Abstract
The purpose of this study was to report our institutional experience with patients with COVID-19 who developed acute limb ischemia during hospitalization and to determine the characteristics and clinical outcomes. Between March 2020 and January 2021, we treated 3 patients who were COVID-19–positive and developed acute limb ischemia after they received thromboprophylaxis. We performed an embolectomy by exposing the popliteal artery below the knee to treat an occlusion of the popliteal and tibial arteries. An infusion of unfractionated heparin was initiated immediately after surgery, maintaining a partial thromboplastin time ratio > 2.5 times the normal value and transferred the patients to the intensive care unit. However, after these patients developed recurrent acute limb ischemia in the same leg, we decided to perform an embolectomy of popliteal and tibial arteries at the ankle and created an arteriovenous fistula (AVF) with tibial veins using polypropylene 7-0. The first patient died from pneumonia after 3 weeks in the intensive care unit; at that time, the foot was viable with triphasic flow in the distal posterior tibial artery and the AVF was patent. The second and third patients are doing well, they can walk without any problems, and the tibial arteries and AFV were patent on duplex ultrasound after 6 months. The AVF allowed part of the flow of tibial arteries to divert into the small veins of the foot that have a low resistance to maintain patency of tibial vessels, despite a hypercoagulable state and extensive thrombotic microangiopathy in patients with COVID-19.
1. Introduction
The novel coronavirus, SARS-CoV-2, emerged in late 2019 and the resulting illness, COVID-19, was declared a pandemic by the World Health organization on March 11, 2020 [1]. Coagulopathy, in the form of venous and arterial thromboembolism, is considered as one of the most severe sequelae of the disease. This phenomenon has been found even in the presence of anticoagulation and has been prognostic of worse outcomes [2]. The incidence of arterial thrombosis in patients with COVID-19 who require hospitalization ranges between 3% and 15%, according to a systematic review of the literature [3]. Reports of arterial thrombosis manifesting as ischemic stroke, myocardial infarction, aortic thrombus, and acute limb ischemia (ALI), as well as arterial thrombi in unusual sites resulting in acute mesenteric ischemia and splenic infarct, have been described [4], [5], [6], [7].
Several pathophysiologic mechanisms have been implicated in the hypercoagulable state that leads to diffuse thrombosis, including direct viral-related endothelial injury and viral invasion of endothelial cells via angiotensin-converting enzyme 2 receptors; excessive cytokine release is postulated to cause severe illness and activation of endothelium, monocytes, and neutrophils; leukocyte- and cytokine-mediated platelet activation, unchecked complement activation, and more recently, elevated Von Willebrand factor antigen to ADAMTS13 (a disintegrin and metallo-proteinase with a thrombospondin type 1 motif, member 13) activity ratio [8], [9], [10], [11]. Several clinical reports have demonstrated evidence of a thrombotic microangiopathy associated with COVID-19 infection. ALI is defined as a sudden decrease in arterial perfusion of an extremity associated with a threat to the viability of the affected extremity. ALI is a surgical emergency and can cause major tissue or limb loss or death if not recognized promptly [6,7]. ALI occurs in approximately 1.5 per 10,000 patients per year. In a report from New York City during the COVID-19 pandemic, ALI occurred 5 times more frequently in COVID-19–positive patients compared with patients who were COVID-19–negative [5]. Characteristics associated with patients with COVID-19 who present with ALI include male sex and presence of at least one cardiovascular risk factor, including hypertension, diabetes, obesity, and cigarette smoking [12], [13], [14].
The purpose of this study was to report on a surgical technique for the management of ALI associated with COVID-19 infection.
2. Methods
Between March 2020 and January 2021, three consecutive patients hospitalized with COVID-19 at Treviso Hospital who developed ALI during hospitalization despite antithrombotic prophylaxis were treated with thromboembolectomy and distal arteriovenous fistula (AVF). All 3 patients presented with thrombosis of the popliteal and tibial arteries in the setting of Rutherford IIb ALI [15]. Urgent embolectomy was performed by exposing the popliteal artery below the knee. Drawing from experience that several patients with COVD-19 developed recurrent ALI requiring reoperation, we decided to perform a distal AVF on the tibial arteries and veins at the ankle, in addition to transpopliteal and tibial embolectomy at the ankle. The AVF is an established technique for improving bypass patency when small tibial vessels are heavily diseased and anastomosis of a bypass graft to these vessels is associated with diminished graft patency rates [16], [17], [18], [19], [20]. The failure mode in this situation is thought to be related to the high-resistance, low-flow state seen in the bypass, which contributes to graft occlusion. The fashioning of an AVF at the distal anastomosis is hypothesized to counteract this flow-limiting state [21,22].
Likewise, we hypothesized that extensive thrombotic microangiopathy at the foot causes high peripheral resistance, leading to re-occlusion after open thrombectomy in patients with COVID-19–associated ALI. For creating the AVF, we used the “common ostium” technique, mobilizing approximately 2 cm of the anterior and/or posterior tibial artery and vein, performing a parallel arteriotomy and venotomy about 6 to 7 mm. The side to side anastomosis of the artery to the vein is established with a continuous 7-0 polypropylene suture. The time to perform the AVF is approximately 10 to 15 minutes and the diameter of tibial vein must be at least 2 mm in diameter.
2.1. Case 1
The first COVID-19 patient with ALI was a man in his 60s with hypertension and an otherwise unremarkable medical history. He presented with acute right leg pain, cyanosis of the forefoot, and motor and sensory impairment, and was found to have acute occlusion of the popliteal and tibial arteries (Rutherford score IIb). An embolectomy was performed by exposing the popliteal artery below the knee and removing fresh thrombus. Anterior and posterior tibial pulses were restored at the end of the case. An infusion of unfractionated heparin was initiated immediately after surgery, maintaining a partial thromboplastin time ratio > 2.5 times the normal value. Two days later, the patient was transferred to the intensive care unit (ICU) because of worsening of COVID-19 pneumonia, during which time he developed recurrent ALI in the same leg, despite being on therapeutic anticoagulation. We performed a cut-down and embolectomy on the popliteal and tibial arteries. Palpable pulses were present at the end of the operation and heparin infusion was initiated after surgery. Eight hours later, however, the patient developed recurrent ALI for a third time, again involving the popliteal and tibial arteries. With a working diagnosis of no-reflow phenomenon (due to thrombosis of the small vessels of the foot), we decided to create a distal AVF at the same time as repeating embolectomy of the popliteal and tibial arteries. We prepared the posterior tibial artery and vein at the ankle and performed an embolectomy of the plantar arch and created an AVF with tibial vein using 7-0 polypropylene. It was not possible to perform AVF on the anterior tibial artery and vein because the patient's respiratory status was deteriorating and he required immediate transfer from the operating room to the ICU. Therapy with heparin was continued perioperatively. Unfortunately, the patient died from COVID-19 pneumonia after 3 weeks in the ICU. At the time of his death, the foot was viable with triphasic flow in the distal posterior tibial artery and the AVF was patent based on duplex ultrasound.
2.2. Case 2
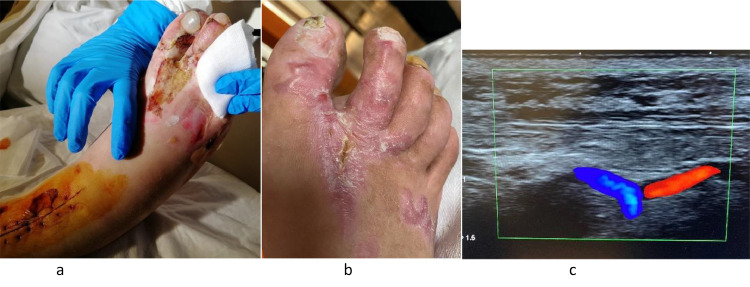
The second patient with COVID-19 with ALI was an obese man in his 50s who presented with pneumonia and acute right leg ischemia with rest pain, cyanosis of the forefoot, and motor and sensory impairment 3 days after hospitalization (Rutherford score IIb). He underwent a transpopliteal embolectomy below the knee of the left leg, pulling out fresh thrombus and, after surgery, had triphasic flow in the tibial arteries on duplex ultrasound. He was transferred to the ICU because of worsening of COVID-19–related pneumonia, and therapy with unfractionated heparin was started. The day after surgery, ALI recurred on the same leg. This time, it was not possible to perform surgery because he was critically ill from pneumonia. After a 2-day delay, the patient's clinical situation improved and he underwent a transpopliteal embolectomy below the knee, embolectomy of the tibial arteries at the ankle, embolectomy of the pedal arteries of the foot, and an AVF between both the anterior tibial vein and artery and posterior tibial vein and artery at the ankle. Unfractionated heparin was continued through surgery, with the addition of an iloprost infusion for 7 days, after which unfractionated heparin was replaced with low-molecular-weight heparin and iloprost continued for 3 weeks. The patient was discharged from the ICU after 3 weeks and ultimately discharged from the hospital after 2 months on acetylsalicylic acid 100 mg monotherapy. At the time of discharge, the foot was viable with triphasic flow in the distal anterior and posterior tibial arteries on duplex ultrasound. He developed a foot drop related to peroneal nerve injury. The ulcers of the forefoot, developed due to protracted ischemia, healed within 2 months. At 3-month clinic follow-up, he was walking without a brace, the wounds were healed, and AVF was patent on duplex ultrasound (Fig. 1 ).
Fig. 1.
(A) The foot of patient 15 days after revascularization. (B) The foot of the same patient after 3 months, completely healed. (C) Outpatient duplex ultrasound during clinic follow-up showing patent arteriovenous fistula between anterior tibial artery and vein on the foot.
2.3. Case 3
The third patient with COVID-19 with ALI was a man in his 70s who was an active smoker but had no other risk factors. He developed ALI of the right leg a few days after admission for COVID-19, despite receiving prophylaxis with low-molecular-weight heparin; he had pain and motor and sensory impairment of the toes (Rutherford score IIb). He underwent thromboendarterectomy for in situ thrombosis of the superficial femoral artery. The soft clot removed was more consistent with a hypercoagulable state than traditional atherosclerotic disease. A transpopliteal embolectomy was performed below the knee, along with embolectomy of the posterior tibial artery at the ankle, retrieving fresh thrombus. At the end of the surgery, he had a palpable posterior tibial artery pulse and was started on therapeutic low-molecular-weight heparin. Five days later, recurrent ALI developed with popliteal occlusion affecting the same leg. We performed a popliteal embolectomy below the knee and posterior tibial artery at the ankle. We also explored the anterior tibial artery at the ankle, but it was chronically occluded, so we performed an AVF between the posterior tibial artery and vein. We discharged the patient with warfarin after 10 days. The patient had triphasic flow in the distal posterior tibial arteries and the AVF was patent on duplex ultrasound at the time of his discharge and at clinic follow-up.
2.4. Follow-up
Our follow-up protocol consists of diagnostic Doppler ultrasonography and clinical evaluation at 1, 3, and 6 months postoperatively. The 2 patients who survived their hospitalization were seen in clinic for regular follow-up and are doing well. At 6-month follow-up, both patients can walk without claudication and their tibial arteries and AVF were patent on duplex ultrasound. The lesions of the toes in the second patient healed in 2 months.
3. Discussion
Low rates of successful revascularization and high mortality in patients with COVID-19 with ALI have been reported due to systemic illness, hypercoagulable state, and extensive thrombotic microangiopathy that involves extrapulmonary organs [23]. Patients with COVID-19 do not have typical causes of ALI and the vessels at surgery tend to be relatively healthy with no atherosclerotic disease [2]. Treatment methods for ALI in general include surgical treatment (such as thromboembolectomy and bypass surgery), endovascular treatment (such as catheter-directed thrombolysis, percutaneous thrombus aspiration, and stent placement), and hybrid treatment that combines both therapies. A meta-analysis of randomized controlled trials performed in 2018 investigated whether surgical or endovascular treatment (catheter-directed thrombolysis) should be performed as the first-line of treatment for ALI. Although there was no significant difference between the 2 groups in terms of limb salvage and mortality rates, the endovascular treatment group showed a significantly higher incidence of stroke and bleeding within 30 days of treatment [24]. Furthermore, although thrombolysis using tissue plasminogen activator remains an effective treatment option for patients presenting with mild and moderate lower extremity ALI, according to Rutherford classification, patients with poor pedal outflow may benefit from alternative revascularization strategies [25]. Our 3 patients presented with Rutherford IIb ALI and we performed emergent surgical embolectomy to resolve the ischemia. Our group in Treviso is very comfortable with surgical revascularization techniques and we sought to avoid catheter-directed thrombolysis, given the current paucity of high-quality data to support catheter-directed thrombolysis in patients with COVID-19.
The usual indications for creating an adjunct AVF is the use of prosthetic or nonautologous biological graft to perform femorotibial reconstructions for atherosclerotic disease. If a borderline saphenous vein is used and the runoff is poor or otherwise compromised, use of an AVF can be beneficial [18]. Contraindications for performing AVF include undersized venous comitantes (smaller than the artery) and a poor deep venous system, such as phlebosclerosis and/or deep vein thrombosis. The location of the fistula is most frequently performed in the middle and distal one-third of the limb to any one of the tibial vessels, although exceptions are possible. The AVF enables modulation of blood inflow into a high-resistance, low-capacitance circulatory bed. As a consequence of the additional flow into the low-resistance, high-capacitance venous circulation, augmentation of blood flow (volume and velocity) occurs above the critical thrombotic threshold velocity level of the bypass. During the immediate postoperative period, mean estimated blood flow through the bypass was 264 mL/min, through the AVF was 157 mL/min, and through the distal artery was 19 mL/min. Unlike an AVF created at the level of the distal bypass anastomosis, a remote distal AVF not only increases graft blood flow but augments native arterial blood flow and improves distal perfusion. One study used capillary microscopy to allow an objective evaluation of clinical outcomes on follow-up. In a patient with an AFV, the red blood cell velocity, peak red blood cell velocity, and time to peak red blood cell velocity were significantly higher compared with those in patients without an AVF. In addition, the individual data showed that microcirculatory blood flow correlated with patency of the graft [26].
We applied our experience using AVF to improve patency of peripheral femorotibial bypass in patients with COVID-19–associated ALI with no-reflow phenomenon. The AVF performed at the ankle diverts tibial artery flow into the small veins of the foot that have a low resistance, which helps maintain patency when runoff is compromised, as occurs with COVID-19–related microangiopathy. Our small case series reports anecdotal success with distal AVF in the setting of recurrent COVID-19 ALI. Based on our early success with this technique, we believe that creation of an AVF can be considered as an adjunctive option to maintain arterial patency in patients with COVID-19–associated ALI.
Declaration of Competing Interest
The authors have no conflict of interest to declare in relation to the this work.
REFERENCES
- 1.Warkentin TE, Kaatz S. COVID-19 versus HIT hypercoagulability. Thromb Res. 2020;196:38–51. doi: 10.1016/j.thromres.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou-Ismail MY, Diamond A, Kapoor S, et al. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management [published correction appears in Thromb Res 2021;204:146] Thromb Res. 2020;194:101–105. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19: acute limb ischemia. UpToDate. Accessed July 31, 2021. Available at: https://www.uptodate.com/contents/covid-19-acute-limb-ischemia
- 4.Stephen E, Al-Adawi SSH, Abdelhady I, et al. Managing vascular surgery emergencies and referrals during the COVID-19 pandemic at a tertiary centre in Oman. Sultan Qaboos Univ Med J. 2021;21:e116–e119. doi: 10.18295/squmj.2021.21.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass DI, Meyer RM, Barros G, et al. The impact of the COVID-19 pandemic on cerebrovascular disease. Semin Vasc Surg. 2021;34:20–27. doi: 10.1053/j.semvascsurg.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma H, George S. Early left ventricular thrombus formation in a COVID-19 patient with ST-elevation myocardial infarction. Case Rep Cardiol. 2020;2020 doi: 10.1155/2020/8882463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilonzo N, Judelson D, Al-Jundi W, et al. A review of acute limb ischemia in COVID-positive patients. Semin Vasc Surg. 2021;34:8–12. doi: 10.1053/j.semvascsurg.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joly BS, Darmon M, Dekimpe C, et al. Imbalance of von Willebrand factor and ADAMTS13 axis is rather a biomarker of strong inflammation and endothelial damage than a cause of thrombotic process in critically ill COVID-19. J Thromb Haemost. 2021;19:2193–2198. doi: 10.1111/jth.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martín-Rojas RM, Chasco-Ganuza M, Casanova-Prieto S, et al. A mild deficiency of ADAMTS13 is associated with severity in COVID-19: comparison of the coagulation profile in critically and noncritically ill patients [published online ahead of print July 23, 2021]. Blood Coagul Fibrinolysis doi: 10.1097/MBC.0000000000001068. [DOI] [PMC free article] [PubMed]
- 11.Jayarangaiah A, Kariyanna PT, Chen X, et al. COVID-19-associated coagulopathy: an exacerbated immunothrombosis response. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620943293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etkin Y, Conway AM, Silpe J, et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City Area. Ann Vasc Surg. 2021;70:290–294. doi: 10.1016/j.avsg.2020.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozzani A, Arici V, Tavazzi G, et al. Acute arterial and deep venous thromboembolism in COVID-19 patients: risk factors and personalized therapy. Surgery. 2020;168:987–992. doi: 10.1016/j.surg.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dardik H, Berry SM, Dardik A, et al. Infrapopliteal prosthetic graft patency by use of the distal adjunctive arteriovenous fistula. J Vasc Surg. 1991;13:685–691. [PubMed] [Google Scholar]
- 17.Neville RF, Dy B, Singh N, et al. Distal vein patch with an arteriovenous fistula: a viable option for the patient without autogenous conduit and severe distal occlusive disease. J Vasc Surg. 2009;50:83–88. doi: 10.1016/j.jvs.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 18.Dardik H, Silvestri F, Alasio T, et al. Improved method to create the common ostium variant of the distal arteriovenous fistula for enhancing crural prosthetic graft patency. J Vasc Surg. 1996;24:240–248. doi: 10.1016/s0741-5214(96)70099-x. [DOI] [PubMed] [Google Scholar]
- 19.Paty PS, Shah DM, Saifi J, et al. Remote distal arteriovenous fistula to improve infrapopliteal bypass patency. J Vasc Surg. 1990;11:171–178. [PubMed] [Google Scholar]
- 20.Ricco JB, Gauthier JB, Richer JP, et al. Remote arteriovenous fistula with infrapopliteal polytetrafluoroethylene bypass for critical ischemia. Ann Vasc Surg. 1991;5:525–528. doi: 10.1007/BF02015276. [DOI] [PubMed] [Google Scholar]
- 21.Aherne T, Kheirelseid E, O'Neill D, et al. The use of arteriovenous fistula as an adjunct to peripheral arterial by-pass: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2016;51:707–717. doi: 10.1016/j.ejvs.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Dardik H. Update on the role of the distal arteriovenous fistula as an adjunct for improving graft patency and limb salvage rates after crural revascularization. Ann Vasc Surg. 2015;29:1022–1028. doi: 10.1016/j.avsg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Ng JJ, Choong AMTL. Thromboembolic events in patients with SARS-CoV-2. J Vasc Surg. 2020;72:760–761. doi: 10.1016/j.jvs.2020.04.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obara H, Matsubara K, Kitagawa Y. Acute limb ischemia. Ann Vasc Dis. 2018;11:443–448. doi: 10.3400/avd.ra.18-00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne RM, Taha AG, Avgerinos E, et al. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014;59:988–995. doi: 10.1016/j.jvs.2013.10.054. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs MJ, Reul GJ, Gregoric ID, et al. Creation of a distal arteriovenous fistula improves microcirculatory hemodynamics of prosthetic graft bypass in secondary limb salvage procedures. J Vasc Surg. 1993;18:1–9. doi: 10.1067/mva.1993.41521. [DOI] [PubMed] [Google Scholar]