64, 65]. In a recent study, a combined treatment featuring OSM and BMSCs was shown to upregulate SDF-1 to improve BMSC migration and neurofunctional recovery in a rat model of ischemic stroke by promoting the expression of VEGF and MMP-2 and by reducing the expression of inflammatory mediators [66]. In addition, researchers have shown that painting cell membranes with palmitic acid-peptide enhanced the targeted migration of MSCs to ischemic brain tissue and reduced the accumulation of cells in peripheral organs, thus leading to improved therapeutic efficiency [67]. These results indicate that modification of the cell surface with targeting peptides may offer a novel approach to increase the efficacy of MSCs in ameliorating ischemic brain injury.
Adjusting the permeability of the BBB is a novel method to help MSCs reach injured areas of brain tissue. Using a rat model of MCAO, researchers have shown that the combination of mannitol and temozolomide improved the efficacy of human umbilical cord-derived MSCs (hUC-MSCs) in the treatment of chronic cerebral ischemia and ameliorated behavioral deficits, possibly by improving neural regeneration and angiogenesis [68]. The principal mechanism underlying the effects induced by this combined drug treatment is likely to involve increased BBB permeability. However, the increased level of permeability in the BBB that results from ischemic damage during acute ischemic stroke is commonly considered to be the primary cause of hemorrhagic transformation and is associated with a worse outcome [69, 70]. Therefore, increasing BBB permeability could be a controversial way of overcoming obstacles to the clinical application of MSCs in patients with acute ischemic stroke.
Activating MSCs to promote neurogenesis and angiogenesis more effectively is another novel approach that could be deployed to improve the efficiency of transplantation treatment. Bone marrow stromal cells that are isolated from rat models of stroke are superior to those from normal rats when used for the transplantation treatment of cerebral ischemia; this may be due to the enhanced expression of trophic factors and the increased angiogenic characteristics of BMSCs from the rat model of stroke [71]. These findings suggest that serum obtained from the rat model of stroke activates allogeneic MSCs. Moon et al. demonstrated that culture methods for MSCs using serum acquired from stroke patients were able to improve the behavioral outcome in a rat model of stoke stroke by promoting neurogenesis and angiogenesis [72]. These effects may have been mediated by the increased expression levels of VEGF, glial cell-derived neurotrophic factor (GCNF), and fibroblast growth factor (FGF), in MSCs that were cultured with serum from stroke patients. Furthermore, another study found that transplanting a plasma-derived scaffold combined with BMSCs into the cystic cavity led to significantly reduced atrophy volume and improved motor function in a rat model of MCAO, as compared with rats treated with a vehicle, the scaffold, or BMSCs only [73]. These results suggest that components of the blood (such as the plasma-derived scaffold and serum from stroke patients) may offer a promising means to improve the efficiency of MSC transplantation in the treatment of cerebral infarction. The main mechanisms of action for the chemical agents described herein are summarized in Table 2 and Figure 1.
Table 2.
Recent studies reporting chemical agents that can improve the efficiency of MSC transplantation in ischemic stroke models.
| Chemical agents | Possible mechanisms | Animal model | Source of MSC | Research time | References |
|---|---|---|---|---|---|
| VX-765 | Improving the cell survival by upregulating of autophagy via AMPK/mTOR | Rat cerebral infarction model | Umbilical cord (human) | 2020 | Sun et al. [56] |
| Oncostatin M | Promoting migration ability of MSCs by upregulating SDF-1 | Rat cerebral infarction model | Bone marrow (rat) | 2019 | Han et al. [66] |
| Tetramethylpyrazine | Promoting cell homing by CXCL12/CXCR4 signaling pathway. | Rat cerebral infarction model | Bone marrow (rat) | 2019 | Li et al. [53] |
| Minocycline | Upregulating NeuN and VEGF in ischemic area boundary | Rat cerebral infarction model | Bone marrow (human) | 2018 | Cho et al. [58] |
| Mannitol and temozolomide | Increasing the BBB permeability | Rat cerebral infarction model | Umbilical cord (human) | 2018 | Choi et al. [68] |
| Stroke patient serum | Promoting neurogenesis and angiogenesis | Rat cerebral infarction model | Bone marrow (human) | 2018 | Moon et al. [72] |
| Palmitic acid-peptide | Promoting migration ability of MSCs | Rat cerebral infarction model | Bone marrow (rat) | 2017 | Huang et al. [67] |
| Tetramethylpyrazine | Promoting survival of MSCs against H2O2-induced apoptosis by PI3K/AKT and ERK1/2 pathways | Cell model of cerebral ischemia | Bone marrow (rat) | 2017 | Fang et al. [52] |
| Valproate and lithium | Promoting cell homing and migration ability by activating CXCL12/CXCR4 and MMP9 | Rat cerebral infarction model | Not mentioned | 2011 | Tsai et al. [60] |
4. Traditional Chinese Medicine and Extracts for Improving the Efficiency of MSC Transplantation
Traditional Chinese medicine (TCM) has been widely used in China for treating a variety of diseases. TCM preparations (Chinese materia medica) and their active compounds have been shown to ameliorate brain damage induced by ischemia [74]. Over recent years, several studies have investigated the beneficial effects of TCM with regard to the efficiency of MSC transplantation treatment for cerebral infarction.
In addition to playing a protective role in ischemic stroke, Buyang Huanwu decoction (BHD) has also been shown to protect the BBB by inactivating glycogen synthase kinase 3 (GSK-3) and Tau [75, 76]. The combination of BHD and MSC transplantation was also shown to be more effective at treating injured blood vessels and ischemic tissues and acted via the upregulation of VEGF and Ki-67 expression [77]. Furthermore, BHD is known to augment angio-miRNA in MSC-secreted exosomes in vitro and promote angiogenesis, as observed in a rat model of ischemic stroke [78]. Collectively, these results suggest that the combination of BHD and MSCs could represent a useful adjuvant therapy for ischemic stroke.
Salvia miltiorrhiza (SM) is a popular plant that is widely used in TCM for the treatment of various diseases. SM was recently shown to ameliorate ischemic brain damage, possibly via multiple processes including antiplatelet aggregation, and both anti-inflammatory and antioxidative effects [79]. In a study involving the treatment of cerebral infarction with MSC transplantation, SM was shown to promote the antiapoptotic ability and survival of MSCs, improve transplantation efficiency, and significantly improve recovery from ischemic stroke [80]. Astragaloside, the main active substance of Astragalus, has also been shown to improve the antiapoptotic and anti-inflammatory abilities of MSCs under inflammatory conditions when combined with baicalin [81]. Furthermore, the combination of astragaloside IV and tanshinone IIA was shown to improve the migration of MSCs and promote the homing of MSCs in vitro, possibly via the modulation of CXCR4 expression [82].
In addition, the combination of borneol and MSC transplantation has been shown to effectively suppress apoptosis, reduce infarct volume, and promote neurogenesis, thus providing a neuroprotective effect during functional recovery in a rat model of MCAO [83]. Angelica gigas (AG) is a widely used herbal medicine; the extract of AG has been shown to exert a neuroprotective effect on ischemic stroke-related injury in the brain [84]. In a rat model of MCAO, Kim et al. found that AG extract and MSCs act synergistically to increase the overall therapeutic effect by enhancing neovascularization [85].
Collectively, these results suggest that the addition of TCM enhances the efficiency of MSC transplantation treatment by enhancing the homing ability or survival of the transplanted MSCs. However, the putative ability of TCM to improve the therapeutic efficacy of MSCs still needs to be supported by further evidence. Although these TCM treatments still require more in vivo data to confirm their efficacy, they are expected to become novel and effective methods for improving the efficiency of MSC transplantation in the treatment of ischemic stroke. The main mechanisms of action related to these TCM approaches are summarized in Table 3 and Figure 1.
Table 3.
Recent studies reporting TCM and extracts that can improve the efficiency of MSC transplantation in ischemic stroke models.
| Drug name | Extraction source | Possible mechanisms | Animal model | Source of MSC | Research time | References |
|---|---|---|---|---|---|---|
| Salvia miltiorrhiza | Salvia miltiorrhiza Bunge | Promoting the antiapoptotic ability and survival of MSCs | Rat cerebral infarction model | Bone marrow (rat) | 2018 | Kim et al. [80] |
| Angelica gigas extract | Angelica gigas | Enhancing neovascularization | Rat cerebral infarction model | Bone marrow (rat) | 2018 | Kim et al. [85] |
| Borneol | Artemisia and Dipterocarpaceae | Suppressing apoptosis and promoting neurogenesis | Mouse cerebral infarction model | Fetal mice | 2017 | Zhang et al. [83] |
| Astragaloside and baicalin | Improving the antiapoptosis and anti-inflammatory ability of MSCs by MAPK/ERK pathway | Cell inflammatory model induced by lipopolysaccharide | Bone marrow (mice) | 2017 | Zhu et al. [81] | |
| Buyang Huanwu decoction | TCM prescription | Augmenting angio-miRNA in MSC-secreted exosomes | Rat cerebral infarction model | Not mentioned | 2015 | Yang et al. [78] |
| Buyang Huanwu decoction | TCM prescription | Upregulating VEGF and Ki-67 | Rat cerebral infarction model | Bone marrow (rat) | 2010 | Zhang et al. [77] |
5. Genetic Modification Approaches to Improve the Efficiency of MSC Transplantation
Improving the homing ability of MSCs is an important key goal of gene editing for transplantation treatment. Neural-induced MSCs have been shown to be more effective than parental MSCs with regard to neurological recovery in a rat model of MCAO [86]. Moreover, neural induction via a retroviral vector expressing the neurogenic transcription factor neurogenin-1 (Ngn1) was shown to increase the homing ability of MSCs and improve their engraftment efficiency in a rat model of MCAO [87]. These results suggest that the neural induction of MSCs by genetic modification may provide a novel method for improving the efficiency of MSC transplantation. Fractalkine (FKN), also known as CX3CL1, is a chemokine that is constitutively expressed in the brain [88]. Zhang et al. found that the FKN–CX3CR1 chemokine axis could activate Jak2–Stat5–ERK1/2 signaling and improve the directional migration of MSCs towards ischemic cerebral lesions [89]. These results indicate that the combination of gene-modified MSCs with chemokines could provide a novel approach to improve the efficiency of MSC transplantation treatment. Recent experimental evidence now supports the fact that the overexpression of CCR2 can enhance the targeted migration of MSCs to ischemic brain tissue and improve their therapeutic efficiency by reducing ischemic lesions and improving neurological recovery [90]. These effects have been attributed to alleviated inflammatory infiltration and PRDX4-mediated antioxidant effects that mediate BBB preservation.
Activating MSCs to resist harsh environments more effectively and to exhibit enhanced levels of functionality is the key aim of genetically modifying MSCs. Hypoxia-inducible factor 1 (HIF-1) is an important transcription factor that plays a key role in the cellular response to hypoxic environments [91]. HIF-1α, a subunit of HIF-1, modulates several target genes related to angiogenesis, energy metabolism, apoptosis, autophagy, and other adaptive responses to hypoxia [92, 93]. The transplantation of BMSCs stably expressing mutant HIF-1α (P564A and N803A) was found to efficiently promote functional recovery and reduce infarct volume in a rat model of MCAO, possibly by increasing the expression levels of VEGF [94]. Therefore, the mutation of HIF-1α may represent a novel target for improving the treatment of stroke with MSC transplantation. Moreover, Lv et al. used MSCs that had been modified with the HIF-1α gene and reported that the overexpression of HIF-1α protects MSCs against oxygen-glucose deprivation- (OGD-) induced injury by promoting cell viability, suppressing apoptosis, and by activating autophagy-related signaling pathways (PI3K/AKT/mTOR); these effects were reversed by knocking down HIF-1α [95, 96]. Further experiments, using a rat model of MCAO, showed that the overexpression of HIF-1α in MSCs reduced infarct volume and improved the neurobehavioral outcome when compared with control MSCs; these effects occurred by the inhibition of proinflammatory cytokines and the promotion of autophagy and neurotrophin secretion [95].
FGF-21, a metabolic regulator involved in the regulation of gluconeogenesis and lipid metabolism, has been shown to protect cells from the excitotoxicity induced by ischemic stroke [97, 98]. Linares et al. found that the overexpression of FGF-21 improved the survival of transplanted MSCs and reduced apoptosis under the harsh microenvironment associated with oxidative stress [99]. However, more experimental data are required to confirm that FGF-21 improves the efficiency of stroke treatment involving the transplantation of MSCs. Survivin (SVV), an inhibitor of apoptosis, has been shown to play a protective role in many cellular metabolic activities [100, 101]. Furthermore, SVV-engineered MSCs were found to not only improve cardiac performance in a rat model of myocardial infarction [102], but also promoted the survival of MSCs during transplantation treatment for stroke [103]. These authors also found that, when compared with parental MSCs, SVV gene-modified MSCs improved the functional recovery of a rat model of MCAO and reduced cerebral infarct volume. In vitro experiments further suggested that these effects were mediated by the increased secretion of protective cytokines (VEGF and bFGF) from the gene-modified MSCs [103]. Erythropoietin (EPO) is a type of glycoprotein hormone that stimulates and promotes the production of red blood cells. EPO may exert a neuroprotective effect on ischemic stroke and protect the BBB from ischemic damage by increasing the expression of p-Connexin43 [104, 105]. Further experiments have demonstrated that MSCs transfected with the EPO gene are able to continuously secrete EPO and various neurotrophic factors, thus improving the viability of neural cells and transplanted MSCs [106]. Furthermore, the implantation of EPO-MSCs was found to improve neurological deficits and reduce infarct volume in a rat model of MCAO [106].
In addition, approaches to editing telomerase and RNA have also become significant research topics in the application of MSC transplantation. As the telomeres in hMSCs gradually become shorter during the process of continuous replication, the ability of hMSCs to self-renew is limited; this may affect the stability of the genome and the metabolic function of the hMSCs [107]. Li et al. attempted to immortalize hMSCs using a human telomerase reverse transcriptase and found that immortalized cells performed better during the treatment of transient cerebral ischemia than control hMSCs; the immortalized hMSCs reduced apoptosis in brain cells, reduced infarct volume and brain edema, and improved neurological recovery [108]. MicroRNAs (miRNAs) are a form of single-stranded and noncoding RNA that exert a crucial regulatory effect on molecular processes following ischemic stroke [109, 110]. Moreover, a large number of miRNAs have been shown to regulate the migration of MSCs in animal models of a variety of diseases; these effects occur via a range of mediatory mechanisms including cytokine–receptor interaction, cytoskeleton remodeling, intracellular signaling, and MSC-derived exosomes [111]. The treatment of a rat model of MCAO with miR-133b-positive MSCs was shown to improve functional recovery when compared with rats that received treatment with naive MSCs, while also promoting axonal plasticity and neurite remodeling in the ischemic boundary zone; these effects were reversed by the administration of miR-133b-negative MSCs [112]. The mechanism underlying these effects may relate to the mediation of miR-133b transfer to astrocytes and neurons via exosomes from the MSCs. Long noncoding RNAs (lncRNAs), a class of noncoding mRNA transcripts that are >200 nucleotides in length and do not encode proteins [113], have also become a significant focus of research with regard to the use of gene editing approaches to improve the efficiency of MSC transplantation for the treatment of stroke. Li et al. conducted a series of experiments using lncRNAs and found that the silencing of SNHG12 in MSCs promoted cell proliferation and reduced apoptosis in cocultured brain microvascular endothelial cells; these effects occurred via the activation of the PI3K/AKT/mTOR signaling pathway [114]. Furthermore, compared with control MSCs, SNHG12-silenced MSCs reduced the infarct volume and significantly reduced apoptosis in a rat model of MCAO [114]. The main mechanisms of action involved in these genetic modification approaches are summarized in Table 4 and Figure 1.
Table 4.
Recent studies reporting genetic modification approaches that can improve the efficiency of MSC transplantation in ischemic stroke models.
| Genetic modification approaches | Possible mechanisms | Animal model | Source of MSC | Research time | References |
|---|---|---|---|---|---|
| FGF-21 overexpression | Improving the survival of MSCs and reducing apoptosis | Cell oxidative stress and inflammation model | Bone marrow (mice) | 2020 | Linares et al. [99] |
| Neurogenin-1 overexpression | Increasing the homing ability and engraftment efficiency of MSCs | Rat cerebral infarction model | Bone marrow (human) | 2020 | Kim et al. [87] |
| Immortalized by human telomerase reverse transcriptase | Reducing apoptosis in brain | Rat cerebral infarction model | Bone marrow (human) | 2019 | Li et al. [108] |
| SNHG12 silencing | Promoting the cell proliferation and reduce apoptosis of brain microvascular endothelial cells | Rat cerebral infarction model | Bone marrow | 2019 | Li et al. [114] |
| CCR2-overexpression | Promoting migration ability of MSCs | Rat cerebral infarction model | Bone marrow (human) | 2018 | Huang et al. [90] |
| HIF-1α gene-modified | Promoting viability, suppressing apoptosis, and activating autophagy-related signaling pathway | Rat cerebral infarction model and cell OGD model | Bone marrow (rat) | 2017 | Lv et al. [95, 96] |
| VEGF gene-modified | Improving the survival of MSCs, increasing expression and secretion of VEGF and BDNF | Rat cerebral infarction model | Bone marrow | 2017 | Zong et al. [137] |
| CX3CL1 gene-modified | Promoting migration ability of MSCs | Rat cerebral infarction model | Bone marrow (rat) | 2015 | Zhang et al. [89] |
| MiR-133b gene-modified | Exosomes from MSCs mediating the miR-133b transfer to astrocytes and neurons | Rat cerebral infarction model | Bone marrow (rat) | 2013 | Xin et al. [112] |
| SVV gene-modified | Improving the survival of MSCs and upregulating the expression of protective cytokines | Rat cerebral infarction model | Bone marrow (rat) | 2011 | Liu et al. [103] |
| Erythropoietin-transduced | Improving the survival of MSCs | Rat cerebral infarction model | Bone marrow (human) | 2010 | Cho et al. [106] |
6. Clinical Trials of MSC Therapy for Ischemic Stroke
First application of autologous BMSCs transplantation in patients with ischemic stroke is reported in 2005 [115]. In this study, the Barthel index and modified Rankin score of the MSC group (5 patients) improved consistently during the follow-up period and no adverse reactions were reported [115]. In 2010, Lee et al. reported that intravenous injection of BMSCs resulted in better recovery and reduced mortality for up to 5 years from treatment initiation, compared with randomized controls [116]. Another study evaluated efficacy of administration of serum-expanded autologous BMSCs to chronic stroke patients and got similar results in 2011 [117]. Furthermore, Steinberg et al. conducted a phase I/II study of intracerebral cell transplantation in patients with chronic stroke and reported that intracerebral transplantation of genetically modified MSCs significantly improved neurological function [118, 119], whereas Jaillard et al. did not report an overall benefit in a single-center, open-label randomized controlled trial study [120]. In 2019, Levy et al. completed a separate phase I/II trial in which patients received intravenous injection of allogeneic single-donor BMSCs [121]. Excellent functional outcome was reported in 35.5% of patients at 12-month postinfusion, compared to in 11.4% at baseline; however, there was no control group included in this study. Although MSC administration has been shown to be safe and feasible in small, early phase trials, questions about the efficacy of MSC treatment for ischemic stroke remain [122]. No significant improvement was observed in a randomized controlled intravenous phase II trial [122]. In 2021, Chung et al. reported that MSC treatment is not associated with improvements in the 3-month modified Rankin score, and their study provided class III evidence that autologous MSCs do not improve 90-day outcomes in patients with chronic stroke [123].
In summary, these studies support the safety of MSCs for transplantation in patients with ischemic stroke, but the therapeutic efficacy remains controversial. More optimized and well-designed large sample multicenter studies are needed to determine the effect of MSC therapy in the treatment of ischemic stroke. The identification of optimal transplantation protocol for routine clinical applications, including the route of administration, the concentration of cells, the timing, patient selection criteria, and combination therapies, will help us to further identify effective and safe therapeutic methods for ischemic stroke.
7. Limitations and Future Directions
Although MSC transplantation has demonstrated good prospects for the treatment of cerebral ischemia, there are still clear limitations with regard to the treatment of ischemic stroke. First, the administration of BMSCs primarily involves intracranial transplantation via stereotactic delivery, intravenous injection, or intra-arterial injection. Intracranial transplantation is an invasive treatment that can cause additional damage to the ischemic brain; in particular, infarct areas often require multiple injections. Although intravascular injection is a minimally invasive treatment option, this type of transplantation prevents many MSCs from reaching the area of cerebral ischemia, as most of the transplanted cells tend to accumulate in the peripheral organs [124, 125]. Second, the potential tumorigenic risks of BMSC transplantation require attention and further investigation [126, 127]. Third, there are also limitations related to the sources of MSCs that are currently available. Cerebral infarction often occurs in elderly patients; it is difficult to obtain a sufficient number of healthy autologous MSCs from elderly patients or from patients with a range of severe diseases [128]. Fourth, whether the transplanted MSCs can successfully differentiate into fully functional neurons has yet to be determined [129]. Finally, the limited replicative lifespan of MSCs also limits the efficacy of transplantation therapy [130]. Therefore, if we are to improve the efficacy of MSC transplantation, a series of targeted studies will need to be carried out. For example, it is important that we fully evaluate methods that could enhance the homing efficiency and survival rate of MSCs. In addition, future studies should also focus on improving the probability of successful differentiation and expanding the replicative lifespan of MSCs.
During preclinical studies, the transplantation of MSCs has demonstrated beneficial effects in several neurological and motor skills tests. However, the encouraging data derived from animal models cannot necessarily be translated into clinical practice [131]. Studies involving preclinical experiments are usually performed by applying standardized protocols for lesions in each study group. These conditions cannot be fully replicated in human patients suffering from ischemic stroke. Most strokes that occur in humans, particularly those that are treatable, are significantly smaller in size than those observed in preclinical models [132, 133]. Different types of donor cells may have also contributed to the differential results arising from preclinical and clinical studies. For example, most studies involving animal models used fresh MSCs that were isolated from healthy and young donors. However, many of the clinical studies used autologous MSCs instead of those derived from healthy individuals [131]. MSCs derived from healthy donors have been demonstrated to be more efficacious than those isolated from nonhealthy donors [134, 135].
In addition, future research should focus on the timing of MSC transplantation; this may constitute a significant challenge with regard to translating basic science studies into clinical practice. Most studies involving preclinical experiments have focused on the acute administration of MSCs. However, all the patients involved in clinical studies were administered with MSC therapy during the subacute or chronic phase following stroke [131]. Moreover, patients who have experienced cerebral infarction often have comorbidities such as hypertension and diabetes. Consequently, these patients need to take antidiabetic medicine and antiplatelet drugs; these drugs could influence the functionality of the MSCs, thus limiting their therapeutic effects [136]. Finally, senescence may also exert influence upon the therapeutic effects of MSCs; this is related to the limited number of passages used in the isolation of these cells. Extending the time available for extension will inevitably lead to replicative senescence [130]. Therefore, future studies should also focus on developing strategies that can address senescence in MSCs.
8. Conclusion
Currently, targeted treatments for cerebral infarction are associated with limited efficacy. As such, there is a clear need to identify novel treatment methods. MSC transplantation has demonstrated significant potential in animal models of MCAO, although efficacy still needs to be improved further. In this article, we reviewed recent progress in research studies aiming to improve the efficacy of MSC transplantation to treat the poststroke brain. A number of approaches have been proposed, including physical methods, chemical agents, traditional Chinese medicines and extracts, and genetic modifications. The latest research evidence has identified two key factors that must be considered if we are to improve efficacy: the survival and homing ability of MSCs after transplantation. Moreover, activating MSCs to promote neurogenesis and angiogenesis and adjusting the permeability of the BBB have also been found to be effective treatment methods. The identification of novel methodology and gaining a deeper understanding of the specific mechanisms underlying improved MSC transplantation treatments will help us to further identify effective and safe therapeutic methods for cerebral ischemia.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 81271298).
Additional Points
Highlights. (1) Existing literature was reviewed to identify strategies to improve the efficiency of MSC transplantation for ischemic stroke. (2) We summarize the specific mechanisms underlying the efficacy of strategies to improve the efficiency of MSC transplantation. (3) Improving the survival and homing abilities of MSC posttransplantation is vital if we are to improve treatment efficacy.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contributions
Y.L. developed the concept and design of the review and drafted the manuscript. W.Z. revised the manuscript. X.T. supervised the work and contributed to the review revision. All authors read and approved the final manuscript.
References
- 1.Poustchi F., Amani H., Ahmadian Z., et al. Combination therapy of killing diseases by injectable hydrogels: from concept to medical applications. Advanced Healthcare Materials. 2021;10(3, article e2001571) doi: 10.1002/adhm.202001571. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W., Kaste M., Bluhmki E., et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England Journal of Medicine. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Hafez S., Hoda M. N., Guo X., Johnson M. H., Fagan S. C., Ergul A. Comparative analysis of different methods of ischemia/reperfusion in hyperglycemic stroke outcomes: interaction with tPA. Translational Stroke Research. 2015;6(3):171–180. doi: 10.1007/s12975-015-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amani H., Habibey R., Shokri F., et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Scientific Reports. 2019;9(1):p. 6044. doi: 10.1038/s41598-019-42633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amani H., Shahbazi M. A., D'Amico C., Fontana F., Abbaszadeh S., Santos H. A. Microneedles for painless transdermal immunotherapeutic applications. Journal of Controlled Release. 2021;330:185–217. doi: 10.1016/j.jconrel.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Lindvall O., Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441(7097):1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya N., Ueda M., Igarashi H., et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sciences. 2008;83(11-12):433–437. doi: 10.1016/j.lfs.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Brenneman M., Sharma S., Harting M., et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. Journal of Cerebral Blood Flow and Metabolism. 2010;30(1):140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komatsu K., Honmou O., Suzuki J., Houkin K., Hamada H., Kocsis J. D. Therapeutic time window of mesenchymal stem cells derived from bone marrow after cerebral ischemia. Brain Research. 2010;1334:84–92. doi: 10.1016/j.brainres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Choi J. R., Yong K. W., Choi J. Y. Effects of mechanical loading on human mesenchymal stem cells for cartilage tissue engineering. Journal of Cellular Physiology. 2018;233(3):1913–1928. doi: 10.1002/jcp.26018. [DOI] [PubMed] [Google Scholar]
- 11.Wan Safwani W. K. Z., Choi J. R., Yong K. W., Ting I., Mat Adenan N. A., Pingguan-Murphy B. Hypoxia enhances the viability, growth and chondrogenic potential of cryopreserved human adipose-derived stem cells. Cryobiology. 2017;75:91–99. doi: 10.1016/j.cryobiol.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells (Dayton, Ohio) 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 13.Ye X., Zhang C. Effects of hyperlipidemia and cardiovascular diseases on proliferation, differentiation and homing of mesenchymal stem cells. Current Stem Cell Research & Therapy. 2017;12(5):377–387. doi: 10.2174/1574888X12666170316105805. [DOI] [PubMed] [Google Scholar]
- 14.Sherman L. S., Condé-Green A., Sandiford O. A., Rameshwar P. A discussion on adult mesenchymal stem cells for drug delivery: pros and cons. Therapeutic Delivery. 2015;6(12):1335–1346. doi: 10.4155/tde.15.80. [DOI] [PubMed] [Google Scholar]
- 15.Hsuan Y. C.-Y., Lin C. H., Chang C. P., Lin M. T. Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke. Brain and Behavior: A Cognitive Neuroscience Perspective. 2016;6(10, article e00526) doi: 10.1002/brb3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham C. J., Redondo-Castro E., Allan S. M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. Journal of Cerebral Blood Flow & Metabolism. 2018;38(8):1276–1292. doi: 10.1177/0271678X18776802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prockop D. J. "Stemness" does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clinical Pharmacology and Therapeutics. 2007;82(3):241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 18.Munoz J. R., Stoutenger B. R., Robinson A. P., Spees J. L., Prockop D. J. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(50):18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernstock J. D., Peruzzotti-Jametti L., Ye D., et al. Neural stem cell transplantation in ischemic stroke: a role for preconditioning and cellular engineering. Journal of Cerebral Blood Flow & Metabolism. 2017;37(7):2314–2319. doi: 10.1177/0271678X17700432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nature Medicine. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal S., Pittenger M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 22.Ma S., Zhong D., Chen H., et al. The immunomodulatory effect of bone marrow stromal cells (BMSCs) on interleukin (IL)-23/IL-17-mediated ischemic stroke in mice. Journal of Neuroimmunology. 2013;257(1-2):28–35. doi: 10.1016/j.jneuroim.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y., Guan Y. M., Huang H. L., Wang Q. S. Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits. Acta Pharmacologica Sinica. 2014;35(5):585–591. doi: 10.1038/aps.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J., Zhu H., Liu Y., et al. Human mesenchymal stem cell transplantation protects against cerebral ischemic injury and upregulates interleukin-10 expression in Macaca fascicularis. Brain Research. 2010;1334:65–72. doi: 10.1016/j.brainres.2010.03.080. [DOI] [PubMed] [Google Scholar]
- 25.Walker P. A., Harting M. T., Jimenez F., et al. Direct intrathecal implantation of mesenchymal stromal cells leads to enhanced neuroprotection via an NFkappaB-mediated increase in interleukin-6 production. Stem cells and development. 2010;19(6):867–876. doi: 10.1089/scd.2009.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luz-Crawford P., Djouad F., Toupet K., et al. Mesenchymal stem cell-derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells. 2016;34(2):483–492. doi: 10.1002/stem.2254. [DOI] [PubMed] [Google Scholar]
- 27.Corcione A., Benvenuto F., Ferretti E., et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 28.Sato K., Ozaki K., Oh I., et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Ramos J., Song S., Cardozo-Pelaez F., et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Experimental neurology. 2000;164(2):247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 30.Li J., Zhang Q., Wang W., Lin F., Wang S., Zhao J. Mesenchymal stem cell therapy for ischemic stroke: a look into treatment mechanism and therapeutic potential. Journal of Neurology. 2020 doi: 10.1007/s00415-020-10138-5. [DOI] [PubMed] [Google Scholar]
- 31.Yang M., Wei X., Li J., Heine L. A., Rosenwasser R., Iacovitti L. Changes in host blood factors and brain glia accompanying the functional recovery after systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transplantation. 2010;19(9):1073–1084. doi: 10.3727/096368910X503415. [DOI] [PubMed] [Google Scholar]
- 32.Song M., Mohamad O., Gu X., Wei L., Yu S. P. Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplantation. 2013;22(11):2001–2015. doi: 10.3727/096368912X657909. [DOI] [PubMed] [Google Scholar]
- 33.Wakabayashi K., Nagai A., Sheikh A. M., et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. Journal of Neuroscience Research. 2010;88(5):1017–1025. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., Li Y., Chen J., et al. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Research. 2004;1030(1):19–27. doi: 10.1016/j.brainres.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C., Li Y., Chen J., et al. Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience. 2006;141(2):687–695. doi: 10.1016/j.neuroscience.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 36.Brahimi-Horn M. C., Pouyssegur J. Oxygen, a source of life and stress. FEBS Letters. 2007;581(19):3582–3591. doi: 10.1016/j.febslet.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Harrison J. S., Rameshwar P., Chang V., Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99(1):p. 394. doi: 10.1182/blood.V99.1.394. [DOI] [PubMed] [Google Scholar]
- 38.Hu C., Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. Journal of Cellular and Molecular Medicine. 2018;22(3):1428–1442. doi: 10.1111/jcmm.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Y., Chen W., Wu L., Jiang L., Qin H., Tang N. Hypoxic preconditioning improves the survival and neural effects of transplanted mesenchymal stem cells via CXCL12/CXCR4 signalling in a rat model of cerebral infarction. Cell Biochemistry and Function. 2019;37(7):504–515. doi: 10.1002/cbf.3423. [DOI] [PubMed] [Google Scholar]
- 40.Wei L., Fraser J. L., Lu Z. Y., Hu X., Yu S. P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiology of Disease. 2012;46(3):635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J., Yang Y., Shen L., et al. Hypoxic preconditioning augments the therapeutic efficacy of bone marrow stromal cells in a rat ischemic stroke model. Cellular and Molecular Neurobiology. 2017;37(6):1115–1129. doi: 10.1007/s10571-016-0445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y. S., Noh M. Y., Cho K. A., et al. Hypoxia/reoxygenation-preconditioned human bone marrow-derived mesenchymal stromal cells rescue ischemic rat cortical neurons by enhancing trophic factor release. Molecular Neurobiology. 2015;52(1):792–803. doi: 10.1007/s12035-014-8912-5. [DOI] [PubMed] [Google Scholar]
- 43.Wang J. W., Qiu Y. R., Fu Y., Liu J., He Z. J., Huang Z. T. Transplantation with hypoxia-preconditioned mesenchymal stem cells suppresses brain injury caused by cardiac arrest-induced global cerebral ischemia in rats. Journal of Neuroscience Research. 2017;95(10):2059–2070. doi: 10.1002/jnr.24025. [DOI] [PubMed] [Google Scholar]
- 44.Yu J., Liu X. L., Cheng Q. G., et al. G-CSF and hypoxic conditioning improve the proliferation, neural differentiation and migration of canine bone marrow mesenchymal stem cells. Experimental and Therapeutic Medicine. 2016;12(3):1822–1828. doi: 10.3892/etm.2016.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burgess A., Shah K., Hough O., Hynynen K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Review of Neurotherapeutics. 2015;15(5):477–491. doi: 10.1586/14737175.2015.1028369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian J., Wang L., Li Q., et al. Ultrasound-targeted microbubble enhances migration and therapeutic efficacy of marrow mesenchymal stem cell on rat middle cerebral artery occlusion stroke model. Journal of Cellular Biochemistry. 2019;120(3):3315–3322. doi: 10.1002/jcb.27600. [DOI] [PubMed] [Google Scholar]
- 47.Chang F., Xiong W., Wang D., et al. Facilitation of ultrasonic microvesicles on homing and molecular mechanism of bone marrow mesenchymal stem cells in cerebral infarction patients. European Review for Medical and Pharmacological Sciences. 2017;21(17):3916–3923. [PubMed] [Google Scholar]
- 48.Yuan X., Rosenberg J. T., Liu Y., Grant S. C., Ma T. Aggregation of human mesenchymal stem cells enhances survival and efficacy in stroke treatment. Cytotherapy. 2019;21(10):1033–1048. doi: 10.1016/j.jcyt.2019.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paudyal A., Ghinea F. S., Driga M. P., et al. p 5 peptide-loaded human adipose-derived mesenchymal stem cells promote neurological recovery after focal cerebral ischemia in a rat model. Translational Stroke Research. 2020;12(1):125–135. doi: 10.1007/s12975-020-00805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo M., Liu Y., Shi D. Cardiovascular actions and therapeutic potential of tetramethylpyrazine (active component isolated from Rhizoma Chuanxiong): roles and mechanisms. BioMed Research International. 2016;2016:9. doi: 10.1155/2016/2430329.2430329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ran X., Ma L., Peng C., Zhang H., Qin L. P. Ligusticum chuanxiong Hort: a review of chemistry and pharmacology. Pharmaceutical Biology. 2011;49(11):1180–1189. doi: 10.3109/13880209.2011.576346. [DOI] [PubMed] [Google Scholar]
- 52.Fang Y., Chu L., Li L., et al. Tetramethylpyrazine protects bone marrow-derived mesenchymal stem cells against hydrogen peroxide-induced apoptosis through PI3K/Akt and ERK1/2 pathways. Biological & Pharmaceutical Bulletin. 2017;40(12):2146–2152. doi: 10.1248/bpb.b17-00524. [DOI] [PubMed] [Google Scholar]
- 53.Li L., Chu L., Ren C., et al. Enhanced migration of bone marrow-derived mesenchymal stem cells with tetramethylpyrazine and its synergistic effect on angiogenesis and neurogenesis after cerebral ischemia in rats. Stem Cells and Development. 2019;28(13):871–881. doi: 10.1089/scd.2018.0254. [DOI] [PubMed] [Google Scholar]
- 54.Boxer M. B., Quinn A. M., Shen M., et al. A highly potent and selective caspase 1 inhibitor that utilizes a key 3-cyanopropanoic acid moiety. ChemMedChem. 2010;5(5):730–738. doi: 10.1002/cmdc.200900531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bialer M., Johannessen S. I., Levy R. H., Perucca E., Tomson T., White H. S. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI) Epilepsy Research. 2013;103(1):2–30. doi: 10.1016/j.eplepsyres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Sun Z., Gu L., Wu K., et al. VX-765 enhances autophagy of human umbilical cord mesenchymal stem cells against stroke-induced apoptosis and inflammatory responses via AMPK/mTOR signaling pathway. CNS Neuroscience & Therapeutics. 2020;26(9):952–961. doi: 10.1111/cns.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y., Candelario-Jalil E., Thompson J. F., et al. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. Journal of Neurochemistry. 2010;112(1):134–149. doi: 10.1111/j.1471-4159.2009.06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho D. Y., Jeun S. S. Combination therapy of human bone marrow-derived mesenchymal stem cells and minocycline improves neuronal function in a rat middle cerebral artery occlusion model. Stem Cell Research & Therapy. 2018;9(1):p. 309. doi: 10.1186/s13287-018-1011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang S. S., Kook J. H., Hwang S., Park S. H., Nam S. C., Kim J. K. Inhibition of matrix metalloproteinase-9 attenuated neural progenitor cell migration after photothrombotic ischemia. Brain Research. 2008;1228:20–26. doi: 10.1016/j.brainres.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 60.Tsai L. K., Wang Z., Munasinghe J., Leng Y., Leeds P., Chuang D. M. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke. 2011;42(10):2932–2939. doi: 10.1161/STROKEAHA.110.612788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koch-Weser J., Browne T. R. valproic acid. The New England Journal of Medicine. 1980;302(12):661–666. doi: 10.1056/NEJM198003203021204. [DOI] [PubMed] [Google Scholar]
- 62.Price L. H., Heninger G. R. Lithium in the treatment of mood disorders. The New England Journal of Medicine. 1994;331(9):591–598. doi: 10.1056/NEJM199409013310907. [DOI] [PubMed] [Google Scholar]
- 63.Chen S. H., Benveniste E. N. Oncostatin M: a pleiotropic cytokine in the central nervous system. Cytokine & Growth Factor Reviews. 2004;15(5):379–391. doi: 10.1016/j.cytogfr.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Lan Y. W., Theng S. M., Huang T. T., et al. Oncostatin M-preconditioned mesenchymal stem cells alleviate bleomycin-induced pulmonary fibrosis through paracrine effects of the hepatocyte growth factor. Stem Cells Translational Medicine. 2017;6(3):1006–1017. doi: 10.5966/sctm.2016-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albiero M., Poncina N., Ciciliot S., et al. Bone marrow macrophages contribute to diabetic stem cell mobilopathy by producing oncostatin M. Diabetes. 2015;64(8):2957–2968. doi: 10.2337/db14-1473. [DOI] [PubMed] [Google Scholar]
- 66.Han J., Feng Z., Xie Y., et al. Oncostatin M-induced upregulation of SDF-1 improves bone marrow stromal cell migration in a rat middle cerebral artery occlusion stroke model. Experimental Neurology. 2019;313:49–59. doi: 10.1016/j.expneurol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Huang B., Jiang X. C., Zhang T. Y., et al. Peptide modified mesenchymal stem cells as targeting delivery system transfected with miR-133b for the treatment of cerebral ischemia. International Journal of Pharmaceutics. 2017;531(1):90–100. doi: 10.1016/j.ijpharm.2017.08.073. [DOI] [PubMed] [Google Scholar]
- 68.Choi C., Kim H. M., Shon J., et al. The combination of mannitol and temozolomide increases the effectiveness of stem cell treatment in a chronic stroke model. Cytotherapy. 2018;20(6):820–829. doi: 10.1016/j.jcyt.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Brouns R., Wauters A., de Surgeloose D., Mariën P., de Deyn P. P. Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. European Neurology. 2011;65(1):23–31. doi: 10.1159/000321965. [DOI] [PubMed] [Google Scholar]
- 70.Lorberboym M., Lampl Y., Sadeh M. Correlation of 99mTc-DTPA SPECT of the blood-brain barrier with neurologic outcome after acute stroke. Journal of Nuclear Medicine. 2003;44(12):1898–1904. [PubMed] [Google Scholar]
- 71.Zacharek A., Shehadah A., Chen J., et al. Comparison of bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke. 2010;41(3):524–530. doi: 10.1161/STROKEAHA.109.568881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moon G. J., Cho Y. H., Kim D. H., et al. Serum-mediated activation of bone marrow-derived mesenchymal stem cells in ischemic stroke patients: a novel preconditioning method. Cell Transplantation. 2018;27(3):485–500. doi: 10.1177/0963689718755404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H., Sun F., Wang J., et al. Combining injectable plasma scaffold with mesenchymal stem/stromal cells for repairing infarct cavity after ischemic stroke. Aging and Disease. 2017;8(2):203–214. doi: 10.14336/AD.2017.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun K., Fan J., Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharmaceutica Sinica B. 2015;5(1):8–24. doi: 10.1016/j.apsb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H. W., Liou K. T., Wang Y. H., et al. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. Journal of Ethnopharmacology. 2011;138(1):22–33. doi: 10.1016/j.jep.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 76.Chen H. J., Shen Y. C., Shiao Y. J., et al. Multiplex brain proteomic analysis revealed the molecular therapeutic effects of Buyang Huanwu decoction on cerebral ischemic stroke mice. PLoS One. 2015;10(10, article e0140823) doi: 10.1371/journal.pone.0140823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y. K., Han X. Y., Che Z. Y. Effects of Buyang Huanwu Tang Combined with Bone Marrow Mesenchymal Stem Cell Transplantation on the Expression of VEGF and Ki-67 in the Brain Tissue of the Cerebral Ischemia-Reperfusion Model Rat. Journal of Traditional Chinese Medicine. 2010;30(4):278–282. doi: 10.1016/S0254-6272(10)60056-8. [DOI] [PubMed] [Google Scholar]
- 78.Yang J., Gao F., Zhang Y., Liu Y., Zhang D. Buyang Huanwu decoction (BYHWD) enhances angiogenic effect of mesenchymal stem cell by upregulating VEGF expression after focal cerebral ischemia. Journal of Molecular Neuroscience. 2015;56(4):898–906. doi: 10.1007/s12031-015-0539-0. [DOI] [PubMed] [Google Scholar]
- 79.Lin T. H., Hsieh C. L. Pharmacological effects of Salvia miltiorrhiza (Danshen) on cerebral infarction. Chinese Medicine. 2010;5(1):p. 22. doi: 10.1186/1749-8546-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim R., Lee S., Lee C. Y., et al. Salvia miltiorrhiza enhances the survival of mesenchymal stem cells under ischemic conditions. The Journal of Pharmacy and Pharmacology. 2018;70(9):1228–1241. doi: 10.1111/jphp.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu L., Liu Y. J., Shen H., Gu P. Q., Zhang L. Astragalus and baicalein regulate inflammation of mesenchymal stem cells (MSCs) by the mitogen-activated protein kinase (MAPK)/ERK pathway. Medical Science Monitor. 2017;23:3209–3216. doi: 10.12659/MSM.902441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie J., Wang H., Song T., et al. Tanshinone IIA and astragaloside IV promote the migration of mesenchymal stem cells by up-regulation of CXCR4. Protoplasma. 2013;250(2):521–530. doi: 10.1007/s00709-012-0435-1. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X. G., Shan C., Zhu J. Z., et al. Additive neuroprotective effect of borneol with mesenchymal stem cells on ischemic stroke in mice. Frontiers in Physiology. 2017;8:p. 1133. doi: 10.3389/fphys.2017.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oh T. W., Park K. H., Jung H. W., Park Y. K. Neuroprotective effect of the hairy root extract of Angelica gigas NAKAI on transient focal cerebral ischemia in rats through the regulation of angiogenesis. BMC Complementary and Alternative Medicine. 2015;15(1):p. 101. doi: 10.1186/s12906-015-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim R., Kim P., Lee C. Y., et al. Multiple combination of Angelica gigas extract and mesenchymal stem cells enhances therapeutic effect. Biological & Pharmaceutical Bulletin. 2018;41(12):1748–1756. doi: 10.1248/bpb.b18-00193. [DOI] [PubMed] [Google Scholar]
- 86.Kim S. S., Yoo S. W., Park T. S., et al. Neural induction with neurogenin 1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008;26(9):2217–2228. doi: 10.1634/stemcells.2008-0108. [DOI] [PubMed] [Google Scholar]
- 87.Kim G. H., Subash M., Yoon J. S., et al. Neurogenin-1 overexpression increases the therapeutic effects of mesenchymal stem cells through enhanced engraftment in an ischemic rat brain. Int J Stem Cells. 2020;13(1):127–141. doi: 10.15283/ijsc19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cardona A. E., Pioro E. P., Sasse M. E., et al. Control of microglial neurotoxicity by the fractalkine receptor. Nature Neuroscience. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y., Zheng J., Zhou Z., et al. Fractalkine promotes chemotaxis of bone marrow-derived mesenchymal stem cells towards ischemic brain lesions through Jak2 signaling and cytoskeletal reorganization. The FEBS journal. 2015;282(5):891–903. doi: 10.1111/febs.13187. [DOI] [PubMed] [Google Scholar]
- 90.Huang Y., Wang J., Cai J., et al. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics. 2018;8(21):5929–5944. doi: 10.7150/thno.28029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Badowska-Kozakiewicz A., Sobol M., Patera J. Expression of hypoxia-inducible factor 1α in invasive breast cancer with metastasis to lymph nodes: correlation with steroid receptors, HER2 and EPO-R. Advances in Clinical and Experimental Medicine. 2016;25(4):741–750. doi: 10.17219/acem/63143. [DOI] [PubMed] [Google Scholar]
- 92.Brown J. M., Wilson W. R. Exploiting tumour hypoxia in cancer treatment. Nature Reviews Cancer. 2004;4(6):437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 93.Mucaj V., Shay J. E. S., Simon M. C. Effects of hypoxia and HIFs on cancer metabolism. International Journal of Hematology. 2012;95(5):464–470. doi: 10.1007/s12185-012-1070-5. [DOI] [PubMed] [Google Scholar]
- 94.Yang C., Liu H., Liu D. Mutant hypoxia-inducible factor 1α modified bone marrow mesenchymal stem cells ameliorate cerebral ischemia. International Journal of Molecular Medicine. 2014;34(6):1622–1628. doi: 10.3892/ijmm.2014.1953. [DOI] [PubMed] [Google Scholar]
- 95.Lv B., Li F., Han J., et al. Hif-1α overexpression improves transplanted bone mesenchymal stem cells survival in rat MCAO stroke model. Frontiers in Molecular Neuroscience. 2017;10:p. 80. doi: 10.3389/fnmol.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lv B., Hua T., Li F., et al. Hypoxia-inducible factor 1 alpha protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. American Journal of Translational Research. 2017;9(5):2492–2499. [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Z., Leng Y., Wang J., et al. Tubastatin A, an HDAC6 inhibitor, alleviates stroke-induced brain infarction and functional deficits: potential roles of α-tubulin acetylation and FGF-21 up-regulation. Scientific Reports. 2016;6(1):p. 19626. doi: 10.1038/srep19626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Staiger H., Keuper M., Berti L., Hrabě de Angelis M., Häring H. U. Fibroblast growth factor 21-metabolic role in mice and men. Endocrine Reviews. 2017;38(5):468–488. doi: 10.1210/er.2017-00016. [DOI] [PubMed] [Google Scholar]
- 99.Linares G. R., Leng Y., Maric D., Chuang D. M. Overexpression of fibroblast growth factor-21 (FGF-21) protects mesenchymal stem cells against caspase-dependent apoptosis induced by oxidative stress and inflammation. Cell Biology International. 2020;44(10):2163–2169. doi: 10.1002/cbin.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abbate A., Scarpa S., Santini D., et al. Myocardial expression of survivin, an apoptosis inhibitor, in aging and heart failure. An experimental study in the spontaneously hypertensive rat. International Journal of Cardiology. 2006;111(3):371–376. doi: 10.1016/j.ijcard.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 101.Shin S., Sung B. J., Cho Y. S., et al. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40(4):1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 102.Fan L., Lin C., Zhuo S., et al. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. European Journal of Heart Failure. 2009;11(11):1023–1030. doi: 10.1093/eurjhf/hfp135. [DOI] [PubMed] [Google Scholar]
- 103.Liu N., Zhang Y., Fan L., et al. Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. Journal of Translational Medicine. 2011;9(1):p. 105. doi: 10.1186/1479-5876-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y., Lu Z., Keogh C. L., Yu S. P., Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. Journal of Cerebral Blood Flow and Metabolism. 2007;27(5):1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- 105.Zhou Z., Wei X., Xiang J., et al. Protection of erythropoietin against ischemic neurovascular unit injuries through the effects of connexin43. Biochemical and Biophysical Research Communications. 2015;458(3):656–662. doi: 10.1016/j.bbrc.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 106.Cho G. W., Koh S. H., Kim M. H., et al. The neuroprotective effect of erythropoietin-transduced human mesenchymal stromal cells in an animal model of ischemic stroke. Brain Research. 2010;1353:1–13. doi: 10.1016/j.brainres.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 107.Serakinci N., Graakjaer J., Kolvraa S. Telomere stability and telomerase in mesenchymal stem cells. Biochimie. 2008;90(1):33–40. doi: 10.1016/j.biochi.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 108.Li J., Liu W., Yao W. Immortalized human bone marrow derived stromal cells in treatment of transient cerebral ischemia in rats. Journal of Alzheimer's Disease. 2019;69(3):871–880. doi: 10.3233/JAD-190279. [DOI] [PubMed] [Google Scholar]
- 109.Jeyaseelan K., Lim K. Y., Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39(3):959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 110.Uhlmann S., Mracsko E., Javidi E., et al. Genome-wide analysis of the circulating miRNome after cerebral ischemia reveals a reperfusion-induced microRNA cluster. Stroke. 2017;48(3):762–769. doi: 10.1161/STROKEAHA.116.013942. [DOI] [PubMed] [Google Scholar]
- 111.He L., Zhang H. MicroRNAs in the migration of mesenchymal stem cells. Stem Cell Reviews and Reports. 2019;15(1):3–12. doi: 10.1007/s12015-018-9852-7. [DOI] [PubMed] [Google Scholar]
- 112.Xin H., Li Y., Liu Z., et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31(12):2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schaukowitch K., Kim T. K. Emerging epigenetic mechanisms of long non-coding RNAs. Neuroscience. 2014;264:25–38. doi: 10.1016/j.neuroscience.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Y., Guo S., Liu W., et al. Silencing of SNHG12 enhanced the effectiveness of MSCs in alleviating ischemia/reperfusion injuries via the PI3K/AKT/mTOR signaling pathway. Frontiers in Neuroscience. 2019;13:p. 645. doi: 10.3389/fnins.2019.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bang O. Y., Lee J. S., Lee P. H., Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Annals of Neurology. 2005;57(6):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 116.Lee J. S., Hong J. M., Moon G. J., et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 117.Honmou O., Houkin K., Matsunaga T., et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134(6):1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Steinberg G. K., Kondziolka D., Wechsler L. R., et al. Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): a phase 1/2a study. Journal of Neurosurgery. 2018;131(5):1462–1472. doi: 10.3171/2018.5.jns173147. [DOI] [PubMed] [Google Scholar]
- 119.Steinberg G. K., Kondziolka D., Wechsler L. R., et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47(7):1817–1824. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.(for the ISIS-HERMES Study Group), Jaillard A., Hommel M., et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Translational Stroke Research. 2020;11(5):910–923. doi: 10.1007/s12975-020-00787-z. [DOI] [PubMed] [Google Scholar]
- 121.Levy M. L., Crawford J. R., Dib N., Verkh L., Tankovich N., Cramer S. C. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. 2019;50(10):2835–2841. doi: 10.1161/STROKEAHA.119.026318. [DOI] [PubMed] [Google Scholar]
- 122.Hess D. C., Wechsler L. R., Clark W. M., et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet Neurology. 2017;16(5):360–368. doi: 10.1016/S1474-4422(17)30046-7. [DOI] [PubMed] [Google Scholar]
- 123.Chung J. W., Chang W. H., Bang O. Y., et al. Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. 2021;96:e1012–e1023. doi: 10.1212/wnl.0000000000011440. [DOI] [PubMed] [Google Scholar]
- 124.Jin K., Sun Y., Xie L., et al. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiology of Disease. 2005;18(2):366–374. doi: 10.1016/j.nbd.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 125.Horie N., Pereira M. P., Niizuma K., et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29(2):274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karnoub A. E., Dash A. B., Vo A. P., et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 127.Subramanian A., Shu-Uin G., Kae-Siang N., et al. Human umbilical cord Wharton's jelly mesenchymal stem cells do not transform to tumor-associated fibroblasts in the presence of breast and ovarian cancer cells unlike bone marrow mesenchymal stem cells. Journal of Cellular Biochemistry. 2012;113(6):1886–1895. doi: 10.1002/jcb.24057. [DOI] [PubMed] [Google Scholar]
- 128.Zhang J., Huang X., Wang H., et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Research & Therapy. 2015;6(1):p. 234. doi: 10.1186/s13287-015-0240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Strioga M., Viswanathan S., Darinskas A., Slaby O., Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem cells and Development. 2012;21(14):2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 130.Kim H. J., Park J. S. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages. Development & Reproduction. 2017;21(1):1–10. doi: 10.12717/DR.2017.21.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lalu M. M., Montroy J., Dowlatshahi D., et al. From the lab to patients: a systematic review and meta-analysis of mesenchymal stem cell therapy for stroke. Translational Stroke Research. 2020;11(3):345–364. doi: 10.1007/s12975-019-00736-5. [DOI] [PubMed] [Google Scholar]
- 132.Brott T., Marler J. R., Olinger C. P., et al. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke. 1989;20(7):871–875. doi: 10.1161/01.STR.20.7.871. [DOI] [PubMed] [Google Scholar]
- 133.Lyden P. D., Zweifler R., Mahdavi Z., Lonzo L. A rapid, reliable, and valid method for measuring infarct and brain compartment volumes from computed tomographic scans. Stroke. 1994;25(12):2421–2428. doi: 10.1161/01.STR.25.12.2421. [DOI] [PubMed] [Google Scholar]
- 134.van de Vyver M. Intrinsic mesenchymal stem cell dysfunction in diabetes mellitus: implications for autologous cell therapy. Stem Cells and Development. 2017;26(14):1042–1053. doi: 10.1089/scd.2017.0025. [DOI] [PubMed] [Google Scholar]
- 135.Block T. J., Marinkovic M., Tran O. N., et al. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Research & Therapy. 2017;8(1):p. 239. doi: 10.1186/s13287-017-0688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ortega F. J., Jolkkonen J., Mahy N., Rodríguez M. J. Glibenclamide enhances neurogenesis and improves long-term functional recovery after transient focal cerebral ischemia. Journal of Cerebral Blood Flow & Metabolism. 2013;33(3):356–364. doi: 10.1038/jcbfm.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zong X., Wu S., Li F., et al. Transplantation of VEGF-mediated bone marrow mesenchymal stem cells promotes functional improvement in a rat acute cerebral infarction model. Brain Research. 2017;1676:9–18. doi: 10.1016/j.brainres.2017.08.006. [DOI] [PubMed] [Google Scholar]


