Abstract
Toxoid vaccines can provide protective immunity against clostridial diseases. Since the duration of the toxoid vaccine immunogenicity is short, these vaccines need to contain an adjuvant. The nanoparticles of chitosan can stimulate humoral and cell-mediated immune responses. In the present study, the effect of chitosan nanoparticles was investigated on the immunogenicity of the pentavalent clostridial toxoid vaccine containing Clostridium perfringens types D, C, and B, Clostridium septicum, as well as Clostridium novyi. Rabbits were immunized by two injections with 3-week intervals and checked clinically and through autopsy 2 weeks after the last injection. Hematological changes were investigated during immunization, including the changes of white and red blood cell counts, hemoglobin, packed cell volume, platelet, neutrophil, lymphocyte, eosinophil, basophile, monocyte, and Neut/Lymph. Biochemical factors, namely creatinine, blood urea nitrogen, glucose, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total protein, and albumin, were also studied. The changes in immune responses during the immunization period were investigated through indirect enzyme-linked immunosorbent assay (ELISA). The results of ELISA showed that chitosan significantly enhanced immunogenicity when accompanied with in the pentavalent clostridial toxoid vaccine. During the immunogenicity period and following that, no changes were observed in clinical behavior and internal organs after autopsy. The hematological and biochemical factors were reported with no significant pathologic changes during immunization in the control and vaccinated groups (P<0.05). The obtained findings revealed that the toxoid vaccines could not induce significant physiological changes in the body. The vaccine containing chitosan could stimulate humoral immunity 2-3 times higher than the nonchitosan vaccine. The humoral immune response was significantly duplicated due to the chitosan effect. Chitosan not only had no local or general side effects but also could be a good help with the enhancement of the immune system; therefore, it can be recommended as an appropriate safe adjuvant in the development of toxoid vaccines
Keywords: Chitosan nanoparticle, Pentavalent clostridial toxoid vaccine, Clostridium novyi alpha toxin, Clostridium perfringens epsilon toxin, Clostridium perfringens beta toxin
Introduction
Clostridium is a gram-positive, anaerobic, and spore-forming bacterium that causes fatal diseases in sheep, cattle, and goats. The diseases often occur in a peracute form; consequently, the clinical signs of the disease are rarely observed before mortality ( Delano et al., 2002 ). Vaccination is the best way for the prevention of clostridial infectious diseases ( Foumani et al., 2012 ). Routine vaccines utilized to prevent the clostridial diseases caused by Clostridium novyi (C. novyi), Clostridium septicum (C. septicum), and Clostridium perfringens (C. perfringens) types B, C, and D are used as inactivated cultures or toxoid vaccines (Europ ph 2015, Institute, 2015). Vaccine production in inactivated culture form is initiated by the growth of clostridial vaccine strains in suitable media. After the inactivation procedure, the toxoid is produced from toxin after the incubation detoxification period. In addition to toxoid, these vaccines contain the bacterial cell mass and their secretions, with media ingredients. In toxoid vaccines, other extra components are discarded except for toxoid. The advantage of the toxoid vaccine which makes the vaccine more potent than inactivated-culture vaccines is the low dose volume, absence of bacterial cell mass, and media ingredients. Toxoid vaccines induce less immunity than live vaccines; therefore, in order to promote the immunogenicity, toxoid vaccines require containing an adjuvant ( Gerdts, 2015 ). Mineral salts, oil-in-water/water-in-oil emulsions, saponins, toll-like receptor ligands and small molecules, particles in both forms of nanoparticles and microparticles, liposomes, and virosomes are types of adjuvants used in veterinary vaccines ( Gerdts, 2015 ). All the mechanisms of adjuvants are not completely known; however, it seems that they can raise antigen presentation, enhance antigen stability, and act as an immunomodulator ( Spickler and Roth, 2003 ). Chitosan has immunostimulatory properties and can function as an adjuvant for vaccines ( Neimert-Andersson et al., 2011 ). Chitosan could become a successful vaccine carrier in the near future due to the highly bioavailable, nontoxic, mucoadhesive, and biodegradable nature ( Islam et al., 2012 ). Several studies conducted on various animals showed that the local administration of chitosans, similar to intranasal, subcutaneous, ocular, or topical uses, was generally safe or with some mild reactions ( Heffernan et al., 2011 ). Multiple studies carried out in veterinary medicine on chitosan and its derivatives as adjuvants demonstrated a significant increase in both humoral and cell-mediated immunity responses ( Rezaei and Alonso, 2006 ; Arca et al., 2009 ; Oliveira et al., 2012 ; Khalili et al., 2015 ). Chitosan has also been considered a perfect adjuvant in injectable vaccines due to its high viscosity, biodegradability, and immunological activity ( Kumar, 2000 ; Heffernan et al., 2011 ). Hepatitis B vaccine ( AbdelAllah et al., 2016 ), influenza vaccine ( Spinner et al., 2015 ; Dabaghian et al., 2018 ), Newcastle disease virus DNA vaccine ( Zhao et al., 2014 ), and some other vaccines were accompanied with chitosan as a suitable adjuvant. Due to antifungal, antibacterial, and antioxidant activities ( Cheung et al., 2015 ), chitosan is a good help to prolong the shelf life of the vaccine. Nanoparticle technologies open new windows to program-specific immune responses for vaccines and immunotherapy ( Irvine et al., 2015 ). The manipulation of chitosan particle size can customize protein release profiles ( Koppolu et al., 2014 ; Caetano et al., 2016 ). The prepared particle of vaccine-loaded chitosan nanoparticles is more permeable, stable, and bioactive ( Ahmed and Aljaeid, 2016 ). In the present study, chitosan nanoparticles were used for the stimulation of immune system as adjuvants in rabbit immunization by the pentavalent clostridial toxoid vaccine containing C. novyi, C. septicum, and C. perfringens types B, C, and D. In addition, immunological, biochemical, and hematological changes were investigated in rabbits during the vaccination.
Material and Methods
Vaccine preparation
A pentavalent clostridial toxoid vaccine was used with and without chitosan nanoparticles as adjuvants. The vaccine contains toxoids of C. septicum, C. novyi type B, as well as C. perfringens types B, C, and D, prepared as a trial vaccine by researchers in Razi Vaccine and Serum Research Institute of Mashhad, Iran. Briefly, after the cultivation of each vaccine strains and inactivation, the ingredients of media and bacterial mass were discarded by precipitation and filtration methods. In toxoid production, formalin (2.8 mg/L) was used for inactivation followed by incubation detoxification periods. The pentavalent vaccine was formulated with C. perfringens type D (60%), type B (10%), type C (10%), C. novyi (10%), and C. septicum (10%) (the detail of this part is not available due to industrial application).
Preparation of chitosan adjuvant
The nanoparticles of chitosan (Sigma-Aldrich, St. Louis, MO, USA) were prepared base on the modified ionic gelation method with some modification ( Khalili et al., 2015 ). Briefly, after the detection of chitosan deacetylation and molecular weight, the chitosan solution was prepared by being dissolved in acetic acid (0.1 M) and heated at 37°C. The solution was filtered using prefilter membranes under vacuum, and acetate sodium (0.1 M) was added. Chitosan derivatives in 0.2 M glutamate buffer were dissolved in acetic acid, and the pH reached 4.6-4.8 using sodium hydroxide. Tween 80 (0.01%) (Sigma-Aldrich, St. Louis, MO, USA) was added to the emulsion. Then, sodium tripolyphosphate (TPP) was separately dissolved in deionized water (0.1% w/v). The nanoparticles were formed while the TPP solution was dropwise pumped to the chitosan solution and gently stirred for 60 min. After three times washing with distilled water to discard the acid, the chitosan was added to the pentavalent clostridial toxoid vaccine (1/10) while the suspension was gently stirred for 30 min.
Immunization procedure
New Zealand rabbits were chosen as animal models for the study on the clostridial vaccine. A total of 30 3- to 6-month-old rabbits were used in three groups of ten. Groups one and two were immunized by the pentavalent clostridial toxoid vaccine with and without chitosan, respectively. Moreover, 10 rabbits in group three were injected with sterile normal saline as the control group. Then, 2 ml of the pentavalent clostridial toxoid vaccine with and without chitosan was injected back to the top area subcutaneously in two groups of rabbits. In the control group, 2 ml of sterile normal saline was also subcutaneously injected. The booster dose of vaccines and placebo was administrated in a similar route 21 days after the first injection. Immunization protocol was followed according to the European Pharmacopoeia (EP) 2015.
Collection of blood samples
The blood samples of the rabbits were collected from all the groups before the first injection (D0) for internal control. The blood samples were gathered 3 weeks after the first injection (D21) before the booster dose administration and finally 2 weeks after the second vaccination (D35). Blood collection was performed in two tube types with ethylene diamine tetra-acetic acid for hematological assay and through serum separating procedure. The sera were kept at -20°C for enzyme-linked immunosorbent assay (ELISA) and biochemical tests.
Vaccine control tests
Control tests, namely safety, residual toxicity, and potency, were conducted according to the EP (2015).
Safety test. The safety test was carried out to evaluate the effect of the vaccine on the target species under the conditions similar to those encountered while using in the field. The test was conducted on eight sheep in each group. The sheep did not have antibodies against C. perfringens, C. septicum, and C. novyi. The pentavalent clostridial toxoid vaccine with and without chitosan, as well as sterile normal saline (as a control group), were subcutaneously injected twofold the usual dosage (4 ml) behind the shoulder. The second dose was administered after an interval of 21 days. The sheep were daily examined for the general and local side effects of the vaccine for 2 weeks after the last administration.
Residual toxicity test
The residual toxicity test was conducted immediately after the detoxification process on six mice, in three groups of two. The second test is carried out after the incubation detoxification period. The pentavalent clostridial toxoid vaccine with and without chitosan, as well as sterile normal saline (i.e., the control group), were subcutaneously injected in a dose of 0.5 ml. The mice were inspected for healthy and injected sites for a week.
Potency test
According to the EP monograph (no. 0362) for the potency test (2015), the antibody level in the immunized rabbits was identified (refer to the complete protocol of the EP 2015). To determine the level of antibodies against the beta and epsilon toxins of C. perfringens and alpha toxin of C. novyi, indirect ELISA was performed using homologous reference serum calibrated in the international units of their antitoxins. The ELISA was performed in a 96-well plate, which is coated with 100 µl of the vaccine solution. The plate was incubated overnight at 4°C followed by washing three times using a wash buffer (Phosphate Buffered Saline [PBS] containing 0.05% v/v Tween 20). Then, 150 µl of blocking solution (i.e., PBS containing 1% w/v bovine serum albumin) was poured in each well. Following the blocking step, the coated plate was firstly incubated at 37°C for 1 h with each anti-toxin antibody (appropriately diluted in wash buffer). The unbound (low-affinity) antigen-antibody complexes were then removed with three washing steps. Afterward, the plate was incubated with a horseradish peroxidase-conjugated anti-rabbit antibody (appropriately diluted in the wash buffer), which binds to the high-affinity antibody-antigen complex attached to the plate with incubation at 37°C for 1 h. Then, the plate was washed three times to remove the unbound antibody. The assay was performed as described by McGregor et al. (1994). The level of antibodies was compared in all the groups before the administration 3 weeks after the first injection and 2 weeks after the second injection.
Hematological assay
Complete blood count (CBC) was conducted with the cell counter (Celltac α, MEK-6400 series, Nihon Kohden, Japan) immediately after the blood collection. The total count was carried out for each cell group. Hematologic factors were determined, including white blood cells (WBC), red blood cells (RBC), hemoglobin (Hb), packed cell volume (PCV), platelet (Plt), neutrophil, lymphocyte, eosinophil, basophile, monocyte, and Neut/Lymph. The differential count was conducted on Giemsa staining blood film.
Biochemical assay. Creatinine, blood urea nitrogen (BUN), glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein, and albumin were separately measured in each group during the immunization.
Statistical analysis
The Shapiro-Wilk test and Levene’s test were used to determine the normality of the data and equivalence analysis of variances, respectively. In the tests, the p-value was higher than 0.05, and parametric tests were used to analyze the data. The paired sample t-test was used to compare the factors measured in each group, including the group on the day before injection (T0) and 2 weeks after the last injection (T1) (day 35) of different vaccines (i.e., the pentavalent toxoid clostridial vaccine with and without chitosan adjuvant). One-way analysis of variance was used to compare the mean of the measured factors between different groups at any time. If there was a significant difference between the means, the Tukey’s post hoc test was utilized to compare two of them. The data were analyzed by SPSS software (version 22; SPSS Inc., Chicago, IL, USA). In all analyzes, the significance level of the results was considered P < 0.05.
Results
Characterization of chitosan nanoparticles
The morphological characteristics and surface morphology of the chitosan nanoparticles were examined using scanning electron microscopy (SEM). According to data analysis and SEM pictures, all the samples had nanometric sizes and spherical shapes. The size and zeta potential of the samples were measured using a Particle-Size Analyzer (Fritsch, German). The samples had a mean size of less than 1 µm related to preparation conditions changed from 506.8 to 801.07 nm. All the samples had positive zeta potential varied within the range of 38.4-48.6 mV (the obtained data were not presented and summarized). The differences between the morphology of chitosan nanoparticles and zeta potential values were due to some small variations during the preparation method.
Safety test
A safety test should demonstrate a lack or acceptable level of detrimental side effects caused by the use of the vaccine in target animals. The inspection of the sheep during 2 weeks after vaccination did not show any changes regarding temperature, appetite, movement, and other general signs in the two vaccinated groups similar to those reported for the normal saline group. None of the local side effects, such as redness, edema, and necrosis, was observed in the injection site similar to that observed in the control group.
Residual toxicity test
The mice inspected for a week did not demonstrate any pathologic changes in the behavior and local site of vaccines, compared to the control group.
Potency test
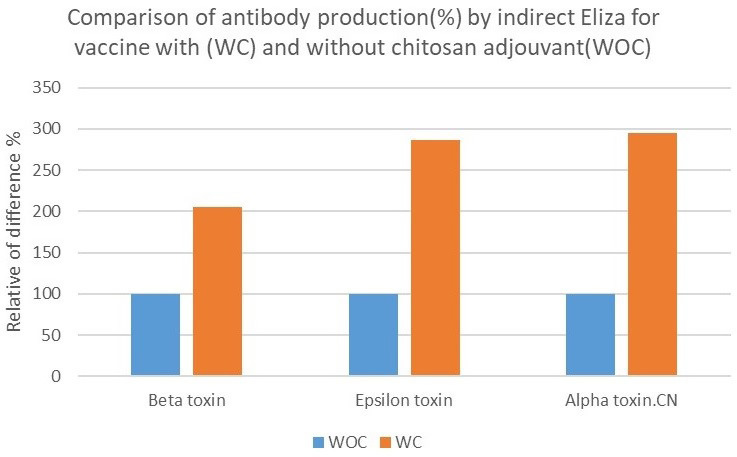
The results of ELISA for the detection of anti-alpha toxin antibody of C. novyi, as well as anti-epsilon and anti-beta toxins of C. perfringens, in the sera of the rabbits indicated that maximum antibody production was on the day 35, 2 weeks after the second administration. The obtained results revealed that the vaccine with chitosan induced more immune protection than the vaccine without chitosan. The statistical analysis showed that chitosan could increase antibody production 2-3 times higher than the vaccine without chitosan. The adjuvant significantly amplified the immunity response level of the vaccine. The obtained results of antibody production in OD 495 were presented for different toxins with (WC) and without chitosan adjuvant (WOC) (Figure 1) and as the percentage increased over the mean of without chitosan adjuvant (WOC) (Figure 2).
Figure 1.
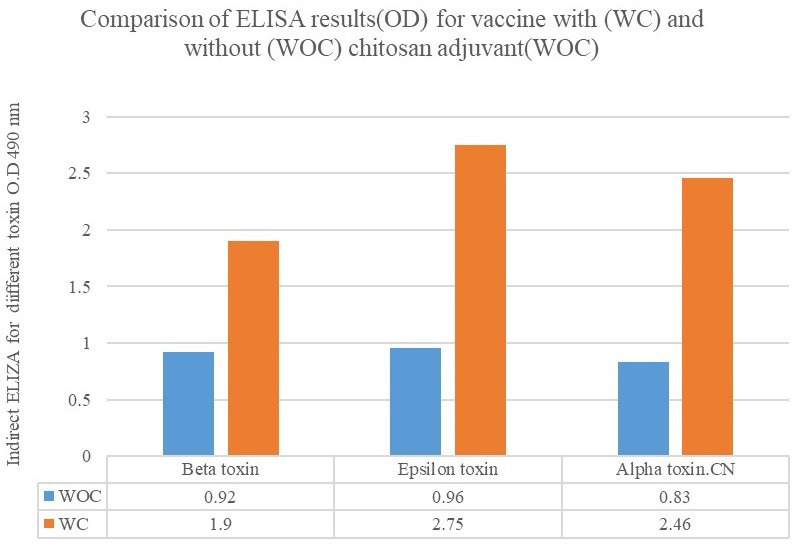
Figure 2.
Hematological assay
Table 1 tabulates the results of CBC changes in the rabbits on the day before injection (T0) and 2 weeks after the last injection (T1) as mean±standard deviation and 95% CI in pentavalent toxoid clostridial vaccination with and without the nanoparticles of chitosan and in the control group. Some variations can be observed in some of the factors after the vaccination and in the control group. The variations can be ignored with regard to the accuracy of the tests. There were no pathologic significant index changes in WBC, RBC, Hb, PCV, Plt, neutrophil, lymphocyte, eosinophil, basophile, monocyte, and Neut/Lymph after pentavalent toxoid vaccination on the day 35.
Table 1.
Results of complete blood count changes in rabbits on the day before injection (T0) and 2 weeks after the last injection (T1) as mean±standard deviation and 95% CI in pentavalent toxoid clostridial vaccination with and without nanoparticles of chitosan and control groups
| Factor | P-value | Without chitosan | With chitosan | Control | Mean | Time |
|---|---|---|---|---|---|---|
| White blood cells (×103/µl) | 0.06 | 7.76±0.70 | 7.83±1.38 | 7.43±0.60 | Mean±standard deviation | T0 |
| 7.25-8.26 | 6.83-8.82 | 6.99-7.86 | 95% CI | |||
| 0.005 | 7.88±1.91ab | 8.80±1.78b | 6.67±1.98ab | Mean±standard deviation | T1 | |
| 6.65-9.59 | 7.52-10.07 | 5.15-8.20 | 95% CI | |||
| - | 0.74 | 0.12 | 0.39 | *P-value | ||
| b, ab Significant increase in mean of white blood cells 35 days after vaccination accompanied with chitosan; *P<0.05 | ||||||
| Red blood cells (×106/µl) | 0.28 | 5.75±0.50 | 5.37±0.38 | 5.29±0.79 | Mean±standard deviation | T0 |
| 5.39-6.11 | 5.09-5.64 | 4.72-5.86 | 95% CI | |||
| 0.23 | 5.42±0.81 | 5.10±0.59 | 5.61±1.09 | Mean±standard deviation | T1 | |
| 4.79-6.04 | 4.68-5.53 | 4.77-6.46 | 95% CI | |||
| 0.3 | 0.16 | 0.66 | *P-value | |||
| No significant change; *P<0.05 | ||||||
| Hemoglobin (gm/dl) | 0.61 | 13.13±0.66 | 12.56±1.001 | 12.11±1.30 | Mean±standard deviation | T0 |
| 12.65-13.6 | 11.84-13.27 | 11.18-13.04 | 95% CI | |||
| 0.26 | 12.44±2.24 | 12.28±1.38 | 12.14±1.91 | Mean±standard deviation | T1 | |
| 10.72-14.16 | 11.29-13.26 | 10.67-13.61 | 95% CI | |||
| - | 0.40 | 0.52 | 0.88 | *P-value | ||
| No significant change; *P<0.05 | ||||||
| Packed cell volume (%) | 0.62 | 41.50±3.09 | 40.17±2.60 | 38.53±2.27 | Mean±standard deviation | T0 |
| 39.28-43.71 | 38.30-42.03 | 36.90-40.15 | 95% CI | |||
| 0.12 | 40.18±6.48 | 40.10±3.23 | 39.80±5.76 | Mean±standard deviation | T1 | |
| 35.20-45.17 | 37.78-42.41 | 34.59-45 | 95% CI | |||
| - | 0.48 | 0.93 | 0.68 | *P-value | ||
| No significant change; *P<0.05 | ||||||
| Platelet (×µl.103) | 0.10 | 429.60±49.46 | 489.10±51.77 | 442.30±65.55 | Mean±standard deviation | T0 |
| 394.21-464.98 | 452.06-526.13 | 395.40-489.19 | 95% CI | |||
| 0.03 | 394.55±139.50ab | 504.80±150.86b | 350.77±90.78a | Mean±standard deviation | T1 | |
| 287.32-501.78 | 396.87-612.72 | 280.99-420.56 | 95% CI | |||
| - | 0.62 | 0.78 | 0.10 | *P-value | ||
| a, b, ab Significant increase in mean of platelet 35 days after vaccination; *P<0.05 | ||||||
| Heterophile | 0.56 | 70.45±5.20 | 98.20± 4.44 | 62.70- 4.45 | Mean±standard deviation | T0 |
| 97.41-49.42 | 76.63-47.40 | 39.005-49.42 | 95% CI | |||
| 0.0001 | 40.24±4.58bc | 81.70±5.37c | 87.00±1.150a | Mean±standard deviation | T1 | |
| 91.43-96.43 | 85.54-41.33 | 43.56-51.48 | 95% CI | |||
| 0.06 | 0.008 | 0.04 | *P-value | |||
| a, b, ab Significant decrease in mean of heterophile 35 days after vaccination; *P<0.05 | ||||||
| Lymphocyte | 0.25 | 50.50±4.48 | 39.80±4.50 | 82.20±3.48 | Mean±standard deviation | T0 |
| 72.27-51.45 | 94.65-53.47 | 93.46-50.45 | 95% CI | |||
| 0.0001 | 86.22±3.50ab | 69.10±6.56 b | 20.1±2.44 a | Mean±standard deviation | T1 | |
| 19.25-53.47 | 88.31-60.51 | 66.55-45.42 | 95% CI | |||
| 0.35 | 0.055 | 0.53 | *P-value | |||
| a, b, ab Significant increase in mean of lymphocyte 35 days after vaccination; *P<0.05 | ||||||
| Eosinophil | 0.62 | 50.00±1.2 | 31.80±1.1 | 97.5±0.2 | Mean±standard deviation | T0 |
| 75.24-2.1 | 74.85-2.0 | 19.80-3.1 | 95% CI | |||
| 0.31 | 10.88±1.1 | 8.10±0.1 | 79.33±0.1 | Mean±standard deviation | T1 | |
| 70.70-3.0 | 88.31-1.0 | 10.65-2.0 | 95% CI | |||
| 0.87 | 0.32 | 0.01 | *P-value | |||
| Significant decrease of eosinophil in control group;*P<0.05 | ||||||
| Basophile | 0.85 | 60.07±1.1 | 30.20±1.1 | 97.50±0.1 | Mean±standard deviation | T0 |
| 83.36-0.2 | 93.46-1.03 | 19.80-20 | 95% CI | |||
| 0.01 | 95.33±0.1ab | 45.90±0.0 b | 33.44±1.2 a | Mean±standard deviation | T1 | |
| 0.2-19.47 | 68.11-1.0 | 46.41-3.1 | 95% CI | |||
| 0.83 | 0.43 | 0.15 | *P-value | |||
| a, b, ab Significant difference in mean of basophile after vaccination; *P<0.05 | ||||||
| Monocyte | 0.98 | 13.20±1.2 | 24.00±1.2 | 10.10±1.2 | Mean±standard deviation | T0 |
| 3.01-3.1 | 81.1-2.1 | 88.31-2.1 | 95% CI | |||
| 0.20 | 72.11±3.6b | 20.57± 2.04ab | 26.1±11.2a | Mean±standard deviation | T1 | |
| 32.89-3.8 | 2.6-35.04 | 3.1-13/08 | 95% CI | |||
| 0.006 | 0.04 | 0.84 | *P-value | |||
| a, b, ab Significant difference in monocyte after vaccination; *P<0.05 | ||||||
| Neut/Lymph | 0.36 | 0.95±0.19 | 0.88±0.17 | 0.95±0.16 | Mean±standard deviation | T0 |
| 0.81-1.09 | 0.75-1.008 | 0.84-1.07 | 95% CI | |||
| 0.0001 | 0.81±0.12 | 0.69±0.18 | 1.13±0.08 | Mean±standard deviation | T1 | |
| 0.71-0.90 | 0.56-0.82 | 0.07-1.19 | 95% CI | |||
| 0.11 | 0.02 | 0.04 | *P-value | |||
| Significant decrease of Neut/Lymph after vaccination; *P<0.05 | ||||||
Biochemical assay
Table 2 shows the results of the biochemical changes in the rabbits on the day before injection (T0) and 2 weeks after the last injection (T1) as mean±standard deviation and 95% CI in pentavalent toxoid clostridial vaccination with and without the nanoparticles of chitosan and in the control group. Although some changes can be observed in some of the factors, the variations can be ignored due to the accuracy of the tests. The pentavalent clostridial toxoid vaccination did not pathologically alter creatinine, BUN, glucose, ALT, AST, ALP, total protein, and albumin.
Table 2.
Results of biochemical changes in rabbits on the day before injection (T0) and 2 weeks after the last injection (T1) as mean±standard deviation and 95% CI in pentavalent toxoid clostridial vaccination with and without nanoparticles of chitosan and control groups
| Factor | P-value | Without chitosan | With chitosan | Control | Mean | Time |
|---|---|---|---|---|---|---|
| Creatinine (mg/dL) | 0.08 | 1.36±0.17 | 1.61±0.52 | 1.65±0.32 | Mean±standard deviation | T0 |
| 1.23-1.49 | 1.53-2.18 | 1.40-1.90 | 95% CI | |||
| 0.06 | 1.36±0.15b | 1.71±0.36b | 1.99±0.56a | Mean±standard deviation | T1 | |
| 1.24-1.48 | 1.44-1.97 | 1.77-2.20 | 95% CI | |||
| - | 0.99 | 0.62 | 0.07 | *P-value | ||
| a, b Significant difference in chitosan and control groups on day 35; *P<0.05 | ||||||
| Blood urea nitrogen (mg/dL) | 0.08 | 29.77±2.72 | 30.00±3.39 | 29.88±2.36 | Mean±standard deviation | T0 |
| 27.68-31.87 | 27.56-32.43 | 28.06-31.70 | 95% CI | |||
| 0.21 | 31.66±1.41 | 29.90±2.96 | 32.55±3.12 | Mean±standard deviation | T1 | |
| 30.57-32.75 | 27.78-32.01 | 30.15-34.95 | 95% CI | |||
| - | 0.08 | 0.92 | 0.01 | *P-value | ||
| Significant increase of blood urea nitrogen in control group; *P <0.05 | ||||||
| Glucose (mg/dl) | 0.09 | 138.77±9.88 | 134.30±13.34 | 123.88±7.40 | Mean±standard deviation | T0 |
| 131.18-146.37 | 124.75-143.84 | 118.19-129.58 | 95% CI | |||
| 0.21 | 121.25±10.64 | 138.40±27.26 | 138.44±24.73 | Mean±standard deviation | T1 | |
| 112.34-130.15 | 118.89-157.90 | 119.43-157.45 | 95% CI | |||
| - | 0.02 | 0.72 | 0.13 | *P-value | ||
| Significant decrease of glucose in without chitosan group after vaccination; *P<0.05 | ||||||
| Alanine aminotransferase (IU/L) | 0.09 | 19.33±6.02 | 18.10±5.06 | 16.55±3.39 | Mean±standard deviation | T0 |
| 14.70-23.96 | 13.04-23.15 | 13.94-19.16 | 95% CI | |||
| 0.055 | 18.33±4.21 | 21.60±6.59 | 23.55±6.87 | Mean±standard deviation | T1 | |
| 15.09-21.57 | 14.73-28.46 | 18.27-28.84 | 95% CI | |||
| - | 0.72 | 0.38 | 0.053 | *P-value | ||
| No significant difference; *P<0.05 | ||||||
| Aspartate aminotransferase (IU/L) | 0.18 | 28.00±10.80 | 25.88±8.17 | 34.33±8.36 | Mean±standard deviation | T0 |
| 19.69-36.30 | 19.60-32.17 | 27.90-40.76 | 95% CI | |||
| 0.14 | 24.22±7.74 | 30.50±9.39 | 29.14±7.42 | Mean±standard deviation | T1 | |
| 18.27-30.17 | 23.77-37.22 | 22.27-36.01 | 95% CI | |||
| - | 0.54 | 0.051 | 0.30 | *P-value | ||
| No significant difference; *P<0.05 | ||||||
| Alkaline phosphatase (IU/L) | 0.07 | 203±48.10 | 165.30±47.59 | 155.00±15.14 | Mean±standard deviation | T0 |
| 109.27-216.95 | 131.25-199.34 | 138.36-161.63 | 95% CI | |||
| 0.01 | 190.66±29.66 | 180.60±26.01 | 129.55±12.12 | Mean±standard deviation | T1 | |
| 167.86-213.46 | 133.37-227.82 | 120.23-138.87 | 95% CI | |||
| - | 0.10 | 0.49 | 0.94 | *P-value | ||
| Significant increase between without chitosan and control groups; *P<0.05 | ||||||
| Total protein (g/dl) | 0.65 | 6.76±0.45 | 6.56±0.93 | 6.28±0.82 | Mean±standard deviation | T0 |
| 6.42-7.11 | 5.89-7.23 | 5.64-6.91 | 95% CI | |||
| 0.10 | 5.79±1.02 | 6.39±1.03 | 6.98±0.53 | Mean±standard deviation | T1 | |
| 5.01-6.58 | 5.65-7.13 | 6.57-7.39 | 95% CI | |||
| - | 0.01 | 0.56 | 0.01 | *P-value | ||
| Significant decrease in without chitosan group after vaccination in comparison to that reported for control group; *P<0.05 | ||||||
| Albumin (g/dl) | 0.07 | 4.67±0.35 | 4.49±0.59 | 4.27±0.49 | Mean±standard deviation | T0 |
| 4.40-4.94 | 4.06-4.92 | 3.88-4.65 | 95% CI | |||
| 0.36 | 4.22±0.48 | 4.61±0.89 | 4.67±0.35 | Mean±standard deviation | T1 | |
| 3.84-4.59 | 3.98-5.25 | 4.39-4.94 | 95% CI | |||
| - | 0.01 | 0.54 | 0.02 | *P-value | ||
| Significant decrease in without chitosan group after vaccination in comparison to that reported for control group; *P<0.05 | ||||||
Discussion
Chitosan is derived from the alkaline deacetylation of chitin, which is the most common biopolymer in nature after cellulose ( Arca et al., 2009 ; Jamialahmadi et al., 2011 ). Chitosan with anti-oxidant activity and some other good properties has different applications in agriculture, environment, food, health, and medical sciences ( Rezaei and Alonso, 2006 ; Rezaee et al., 2018 ). Some studies showed that chitosan, a substance slowly dissolved in an acidic environment, is an ideal candidate for controlling the release of drugs in the body ( Kumar, 2000 ). Chitosan has different applications in veterinary medicine, such as in a gel or sponge form, to deliver antigens via rectal or nasal administration to sheep. The significant levels of immunoglobulin (Ig) G1, IgG2, and IgA have been detected in local mucosal tissues and lymph nodes. In addition, when chitosan is intranasally used with the purified antigen, a high level of Ig was detected ( (Arca et al., 2009 ). In a study carried out by Rezaei and Alonso (2006), it was shown that chitosan associated with a toxoid (as a mucosal vaccine) induced higher antibody titers than an injectable vaccine. In another study, chitosan was used with an injectable vaccine, and it could improve humoral and cell-mediated immune responses due to antigen depot at the site of injection ( Zaharoff et al., 2007 ). Gong et al. (2015) demonstrated that chitosan (as an adjuvant) exhibited a similar effect to that reported for cholera toxin (as a strong adjuvant). Neimert-Andersson et al. (2011) also indicated that chitosan can increase the interleukins and consequently increase the vaccine immunization effect.
In the present study, chitosan was accompanied by the pentavalent clostridial toxoid vaccine to show the immunity response variation with/without adjuvant. According to international protocols, especially the EP protocol, the detection of antibody level can be used instead of last potency procedures, and in some studies, the validity of ELISA was considered similar to that reported for the neutralization test on laboratory animals ( Ebert et al., 1999 ; Borrmann et al., 2006 ; Draayer, 2011 ). The obtained results of the present study emphasized that the use of chitosan nanoparticles as adjuvants could generate more Ig than the administration of the toxoid vaccine alone. There was a significant difference in the serum antibody level when the toxoid vaccine was used with chitosan, compared to that reported for the nonchitosan toxoid vaccine. Chitosan could increase antibody production 2-3 times more than the nonchitosan vaccine. The humoral immunity response was significantly duplicated due to the chitosan effect. In the present study, a suitable technique was chosen to produce the nanoparticle of chitosan. The bioactive nanoparticle of chitosan could penetrate, regulate the release of the antigen, and multiply the immune response of the vaccination. In a study carried out by Saleh et al. (2011), clinicopathological and immunological effects were identified after the injection of a clostridial toxoid vaccine and bacterial challenge in the fowl. They can induce the disease by the oral administration of C. perfringens. By the challenging effect on poultry, some blood parameters, such as RBC, hematocrit, Hb, ALT, AST, and BUN, significantly changed after the challenge with the bacterium ( Saleh et al., 2011 ). In the present study, no abnormalities were detected clinically, as well as in the autopsy and clinical pathology (the data are not shown). There were no significant pathological changes in the blood parameters, such as RBC, WBC, hematocrit, and Hb. No biochemical variations were observed between the treatment and control groups in terms of ALT, AST, BUN, total protein, and albumin. However, there have been no challenges in the routine use of these particles in vaccine production at an industrial level in the Razi Vaccine and Serum Research Institute.
Chitosan not only has no side effects on local or general tissues but also can be a good help with the stimulation of the immune system. In addition, chitosan can be considered a safe perfect adjuvant. The obtained findings suggested that toxoid vaccines do not significantly alter the biochemical and hematological factors pathologically. Toxoids are easily recognized by the immune system and provide the desired immunity without any major changes in general system factors.
Ethics
We hereby declare all ethical standards have been respected in preparation of the submitted article.
Grant Support
This study was supported by the Razi Vaccine and Serum Research Institute of Mashhad and Clinical Pathology Department of Tehran University in Tehran, Iran.
Authors’ Contribution
Study concept and design: Hemmaty, M., Fathi Najafi, M.
Acquisition of data: Rahman Mashhadi, M., Hemmaty, M.
Analysis and interpretation of data: Hemmaty, M., Fathi Najafi, M., Rahman Mashhadi, M.
Drafting of the manuscript: Hemmaty, M., Fathi Najafi, M., Rahman Mashhadi, M
Critical revision of the manuscript for important intellectual content: Hemmaty, M., Fathi Najafi, M.
Statistical analysis: Rahman Mashhadi, M., Hemmaty, M.
Administrative, technical, and material support:
Fathi Najafi, M., Hemmaty, M., Rahman Mashhadi, M.,
Conflict of Interest:
The authors declare that they have no conflict of interest.
Acknowledgement
The authors would like to express their appreciation to Behdjat Majidi who provided expertise that greatly assisted in performing the project.
References
- 1.AbdelAllah NH, Abdeltawab NF, Boseila AA, Amin MA. Chitosan and Sodium Alginate Combinations Are Alternative, Efficient, and Safe Natural Adjuvant Systems for Hepatitis B Vaccine in Mouse Model. Evid Based Complement Alternat Med. 2016;2016:7659684. doi: 10.1155/2016/7659684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed TA, Aljaeid BM. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des Devel Ther. 2016; 10: 483–507. doi: 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arca HC, Gunbeyaz M, Senel S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev Vaccines. 2009; 8: 937–953. doi: 10.1586/erv.09.47. [DOI] [PubMed] [Google Scholar]
- 4.Borrmann E, Schulze F, Cussler K, Hänel I, Diller R. Development of a cell culture assay for the quantitative determination of vaccination-induced antibodies in rabbit sera against Clostridium perfringens epsilon toxin and Clostridium novyi alpha toxin. Vet Microbiol. 2006;114(1-2):41–50. doi: 10.1016/j.vetmic.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Caetano LA, Almeida AJ, Goncalves LM. Effect of Experimental Parameters on Alginate/Chitosan Microparticles for BCG Encapsulation. Mar Drugs. 2016; 14: 90. doi: 10.3390/md14050090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung RC, Ng TB, Wong JH, Chan WY. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar Drugs. 2015;13(8):5156–86. doi: 10.3390/md13085156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabaghian M, Latifi AM, Tebianian M, NajmiNejad H, Ebrahimi SM. Nasal vaccination with r4M2e. HSP70c antigen encapsulated into N-trimethyl chitosan (TMC) nanoparticulate systems: Preparation and immunogenicity in a mouse model. Vaccine. 2018;36(20):2886–2895. doi: 10.1016/j.vaccine.2018.02.072. [DOI] [PubMed] [Google Scholar]
- 8.Delano ML, Mischler SA, Underwood WJ. Biology and diseases of ruminants: sheep, goats, and cattle. Laboratory animal medicine, Elsevier, New York; 2002 pp. 519-614 [Google Scholar]
- 9.Draayer H. Overview of currently approved veterinary vaccine potency testing methods and methods in development that do not require animal use. Proc Vaccinol. 2011; 5: 171–174. [Google Scholar]
- 10.Ebert E, Oppling V, Werner E, Cussler K. Development and prevalidation of two different ELISA systems for the potency testing of Clostridium perfringens beta and epsilon-toxoid containing veterinary vaccines. FEMS Immunol Med Microbiol. 1999;24(3):299–311. doi: 10.1111/j.1574-695X.1999.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 11.Foumani M, Asadpour L, Azizi Saraji A, Sharifat Salmani A, Aghasadeghi M. Adjuvants and their mechanisms of action. J Ardabil Univ Med Sci. 2012; 12: 276–291. [Google Scholar]
- 12.Gerdts V. Adjuvants for veterinary vaccines--types and modes of action. Berl Munch Tierarztl Wochenschr. 2015; 128: 456–463. [PubMed] [Google Scholar]
- 13.Gong Y, Tao L, Wang F, Liu W, Jing L, Liu D, Hu S, Xie Y, Zhou N. Chitosan as an adjuvant for a Helicobacter pylori therapeutic vaccine. Mol Med Rep. 2015;12(3):4123–4132. doi: 10.3892/mmr.2015.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heffernan MJ, Zaharoff DA, Fallon JK, Schlom J, Greiner JW. In vivo efficacy of a chitosan/IL-12 adjuvant system for protein-based vaccines. Biomaterials. 2011;32(3):926–32. doi: 10.1016/j.biomaterials.2010.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem Rev. 2015;115(19):11109–46. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam MA, Firdous J, Choi YJ, Yun CH, Cho CS. Design and application of chitosan microspheres as oral and nasal vaccine carriers: an updated review. Int J Nanomedicine. 2012;7:6077–93. doi: 10.2147/IJN.S38330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamialahmadi K, Behravan J, Fathi Najafi M, Tabatabaei Yazdi M, Shahverdi A, Faramarzi M. Enzymatic production of N-acetyl-D-glucosamine from chitin using crude enzyme preparation of Aeromonas sp. PTCC1691. Biotechnology. 2011; 10: 292–297. [Google Scholar]
- 18.Khalili I, Ghadimipour R, Sadigh Eteghad S, Fathi Najafi M, Ebrahimi MM, Godsian N, Sefidi Heris Y, Khalili MT. Evaluation of Immune Response Against Inactivated Avian Influenza (H9N2) Vaccine, by Using Chitosan Nanoparticles. Jundishapur J Microbiol. 2015;8(12):e27035. doi: 10.5812/jjm.27035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koppolu BP, Smith SG, Ravindranathan S, Jayanthi S, Suresh Kumar TK, Zaharoff DA. Controlling chitosan-based encapsulation for protein and vaccine delivery. Biomaterials. 2014;35(14):4382–9. doi: 10.1016/j.biomaterials.2014.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar MNR. A review of chitin and chitosan applications. React Functional Polymers. 2000; 46: 1–27. [Google Scholar]
- 21.McGregor DP, Molloy PE, Cunningham C, Harris WJ. Spontaneous assembly of bivalent single chain antibody fragments in Escherichia coli. Mol Immunol. 1994;31(3):219–26. doi: 10.1016/0161-5890(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 22.Neimert-Andersson T, Hällgren AC, Andersson M, Langebäck J, Zettergren L, Nilsen-Nygaard J, et al. Improved immune responses in mice using the novel chitosan adjuvant ViscoGel, with a Haemophilus influenzae type b glycoconjugate vaccine. Vaccine. 2011;29(48):8965–73. doi: 10.1016/j.vaccine.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira CR, Rezende CM, Silva MR, Pêgo AP, Borges O, Goes AM. A new strategy based on SmRho protein loaded chitosan nanoparticles as a candidate oral vaccine against schistosomiasis. PLoS Negl Trop Dis. 2012;6(11):e1894. doi: 10.1371/journal.pntd.0001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezaee M, Askari G, EmamDjomeh Z, Salami M. Effect of organic additives on physiochemical properties and anti-oxidant release from chitosan-gelatin composite films to fatty food simulant. Int J Biol Macromol. 2018;114:844–850. doi: 10.1016/j.ijbiomac.2018.03.122. [DOI] [PubMed] [Google Scholar]
- 25.Rezaei MA, Alonso M. Preparation and evaluation of chitosan nanoparticles containing diphtheria toxoid as new carriers for nasal vaccine delivery in mice. Arch Razi Inst. 2006; 61: 13–25. [Google Scholar]
- 26.Saleh N, Fathalla SI, Nabil R, Mosaad AA. Clinicopathological and immunological studies on toxoids vaccine as a successful alternative in controlling clostridial infection in broilers. Anaerobe. 2011;17(6):426–30. doi: 10.1016/j.anaerobe.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Spickler AR, Roth JA. Adjuvants in veterinary vaccines: modes of action and adverse effects. J Vet Intern Med. 2003; 17: 273–281. doi: 10.1111/j.1939-1676.2003.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 28.Spinner JL, Oberoi HS, Yorgensen YM, Poirier DS, Burkhart DJ, Plante M, Evans JT. Methylglycol chitosan and a synthetic TLR4 agonist enhance immune responses to influenza vaccine administered sublingually. Vaccine. 2015;33(43):5845–5853. doi: 10.1016/j.vaccine.2015.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25(11):2085–94. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao K, Zhang Y, Zhang X, Li W, Shi C, Guo C, Dai C, Chen Q, Jin Z, Zhao Y, Cui H, Wang Y. Preparation and efficacy of Newcastle disease virus DNA vaccine encapsulated in chitosan nanoparticles. Int J Nanomedicine. 2014;9:389–402. doi: 10.2147/IJN.S54226. [DOI] [PMC free article] [PubMed] [Google Scholar]


