Abstract
Cumulative evidence shows that gut microbiome can influence brain function and behavior via the inflammatory processes. However, the role of interaction between gut dysbiosis and C-reactive protein (CRP) in the development of anxiety and depression remains to be elucidated. In this study, a total of 3321 independent single nucleotide polymorphism (SNP) loci associated with gut microbiome were driven from genome-wide association study (GWAS). Using individual level genotype data from UK Biobank, we then calculated the polygenetic risk scoring (PRS) of 114 gut microbiome related traits. Moreover, regression analysis was conducted to evaluate the possible effect of interaction between gut microbiome and CRP on the risks of Patient Health Questionnaire-9 (PHQ-9) (N = 113,693) and Generalized Anxiety Disorder-7 (GAD-7) (N = 114,219). At last, 11 candidate CRP × gut microbiome interaction with suggestive significance was detected for PHQ-9 score, such as F_Ruminococcaceae (β = − 0.009, P = 2.2 × 10–3), G_Akkermansia (β = − 0.008, P = 7.60 × 10–3), F_Acidaminococcaceae (β = 0.008, P = 1.22 × 10–2), G_Holdemanella (β = − 0.007, P = 1.39 × 10–2) and O_Lactobacillales (β = 0.006, P = 1.79× 10–2). 16 candidate CRP × gut microbiome interaction with suggestive significance was detected for GAD-7 score, such as O_Bacteroidales (β = 0.010, P = 4.00× 10–4), O_Selenomonadales (β = − 0.010, P = 1.20 × 10–3), O_Clostridiales (β = 0.009, P = 2.70 × 10–3) and G_Holdemanella (β = − 0.008, P = 4.20 × 10–3). Our results support the significant effect of interaction between CRP and gut microbiome on the risks of anxiety and depression, and identified several candidate gut microbiomes for them.
Keywords: Gut microbiome, C-reactive protein (CRP), Depression, Anxiety
Introduction
As common psychiatric disorders, the amount of people with depression and anxiety has increased over the past several decades leading to a growing concern in mental health research around the world [1]. According to the report of WHO, the global population suffering from depression was estimated to be 322 million, while anxiety disorders affected more than 260 million people, accounting for 4.4% and 3.6% of the global population respectively that resulted in a surge in suicide rates as well as a huge social and economic burden [2–4]. However, there are elusive pathogenesis and lackluster treatments in depression and anxiety.
Various gut microbiome in the human intestine harbors forms a symbiotic relationship with the host and plays a vital role in both health and disease [5]. The dysbiosis of gut microbiome has been closely linked to increased risks of mental disorders [6]. The findings for microbiome-gut-brain axis indicated a complex multiorgan bidirectional signaling system between the gut microbiome and the brain [7]. Thereby, gut microbiome has the potential to influence brain activity and ultimately, mental health. It is demonstrated that host-associated microbial communities could affect basic developmental processes of the brain through the immune, metabolic or endocrine systems directly or indirectly [8]. Besides, growing evidence indicated that alterations in the gut microbiome were associated with anxiety and depressive disorders [9–11]. For example, changes in the gut microbiome were likely to modulate the expression of the gut-derived peptides which were widely expressed in the brain and played well-established roles in the neurobiology of anxiety and depression [12]. Fecal transplants from anxious-type mice into a more resilient strain increasing anxiety-like behaviors in the resilient strain, and vice versa [13]. Individuals with depression could be identified from healthy subjects by single nucleotide exact amplicon sequence variants of gut microbiome [9].
As an acute-phase protein, C-reactive protein (CRP) is associated with both pro-inflammatory and anti-inflammatory properties [14, 15]. It plays a role in the recognition and clearance of foreign pathogens and damaged cells [16]. CRP also could activate the classic complement pathway and phagocytic cells [16]. The associations between inflammation and multiple psychiatric disorders are clinically relevant. Parallel neural, humoral, and cellular interoceptive pathways can transmit inflammatory mediators to the brain to trigger alterations in mood and cognition motivation, and amplify behavioral stress responses [17]. Inflammatory markers are well-known etiological factors for psychiatric disorders, which could promote sickness behavior [5, 18]. CRP is a marker of acute phase response which has been used most extensively as a measure of low-grade inflammation in psychiatric and physical conditions [19]. Increased peripheral blood CRP has been related to reduced functional connectivity between the left ventral striatum and ventromedial prefrontal cortex that correlated with the severity of anhedonia [20]. People with symptoms of depression or anxiety frequently have an increased level of CRP [21–23]. However, the biological mechanism of CRP affecting the development of psychiatric disorders remains largely unknown now.
Gut microbiome affects inflammation status. Certain species of gut microbiome could produce specific enzymes that enable fermentation of nutrients into absorbable forms, including that of indigestible carbohydrates into short-chain fatty acids (SCFAs) which may have anti-inflammatory and immunomodulatory [24]. In addition to specific enzymes produced, some components of the bacteria, such as lipopolysaccharide (LPS), cell capsule carbohydrates and other endotoxins, may release and result in inflammatory response in the host [24]. The activation of innate immune response leads to chronically high levels of inflammation mediators that are known to cause diseases, including a broad spectrum of psychiatric diseases [25]. These inflammation mediators, in turn, attacked bacteria, causing gut dysbiosis. Therefore, the relationship between gut microbiome and inflammation is very complicated. For example, certain gut microbiome alterations (or disturbances) could secrete a pro-inflammatory zinc-dependent metalloprotease toxin and lead to colitis with severe inflammation and overproduction of interleukin-17, a central regulator of inflammation and autoimmunity [26]. There was also evidence linking high levels of IL-17 to depression [27]. A pecious study found the proportion of Akkermansia muciniphila declined in obese mice with elevated plasma levels of CRP [24]. The abundance of Faecalibacterium was inversely correlated with levels of CRP [28]. However, whether CRP modulates the gut microbiome, or whether the gut microbiome contributes to CRP elevation and its exact mechanism remains unclear now. Further explorations are needed to draw a definitive conclusion.
In this study, data from UK biobank were applied to evaluate the influence of interactions between CRP and gut microbiome on anxiety and depression. Based on the significant single nucleotide polymorphisms (SNPs) associated with gut microbiome, we calculated PRS firstly. Then conducted linear regression to evaluate the influence of CPRxgut microbiome interactions on the risks of anxiety and depression.
Materials and methods
UK Biobank cohort
Our study utilized the UK Biobank cohort (https://www.ukbiobank.ac.uk/), a prospective cohort study with a number of physical, health, and genetic data from approximately 500,000 individuals aged 40–69. This large-scale biomedical database includes detailed lifestyle information as well as blood, urine, and saliva samples of participants. The UK Biobank genetic data contains genotypes of 488,377 participants. These were assayed using the UK BiLEVE Axiom array and UK Biobank Axiom array. Marker-based quality control was performed by using statistical tests designed primarily to check for consistency of genotype calling across experimental factors to identify poor quality markers. SNPs with calling rate < 98.5%, MAF < 0.01 were removed. Samples with calling rate < 98.0% and mismatch between inferred sex and self-reported sex were removed. Imputation was carried out by IMPUTE4 (https://jmarchini.org/software/). Details of the array design, genotyping, and quality control procedures have been described previously [29]. All data usage in this article is approved by UK Biobank (application 46,478) and the Ethics Advisory Committee (EAC).
CRP measures in UK Biobank
Our study contains 376,802 participants from UK Biobank with CRP data. The CRP was measured by immunoturbidimetric—high sensitivity analysis on a Beckman Coulter AU5800 when the participants were recruited and consent.
Definition of depression and anxiety
In this study, two common psychiatric disorders were analyzed, including depression and anxiety. We measured depression based on Patient Health Questionnaire-9 (PHQ-9) which is a classification algorithm used to screen for and measure depression severity [30]. It focuses on nine depressive symptoms and signs, for example, Lack of interest or pleasure in doing things 20,514, Recent feelings of depression 20,510, Trouble falling or staying asleep, or sleeping too much 20,517, etc. The total score of it is 0–27. Meanwhile, anxiety severity was measured by general anxiety disorder-7 (GAD-7) with a total score (0–21) [31]. It focuses on seven anxious symptoms and signs, for example, recent feelings or nervousness or anxiety 20,506, Recent inability to stop or control worrying 20,509, Recent worrying too much about different things 20,520, etc. We provide a detailed definition in the supplement. PHQ-9 score and GAD-7 score were used as continuous variables in this study.
GWAS data of gut microbiome
The GWAS summary data sets of gut microbiome were derived from a recent large-scale study which included 114 gut microbiome related traits [32]. Briefly, they carried out the 515F/806R primer pair to amplify the V4 region of the 16S rRNA gene for Flemish Gut Flora Project (FGFP) cohort individuals at first. Then carried out sequencing on the Illumina HiSeq platform. Fastq sequences were further analyzed per sample using the DADA2 pipeline (v.1.6). Linear models were fit with age, sex and the top ten principle components as covariates, along with each microbial trait analyzed in the GWAS. Genotyping was conducted on two different arrays—the Human Core Exome v1.0 and the Human Core Exome v1.1. For quality control, the SNPs with call rate < 95%, MAF < 0.01 and Hardy–Weinberg equilibrium deviations P < 1 × 10–5 were removed. FGFP genotype data was phased using SHAPEIT3 and imputed with IMPUTE2 using UK10K and all 1000 Genome Project phase 3 samples as the reference panel. After association analyses, 3,321 LD independent loci associated with 16S gut microbiome phenotypes were identified. Specific for this study, the SNPs with P < 1.0 × 10−4 were selected for subsequent PRS analysis. Details of the array design, genotyping, and quality control procedures have been described previously [32].
Gut microbiome related PRS calculation and association analysis
In this study, we calculated the gut microbiome related PRS of each subject by using individual SNP genotype data of the UK Biobank. Based on self-reported ethnicity (UK Biobank data field: 21,000), the individuals were restricted to only “White British”. Let PRSn denote the PRS value of gut microbiome for the nth subject, defined as:
where l denotes the total number of gut microbiome analyzed in this study; Ei denotes the effect size of significant gut microbiome associated SNPi; Din denotes the dosage of the risk allele of the ith SNP for the nth individual (0 is coded for homozygous protective genotype, 1 for heterozygous and 2 for homozygous polymorphic genotypes) [33]. We used PLINK 2.0 to perform the PRS analysis. Then established a linear regression model to evaluate the possible associations among each gut microbiome PRS, CRP, and two psychiatric disorders by R software (https://www.r-project.org/). The PRSs of gut microbiome, CRP, and interaction of them were set as instrumental variables. PHQ-9 score or GAD-7 score were the outcomes. Age, sex, Townsend deprivation index, and 10 principal components of population structure were used as covariates. In this study, the significant association thresholds should be P < 2.19 × 10–4 [0.05/(114 × 2)] after strict Bonferroni correction. The suggestive significance threshold was set as P < 0.05.
Results
Descriptive characteristics of study participants
For the PHQ-9 score, 113,693 participants were selected; 55.7% of them were women, mean age was 56.23 years, and mean PHQ-9 score (SD) was – 2.71 (3.64). For the GAD-7 score, 114,219 participants were selected; 55.7% of them were women, mean age was 56.22 years, and mean GAD-7 score (SD) was − 0.28 (1.05).
Interactions of gut microbiome and CRP for PHQ-9 score
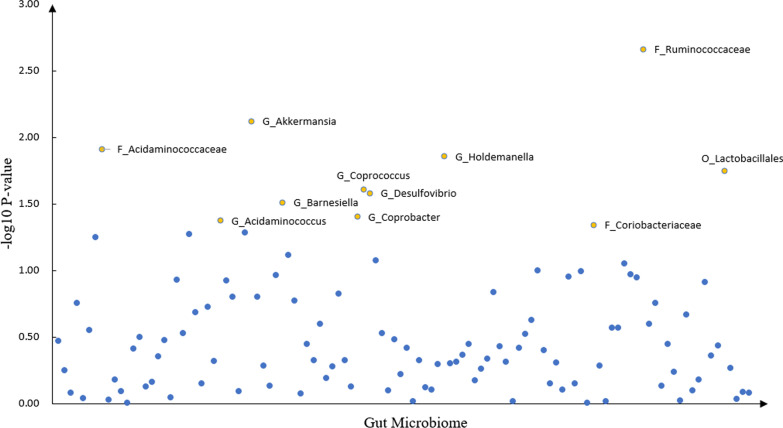
We detected 11 CRP × gut microbiome interaction with suggestive significance for PHQ-9 score, such as F_Ruminococcaceae (β = − 0.009, P = 2.2 × 10–3), G_Akkermansia (β = − 0.008, P = 7.60 × 10–3), F_Acidaminococcaceae (β = 0.008, P = 1.22 × 10–2), G_Holdemanella (β = − 0.007, P = 1.39 × 10–2) and O_Lactobacillales (β = 0.006, P = 1.79 × 10–2). The details were shown in Table 1 and Fig. 1.
Table 1.
Association between PHQ score and GUT microbiota × CRP
| Instrumental | GUT microbiota × CRP | ||
|---|---|---|---|
| GUT microbiota | |||
| Beta | T | P-value | |
| F_Ruminococcaceae | − 0.009 | − 3.07 | 0.0022 |
| G_Akkermansia | − 0.008 | − 2.67 | 0.0076 |
| F_Acidaminococcaceae | 0.008 | 2.51 | 0.0122 |
| G_Holdemanella | − 0.007 | − 2.46 | 0.0139 |
| O_Lactobacillales | 0.006 | 2.37 | 0.0179 |
| G_Coprococcus | − 0.007 | − 2.25 | 0.0246 |
| G_Desulfovibrio | 0.007 | 2.22 | 0.0263 |
| G_Barnesiella | − 0.006 | − 2.16 | 0.0309 |
| G_Acidaminococcus | 0.006 | 2.03 | 0.0422 |
| G_Coprobacter | 0.005 | 2.06 | 0.0394 |
| F_Coriobacteriaceae | − 0.006 | − 2.00 | 0.0455 |
O order, F family, G genus
Fig. 1.
The scatter plot of the gut microbiome interacting with CRP in depression
Interactions of gut microbiome and CRP for GAD-7 score
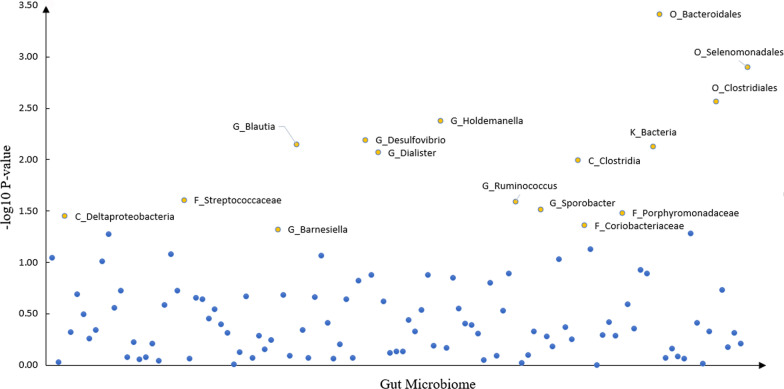
We detected 16 CRP × gut microbiome interaction with suggestive significance for anxiety GAD-7 score, like O_Bacteroidales (β = 0.010, P = 4.00 × 10–4), O_Selenomonadales (β = − 0.010, P = 1.20 × 10–3), O_Clostridiales (β = 0.009, P = 2.70 × 10–3) and G_Holdemanella (β = − 0.008, P = 4.20 × 10–3). The details were shown in Table 2 and Fig. 2.
Table 2.
Association between GAD score and GUT microbiota × CRP
| Instrumental | GUT microbiota × CRP | ||
|---|---|---|---|
| GUT microbiota | |||
| Beta | T | P-value | |
| O_Bacteroidales | 0.010 | 3.55 | 0.0004 |
| O_Selenomonadales | − 0.010 | − 3.23 | 0.0012 |
| O_Clostridiales | 0.009 | 3.00 | 0.0027 |
| G_Holdemanella | − 0.008 | − 2.86 | 0.0042 |
| G_Desulfovibrio | 0.008 | 2.73 | 0.0064 |
| G_Blautia | 0.008 | 2.69 | 0.0071 |
| K_Bacteria | 0.008 | 2.68 | 0.0074 |
| G_Dialister | − 0.008 | − 2.63 | 0.0085 |
| C_Clostridia | − 0.008 | − 2.57 | 0.0101 |
| G_Ruminococcus | − 0.006 | − 2.23 | 0.0255 |
| F_Streptococcaceae | 0.007 | 2.25 | 0.0248 |
| G_Sporobacter | − 0.007 | − 2.16 | 0.0307 |
| F_Porphyromonadaceae | 0.006 | 2.13 | 0.0330 |
| C_Deltaproteobacteria | − 0.006 | − 2.10 | 0.0354 |
| F_Coriobacteriaceae | − 0.006 | − 2.02 | 0.0436 |
| G_Barnesiella | − 0.006 | − 1.98 | 0.0478 |
K kingdom, P phylum, C class, O order, F family, G genus
Fig. 2.
The scatter plot of the gut microbiome interacting with CRP in anxiety
Common Interactions for both anxiety and depression
We also compared the above association analysis results, found 4 common CRP × gut microbiome interactions for both PHQ-9 score and GAD-7 score: G_Holdemanella (β = − 0.007, P = 1.43 × 10–2 for depression and β = − 0.008, P = 4.30 × 10–3 for anxiety), G_Desulfovibrio (β = 0.007, P = 2.64 × 10–2 for depression and β = 0.008, P = 6.30 × 10–3 for anxiety), F_Coriobacteriaceae (β = − 0.006, P = 4.57 × 10–2 for depression and β = − 0.005, P = 4.46 × 10–2 for anxiety) and G_Barnesiella (β = − 0.006, P = 3.16 × 10–2 for depression and β = − 0.006, P = 4.96 × 10–2 for anxiety).
Discussion
Although previous studies have found the functional relevance of gut microbiome and CRP with the development of anxiety and depression [34, 35], the biological mechanism underlying the effects of interaction between gut microbiome and CRP on the risks of anxiety and depression remains to be elucidated [36]. In this study, we explored the interaction between CRP and 114 gut microbiome-related traits and observed a significant interaction between them for depression and anxiety.
Inflammation takes an indirect role in modulating brain function. For example, several gut microbiomes ferment dietary fibers, producing SCFAs to promote the expression of anti‐inflammatory IL‐10 in macrophages and intestinal dendritic cells to avoid trigger diseases [37–39]. SCFAs also regulate the permeability of the blood–brain barrier and microglia homeostasis [25]. Furthermore, the gut microbiome serves as a barrier to enteropathogen infection [40]. Intestinal permeability defects are believed to be the basis for the chronic low-grade inflammation observed in stress-related psychiatric disorders [21]. Psychological stress activates the hypo-thalamus-pituitary-adrenal axis and results in increased intestinal permeability allowing increased translocation of LPS or Gram-negative bacteria [41, 42]. Once translocated into the lymph nodes or beyond, IgA and IgM responding to the LPS and other antigens of Gram-negative bacteria may be mounted [42]. This peripheral inflammation then can spread to the central nervous system (CNS) in various ways and thus affect mental health by promoting neurotoxins and hindering neurotransmitters [41]. Therefore, some neurological disorders share a common etiology involving gut dysbiosis [41]. As a marker of peripheral and CNS inflammation [43], CRP may be also activated by gut dysbiosis. However, its exact mechanism remains unclear now. Further explorations are needed to draw a definitive conclusion.
In this study, we found 11 significant taxons associated with PHQ-9 score, such as Ruminococcaceae, Akkermansia, Lactobacillales, and Coprococcus. Ruminococcaceae is the most significant taxon associated with PHQ-9 score and could produce SCFAs. Previous studies found Ruminococcaceae was associated with disorders of the CNS [39, 44]. Compared with APOE4 carriers, higher levels of Ruminococcaceae in APOE2/E3 genotype carriers were one of the strongest prevalent risk factors for neuropathology and Alzheimer’s disease [44]. Akkermansia muciniphila (Akk bacteria) could degrade mucin, which is negatively related to inflammation and metabolic disorders [45, 46]. It is demonstrated that genus Akkermansia and family Akkermansiaceae were consistently changed in both idiopathic rapid-eye-movement sleep behavior disorder and Parkinson’s disease [47]. In addition, microbial community profiling revealed reduction (e.g. Akkermansia, Lactobacillus) in the Adrenocorticotrophic hormone-induced depression rat model [48]. Anti-inflammatory properties have been displayed in several strains of Lactobacillus in vitro in human intestinal epithelial cells [49]. Lactobacillus was implicated in gut-brain communication and had positive effects on stress and cognition [50]. Coprococcus was related to the activity of the dopamine pathway, and also led to the production of butyrate [51]. Loss of bacteria that produce the anti-inflammatory, barrier-strengthening molecule butyrate, could lead to a loss of protection against epithelial inflammation and gut barrier disruption [52]. Furthermore, Coprococcus was associated with higher quality of life indicators and was also depleted in depression [53].
We also found 16 significant taxons associated with GAD-7 score. Bacteroidales is the most common microbial category in the human gut. It takes significant roles in metabolic pathways and immune system [54]. Previous studies reported that acquired inter bacterial defense gene clusters in Bacteroidales species reside in the human gut microbiome. In a mouse model, taking oral human commensal Bacteroides fragilis corrected gut permeability, altered gut microbiome composition, and ameliorated defects in communicative, stereotypic, anxiety-like, and sensorimotor behaviors [55]. Besides, in the healthy human colon, Bacteroidales accounted for the majority of the Gram-negative bacteria [56]. It was demonstrated that neuropsychiatric disorders were accompanied by higher serum IgM/IgA response to LPS of Gram-negative bacteria [42]. Individuals with major depressive disorder (MDD) showed enriched species for Bacteroides and depleted species for Blautia [54]. Furthermore, Blautia can mediate beneficial anti-inflammatory effects [54].
We observed 4 gut microbiome PRS interacting with CRP were associated with both PHQ-9 score and GAD-7 score in our study, which may be related to the pathophysiology of anxiety and depression through the communication of peripheral inflammation to the brain. For example, 3-hydroxyoctadecaenoic acid (C18-3OH) is an agonist of peroxisome proliferator activated receptor gamma. The production of it by bacteria could be one of the mechanisms implicated in the anti-inflammatory properties of probiotics. In addition, C18-3OH correlated with an increase in the abundance in Holdemanella [57]. In a previous animal study, higher loading of Holdemanella and Desulfovermiculus were found in Obsessive–compulsive patients [58]. The over-representation of Desulfovibrio is associated with gut mucosal injury and inflammatory pathology through releasing hydrogen sulfide [58]. In addition, Desulfovibrio competes with butyrate-producing bacteria for the lactate which results in the production of higher amounts of propionic acid [59]. This phenomenon led to autism-like manifestations in animals [59]. Moreover, previous studies also observed higher abundance of Desulfovibrio in MDD [11].
To the best of our knowledge, this is a novel study to explore the relationship between psychiatric disorders and the interaction of gut microbiome and CRP. Our study is based on a large cohort study with a long follow-up as well as representative samples. However, several limitations should be pointed out. First, owing to all samples in this study are from European ancestry, the findings should be inferred to other races with caution. Second, the key elements that influence the accuracy of PRS for a specific trait are SNP heritability, genetic architecture, sample size of the discovery GWAS including insufficiently powered GWAS sample sizes for most complex traits, potential confounding in causal inference, and a lack of ancestral diversity. Due to the related loci relied on previous published GWAS, the results may be affected. Third, based on the results of multiple test corrections, we detected several suggestive associations (P < 0.05) for the effect of interaction between CRP and gut microbiome on the risks of anxiety and depression. Further studies are warranted to validate this finding and to explore its underlying mechanism.
In summary, our results support the significant effect of interaction between CRP and gut microbiome on the risks of anxiety and depression, and identified several candidate gut microbiomes for them. These findings may provide novel therapeutic targets for psychiatric disorders, and give insights into the mechanism of anxiety and depression. Further studies are eager to confirm our findings and clarify the more detailed mechanism of gut microbiome × CRP interaction in psychiatric disorders.
Acknowledgements
We thank Jing Ye, Xiaomeng Chu, Chujun Liang, Bolun Cheng for up-front data collation.
Abbreviations
- CRP
C-reactive protein
- SNP
Single nucleotide polymorphism
- GWAS
Genome-Wide Association Study
- PRS
Polygenetic risk scoring
- PHQ-9
Patient Health Questionnaire-9
- GAD-7
Generalized Anxiety Disorder-7
- SCFAs
Short-chain fatty acids
- LPS
Lipopolysaccharide
- EAC
Ethics Advisory Committee
- FGFP
Flemish Gut Flora Project
- CNS
Central nervous system
- Akk bacteria
Akkermansia muciniphila
- MDD
Major depressive disorder
- C18-3OH
3-Hydroxyoctadecaenoic acid
Authors' contributions
YC and FZ conceived and designed the study; YC and PM wrote the manuscript; All authors collected the data and SC carried out the statistical analyses; CL, CP, HZ, JZ, ZZ, YW and YJ made preparations for the manuscript at first. All authors reviewed and approved the final manuscript.
Funding
This study was supported by the National Natural Scientific Foundation of China (Grant Nos. 81922059), the Natural Science Basic Research Plan in Shaanxi Province of China (Grant No. 2017JZ024).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There’s no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yujing Chen and Peilin Meng have contributed equally to this work
References
- 1.Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, et al. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am J Psychiatry. 2017;174(7):640–648. doi: 10.1176/appi.ajp.2017.16010034. [DOI] [PubMed] [Google Scholar]
- 2.Hedegaard H, Curtin SC, Warner M. Suicide rates in the United States continue to increase. NCHS Data Brief. 2018;309:1–8. [PubMed] [Google Scholar]
- 3.Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Friedrich MJ. Depression is the leading cause of disability around the World. JAMA. 2017;317(15):1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 5.Ihekweazu FD, Versalovic J. Development of the pediatric gut microbiome: impact on health and disease. Am J Med Sci. 2018;356(5):413–423. doi: 10.1016/j.amjms.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21(6):738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota–gut–brain axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 8.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens BR, Roesch L, Thiago P, Russell JT, Pepine CJ, Holbert RC, et al. Depression phenotype identified by using single nucleotide exact amplicon sequence variants of the human gut microbiome. Mol Psychiatry. 2020 doi: 10.1038/s41380-020-0652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression: a systematic review. Clin Psychol Rev. 2021;83:101943. doi: 10.1016/j.cpr.2020.101943. [DOI] [PubMed] [Google Scholar]
- 12.Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. 2018;15(1):36–59. doi: 10.1007/s13311-017-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bear TLK, Dalziel JE, Coad J, Roy NC, Butts CA, Gopal PK. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv Nutr. 2020;11(4):890–907. doi: 10.1093/advances/nmaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32(4):274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 15.Brouillet S, Boursier G, Anav M, Gala A, Ferrieres-Hoa A, et al. C-reactive protein and ART outcomes: a systematic review. Hum Reprod Update. 2020;26(5):753–773. doi: 10.1093/humupd/dmaa012. [DOI] [PubMed] [Google Scholar]
- 16.Nehring SM, Goyal A, Bansal P, Patel BC. C reactive protein. Treasure Island: StatPearls; 2021. [Google Scholar]
- 17.Savitz J, Harrison NA. Interoception and inflammation in psychiatric disorders. Biol Psychiatry Cogn Neurosci Neuroimag. 2018;3(6):514–524. doi: 10.1016/j.bpsc.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–286. doi: 10.1016/j.pnpbp.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49(12):1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta ND, Stevens JS, Li Z, Gillespie CF, Fani N, Michopoulos V, et al. Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women. Soc Cogn Affect Neurosci. 2020;15(10):1046–1055. doi: 10.1093/scan/nsz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appleton J. The gut-brain axis: influence of microbiota on mood and mental health. Integr Med (Encinitas) 2018;17(4):28–32. [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. 2018;55(5):4195–4206. doi: 10.1007/s12035-017-0632-1. [DOI] [PubMed] [Google Scholar]
- 23.Jiang HY, Zhang X, Yu ZH, Zhang Z, Deng M, Zhao JH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130–136. doi: 10.1016/j.jpsychires.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The gut microbiota and inflammation: an overview. Int J Environ Res Public Health. 2020;17(20):1. doi: 10.3390/ijerph17207618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Generoso JS, Giridharan VV, Lee J, Macedo D, Barichello T. The role of the microbiota–gut–brain axis in neuropsychiatric disorders. Braz J Psychiatry. 2021;43(3):293–305. doi: 10.1590/1516-4446-2020-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed I, Roy BC, Khan SA, Septer S, Umar S. Microbiome, metabolome and inflammatory bowel disease. Microorganisms. 2016;4(2):1. doi: 10.3390/microorganisms4020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waisman A, Hauptmann J, Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015;129(5):625–637. doi: 10.1007/s00401-015-1402-7. [DOI] [PubMed] [Google Scholar]
- 28.Citronberg JS, Curtis KR, White E, Newcomb PA, Newton K, Atkinson C, et al. Association of gut microbial communities with plasma lipopolysaccharide-binding protein (LBP) in premenopausal women. ISME J. 2018;12(7):1631–1641. doi: 10.1038/s41396-018-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis KAS, Cullen B, Adams M, Brailean A, Breen G, Coleman JRI, et al. Indicators of mental disorders in UK Biobank-a comparison of approaches. Int J Methods Psychiatr Res. 2019;28(3):e1796. doi: 10.1002/mpr.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Hughes DA, Bacigalupe R, Wang J, Ruhlemann MC, Tito RY, Falony G, et al. Genome-wide associations of human gut microbiome variation and implications for causal inference analyses. Nat Microbiol. 2020;5(9):1079–1087. doi: 10.1038/s41564-020-0743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudbridge F. Polygenic epidemiology. Genet Epidemiol. 2016;40(4):268–272. doi: 10.1002/gepi.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lizano P, Lutz O, Xu Y, Rubin LH, Paskowitz L, Lee AM, et al. Multivariate relationships between peripheral inflammatory marker subtypes and cognitive and brain structural measures in psychosis. Mol Psychiatry. 2020;1:1–14. doi: 10.1038/s41380-020-00914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, Li J, Gui X, Shi X, Bao Z, Han H, et al. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol Psychiatry. 2020;25(11):2759–2772. doi: 10.1038/s41380-020-0729-1. [DOI] [PubMed] [Google Scholar]
- 36.Cathomas F, Murrough JW, Nestler EJ, Han MH, Russo SJ. Neurobiology of resilience: interface between mind and body. Biol Psychiatry. 2019;86(6):410–420. doi: 10.1016/j.biopsych.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 39.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017;4:14. doi: 10.1186/s40779-017-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peirce JM, Alvina K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223–1241. doi: 10.1002/jnr.24476. [DOI] [PubMed] [Google Scholar]
- 42.Simeonova D, Stoyanov D, Leunis JC, Carvalho AF, Kubera M, Murdjeva M, et al. Increased serum immunoglobulin responses to gut commensal Gram-negative bacteria in unipolar major depression and bipolar disorder type 1, especially when melancholia is present. Neurotox Res. 2020;37(2):338–348. doi: 10.1007/s12640-019-00126-7. [DOI] [PubMed] [Google Scholar]
- 43.Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2020;25(6):1301–1311. doi: 10.1038/s41380-018-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D'Amato A, Di Cesare ML, Lucarini E, Man AL, Le Gall G, Branca JJV, et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome. 2020;8(1):140. doi: 10.1186/s40168-020-00914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin J, Noh JR, Chang DH, Kim YH, Kim MH, Lee ES, et al. Elucidation of akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol. 2019;10:1137. doi: 10.3389/fmicb.2019.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract Res Clin Gastroenterol. 2017;31(6):637–642. doi: 10.1016/j.bpg.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Nishiwaki H, Hamaguchi T, Ito M, Ishida T, Maeda T, Kashihara K, et al. Short-chain fatty acid-producing gut microbiota is decreased in Parkinson's disease but not in rapid-eye-movement sleep behavior disorder. mSystems. 2020;5(6):1. doi: 10.1128/mSystems.00797-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song J, Ma W, Gu X, Zhao L, Jiang J, Xu Y, et al. Metabolomic signatures and microbial community profiling of depressive rat model induced by adrenocorticotrophic hormone. J Transl Med. 2019;17(1):224. doi: 10.1186/s12967-019-1970-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust N Z J Psychiatry. 2017;51(8):810–821. doi: 10.1177/0004867416686694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107(2):234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu RT, Rowan-Nash AD, Sheehan AE, Walsh RFL, Sanzari CM, Korry BJ, et al. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav Immun. 2020;88:308–324. doi: 10.1016/j.bbi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4(4):623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 54.Yang J, Zheng P, Li Y, Wu J, Tan X, Zhou J, et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci Adv. 2020;6(49):8555. doi: 10.1126/sciadv.aba8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coyne MJ, Comstock LE. Type VI secretion systems and the gut microbiota. Microbiol Spectr. 2019;7(2):7. doi: 10.1128/microbiolspec.PSIB-0009-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pujo J, Petitfils C, Le Faouder P, Eeckhaut V, Payros G, Maurel S, et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut. 2020;70:1088. doi: 10.1136/gutjnl-2020-321173. [DOI] [PubMed] [Google Scholar]
- 58.Scheepers IM, Cryan JF, Bastiaanssen TFS, Rea K, Clarke G, Jaspan HB, et al. Natural compulsive-like behaviour in the deer mouse (Peromyscus maniculatus bairdii) is associated with altered gut microbiota composition. Eur J Neurosci. 2020;51(6):1419–1427. doi: 10.1111/ejn.14610. [DOI] [PubMed] [Google Scholar]
- 59.El Aidy S, Ramsteijn AS, Dini-Andreote F, van Eijk R, Houwing DJ, Salles JF, et al. Serotonin transporter genotype modulates the gut microbiota composition in young rats, an effect augmented by early life stress. Front Cell Neurosci. 2017;11:222. doi: 10.3389/fncel.2017.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.