Abstract
Background:
A specific treatment has not yet developed for cryptosporidiosis, and some of the used drugs had side effects in immunodeficient patients. The goal of an appropriate remedy is to remove symptoms and improve immune responses in hosts. The current study was designed to evaluate the therapeutic efficacy of Artemisia spicigera ethanolic extract in experimentally infected immunosuppressed mice.
Methods:
Thirty six NMRI mice, 4–6 wk old, were randomly divided into six equal groups. C1: uninfected, treated control; C2: infected, untreated control; T1, T2, T3, and P: infected, treated with 0.2, 2, and 20 mg/ml extract, and 5mg/ml paromomycin, respectively. Mice were experimentally infected by oral administration of 104 oocysts/animal of Cryptosporidium parvum and treated orally for eight days per 12h, starting 12h before experimental infection. The presence of oocyst shedding, weight gain/loss, and the histopathology of ileum sections were examined.
Results:
Results revealed that oocyst shedding was significantly (P<0.05) reduced in treatment groups. There was no significant difference between the mean of weight gain/loss in the infected control and treated groups. Histopathological analysis of ileum sections further supported the parasitological findings.
Conclusion:
Artemisia spicigera had acceptable efficacy as a therapeutic agent for cryptosporidiosis.
Keywords: Artemisia spicigera, Extract, Cryptosporidium parvum, Treatment, Mice
Introduction
Cryptosporidium parvum is an obligate, intracellular protozoan parasite in a wide range of hosts that develops within the microvillus layer of epithelial cells throughout the gastrointestinal tract (GI) and even the respiratory tract. Cryptosporidium has a worldwide spread and zoonotic importance (1–3). Cryptosporidiosis is primarily a self-limiting disease in immunocompetent hosts. But, in immunodeficient and immunocompromised hosts and children with malnutrition, this infection could cause an extra-intestinal form, chronic diarrhea, and death (3, 4). In livestock, cryptosporidiosis has both economic and clinical importance. Young animals are more vulnerable to infection, and high mortality rates were reported in neonates of various species, particularly ruminants (1–3).
Effective therapy for cryptosporidiosis is already unspecified. Despite the evaluation of over 200 chemotherapeutic agents for their anti-Cryptosporidium effects, appropriate treatments to eliminate these parasites of the host are unavailable yet (3, 5, 6). In recent years the herbal compositions with anti-Cryptosporidium activity were recognized. They suggested safe and effective in the treatment and control of cryptosporidiosis.
Artemisia genus (Family: Asteraceae) encompasses almost 500 species in the world (7), of which 34 species are in Iran (8). Pharmacological researches have indicated antimalarial (7, 9), antibacterial (10), antifungal (11), antioxidant (10), nematocidal, and cesticidal activities (12, 13), for Artemisia species. Known as «dermane ye sonbolei〉〉 in Persian, A. spicigera C. Koch is growing in Armenia, Middle Anatolia, Northwest and North of Iran (14). This species has traditionally been used in medicine. According to the researches, this species of Artemisia have insecticidal, antibacterial, and antioxidant but not cytotoxic activity. So, it was recommended that this species is suitable to be utilized as a natural insecticide or antibacterial agent (8).
Even though Artemisia species are efficient in parasitic diseases and digestive system disorders (7, 9, 12, 13, 15), no declared factual data are available regarding the anti-Cryptosporidium effect of Artemisia in vivo. Among laboratory animals, immunocompromised or immunosuppressed mice are the best candidate for experimental infection with C. parvum (16).
So, the present study was conducted to appraise the efficacy of A. spicigera ethanolic extract (As-EtOH) compared to paromomycin as a commercial drug against cryptosporidiosis in an immunosuppressed mouse model.
Materials and Methods
Preparation of Cryptosporidium parvum oocysts
Oocysts of C. parvum were obtained from naturally infected calves (Aminabad Institute, Tehran University, Tehran, Iran) and microscopically examined using the modified Ziehl–Neelsen (MZN) staining, and then purified (17). The isolated oocysts were treated in 10 % sodium hypochlorite. Afterward, they were washed three times in double distilled water, and then were diluted to the needed concentration in PBS and stored at 4 °C until use (18). Species confirmation of the collected oocysts carried out using PCR assay. After the extraction of DNA using the DNA extraction kit (MBST, Tehran, Iran), DNA was amplified using a pair of primer obtained from the entire 18S rRNA gene of C. parvum isolate, according to Sturbaum et al. 2001 (19) that amplify an 840-bp fragment from C. parvum genotype 2.
Preparation of Artemisia spicigera ethanolic extract and analysis
The plant material for this study was collected from East Azerbaijan province, Iran. For this collection, a Voucher specimen (14966) was deposited at the Herbarium of the Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran. After collection in a dark room with fresh air circulation, the plant leaf, root, and stalk were separated, cleaned, and air-dried. Then, all the parts were ground, packed individually, and cached in a dark place. The ethanolic extract was prepared by percolation method as previously described (20). Next, phenol and flavonoids contents measured (21). All the extracts were dissolved in distilled water to reach the final concentrations of 0.2, 2, and 20 mg/ml and were stored at 4 °C until use.
Paromomycin
Paromomycin (125 mg/ 5ml syrup, Alhavi CO., Tehran, Iran) was purchased and used as a standard control drug.
Animals and housing
Thirty six female NMRI mice aged four to six weeks (Pasteur Institute, Tehran, Iran) were divided into six groups: C1 (negative control): uninfected, treated group, C2 (positive control): infected, untreated group, T1–T3: infected, treated groups with AS-EtOH extract and P: infected, treated group with paromomycin. Six mice of equal mean body weight housed per cage. Before beginning the study, all the mice kept for ten days to adapt to the keeping place (animal house, University of Tabriz) and new conditions. In this period, they were evaluated for GI parasite infections using fecal smears and flotation methods, and they resulted in free from endo-parasites. Also, the result of Cryptosporidium oocysts in the examination of fecal smears stained with modified Ziel-Neelsen acid-fast staining in mice was negative. The animals were maintained under controlled temperatures of about 24 °C, with a photoperiod of 12 h light/dark and with humidity at approximately 45–50%.
Experimental infection and treatment
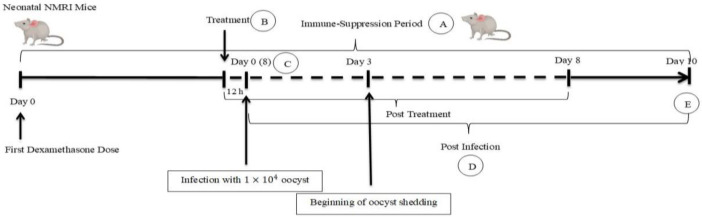
NMRI female mice from all the groups were dosed on alternate days with 120 mg tetracycline or 7.2 ml of 1% dexamethasone 21-phosphate solution in 250 ml drinking water per cage (16). After completing the fourth dexamethasone dose, the mice in five groups (C2, T1, T2, T3, and P) were orally inoculated with 10 4 C. parvum oocysts in 100 ml PBS (18). After the experimental infection, mice in T1–T3 groups were treated with 250 μl of 0.2 mg/ml, 2mg/ml, and 20 mg/ml (5mg/dose) of A. spicigera extract, respectively. Uninfected mice in the C1 group received 20 mg/ml of AS extract, and infected control group (C2) received only distilled water. The P group was treated with 200 μl of paromomycin (5 mg/dose). Administration of herbal extract, distilled water, and paromomycin was performed 12h before oocyst inoculation, and lasted per 12 h for the next eight days (18, 22, 23). The mouse challenge graphically was shown in short in Fig. 1.
Fig. 1:
Experimental infection and treatment in NMRI mice model.
A: Dexamethasone and tetracycline were dosed on alternate days. B: First dose of treatment that repeated per 12 h. C: Day 8 of Immune-Suppression Period. D: Oocyst counting and weight measuring daily. E: Histopathological evaluation
Diagnostic methods
Oocyst counts and body weight gain
The fecal pellets were gathered daily from each group for ten days. These fecal samples were suspended in 50 ml of distilled water and were left at room temperature for 5 min. Next, 1ml of the upper suspension was put into a 1.5-ml Eppendorf tube. Then, this part of the suspension was centrifuged at 8,000×g for 3 min, and the supernatant was removed. Besides, the pellet was re-suspended in a 1 ml solution of sucrose (1.2 g/cm3). The oocysts were counted by a light microscope at a magnification of 1×400 and a hemocytometer. Furthermore, the number of oocysts per gram (OPG) was determined (24). Throughout the study, each of the mice was weighed daily, and the mean was used in data analysis.
Histopathological analysis
On day 10 post-infection (3 days after the end of treatment), using a diethyl ether overdose, all mice of the groups were humanely killed and then necropsied. Additionally, for histological examination, the intestine was removed and placed in 10% formalin. The intestines were paraffin-embedded, and 3-μm sections were prepared, and then were studied by hematoxylin–eosin (H&E) staining. Using a digital-camera microscope (EclipseE200-coolpix-4500, Nikon, Tokyo, Japan), the ileum terminal portion (2 cm before the ileocecocolic valve) of the sections were observed and photographed.
Statistical analysis
Statistical significance was determined by the one-way analysis of variance as well as t-tests. Using Statistical SPSS for Windows, issue 22, the data are presented as means ± standard error (SE) with P ≤0 .05 as the significant level.
Ethics approval
This study was undertaken by the bioethics committee of the University of Tabriz (http://ethics.research.ac.ir/IR.TABRIZU.REC.1398.018) and approved with code no. IR.TABRIZU.REC.1398.018
Results
Microscopic and Molecular analysis of Cryptosporidium oocysts
As expected, the Cryptosporidium oocysts were observed in the obtained feces by MZN staining. In the PCR, the primer set amplified 840bp fragments (Fig. 2).
Fig. 2:
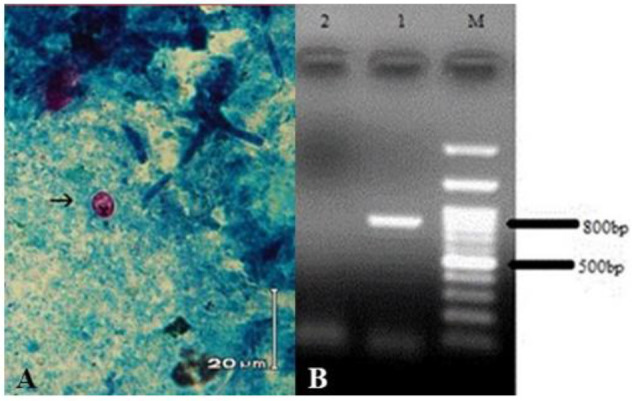
Microscopic and Molecular analysis of Cryptosporidium oocysts. A: The oocyst in MZN staining showed by an arrow. B: PCR amplification of the 18S rRNA gene of Cryptosporidium; Lanes 1: C. parvum with 840 bp fragment, Lanes 2: negative control, Lanes M: 100-bp molecular marker (CinnaGen Co.Iran)
Evaluation of A. spicigera extract on fecal oocyst counts and mean body weight
All the examined mice from infected groups (C2, T1, T2, T3, and P) were considered Cryptosporidium-positive, but symptoms such as diarrhea, were not seen in any of the infected mice. During or after the treatment period, no mouse died or exhibited drug toxicity symptoms or side effects. Three days after oocyst inoculation, oocyst shedding in infected test and control groups (C2, T1, T2, T3, and P) was determined and continued until the end of the experiment. The results demonstrated that at day four post-treatment, the test groups presented a decrease of the mean oocysts number in comparison to day 3 (P<0.05), which remained constant throughout the treatment period. However, the C2 group exhibited a high number of mean oocysts until day 10 (Table 1). Although parasite eradication could not be shown in any of the groups, the excreted oocysts number in the test groups was significantly lower than in the C2 group from day 3 to day 10 post-infection (P< 0.05; Table 1). The T1 and T2 groups had a lower number of mean oocysts than that of group C2 from day 3 (P<0.05), but a higher number of mean oocysts than those of P and T3 groups from day 6 to 10 (P<0.05) (Table 1). On the last day of treatment (day 8), T3 and P groups exhibited a lower number of mean oocysts than those of C2, T1, and T2 groups (P<0.05), and the percentages of decreased oocysts shedding were achieved to be over 70% for these groups. But, they resumed increasing in oocyst shedding after the end of treatment (days 9 and 10) (Table 1). In the uninfected control group (C1), no excretion of oocyst was observed throughout the experiment.
Table 1:
Fecal oocyst counts (Mean ± SE) in mice groups
| *Day 3 | *Day 4 | *Day 6 | *Day 8 | *Day 9 | *Day 10 | |
|---|---|---|---|---|---|---|
| **C1 | 0 | 0 | 0 | 0 | 0 | 0 |
| C2 | 5.40±4.59a | 5.45±4.60a1 | 5.51±4.50a12 | 5.53±4.52a123 | 5.59±4.38a234 | 5.6±4.6a12345 |
| T1 | 5.22±4.08a | 5.16±4.29ab1 | 5.10±4.20ab12 | 4.95±4.34ab123 | 5.08±4.53ab1234 | 5.12±4.22ab12345 |
| T2 | 5.20±4.36abc | 5.10±4.38abc1 | 5±4.35abc12 | 4.86±4.08bc123 | 4.9±4.25abc1234 | 4.93±4.33bc2345 |
| T3 | 4.98±4.08abcd12345 | 4.93±4.28abc1 | 4.84±4.16abcd12 | 4.61±4.14abcd123 | 4.77±4.35abcd1234 | 4.74±4.19abcd12345 |
| P | 4.86±4.08abcde12345 | 4.71±3.97ab1 | 4.52±3.69abcde12 | 4.12±3.32abde123 | 4.77±4.58abcde1234 | 4.42±3.39abcde12345 |
Data are shown as Mean ± SE (in log 10).
Days post-infection.
: Groups a,b,c,d,e: P<0.05 comparison of the same day of treatment in the different groups
a. The statistical difference with the group C1; b. Statistical difference with the group C2; c. The statistical difference with the group T1; d. Statistical difference with the group T2; e. The statistical difference with the group T3; 1, 2, 3, 4, 5: P<0.05 comparison between days of treatment in the same group
Body weight gain/loss: Infected mice in the test and the C2 control groups showed a significant reduction in mean body weight compared to the healthy control (C1) (P< 0.05). No significant decrease in mean body weight was noticed in treated groups compared to the C2 group (P> 0.05).
Histopathological analysis
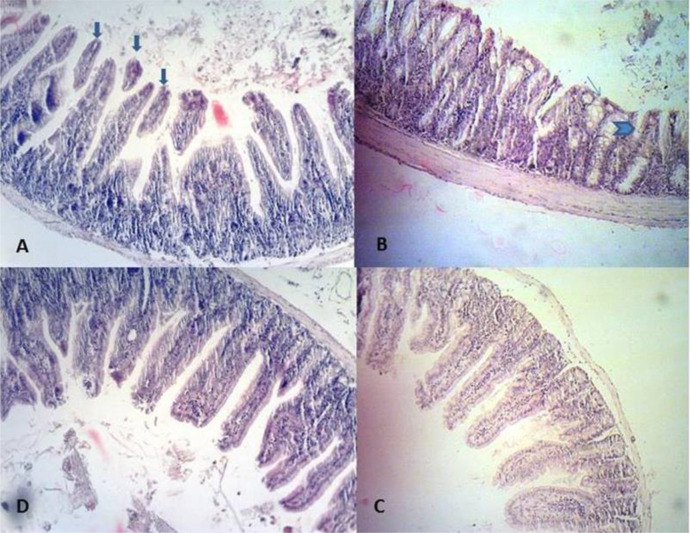
Histopathological examination of intestinal mucosa in 6 groups revealed differences concerning villus/crypt (V/C) length ratios. The ileal sections of the infected control group (C2) demonstrated the existence of Cryptosporidium oocysts on the luminal surface of the epithelium lining the villi. These sections also exhibited altered mucosal architecture, with blunting, widening, and shortening of the intestinal villi and goblet cell hyperplasia (Fig. 3 A-B). In any infected groups, the parasite was not completely eradicated from the intestine; however, ileal sections of infected, treated mice (T1, T2, T3, and P groups) showed a reduced number of Cryptosporidium oocysts. It was observed that the intestinal sections of all extract treated groups regained a typical architecture with standard villus/crypt length ratios, and brush borders were restored. (Fig. 3C). No pathological changes and no parasites were identified in the intestinal sections of the C1 group (uninfected, treated control group), which indicates that A. spicigera extract at the highest dose in this study (20mg/ml) did not negatively affect the health of the intestine (Fig. 3D).
Fig. 3:
Ileum histopathological findings in different experimental mice groups: In the C2, A: Desquamation (big arrows) B: villous atrophy (V/C< 1/3) (head arrow) and hyperplasia of goblet cells (little arrow) (B) are observed. C: In the T3, the villus/crypt length ratios are standard (V/C: 1/3) (C). D: In the C1, the villus/crypt length ratios are standard (V/C: 1/3)
Discussion
Finding a natural product with anti-Cryptosporidium properties used as a feed additive, was the initial hope underlying this experiment. Since, in cryptosporidiosis treatment, the possibility of using plant extracts and derivatives has been studied, such as Pine bark (25), Mangiferin (23), Allium sativum (garlic) (26), Punica granatum (27), Egyptian propolis (28), Oliva europaea (29) and curcumin (30,31).
The therapeutic potential of A. spicigera in the present study was assessed based on oocyst shedding pattern, histopathological findings, and weight gain/loss. The doses of A. spicigera used in this experiment were effective in treating mice infected with C. parvum and resulted in no side effects. No changes in weight gain, behavior, or intestinal histopathology were observed in uninfected mice treated with A. spicigera (C2).
Our results revealed that A. spicigera at 20 mg/ml has remarkable anti-Cryptosporidium activity in mice. Furthermore, its activity was similar to that proved by the proposed dose of paromomycin in the past works (5, 32). In agreement with some previous researches, our findings showed that paromomycin and Artemisia could not eliminate Cryptosporidium infection. When garlic was used to treat HIV patients with confirmed cryptosporidiosis, complete and partial remission occurred in some patients (33). Both paromomycin and mangiferin could not inhibit the intestinal colonization of C. parvum completely, but they reduced it (23). Furthermore, water and ethanol extracts of propolis failed to eradicate the infection, but they reduced the oocyst shedding (28). These results are contrary to findings in other former studies into the anti-Cryptosporidium activity of paromomycin and herbal extracts, which showed complete remission of infection (26–27, 30–31). In comparison to the untreated mice, oocyst shedding was reduced in Artemisia-treated groups with time and dose-dependent patterns. The effect of time and dose of treatment on the reduction or elimination of Cryptosporidium infection has been shown in previous studies (23, 25, 26, 30,31). Oocyst shedding was significantly decreased in the P. granatum-treated mice by day 14 PI (P < .05) and was removed by day 28 PI (27). Moreover, the current study, like some previous researches, confirmed that the beginning and duration of treatment and intervals of repetitions affected the results (26, 28). Propolis extracts exhibited more efficacies in reducing oocysts excretion when given three days before the infection and continued for seven successive days (28). Riad et al demonstrated the strong prophylactic effect of garlic when given before the infection (34).
C. parvum infection negatively affects weight gain and feeding in infants and nursling animals like calves (5, 35). Low absorption arising from mucosal surface loss and chronic malabsorption may be the cause of weight loss (36). Despite more reduction in weight gain in the infected/untreated group (C2), extract treated mice showed no significant weight gains during the treatment period (P ≥ .05). This finding is against another study (27), and it maybe because of the experimentation’s limited length.
In the present study, histopathological alterations included atrophy of the villi, increased desquamation, and goblet cell hyperplasia. Some previous studies indicated similar structural abnormalities in the ileal villi in Cryptosporidium infection in mice, rats, pigs, cats, man, lambs, and calves (26–28, 37, 38). Goblet cell hyperplasia could be a sign of infection because the cells have a crucial role in producing antimicrobial antibodies (39). Similarly, the villi from infected/extract-treated mice exhibited decreased atrophy and hyperplasia and enhanced architectural symmetry (26, 27). Conversely, some examined extracts could not restore the symmetry of ileal villi and mucosa (28).
The anti-coccidial activity of Artemisia species has been demonstrated previously (40). Artemisia species contain considerable amounts of artemisinin and polymethoxy flavonoids that they are important for the overall bioactivity of Artemisia plants (41). Like A. indica and A. abrotanum and some other species, artemisinin was not detected in A. spicigera extract (42, 7). Also, artemisinin and its derivatives previously showed ineffectiveness against C. parvum and exhibited toxicity when given approximately 200 mg/kg to neonatal mice intra-rectally or subcutaneously (43). In this way, Artemisia species may have alternative sources of other useful compounds with diverse modes of action.
All solvent extracts of A. indica were found to contain typical poly methoxy flavonoids, and the highest levels of these contents were detected in the EtOH extract (42). In A. abrotanum L., a range of several flavonoids, coumarins and sesquiterpenes have been detected (7). In essential oil and extracts of A. spicigera, significant amounts of phenol, flavonoids contents have been detected (14, 15, 44). A positive result was seen between total phenol and flavonoid levels of samples and the free radical scavenging activity potential (44, 28). Phenolic compounds are the major antioxidants of some herbal extracts like Propolis against Cryptosporidium (28). Antibacterial and antifungal activity of A. spicigera is related to high levels of these compounds (11, 14), found in this study as major compounds in As –EtOH extract. Therefore the activity of A. spicigera ethanolic extract against Cryptosporidium may be owing to its rich contents of phenolic compounds and flavonoids, demonstrated to be responsible for the anti-protozoal effect by enhancing oxidative defense mechanisms.
Conclusion
According to the pharmacological properties resulted from A. spicigera C. Koch in the current study, we proposed that it is a safe and effective treatment for cryptosporidiosis in a mouse model. Considering that Artemisia is native in Iran, and because of its easy and cheap accessibility, the use of this plant can propose. Nevertheless, more studies are necessary to assess the applications of Artemisia spp. as complementary medicine in the management of Cryptosporidium infections.
Acknowledgments
The authors are grateful to the University of Tabriz for financial supporting and Mahmoudi R. for technical and scientific assistance. This work was supported by the Research Deputy of the University of Tabriz (number: 2233405).
Footnotes
Conflicts of interest
The authors declare that there is no conflict of interests.
References
- 1.O’Donoghue PJ. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995; 25(2):139–95. [DOI] [PubMed] [Google Scholar]
- 2.Fayer R, Xiao L. (2nd ed)Cryptosporidium and Cryptosporidiosis. 2007; CRC Press, New York, USA. [Google Scholar]
- 3.Ryan U, Zahedi A, Paparin A. Cryptosporidium in humans and animals - a one health approach to prophylaxis. Parasite Immunol. 2016; 38(9):535–47. [DOI] [PubMed] [Google Scholar]
- 4.Hunter RP, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002; 15 (1): 145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theodos CM, Griffiths JK, D’Onfro J, et al. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob Agents Chemother. 1998; 42: 1959–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparks H, Nair G, Castellanos-Gonzalez A, et al. Treatment of Cryptosporidium: What We Know, Gaps, and the Way Forward. Curr Trop Med Rep. 2015; 2(3): 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellicera J, Saslis-Lagoudakisb CH, Carrióc E, et al. A phylogenetic road map to antimalarial Artemisia species. J Ethnopharmacol.2018; 225:1–9. [DOI] [PubMed] [Google Scholar]
- 8.Naghavi MR, Alaeimoghadam F, Ghafoori H. Artemisia Species from Iran as Valuable Resources for Medicinal Uses. International Scholarly and Scientific Research & Innovation. 2014; 8(11). [Google Scholar]
- 9.Willcox M. Improved traditional phytomedicines in current use for the clinical treatment of malaria. Planta Med. 2011; 77(6): 662–71. [DOI] [PubMed] [Google Scholar]
- 10.Juteau F, Masotti V, Bessiere JM, et al. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia. 2002; 73(6):532–5. [DOI] [PubMed] [Google Scholar]
- 11.Kordali S, Kotan R, Mavi A, et al. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J Agric Food Chem. 2005; 53(24):9452–8. [DOI] [PubMed] [Google Scholar]
- 12.Yildiz K, Basalan M, Duru O, et al. Antiparasitic efficiency of Artemisia absinthium on Toxocara cati in naturally infected cats. Turkiye Parazitol Derg. 2011; 35(1): 10–4. [DOI] [PubMed] [Google Scholar]
- 13.Shahbazi P, Arshadi S. Effect of Artemisia spicigera ethanolic extract on digestive system parasitic worms in mice. JSSU. 2018; 26(2): 141–150. [Google Scholar]
- 14.Chehregani A, Atri M, Yousefi S, et al. “Essential oil variation in the populations of Artemisia spicigera from northwest of Iran: chemical composition and antibacterial activity”. Pharm Biol. 2013; 51(2):246–52. [DOI] [PubMed] [Google Scholar]
- 15.Abad MJ, Bedoya LM, Apaza L, et al. The Artemisia L. genus: a review of bioactive essential oils. Molecules. 2012; 17(3):2542–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller TA, Schaeder FW. Changes in mouse circulating leukocyte numbers in C57BL/6 mice immunosuppressed with dexamethasone for Cryptosporidium parvum oocyst production. Vet Parasitol. 2007; 149(3–4): 147–57. [DOI] [PubMed] [Google Scholar]
- 17.Rossi P, Pozio E, Besse MG, et al. Experimental cryptosporidiosis in hamsters. J Clin Microbiol. 1990; 28(2):356–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omidian Z, Ebrahimzadeh E, Shahbazi P, et al. Application of recombinant Cryptosporidium parvum P23 for isolation and prevention. Parasitol Res. 2014; 113(1):229–37. [DOI] [PubMed] [Google Scholar]
- 19.Sturbaum GD, Reed C, Hoover PJ, et al. Species-specific, nested PCR-restriction fragment length polymorphism detection of single Cryptosporidium parvum oocysts. Appl Environ Microbiol. 2001; 67(6): 2665–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmoudi R, Amini K, Fakhri O, et al. Aroma profile and antimicrobial properties of alcoholic and aqueous extracts from root, leaf and stalk of nettle (Urtica dioica L). J Microbiol, Biotechnol Food Sci. 2014; 4(3): 220–224. [Google Scholar]
- 21.Ghajarbeygi P, Mohammadi A, Mahmoudi R, et al. Artemisia spicigera Essential Oil: Assessment of Phytochemical and Antioxidant Properties. Biotechnol Health Sci. 2015; 2(4): 11–16. [Google Scholar]
- 22.Jenkins MC, O‘Brien C, Trout J, et al. Hyperimmune bovine colostrum specific for recombinant Cryptosporidium parvum antigen confers partial protection against cryptosporidiosis in immunosuppressed adult mice. Vaccine. 1999; 17(19):2453–60. [DOI] [PubMed] [Google Scholar]
- 23.Perruci S, Fichi G, Buggiani C, et al. Efficacy of mangiferin against Cryptosporidium parvum in a neonatal mouse model. Parasitol Res. 2006; 99(2):184–8. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi C, Yokoyama H, Neguyen S A, et al. Effect of egg yolk antibody on experimental Cryptosporidium parvum infection in SCID mice. Vaccine. 2004; 23(2):232–5. [DOI] [PubMed] [Google Scholar]
- 25.Kim CH, Healey JM. Effects of pine bark extract administered to immunosuppressed adult mice infected with Cryptosporidium parvum. Am J Chin Med. 2001; 29(3–4): 469–75. [DOI] [PubMed] [Google Scholar]
- 26.Gaafar MR. Efficacy of Allium sativum (garlic) against experimental cryptosporidiosis. Alexandria J Med. 2012; 48: 59–66. [Google Scholar]
- 27.Al-Mathal EM, Alsalem AM. Pomegranate (Punica granatum) peel is effective in a murine model of experimental Cryptosporidium parvum. Exp Parasitol. 2012; 131(3): 350–7. [DOI] [PubMed] [Google Scholar]
- 28.Soufy H, El-Beih MN, Nasr SM, et al. Effect of Egyptian Propolis on cryptosporidiosis in immunosuppressed rats with special emphasis on oocysts shedding, leukogram, protein profile and ileum histopathology. Asian Pac J Trop Med. 2017; 10(3): 253–262. [DOI] [PubMed] [Google Scholar]
- 29.Khater MM, El-Sayed SH, Yousof HAS, et al. Anti-Cryptosporidium efficacy of Olea europaea and Actinidia deliciosa in a neonatal mouse model. Kasr Al Ainy Medical Journal. 2017; 23: 32–37. [Google Scholar]
- 30.Asadpour M, Namazi F, Razavi SM.Comparative efficacy of curcumin and paromomycin against Cryptosporidium parvum infection in a BALB/c model. Vet Parasitol. 2018; 250: 7–14. [DOI] [PubMed] [Google Scholar]
- 31.Asadpour M, Namazi F, Razavi SM, et al. Curcumin: A promising treatment for Cryptosporidium parvum infection in immunosupressed BALB/c mice. Exp Parasitol. 2018; 195:59–65. [DOI] [PubMed] [Google Scholar]
- 32.Healey M, Yang C, Rasmussen S, et al. Therapeutic efficacy of paromomycin in immunosuppressed adult mice infected with Cryptosporidium parvum. J Parasitol. 1995; 81(1):114–6. [PubMed] [Google Scholar]
- 33.Fareed G, Scolaro M, Jordan W, et al. The use of a high-dose garlic preparation for the treatment of Cryptosporidium parvum diarrhea. Int Conf AIDS. 1996; 11:288–92. [Google Scholar]
- 34.Riad NHA, Taha HA, Mahmoud YI. Effects of garlic on albino mice experimentally infected with Schistosoma mansoni: A parasitological and ultrastructural study. Trop Biomed. 2009; 26(1):40–50. [PubMed] [Google Scholar]
- 35.Enemark HL, Bille-Hansen V, Lind P, et al. Pathogenicity of Cryptosporidium parvum-evaluation of an animal infection model. Vet Parasitol. 2003; 113(1):35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sloper KS, Dourmashkin RR, Bird RB, et al. Chronic malabsorption due to cryptosporidiosis in a child with immunoglobulin deficiency. Gut. 1982; 23(1): 80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poonacha K B, Pippin C. Intestinal Cryptosporidiosis in a cat. Vet Pathol. 1982; 19: 708–10. [DOI] [PubMed] [Google Scholar]
- 38.Klein P, Kleinova T, Volek Z, et al. Effect of Cryptosporidium parvum infection on the absorptive capacity and paracellular permeability of the small intestine in neonatal calves. Vet Parasitol. 2008; 152(1–2): 53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourlioux P, Koletzko B, Guarner F, et al. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium ‘The Intelligent Intestine’, held in Paris, June 14, 2002. Am J Clin Nutr. 2003; 78(4): 675–83. [DOI] [PubMed] [Google Scholar]
- 40.Pirali Kheirabadi K, Kaboutari Katadj J, Bahadoran S, et al. Comparison of the anticoccidial effect of granulated extract of Artemisia sieberi with monensin in experimental coccidiosis in broiler chickens. Exp Parasitol. 2014; 141:129–33. [DOI] [PubMed] [Google Scholar]
- 41.Klayman DL, Lin AJ, Acton N, et al. Isolation of artemisinin (qinghaosu) from Artemisia annua growing in the United States. J Nat Prod. 1984; 47: 715–717. [DOI] [PubMed] [Google Scholar]
- 42.Tasdemir D, Tierney M, Sen R, et al. Antiprotozoal Effect of Artemisia indica Extracts and Essential Oil. Planta Med. 2015; 81(12–13):1029–37. [DOI] [PubMed] [Google Scholar]
- 43.Fayer R, Ellis W. Qinghaosu (artemisinin) and derivatives fail to protect neonatal BALB/c mice against Cryptosporidium parvum (Cp) infection. J Eukaryot Microbiol. 1994; 41(5):41S. [PubMed] [Google Scholar]
- 44.Afshar FH, Delazar A, Nazemiyeh H, et al. Comparison of the total phenol, flavonoid contents and antioxidant activity of methanolic extracts of Artemisia spicigera and A. splendens growing in Iran. Pharmaceutical sciences. 2012; 18(3), 165–170. [Google Scholar]