Abstract
Background:
Toxoplasma gondii is a zoonotic obligatory intracellular protozoan parasite that infects a wide range of warm-blooded species. This study aimed to obtain further information on the role of T. gondii infection in ruminant abortion (sheep, goats and cattle) using bioassay and PCR methods in Mazandaran province, northern Iran.
Methods:
Overall, 104 aborted fetuses (52 bovine, 48 ovine, 4 caprine) were collected at different stages of gestation during the lambing seasons in various parts of Mazandaran Province from Mar 2016 to May 2017. Brains of 104 aborted fetuses were bioassayed in female BALB/c mice. DNA was extracted from all brain samples using phenol-chloroform-isoamyl Alcohol instructions. RE gene was used for detection all of T. gondii DNA by conventional PCR assay.
Results:
The results of the bioassayed samples were negative because no tachyzoites or cyst were observed in the peritoneal and brain specimens of the mice. The detection of T. gondii DNA was confirmed by observation of a 529 bp band in 15 out of 104 fetuses (14.4%). The highest prevalence rate of T. gondii detected from sheep (16.6%) followed by cattle (13.4%) and goats (0%). The highest prevalence of the infection was observed in east area, while the lowest prevalence of the infection was observed in west area.
Conclusion:
T. gondii infection may partly be responsible for abortion and economic losses in livestock husbandry in this region. Therefore, further additional researches such as genotyping T. gondii and designing control strategies for improving management in livestock flocks are necessary.
Keywords: Toxoplasma gondii, Aborted fetuses, Sheep, Goats, Cattle, Iran
Introduction
Toxoplasma gondii is a zoonotic obligatory intracellular protozoan parasite that infects a wide range of warm-blooded species (1). Infection usually occurs transplacentally or through ingestion of T. gondii oocysts excreted from infected cats or consumption of undercooked meat containing the parasite cysts and drinking water contaminated with T. gondii oocysts (2). Thus, the oocysts can act as risk factor for the occurrence of abortions and stillbirths associated with T. gondii infection in domestic animals (3). Contaminated animals are usually asymptomatic. However, when infection transplacentally is transmitted from mother to fetus, the protozoan can cause early abortion, stillbirth, fetal mummification, and thus, impose heavy financial burden to animal-husbandry industry.
The accurate diagnosis of abortion is difficult due to the presence of multiple etiological agents and the advanced stages of autolysis in the many fetuses submitted for diagnosis (4). Diagnosis of this parasite is based on molecular detection, bioassay, histopathology and serological examinations. The majority of epidemiological studies dealing with this pathogen in ruminants have been based on serological diagnostic techniques (5, 6). Molecular assays are considered as a specific and sensitive tool used to diagnose small quantities of target DNA. The RE gene because of its high sensitivity and specificity was applied for detection T. gondii (7).
Taking these considerations into account, the purpose of this study was to obtain further information on the role of T. gondii infection in ruminant abortion (sheep, goats and cattle) using bioassay and PCR methods in Mazandaran province, northern Iran.
Materials and Methods
Study area and sample collection
Mazandaran Province is near the Caspian Sea, in northern region of Iran. The climate is temperature humid with an annual rainfall of 500 mm and an average temperature of 17 ºC with moderate summer and winter owing to the proper conditions; in this region of Iran is considered as the most important area for developing of the livestock industry.
In this study, 104 aborted fetuses (52 bovine, 48 ovine, 4 caprine) were collected randomly at different stages of gestation during the lambing seasons in various parts of Mazandaran province from Mar 2016 to May 2017. Each of the samples was submitted to the Mazandaran Provincial Veterinary Department Lab for diagnosis of abortion reasons. A complete necropsy was performed on all submitted fetuses; the skull was also opened under aseptic condition (disinfecting skull surface with 70% ethanol, sterile instruments for cutting, removing tissues, and sterile glass dishes to collect samples) and the brain samples (e.g., cortex, midbrain, medulla, and cerebellum) were subjected for the detection of T. gondii using bioassay and PCR tests.
Ethical issues
All investigations reported here were performed in strict accordance with the guidelines approved by the Animal Ethics Committee of our faculty (No. IR.MAZUMS.REC.96.3018).
Laboratory tests
Bioassay
Brains of 104 aborted fetuses were bioassayed in female BALB/c mice in Institute for Experimental Animal Research, Mazandaran University of Medical Science, Sari, Iran. Briefly, 50 g of each brain were homogenized into the blender and incubated with an acid pepsin solution for 1 h at 37 °C, then filtered through two layers of gauze, pelleted by centrifugation for 10 min at 1200 g, the sediment neutralized and suspended in 5–10 mL antibiotic solution (100 international units (IU/ml) penicillin and 745 IU/ml streptomycin). One ml of this homogenate was inoculated subcutaneously into two mice and also some of them was frozen and stored at −20 °C for performance of PCR. Inoculated mice were followed for 60 d (8, 9). Meanwhile each mouse died, its perituan fluid and brain tissue were examined for detection of tachyzoite and tissue cyst by direct smear and Giemsa staining method. Finally, 60 d after inoculation, all alive mice were also killed and their brains examined for tachyzoite and tissue cyst (9).
DNA extraction and molecular assay
DNA extraction was performed an all brain tissue samples (n=104) collected from aborted fetuses and brains of inoculated mice. For both types of tissues, we used the same protocol (10). Briefly, 80–100 μl homogenized brain samples was transferred to a 2 mL eppendorf tube and were added 800 of DNA digestion buffer (50 mM Tris-HCL, pH8.0; 25 mM EDTA and 400 mM NaCl), 100 μl 10% SDS, 30 μl proteinase K (20 μg/μl) (Fermentas, Hanover, MD, USA) then were vortexed. Suspension was incubated at 55 °C until the tissue was completely lysed (3 h). After that, 300 μl of 6 M NaCl was added to the suspension to precipitate the proteins and cellular debris and kept at 4 °C (15 min). Centrifugation was done at 13,000 g for 15 min, then 500 μl of the supernatant was transferred to a new Eppendorf tube and was added an equal volume of phenol–chloroform–isoamyl alcohol (25:24:1). Following this step, DNA was precipitated in 100 μl sodium acetate and 800 μl cold 100% ethanol and was placed at –70 °C (60 min). Following centrifugation at 13,000 g for 5 min, the columns were then washed twice with 70% ethanol. Finally, the pellet was dried and solubilized in 50 μl TE buffer (10 mM Tris-HCl, 1mM EDTA). Its concentration was estimated by UV spectrophotometric absorbance at 280/260 nm and stored at −20 °C prior to PCR analysis.
The target gene for PCR method was a repetitive 529 bp DNA sequence (Gene RE). Nucleotide sequences of the primers used to detect T. gondii are TOX4; (CGCTGCAGGGAGGAAGACGAAAGTTG) and TOX5; (CGCTGCAGACACAGTGCATCTGGATT). DNA fragments of RE gene were amplified by PCR at a final volume of 25 μl, containing 12.5 μl of commercial premix (Ampliqon, Denmark), 1 μl of total DNA, 0.6 μl of each primer (10 pmol/μl) (Bioneer, Korea) and 10.3 μl of PCR H2O. The reaction mixtures carried out in thermal cycler (BioRad C1000, USA) under the following condition: at 93 °C for five min as initial denaturation fallowed by 35 cycles at 93 °C for 30 sec as denaturation, 55 °C for 30 sec as annealing, 72 °C for 30 sec as extension, followed by a final extension at 72 °C for five minutes. For each reaction, a negative control (1 μl DDW instead of DNA) and a positive control (T. gondii DNA, Accession No: KT715444) were also used. Electrophoretic analysis of the amplified products was performed on a 1.5% agarose gel stained with safe stain under Ultraviolet light (7).
Results
Overall, 104 brain samples were collected from aborted fetuses including 52 cattle, 48 sheep, and 4 goats and from herbs in northern Iran. The mean of age aborted fetuses was 5.1 ± 2.03 months in cattle, 3.3 ± 0.99 in sheep and 3.1 ± 1.03 in goat.
All 208 mice inoculated with brain tissues remained asymptomatic, and tissue cysts were not found in their brains by microscopic observation when euthanized at the termination of experiment.
The detection of T. gondii DNA was confirmed by observation of a 529 bp band in 1.5% electrophoresis gel in 15 out of 104 fetuses (14.4%) (Fig. 1).
Fig. 1:
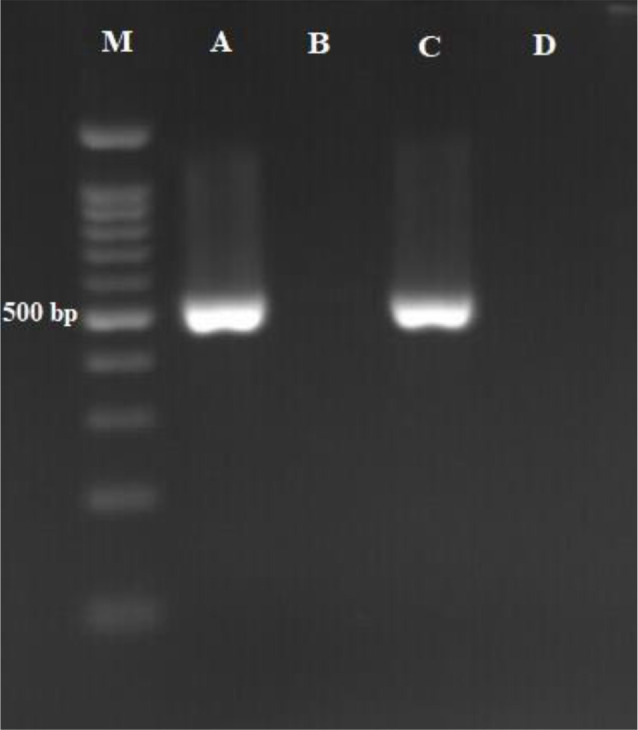
Representative Lanes for T. gondii. Lane M, 100 bp DNA marker (GeneDirex, Cat.No DMoo3); Lane A, Positive control; Lane B, Negative control; Lane C, Positive sample; Lane D, Negative sample
The molecular prevalence rate of T. gondii in sheep (16.6%) was higher than in cattle (13.4%) (Table 1). The highest prevalence of the infection in sheep and cattle was observed in east area of northern Iran, while the lowest prevalence in sheep and cattle was seen in west area (Table 2).
Table 1:
Summary of prevalence of T. gondii in aborted fetuses using PCR from Mazandaran, Iran
| Type of ruminants | No. samples | No. (%) positive |
|---|---|---|
| Cattle | 52 | 7 (13.4) |
| Sheep | 48 | 8 (16.6) |
| Goats | 4 | 0 (0) |
| Total | 104 | 15 (14.4) |
Table 2:
Relationship between prevalence T. gondii and geographical in aborted fetuses from Mazandaran, Iran
| Animals | Type of area | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| East | Center | West | ||||||
| No. | No. Pos (%) | No. | No. Pos (%) | No. | No. Pos (%) | |||
| Cattle | 16 | 3 (18.7) | 26 | 3 (11.5) | 10 | 1 (10) | 52 | 7 (13.4) |
| Sheep | 10 | 2 (20) | 30 | 5 (16.6) | 8 | 1 (12.5) | 48 | 8 (16.6) |
| Goats | 2 | 0 (0) | 2 | 0 (0) | - | - | 4 | 0 (0) |
| Total | 28 | 5 (17.8) | 59 | 8 (13.5) | 18 | 2 (11.1) | 104 | 15 (14.4) |
Discussion
High fetal mortality rate of ruminants is a major cause of economic losses in farming industry especially in developing countries such as Iran (11). In the past decades, T. gondii was not thought to cause significant clinical disease such as fetal mortality in ruminants under field conditions. However today, the congenital toxoplasmosis as a cause of reproductive disorders in sheep and goats (12, 13) has been recognized to occur worldwide. Diagnosis of ruminant’s toxoplasmosis is usually based on serological assay, histopathological examination and isolation of parasite via mouse inoculation (14, 15).
Most of epidemiological studies on T. gondii have been done based on extensive serological tests in all over the world including Iran. Molecular assays are able to detect small quantities of target DNA and is a specific and sensitive technique used to diagnose parasite-specific DNA such as T. gondii (16).
In current study, we used PCR method to amplify a 529 bp fragment of RE gene marker for the diagnosis of T. gondii because of its high sensitivity and specificity (7). According to molecular diagnosis, we observed T. gondii DNA in 15 brain tissue samples of aborted fetuses (14.4%). In this area, parasite was detected by RE gene, where 18.6% (13/70) of ruminant abortions were found positive (17). The prevalence rate of T. gondii in small ruminant aborted fetuses in Iran is variable; 13.5% in Khorasan Razavi, 66% in Qazvin Provinces using PCR method and with aim to amplification of B1 gene marker, and 64% in aborted lambs in Ardebil by the GRA6 gene (18–20).
Our literature review and a comparison of our study with other studies, independently of the used diagnostic technique, shows that the prevalence of T. gondii is different in the world. For example, prevalence rate of T. gondii in small ruminants’ aborted fetuses was 0% in Nigeria (21), 10% in Australia (22), 14% to 18% in USA (23), 23.1% in Spain (24) and 14%–32% in New Zealand (25). The reasons for these differences could be climate and environmental factors and kind of diagnostic methods.
According to our knowledge, the moderate climate is more favorable conditions to the development and maintenance of T. gondii oocysts in the environment. Oocysts of this parasite can survive in the environment for several months, depending on moisture and temperature. The viability of T. gondii oocysts is reliant on the moisture and temperature of the soil and environment (26). Higher prevalence of ovine toxoplasmosis has been reported in the humid and temperate areas compared to dry areas (27–29). Therefore, in an area, suitable weather conditions such as humidity and temperature are the most important factors in the occurrence of small ruminants’ toxoplasmosis.
Another important effective factor on the prevalence of T. gondii in ruminant aborted fetuses is used diagnostic method. In diagnosis of livestock aborted fetuses, there are mainly three main methods such as PCR, pathology and bioassay. In the molecular method, several gene markers are used, referred to as B1 and RE (529 bp). RE gene marker is repeated 200 to 300 times in the genome of T. gondii, while the B1 marker has 35 folds. Therefore, the use of PCR method with RE gene marker is very sensitive and can have a significant effect on the results of the studies (7, 30).
In our study, the highest prevalence rates of T. gondii were observed in the aborted ovine and bovine fetuses, respectively (Table 1). These results are agreement with a study conducted in Italy (31).
The clinical signs of small ruminants’ toxoplasmosis in pregnant animals are influenced by the age and immune system status of fetus. During the first trimester of pregnancy in small ruminants, fetal mortality due to various parasitic infections increases when the immune system is relatively immature. In the middle of pregnancy, the infection may lead to the birth of an immature, weakened, or fetal mummified animal, while infection in a postnatal pregnancy may result in the birth of live, clinically normal but infected small ruminants (32–35).
Conclusion
There is widespread exposure of livestock to T. gondii in northern Iran. T. gondii infection may partly be responsible for abortion and economic losses in livestock husbandry in this region. Therefore, further additional researches such as genotyping of T. gondii and designing of control strategies for improving management in livestock flocks are necessary.
Acknowledgements
This work was supported by grant (No. 3088) from the Deputy of Research, Mazandaran University of Medical Sciences, Sari, Iran. We gratefully acknowledge the supporting the Toxoplasmosis Research Center (TRC) at Mazandaran University of Medical Sciences.
Footnotes
Conflict of interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
References
- 1.Dubey JP. Toxoplasmosis of animals and humans. CRC press; 2016. (2nd ed) CRC Press, Boca Raton, Florida, pp. 1–313. [Google Scholar]
- 2.Heidari H, Gharekhani J, Tavoosidana G. Role of toxoplasmosis in abortion of ewes in western Iran: a serological study. Sci Parasitol. 2013; 14(2):99–103. [Google Scholar]
- 3.Dubey JP, Schares G. Neosporosis in animals—the last five years. Vet Parasitol. 2011; 180(1–2):90–108. [DOI] [PubMed] [Google Scholar]
- 4.Tenter AM. Toxoplasma gondii in animals used for human consumption. Mem Inst Oswaldo Cruz. 2009; 104(2):364–9. [DOI] [PubMed] [Google Scholar]
- 5.Dubey JP. Toxoplasmosis in sheep—the last 20 years. Vet Parasitol. 2009; 163(1–2):1–14. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Wang Z-D, Huang S-Y, et al. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasit Vectors. 2015; 8:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homan W, Vercammen M, De Braekeleer J, et al. Identification of a 200-to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR1. Int J Parasitol. 2000; 30(1):69–75. [DOI] [PubMed] [Google Scholar]
- 8.De Sousa S, Ajzenberg D, Canada N, et al. Biologic and molecular characterization of Toxoplasma gondii isolates from pigs from Portugal. Vet Parasitol. 2006;135(2):133–6. [DOI] [PubMed] [Google Scholar]
- 9.Dubey JP. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet Parasitol. 1998; 74(1):75–7. [DOI] [PubMed] [Google Scholar]
- 10.Biase FH, Franco MM, Goulart LR, et al. Protocol for extraction of genomic DNA from swine solid tissues. Genet Mol Biol. 2002; 25(3):313–5. [Google Scholar]
- 11.Keshavarzi H, Sadeghi-Sefidmazgi A, Kristensen AR, et al. Abortion studies in Iranian dairy herds: I. Risk factors for abortion. Livest Sci. 2017; 195:45–52. [Google Scholar]
- 12.Munday B, Mason R. Toxoplasmosis as a cause of perinatal death in goats. Aust Vet J. 1979; 55(10):485–7. [DOI] [PubMed] [Google Scholar]
- 13.Hartley W, Marshall S. Toxoplasmosis as a cause of ovine perinatal mortality. N Z Vet J. 1957; 5(4):119–24. [Google Scholar]
- 14.Garcia JL, Gennari SM, Machado RZ, et al. Toxoplasma gondii: detection by mouse bioassay, histopathology, and polymerase chain reaction in tissues from experimentally infected pigs. Exp Parasitol. 2006; 113(4):267–71. [DOI] [PubMed] [Google Scholar]
- 15.da Silva AV, Langoni H. The detection of Toxoplasma gondii by comparing cytology, histopathology, bioassay in mice, and the polymerase chain reaction (PCR). Vet Parasitol. 2001; 97(3):191–8. [DOI] [PubMed] [Google Scholar]
- 16.Wastling J, Nicoll S, Buxton D. Comparison of two gene amplification methods for the detection of Toxoplasma gondii in experimentally infected sheep. J Med Microbiol. 1993; 38(5):360–5. [DOI] [PubMed] [Google Scholar]
- 17.Amouei A, Sharif M, Sarvi S, et al. Aetiology of livestock fetal mortality in Mazandaran province, Iran. PeerJ. 2019;6:e5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rassouli M, Razmi G, Bassami M, et al. Study on ovine abortion associated with Toxoplasma gondii in affected herds of Khorasan Razavi Province, Iran based on PCR detection of fetal brains and maternal serology. Parasitology. 2011; 138(6):691–7. [DOI] [PubMed] [Google Scholar]
- 19.Habibi G, Imani A, Gholami M, et al. Detection and identification of Toxoplasma gondii type one infection in sheep aborted fetuses in Qazvin Province of Iran. Iran J Parasitol. 2012; 7(3):64–72. [PMC free article] [PubMed] [Google Scholar]
- 20.Shahbazi G, Rad NH, Madani R, et al. Toxoplasma gondii in Aborted Fetuses of Sheep in Ardebil Area, North-West of Iran. Iran J Parasitol. 2019; 14(3): 430–435. [PMC free article] [PubMed] [Google Scholar]
- 21.Kamani J, Egwu G, Mani A, et al. Survey of Toxoplasma gondii DNA in aborted Ovine and Caprine fetuses by nested PCR in Borno state, Nigeria. Vet World. 2010; 3(8):360–3. [Google Scholar]
- 22.Plant J, Beh K, Acland HM. laboratory findings form ovine abortion and perinatal mortality. Aust Vet J. 1972; 48(10):558–61. [DOI] [PubMed] [Google Scholar]
- 23.Dubey JP, Kirkbride C. Toxoplasmosis and other causes of abortions in sheep from north central United States. J Am Vet Med Assoc. 1990; 196(2):287–90. [PubMed] [Google Scholar]
- 24.Pereira-Bueno J, Quintanilla-Gozalo A, Pérez-Pérez V, et al. Evaluation of ovine abortion associated with Toxoplasma gondii in Spain by different diagnostic techniques. Vet Parasitol. 2004; 121(1–2):33–43. [DOI] [PubMed] [Google Scholar]
- 25.Charleston W. Toxoplasma and other protozoan infections of economic importance in New Zealand. N Z J Zool. 1994; 21:67–81. [Google Scholar]
- 26.Lélu M, Villena I, Dardé M-L, et al. Quantitative estimation of the viability of Toxoplasma gondii oocysts in soil. Appl Environ Microbiol. 2012; 78(15):5127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarado-Esquivel C, Campillo-Ruiz F, Liesenfeld O. Seroepidemiology of infection with Toxoplasma gondii in migrant agricultural workers living in poverty in Durango, Mexico. Parasit Vectors. 2013; 6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamidinejat H, Goraninejad S, Ghorbanpoor M, et al. Role of Toxoplasma gondii in abortion of ewes in Ahvaz (South-West Iran). Bull Vet Inst Pulawy. 2008; 52(3):369–71. [Google Scholar]
- 29.Andrade MMC, Carneiro M, Medeiros AD, et al. Seroprevalence and risk factors associated with ovine toxoplasmosis in Northeast Brazil. Parasite. 2013; 20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fallahi S, Kazemi B, Bandehpour M, et al. Comparison of the RE and B1 gene for detection of Toxoplasma gondii infection in children with cancer. Parasitol Int. 2014; 63(1): 37–41. [DOI] [PubMed] [Google Scholar]
- 31.Masala G, Porcu R, Daga C, et al. Detection of pathogens in ovine and caprine abortion samples from Sardinia, Italy, by PCR. J Vet Diagn Invest. 2007; 19(1):96–8. [DOI] [PubMed] [Google Scholar]
- 32.Buxton D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet Res. 1998; 29(3–4):289–310. [PubMed] [Google Scholar]
- 33.Innes EA, Bartley PM, Buxton D, et al. Ovine toxoplasmosis. Parasitology. 2009; 136(14):1887–94. [DOI] [PubMed] [Google Scholar]
- 34.Scott P, Sargison N, Wilson D. The potential for improving welfare standards and productivity in United Kingdom sheep flocks using veterinary flock health plans. Vet J. 2007; 173(3):522–31. [DOI] [PubMed] [Google Scholar]
- 35.Danehchin L, Razmi G, Naghibi A. Molecular detection of Toxoplasma gondii infection in aborted fetuses in sheep in Khorasan Razavi province, Iran. Iran J Vet Med. 2017; 11(2):147–53. [Google Scholar]


