Abstract
CD1c presents lipid-based antigens to CD1c-restricted T cells which are thought to be a major component of the human T cell pool. However, the study of CD1c-restricted T cells is hampered by the presence of an abundantly expressed, non-T cell receptor (TCR)-ligand for CD1c on blood cells, confounding analysis of TCR-mediated CD1c tetramer staining. Here, we identified the CD36 family (CD36, SR-B1 and LIMP-2) as ligands for CD1c, CD1b and CD1d proteins, and showed that CD36 is the receptor responsible for non-TCR-mediated CD1c tetramer staining of blood cells. Moreover, CD36-blockade clarified tetramer-based identification of CD1c-restricted T cells and improved identification of CD1b- and CD1d-restricted T cells. We used this technique to characterise CD1c-restricted T cells ex vivo and showed diverse phenotypic features, TCR repertoire and antigen-specific subsets. Accordingly, this work will enable further studies into the biology of CD1 and human CD1-restricted T cells.
One Sentence Summary:
CD1 molecules bind CD36 family members and blocking this interaction facilitates staining and study of CD1-restricted T cells.
Introduction:
While the majority of T cells express αTCRs that detect peptide-antigens presented by polymorphic major histocompatibility complex (MHC) class I and II glycoproteins(1), many T cells instead recognise monomorphic MHC class-I-like molecules that present non-peptide antigens(2). These include a broad group of T cells that detect lipids presented by the CD1 family(3). Humans express four distinct cell-surface CD1 molecules including CD1a, CD1b, CD1c, and CD1d, each of which exhibits unique lipid-binding capacity(3, 4). These CD1 isoforms follow different intracellular trafficking routes, collectively sampling and presenting the cellular lipidome for T cell surveillance(5). Amino-acid diversity in the TCR-binding site of each CD1 isoform engenders restriction by a discrete repertoire of T cells(3). Accordingly, CD1a-, CD1b-, CD1c- and CD1d-restricted T cells are thought to play non-redundant roles in health and disease(2).
Most of our knowledge of CD1-restricted T cells pertains to CD1d-restricted natural killer T (NKT) cells that express an invariant TCR-α chain, also known as type-I NKT cells(6). This is largely due to the conservation of CD1d and the type-I NKT TCR between mice and humans, and the discovery of the archetypal CD1d-presented lipid-antigen α-galactosylceramide (α-GalCer)(7) which is recognised by type-I NKT cell TCRs with high affinity(8). This enabled the generation of α-GalCer-loaded CD1d tetramers that bind all type-I NKT cells in humans and mice, facilitating their characterisation(9, 10). Conversely, CD1a-, CD1b- and CD1c are not expressed in mice, there is no single prototypic antigen presented by these molecules to T cells, and consequently, CD1a-, CD1b- and CD1c-restricted T cells are less well-understood. Most studies of these T cells have focussed on their recognition of lipids from mycobacterium tuberculosis (Mtb)(11) and CD1 tetramers loaded with mycobacterial lipids can identify CD1-lipid-specific T cells from human blood(12);(13–15).
In one study using limiting dilution analysis, CD1c-autoreactive T cells accounted for up to 8% of CD4+ T cells in healthy adult donors(16), and at least a subset of CD1-autoreactive T cells are detectable with CD1c-endo tetramers(17). CD1c-reactive T cells also detect CD1c-expressing leukaemias, suggesting a role in surveillance of haematopoetic tumours(18). Furthemore, CD1c is recognised by some Vδ1+ γδ T cells(19) as measured by clonal response assays and CD1c tetramers(20). Despite this progress, detection of CD1c-restricted T cells is complicated by the fact that CD1c tetramers brightly stain some non-T cells in peripheral blood(17). This finding suggests the existence of non-TCR ligands for CD1c and limits confidence in the identification and characterisation of CD1c-restricted T cells using tetramers.
Here, we demonstrated that CD1c bound to members of the CD36 family of lipid scavenger receptors including CD36, SR-B1 (SCARB1/CD36L1) and LIMP-2 (SCARB2/CD36L2). Moreover, CD1b and CD1d also bound to these molecules, albeit to a lesser extent. This interaction was responsible for non-TCR mediated staining of human blood cells, including T cells, by CD1b, CD1c and CD1d tetramers, and blockade with anti-CD36 mAb allowed efficient identification of T cells expressing CD1b-, CD1c- and CD1d-reactive TCRs. Thus, this technique will allow for more research surrounding these cells.
Results:
Non-TCR-mediated CD1c tetramer staining was prevalent in human blood
To investigate CD1c-autoreactive T cells, we used a mammalian expression system to produce fluorescent CD1 tetramers, as previously described(14). These CD1 tetramers furnish a spectrum of endogenous mammalian lipids from the cells which they are derived (21). We refer to these as ‘CD1-endo’ tetramers which can be used to detect ‘CD1-autoreactive’ cells. We generated BV421-conjugated CD1a-, CD1b-, CD1c- and CD1d-endo tetramers, as well as CD1d-α-GalCer and MR1–5-OP-RU tetramers that are used to detect NKT and MAIT cells, respectively(10, 22). Each tetramer was used to stain human PBMC (Fig 1A). As expected(23), MR1–5-OP-RU and CD1d-α-GalCer tetramers stained clearly-defined populations of T cells, whereas CD1a- and CD1b-endo tetramers showed little staining above that of the control (Streptavidin-BV421 alone). Confirming prior reports(17), CD1c-endo tetramers, and to a lesser extent CD1d-endo tetramers, stained both CD3+ and CD3− cells, suggesting that staining is not necessarily TCR-dependent.
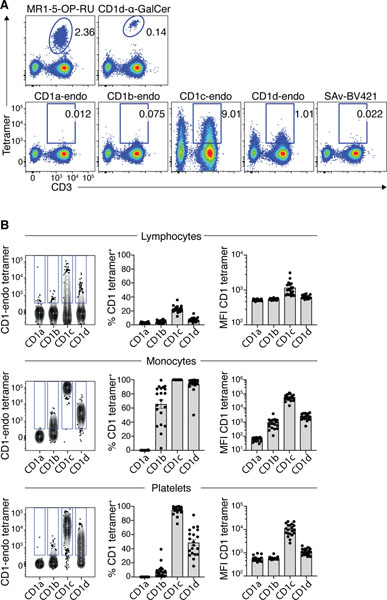
Figure 1: CD1-endo tetramers exhibited non-TCR-mediated binding to diverse blood cells.
A. Representative flow cytometric pseudo-colour plots showing tetramer staining on CD3+ T cells and CD3− lymphocytes using MR1–5-OP-RU, CD1d-αGalCer, CD1a-endo, CD1b-endo, CD1c-endo and CD1d-endo BV421 tetramers. Representative of n=6 donors. B. Comparison of staining profiles of CD1-endo PE tetramers on lymphocytes (CD45+), monocytes (CD14+, SSC-AHI and FSC-AHI) and platelets (CD42b+ SSC-Alow and FSC-Alow). Left panel: contour plots showing concatenated CD1-endo tetramer staining profiles from the same donor. Different CD1-endo tetramers were used to stain separate samples and the data subsequently concatenated. Gates were set based on FMO. Middle panel: Bar graphs showing the proportion of cells staining with each CD1-endo tetramer (n=20). Right panel: Bar graphs showing median fluorescence intensity (MFI) of CD1-endo tetramer positive cells (n=20). SAv = streptavidin.
To investigate TCR-independent reactivity of CD1-endo tetramers, we stained human PBMCs and assessed CD1-endo tetramer staining profiles on distinct cell lineages (Fig. 1B). CD1c, and to a lesser extent CD1d, exhibited staining ranging from near background to very bright on lymphoid cells. A median of 22% (range 12–36%) of lymphoid cells stained with CD1c-endo tetramers, and 6.6% (range 3–13%) with CD1d-endo tetramers. Of the cells that stained positively with the tetramers, CD1c-endo tetramer+ cells stained more brightly than CD1d-endo tetramer+ cells, with the median mean-fluorescence-intensities (MFI) of 988 and 613 respectively. Very little staining above background, as defined by cells not stained with tetramer, was detected with CD1a-endo and CD1b-endo tetramers, with medians of 2.6% and 4.6% respectively. Further specific analysis of NK, B and T lymphocyte subsets all gave similar staining patterns (Fig. S1). Monocytes were intensely stained by CD1c-endo tetramers (MFI=52,000) and, to a lesser extent, CD1d-endo tetramers (MFI=2,431) and CD1b-endo tetramers, the latter with a median of 75.4% (range 3–99%) and MFI of 866 (Fig. 1B). In contrast, less than 1% of monocytes stained with CD1a-endo tetramers. Platelets also showed a similar hierarchy, staining strongly with CD1c-endo, moderately with CD1d-endo tetramers, and slightly with CD1b-endo tetramers, but not CD1a-endo tetramers. Accordingly, CD1c, CD1d and CD1b-endo tetramers, but not CD1a-endo tetramers, bound to an undefined surface ligand(s) that was widely expressed on cells of diverse lineages but was most abundant on monocytes and platelets, and to a lesser extent lymphocytes.
To further investigate T cell staining with CD1c tetramers, we reasoned that if the staining was TCR-mediated, we should be able to use flow cytometry to sort CD1c tetramer+ T cells and expand them in vitro, yielding a population enriched for CD1c-endo tetramer+ T cells. However, after 10 days post-sort, most of the CD1c-endo tetramer staining was lost, despite these cells retaining CD3/TCR on their cell surface (Fig. S2). This suggested that CD1c-endo tetramer staining of T cells was not TCR-mediated, and that the ligand responsible was reduced with in vitro culture and/or expansion. This observation aligned with previous studies with CD1b (24–26) and CD1c(17) tetramers that have successfully used this approach to isolate CD1-reactive TCR-expressing cells from blood, although multiple rounds of purification and enrichment were required.
We next examined the role of the α3 domain of CD1c in binding the undefined TCR-independent ligand(s), given that this domain is the key determinant in mediating MHC-binding to receptors such as LILRB2 (ILT-4)(27). We reasoned that by generating tetramers of chimeric CD1 proteins which have the α1 and α2 domains of CD1c fused to the α3 domain of CD1b, this would retain TCR CD1c-binding capacity, as previously published(28), while reducing any α3 domain-mediated, non-TCR binding. These chimeric tetramers stained Jurkat cells expressing a CD1c-restricted TCR but not a CD1b-restricted TCR (Fig. S3A), confirming that the chimeric protein retained its specificity. However, the staining intensity on PBMCs was similar to that of non-chimeric CD1c tetramers (Fig. S3B), suggesting that non-TCR-mediated binding of CD1c to PBMC was dependent on the α1 and/or α2 domains.
CD36 family members bound CD1 molecules
Given that diverse primary cell types stained with CD1c tetramers, we next tested a panel of cell lines, and found that C1R, 293T, K562, MEG-01 and THP-1 cells all stained brightly with CD1c-endo tetramers, the brightest of which (K562) also stained weakly with CD1d-endo tetramers, suggesting that these cell lines were potential sources of CD1-binding ligand (Fig. 2A). These ligands were not universally expressed by cell lines because Jurkat and MOLT-4 cells did not stain with these tetramers.
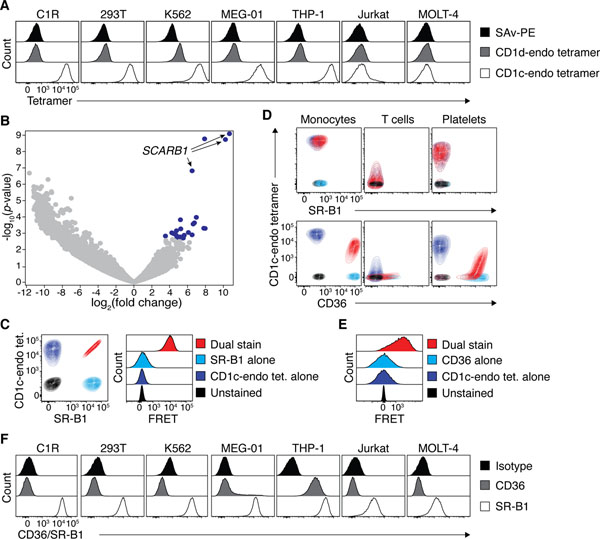
Figure 2: Identification of SR-B1 and CD36 as CD1 binding partners.
A. Representative histogram overlays showing CD1c- and CD1d-endo tetramer staining on diverse cell lines. B. Volcano plot showing relative expression of gRNAs between C1R cells transduced with the GeCKO v1 human CRISPR knockout library versus those enriched for an inability to bind CD1c-endo tetramers. Blue data points represent guides significantly overrepresented with a false discovery rate <0.05 in the enriched pool. Arrows point to guides targeting the SCARB1 gene. C. Left plot: Representative Overlaid contour plots of C1R cells that were unstained (black), single labelled (navy/light blue) or co-labelled (red) with CD1c-endo tetramers and/or anti-SR-B1. Right plot: Representative histogram overlays of these cells showing emission in the FRET channel. D. Representative overlaid contour plots of blood cell subsets that were unstained (black), single labelled (navy/light blue) or co-labelled (red) with CD1c-endo tetramers and/or anti-SR-B1 (top panel) or anti-CD36 (bottom panel). Colour code as per C and E for SR-B1 and CD36 respectively E. Representative histogram overlays of cells in D. stained with anti-CD36, showing emission in the FRET channel. F. Representative histogram overlays showing CD36 and SR-B1 staining on diverse cell lines. All subfigures presenting FACS data are representative of 3 independent experiments.
To establish the identity of these CD1-binding ligand(s), we performed a genome-wide CRISPR/Cas9-based library screen using C1R cells which have previously been used in a similar approach to identify genes that regulate surface expression of MR1(29). After two rounds of FACS-purification of CD1c-endo tetramer− C1R cells (ie. cells where the candidate ligand may have been deleted), these were enriched to ~45% purity (Fig. S4C). Subsequent sequencing and analysis revealed 24 significantly enriched distinct guide RNAs [false discovery rate (FDR) <0.05] (Fig. S4D). Of these, 3 of the top 4 guides targeted the SCARB1 gene, with the remaining guide targeting MBNL1, a gene known to regulate alternative splicing of SCARB1(30) (Fig 2B). The SCARB1 gene encodes the SR-B1 protein (also known as CD36L1 and SCARB1), a surface-expressed member of the CD36 family of scavenger receptors involved in lipid metabolism with diverse roles in human physiology(31). Given the lipid-binding capacity of SR-B1, this seemed like a logical candidate binding partner for the lipid-presenting molecule CD1c. To test this hypothesis, we co-stained C1R cells with CD1c-endo tetramers and a mAb targeting SR-B1 (Fig. 2C, left). C1R cells stained brightly with anti-SR-B1 and the staining intensity was directly proportional to that of CD1c-endo tetramer. Moreover, when assessing Förster resonance energy transfer (FRET) between the donor fluorophore PE (conjugated to CD1c-endo tetramers) and acceptor fluorophore APC (conjugated to anti-SR-B1), a strong FRET signal was detected (Fig. 2C, right). These data suggested that CD1c-endo tetramers and anti-SR-B1 mAb were within 10 nm of each other on the cell surface. This supported the notion that SR-B1 was a direct ligand for CD1c and was responsible for CD1c-endo tetramer staining on C1R cells.
We next applied the same staining approach to PBMC, however, PBMC failed to stain with anti-SR-B1 (Fig. 2D, top). Since the CD36 family includes two related receptors, CD36 (also known as SCARB3) and LIMP-2 (also known as SCARB2 or CD36L2) we reasoned that these may also facilitate binding to CD1. LIMP-2 is not expressed on the cell surface and is restricted to the lysosome, however CD36 is known to be widely expressed on diverse cell types(32). When staining PBMC with anti-CD36, we found that the staining profile on monocytes, T cells and platelets was highly similar to that of CD1c-endo tetramer (Figure 2D, bottom). Furthermore, when co-staining with anti-CD36 and CD1c tetramers, a clear coincident staining pattern was observed on both monocytes and platelets. Of note, in the presence of anti-CD36, CD1c-endo tetramer had reduced staining intensity, suggesting that the anti-CD36 mAb and CD1c may bind to overlapping sites on CD36. This may explain why CD1c-endo tetramer staining was much lower on T cells when they were costained with CD36 compared to those stained with CD1c tetramer alone. Akin to C1R cells, FRET was also observed on monocytes costained with PE-conjugated CD1c-endo tetramer and APC-conjugated anti-CD36 mAb (Fig. 2E). Revisiting the original panel of cell lines (Fig.2A) for CD36 and SR-B1 expression, we found that while they all expressed SR-B1, the 2 lines (Jurkat and MOLT-4) that did not stain with CD1c-endo tetramers had approximately 10-fold less SR-B1 surface expression than the others (Fig. 2F). Only THP-1 cells also stained with anti-CD36. Collectively, these data suggested that CD36 expression on lymphocytes was responsible for non-TCR-mediated CD1c-endo tetramer staining on PBMC.
To confirm that CD36 family members were sufficient for binding to CD1 tetramers, we transiently transfected 293T cells with plasmids encoding CD36 family members and then stained with a panel of CD1-endo tetramers. Since 293T cells already express SR-B1, we first generated a 293T SCARB1 knockout line using CRISPR/cas9, which knocked-down CD1c-endo tetramer staining (Fig. 3A). These 293T.SCARB1−/− cells were then transiently transfected to express full-length CD36 (encoded by CD36), SR-B1 (encoded by SCARB1) or LIMP-2 (encoded by SCARB2), and stained with CD1a, CD1b, CD1c and CD1d-endo tetramers (Fig. 3B). Surface expression of CD36, SR-B1 and LIMP-2 on transfected cells was confirmed with specific mAbs (Fig. S5A). Despite LIMP-2 being a lysosomal protein, some was detected at the surface of SCARB2-transfected cells, which may reflect overexpression in this transfection system. CD36-transfected cells stained clearly with CD1-endo tetramers, with a similar hierarchy to that observed with PBMCs (CD1c-endo > CD1d-endo > CD1b-endo tetramers), while CD1a-endo tetramers failed to stain these cells (Fig. 3B). SCARB1-transfected cells also stained brightly with CD1c- and to a lesser extent CD1d-endo tetramers, but not with CD1b and CD1a-endo tetramers. SCARB2-transfected cells only stained weakly with CD1c-endo tetramers, which may reflect its low surface expression. Control transfections of 293T cells with CD1a, CD1b, CD1c and CD1d-restricted autoreactive TCRs showed appropriate TCR-dependent staining, validating the integrity of the CD1-endo tetramers (Fig. S5B). Thus, all three CD36 family members bound at least one CD1 family member.
Figure 3: CD36 family members bound to CD1 molecules.
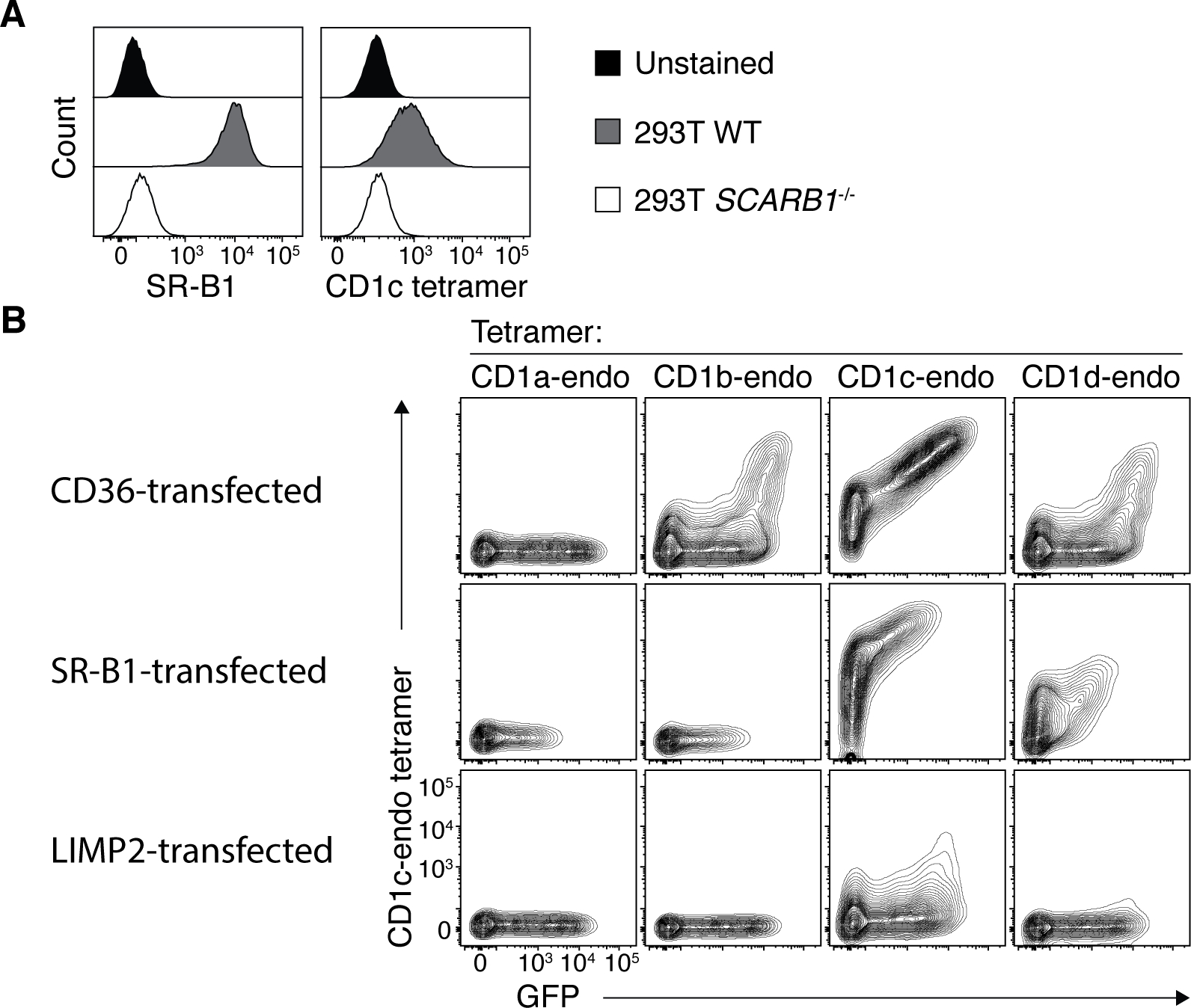
A. Representative histogram overlays showing SR-B1 and CD1c-endo tetramer staining on wildtype and SCARB1-deficient 293T cells. B. Representative contour plots showing CD1-endo tetramer staining on 293T.SCARB1−/− cells transiently transfected to express CD36, SR-B1 or LIMP2. Data are representative of 3 independent experiments each.
CD36 blockade facilitated characterisation of CD1c-restricted T cells
These findings suggested that CD1c tetramers may bind to T cells via two independent mechanisms; CD36-mediated or TCR-mediated binding. We thus reasoned that if we could eliminate the CD36-mediated staining, any residual staining wwould have likely been mediated by CD1c-specific TCRs, thereby providing a method of identifying and isolating CD1c-restricted T cells. Indeed, pre-incubation with increasing amounts of anti-CD36 resulted in a progressive reduction of CD1c-endo tetramer staining. A saturating dose of anti-CD36 blockade was observed at 3 μg/ml which reduced CD1c tetramer+ cells from a median of 40.5% when unblocked, to 0.02% when blocked (Fig. 4A). Residual CD1c-endo tetramer+ T cells were nonetheless detected as clear populations when sufficient cell numbers were acquired (Fig. 4B).
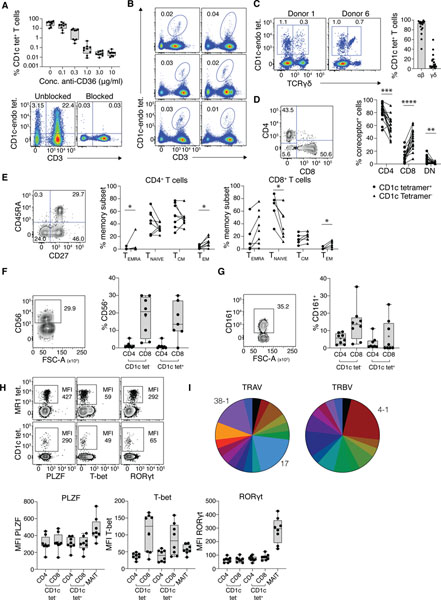
Figure 4: CD36 blockade enabled characterisation of CD1c-reactive T cells.
A. Upper panel: Bar graph showing the proportion of CD3+ T cells in PBMC that stain with CD1c-endo tetramers after blocking with titrating doses of anti-CD36 (n=9). Lower panel: Representative FACS plots from the n=9 donors showing CD1c-endo staining on lymphocytes with and without complete CD36 blockade, gated on CD14−, CD19− viable lymphocytes. B. Representative FACS plots showing CD1-endo tetramer+ T cells on 6 selected PBMC samples after CD36-blockade, gated as per A. C. Left panel: Representative FACS plots from 2 donor samples showing CD1c-endo tetramer staining on CD3+ T cells after magnetic enrichment from PBMC using CD1c-endo tetramers, post CD36 blockade. Gated on CD3+, CD14−, CD19− viable lymphocytes. Right panel: Bar graph showing distribution of γδ and αβ T cells amongst CD1c-endo tetramer+ T cells from n=16 enriched PBMC samples. D. Coreceptor distribution of magnetically-enriched CD1c-endo-restricted αβ T cells. Left panel: representative FACS plot of CD4 and CD8 staining. Right panel: Line graph showing coreceptor distribution from n=16 donors. E. Memory subset distribution of magnetically-enriched CD1c-endo-restricted αβ T cells. Left panel: representative FACS plot of CD27 and CD45RA staining. Middle and right panels: Line graphs showing memory subset distribution of CD4 (middle) and CD8 (right) T cell subsets from n=8 donors. F-G. Innate surface marker expression on magnetically-enriched CD1c-endo-restricted αβ T cells. Left panels: representative FACS plot of CD56 (F) or CD161 (G) staining. Right panel: Bar graph showing CD56 or CD161 expression on n=8 donors. H. Transcription factor expression. Upper panel: Representative FACS plot of transcription factor staining from n=8 donors on MAIT versus non-MAIT cells from the enriched samples. Lower panel: Bar graphs showing transcription factor expression on CD4+ or CD8+ cells from CD1c-endo tetramer+ (CD1c tet+), CD1c-endo tetramer− (CD1c tet−) or MAIT cells in the enriched sample, from n=8 donors. I. Pie charts showing distribution of distinct TRAV (left) and TRBV (right) genes used by n=24 CD1c-endo tetramer+ αβ T cells annotated in table 1. Enriched genes are labelled, remaining genes are differentiated by colour only.
To further confirm that CD36-mediated CD1c-endo tetramer staining on T cells was independent of TCR-specificity, we examined MR1-restricted MAIT cells and butyrophilin 2A1-reactive Vδ2+ γδ T cells; neither of which are CD1c-restricted(2) (Fig. S6A). Both MAIT and Vδ2+ cells expressed CD36, and stained with CD1c-endo tetramers, and this was abrogated by prior blockade with anti-CD36. This showed that T cells that did not express CD1c-restricted TCRs could be stained by CD1c tetramers due to CD36-mediated binding. Taken together, these data suggested that in the absence of CD36 blockade, most T cells identified by CD1c tetramer will not really be CD1c-restricted T cells binding via their TCRs.
Given the low frequency of residual CD1c tetramer+ T cells following CD36 blockade, we used magnetic-activated cell sorting (MACS) to enrich CD1c tetramer+ cells after anti-CD36-blockade from 16 human PBMC samples (Fig. 4C and Fig. S7). Consistent with CD1c being a prominent restriction element for γδ T cells(19, 20), each donor yielded a mix of both αβ and γδ T cells. The rates of CD1c-endo tetramer staining for αβ versus γδ T cells was variable between donors, with a median of 6.3% γδ but a range of 0.6–59.5% (Fig 4C). As expected, following MACS-enrichment, MAIT cells were abundant in the unblocked CD1c-endo tetramer+ cells but not in the CD36-blocked samples, further demonstrating that in the absence of CD36 blockade, CD1c tetramer staining detects T cells that do not express CD1c-reactive TCRs (Fig S6B).
Further characterisation of the CD1c-endo-reactive αβ T cells in the CD36-blocked MACS-enriched samples, revealed diverse CD4/CD8 coreceptor distribution, although relative to CD1c tetramer− cells, they had less CD8+ cells and were enriched for CD4+ and CD4−CD8− (double negative; DN) cells (Fig 4D). Assessment of memory markers on the CD4 and CD8 αβ T cell subsets showed that CD36-blocked CD1c tetramer+ cells were distributed similarly to tetramer− cells across the 4 broad memory subsets found in blood (CD45RA+CD27− TEMRA; CD45RA+CD27+ naïve; CD45RA−CD27+ TCM; CD45RA−CD27− TEM), although some minor but significant differences were apparent (Fig. 4E). Given that some unconventional T cells, such as MAIT cells, express NK cell-associated markers, we assessed expression of CD56 and CD161, but found no difference between CD1c tetramer+ cells and CD1c tetramer− cells (Fig. 4F–G). We also assessed transcription factors including promyelocytic leukaemia zinc finger (PLZF), a key transcription factor for MAIT cells, and T-bet and RORγt, associated with type I and type 17 immunity, respectively (Fig. 4H). CD1c tetramer+ T cells aligned more closely with CD1c tetramer− conventional T cells than with MAIT cells, expressing neither PLZF nor RORγt, while the CD8+ CD1c tetramer+ fraction expressed low and varied amounts of T-bet, similar to CD1c tetramer− CD8+ T cells.
We also compared the phenotype of CD1c tetramer+ T cells with or without CD36 blockade (Fig S6C–F). CD1c tetramer+ CD36-blocked cells were more enriched for γδ T cells and CD4+ αβ T cells compared to CD1c tetramer+ CD36-unblocked cells, but markers such as CD56, T-bet and RORγt were similar between CD36-blocked and unblocked cells, which is consistent with the data above comparing CD1c tetramer+ CD36-blocked T cells to conventional T cells (Fig. 4).
We next assessed the TCR-repertoire of CD1c tetramer+ CD36-blocked T cells using single cell-sorting and TCR sequencing, which revealed diverse TRAV and TRBV gene usage with extensive variability of CDR3α and CDR3β amino acid usage and length (Table 1). Despite this, we detected an enrichment of TRAV17 (5/24 matched pairs) in the TCR-α chain, a variable gene known to be enriched in a population of CD1b-restricted T cells(33, 34), as well as TRAV38–1 (4/24). We also noted an enrichment of TRBV4–1 in the TCR-β chain (5/26), in line with previous studies(35) (Fig. 4I). These results suggested that CD1c-restricted T cells were similar to CD1b-restricted T cells, in that they expressed a broad range of TCRs but included biases toward some αβTCR pairs.
Table 1.
List of TCR sequences derived from CD1c-endo tetramer+ T cells
| TRAV | CDR3α | TRAJ | TRBV | CDR3β | TRBJ | Intensity | Co-receptor | Donor |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 25 | CAGHNYGQNFVF | 26 | 2 | CAIYTDTQYF | 2-3 | HI | CD8 | SJR9 |
| 8* | CWISNSGNTPLVF | 29 | 4-1 | CASSHPVDTQYF | 2-3 | LOW | CD4 | SJR12 |
| 12-2 | CAVNILIQGAQKLVF | 54 | 4-1 | CASSQALGFEKLFF | 1-4 | HI/INT | CD4 | SJR10 |
| 19 | CALGGFRQAGTALIF | 15 | 4-1 | CASSQVAGLYGYTF | 1-2 | LOW | CD4 | SJR1 |
| 36 | CAVEARVGGATNKLIF | 32 | 4-1 | CASSTHLPYREGRADTQYF | 2-3 | LOW | CD4 | SJR2 |
| 38-1 | CAFIAGGTSYGKLTF | 52 | 4-1 | CASSQGKAQGRTGELFF | 2-2 | INT | CD4 | SJR9 |
| 38-2 | CAYRIMGGGATNKLIF | 32 | 4-1 | CASSPSWGVYEQYF | 2-7 | HI | CD4 | SJR1 |
| 9-2 | CALPLNSGGYQKVTF | 13 | 5-4 | CASIAMGAKGANVLTF | 2-6 | LOW | CD4 | SJR2 |
| 8* | CAVITNFGNEKLTF | 48 | 5-6 | CASKTRGSSLYEQYF | 2-7 | HI | CD4 | SJR2 |
| 20 | CAVQEYSGYSTLTF | 11 | 6-1 | CASRSRTVLGNTQYF | 2-3 | LOW | CD4 | SJR12 |
| 29 | CAASALKQANAGGTSYGKLTF | 52 | 6-1 | CASTRDRYNYGYTF | 1-2 | LOW | CD4 | SJR1 |
| 3 | CAVRPLGFQKLVF | 8 | 7-6 | CASSSLQGLGNTIYF | 1-3 | LOW | CD4 | SJR12 |
| 8-6 | CAVSKSGGYQKVTF | 13 | 7-8 | CASSLRRETNNEQFF | 2-1 | LOW | CD4 | SJR2 |
| 26-2 | CIRVKIYNQGGKLIF | 23 | 7-8 | CASTQRWGQGSSYNEQFF | 2-1 | LOW | CD4 | SJR11 |
| 17 | CATAKYSSASKIIF | 3 | 7-9 | CASSSQVWFSVDLSSGNTIYF | 1-3 | HI/INT | DN | SJR9 |
| 17 | CATARMSGYSTLTF | 11 | 7-9 | CASSLGQGAFSSFSYEQYF | 2-7 | HI/INT | CD4 | SJR10 |
| 22 | CAVPTRIYNQGGKLIF | 23 | 14 | CASSLRSWRSYNSPLHF | 1-6 | INT | CD4 | SJR9 |
| 17 | CATDAYNQGGKLIF | 23 | 16 | CASSQSGQGGGRYEQYF | 2-7 | HI/INT | DN | SJR9 |
| 17 | CATDGWEYGNKLVF | 47 | 16 | CASSQGATGPSYEQYF | 2-7 | HI | CD4 | SJR2 |
| 38-1 | CAVMKHVVTGNQFYF | 49 | 16 | CASSQGATGGPSYEQYF | 2-7 | HI | CD4 | SJR2 |
| 21 | CAVKVQRLGGYQKVTF | 13 | 20-1 | CSARTPGQGRIYDTEAFF | 1-1 | INT | CD4 | SJR9 |
| 38-1 | CAVMRYTDKLIF | 34 | 20-1 | CSARKWDLANYGYTF | 1-2 | HI | CD4 | SJR1 |
| 8-1 | CAVKREYGNKLVF | 47 | 23-1 | CASSCRPFDRGNNPDTQYF | 2-3 | HI | CD4 | SJR12 |
| 38-1 | CAFTLYNFNKFYF | 21 | 27 | CASSSRQPRDDQPQHF | 1-5 | INT | CD4 | SJR1 |
| 17 | CATGQGAQKLVF | 54 | 28 | CASSPRRANREYQYF | 2-7 | HI | CD4 | SJR11 |
| 36 | CAVGGSGGSNYKLTF | 53 | 29-1 | CSVGHRGEKLFF | 1-4 | HI/INT | CD4 | SJR10 |
either TRAV8-2 or 8-4
Collectively, these data suggested that CD36 blockade allowed for the identification and isolation of true CD1c-restricted T cells and that these were heterogeneous but moderately enriched for expression of γδTCRs, CD4 and DN subsets, with biases in their TCR repertoire compared to non-CD1c-restricted T cells.
Lipid-antigen discrimination by CD1c-autoreactive T cells
We next attempted to isolate CD1c-autoreactive T cells with specificity toward defined self-lipids. CD1c tetramers were loaded with lipids known to be presented by other CD1 isoforms, including phosphatidylcholine (PC), lysophosphatidylcholine (LPC), sulfatide and GD3 ganglioside(3). These CD1c-lipid-loaded tetramer+ populations were detected with distinct staining hierarchies (Fig 5A). For example, donor 7 had a population of T cells that appeared to stain with all tetramers, suggesting lipid-Ag independent CD1c reactivity, whereas donor 8 had a population that was only detected with CD1c-endo, vehicle, -PC and -LPC loaded tetramers, but not CD1c-sulfatide or -GD3 loaded tetramers. Conversely, donor 9 had the reciprocal staining pattern, with a population that preferentially stained with CD1c-sulfatide and -GD3 tetramers. Different again, donor 10 predominantly stained with CD1c-sulfatide, but not CD1c-GD3, tetramers.
Figure 5: Lipid-antigen discrimination by CD1c-restricted T cells.
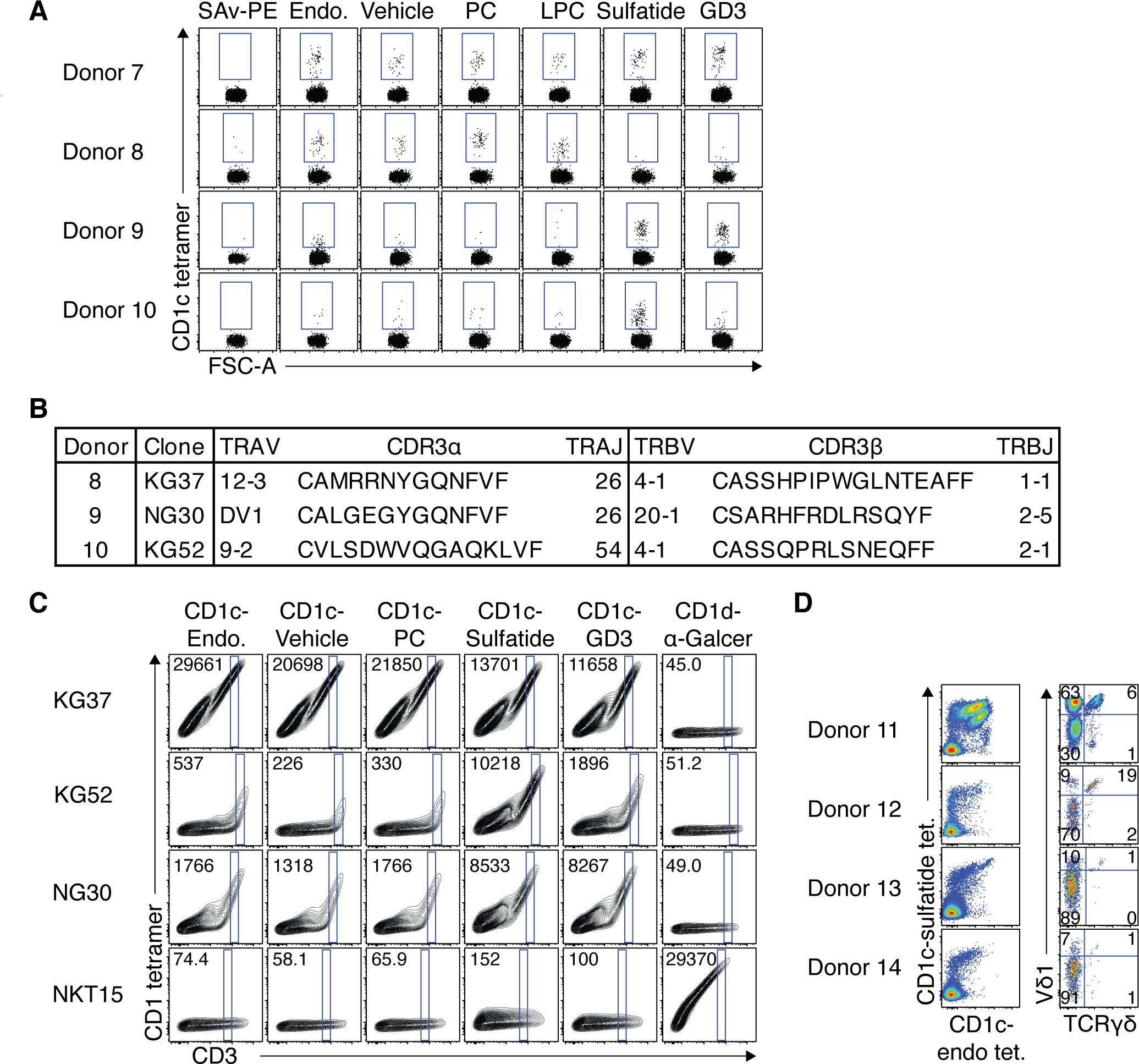
A. Dot plots showing staining of CD3+ T cells from 4 donors with CD1c tetramers loaded with PC, LPC, sulfatide or GD3 compared to CD1c-endo or tyloxapol vehicle control. Streptavidin-PE (SAv-PE) was also used a negative staining control. B. Table showing TCR sequences derived from CD1c tetramer+ populations in figure A. C. Representative contour plots showing CD1-lipid tetramer staining of 293T.SCARB1−/− cells transiently transfected to express CD1c-restricted TCRs from table in B or control NKT TCR clone NKT15. Consistent gates were set on a ‘window’ of CD3 expression across the different tetramer for each transfection, and median fluorescent intensity (MFI) of events in that gate is depicted in the top left of each plot. Experiment was performed 5 times for KG37, KG52 and NKT15 and 3 times for NG30, with similar results in each experiment. D. FACS plots from 4 donor PBMC samples co-stained and magnetically enriched with both CD1c-sulfatide and CD1c-endo tetramers. Left panel shows CD1c tetramer co-staining on enriched CD3+ T cells. Right panel shows TCRγδ and Vδ1 staining on total CD1c tetramer+ T cells.
To validate the antigen-specificity of these populations, we carried out single cell TCR analysis on these cells. While cells from donors 8 and 10 yielded matched TCR-α and TCR-β chain sequences, both of which utilised TRBV4–1 (clones KG37 and KG52 respectively), cells from donor 9 failed to yield a productive TCR-α chain. Noting that TRDV genes can be used by CD1d-restricted αβ T cells (so-called δ/αβ cells)(36), and CD1c tetramers bind polyclonal Vδ1+ γδ T cells(20), we resequenced, incorporating TRDV gene primers. This yielded a productive TRDV1 (encoding Vδ1) rearranged with TRAJ26 and the TRAC TCR-α constant-region gene (clone NG30; Fig 5B), representing the first example of a CD1c-restricted δ/αβ T cell clone. Importantly, by transferring these TCR genes into 293T.SCARB1−/− cells and subsequently staining them with the same panel of CD1c tetramers, we recapitulated the lipid-reactivity observed in the primary cells (Fig. 5C). For example, KG52, derived from CD1c-sulfatide-reactive cells in donor 10, was brightly stained with CD1c-sulfatide and only weakly stained with other tetramers. Similarly, NG30, derived from the CD1c-sulfatide and GD3-reactive δ/αβ TCR+ cells in donor 9, was much more brightly stained by CD1c tetramers loaded with sulfatide and GD3 versus other lipids. Although all CD1c tetramers stained the transfected cells to some extent, this likely reflected TCR overexpression in this transfection system. Collectively, these experiments not only validated the TCR-mediated CD1c-reactivity of CD1c tetramer+ cells after CD36 blockade, but also highlighted a degree of self-lipid-antigen-discrimination in the CD1c-restricted T cell pool.
To further investigate the ability of CD1c-restricted T cells to respond to defined lipid antigens, we enriched T cells from 4 healthy PBMC samples, and stained with both CD1c-sulfatide and CD1c-endo tetramers simultaneously. CD1c tetramer co-staining revealed distinct populations of CD1c-restricted T cells based on varying relative staining intensity with the two tetramers (Fig. 5D, left). For example, donor 11 had two populations of cells which stained brightly with both CD1c-endo and CD1c-sulfatide tetramers, but also some cells which preferentially bound CD1c-sulfatide or CD1c-endo tetramers. Donors 12, 13 and 14 lacked a prominent population of CD1c-endo exclusivity but had populations that appeared to preference CD1c-sulfatide or co-stained with both tetramers. When gating on total CD1c tetramer+ cells and assessing the γδTCR usage (Fig. 5D, right), the distribution of subsets varied between the 4 donors. γδ T cells accounted for 7%, 21%, 2% and 2% for donors 11–14 respectively, and Vδ1+ T cells accounted for 69%, 27%, 11% and 8%. Vδ1+ TCRγδ− (δ/αβ T cells) accounted for 63%, 9%, 10% and 7% of total CD1c tetramer+ cells, and 91%, 47%, 91% and 88% of the Vδ1+ fraction for donors 11–14 respectively. Accordingly, similar to CD1d-restricted T cells(37–39), Vδ1 was used in both the αβ and γδ CD1c-restricted T cell repertoires.
CD36 blockade facilitated isolation of CD1d- and CD1b-restricted T cells
Given CD1d-endo and CD1b-endo tetramers also bind CD36, we reasoned that CD36 blockade may also be a valuable tool for the ex vivo isolation of CD1d-restricted type-II NKT cells and CD1b-restricted T cells, including GMM-reactive germline-encoded mycolyl-lipid-reactive (GEM) T cells(40). Thus, we stained healthy PBMC from 3 donors with CD1d-endo, CD1d-LPC and CD1d-sulfatide tetramers, using CD1d-α-GalCer tetramers as a control, with or without CD36 blockade (Fig. 6A). In line with the staining profile in figure 1, the lipids differentially affected the level of CD36-mediated staining. Thus, CD1d-endo and CD1d-LPC tetramers had substantial CD36-mediated staining on both T and non-T cells, whereas this was less prominent in CD1d tetramers loaded with sulfatide or α-GalCer, suggesting that some bound lipids may inhibit CD36 binding to CD1d. Nonetheless, CD36-blockade prior to tetramer staining resulted in a marked reduction in staining events in each case, revealing residual populations of tetramer+ T cells. Thus, CD36-blockade should facilitate ex vivo analysis of CD1d-restricted cells with type II Ags such as sulfatide and LPC(41). We took a similar approach using CD1b-GMM tetramers (Fig 6B) which also improved the signal to noise ratio for analysis of TRAV1–2+ GEM T cells. For example, donors 18 and 20 had prominent populations of GEM T cells which were more readily defined after CD36-blockade. Reciprocally, the rare TRAV1–2+ cells that stained with CD1b-GMM tetramers in donor 19 where no longer present after CD36-blockade. Accordingly, CD36-blockade was also applicable to analysis of CD1d and CD1b-restricted T cells, particularly rare populations where high signal to noise is required.
Figure 6: CD36-blockade facilitated ex vivo analysis of type II NKT cells and GEM-T cells.
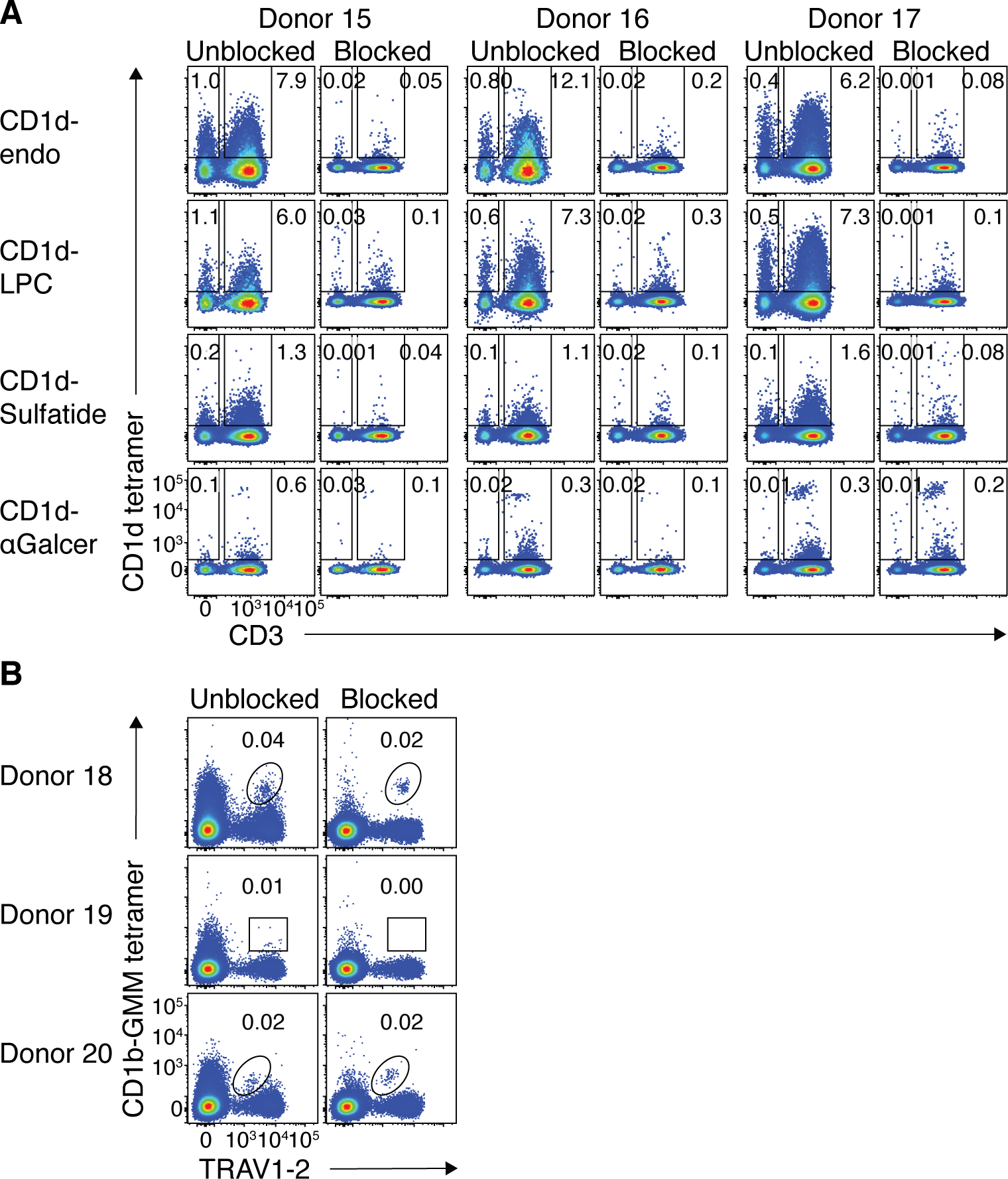
A. FACS plots from 3 healthy donor PBMC samples (representative of a cohort of 8) showing CD1d tetramer staining on lymphocytes with and without CD36-blockade. B. FACS plots from 3 healthy donor PBMC samples showing CD1b-GMM tetramer staining on CD4+ T cells with and without CD36-blockade.
Together, we identified an interaction between CD1c, b and d molecules and the CD36 family, and showed that blocking this interaction facilitated the use of CD1 tetramers to stain and isolate CD1-restricted T cells.
Discussion:
Here, we identified unanticipated interactions between the CD1 and CD36 families of surface receptors. While the functional significance of these interactions remains to be explored, an obvious association is the lipid binding role of both families of molecules. The CD36 family regulates lipid metabolism through binding of cholesterols, fatty acids and normal and modified lipoproteins(31). It is thus tempting to speculate that the CD36 family may have a role in lipid-antigen presentation by CD1 molecules, a process known to involve other lipid scavenger receptors(42). Indeed, apolipoproteins that are scavenged by CD36, are important in the efficient delivery of exogenous lipid-Ags to antigen-presenting cells (APCs) to mediate NKT cell activation(43). Thus, CD36 family members may play a role in shuttling lipids into the lipid-loading pathway of CD1 molecules. It is also possible that these molecules play a more direct role in lipid loading into CD1 molecules. Our data using CD1b/c chimeric tetramers, and that distinct lipids could influence the CD1-CD36 interaction, was consistent with the notion that the CD1 Ag-binding was likely to be involved in this interaction. Moreover LIMP-2, which appears to bind at least to CD1c, is typically retained in lysosomes, through which CD1b, c and d recycle to acquire exogenous lipid Ags(44). In this respect, it is noteworthy that CD1a, the only CD1 molecule that does not traffic to the endo-lysosome as a result of its lack of adaptor protein binding(44), does not bind to CD36 family members. Further studies on the role of the CD36 family in CD1 Ag presentation are clearly warranted.
Our discovery has immediate implications for research into CD1-restricted T cells. Blockade of CD36 greatly improved the sensitivity of CD1c tetramers for the identification and analysis of CD1c-restricted T cells ex vivo. Our data suggests that CD1c-endo-tetramer+ T cells bind ~0.02% of T cells, which contrasts with another study that estimated the frequency of CD1c-autoreactive T cells at ~7% of circulating T cells(16). In that study, large libraries of T cell clones were challenged with CD1-expressing APCs and the frequency of responding cells enumerated. The discrepancy with our tetramer-based analysis may reflect the vastly different approaches. For example, the repertoire of lipids in CD1 tetramers compared to APCs may limit detection of some CD1-restricted T cells. Furthermore, in vitro activation-based assays may be better suited than CD1 tetramer staining to detect low affinity TCR-binding which may be a feature of autoreactive TCRs.
There is some discrepancy in the levels of CD1c tetramer staining on non-anti-CD36-blocked samples between data presented here and that of previous studies(15, 17, 20, 45). This will be influenced by differences in reagents, staining protocols, and instrumentation. A key difference in previous studies may be the pre-enrichment of T cells(20) or overnight resting of the cells prior to staining(15, 45), which aligns with reduced non-TCR-mediated staining with culture(17). Another possible difference may be the PBMC isolation methods which likely result in different levels of platelet contamination. Platelets express high levels of CD36 and can coat the surface of lymphoid and myeloid cells during PBMC preparation, and platelet contaminated cells can stain with platelet markers (46, 47). Because some previous studies of CD1c-restricted T cells have isolated PBMC from platelet-pheresed(20) or leukapheresed(15, 17) samples these will probably have less platelet contamination than our samples which are derived from ficoll-density gradient buffy packs.
In a recent study(17), we provided evidence that some CD1c-reactive T cells recognise CD1c only when it carries lipid-antigens that have unobtrusive headgroups that do not interfere with TCR binding. This may explain how CD1c-endo tetramers, which likely carry a range of lipids, can detect CD1c-autoreactive T cells because the identity of the bound lipids is not critical, providing they stay out of the way of the TCR. Clearly though, CD1c is also capable of presenting specific microbial lipid-antigens such as PM and MPM to lipid-antigen-specific CD1c-restricted T cells(15). Further, CD1c-restricted T cells appear to detect the self-lipid antigen mLPA, associated with leukemia cells(18). It follows that CD1c-endo tetramers may only detect a fraction of the CD1c-autoreactive T cell pool, in which the epitope is likely to be CD1c itself, and loading with specific self-lipids may allow detection of T cells that recognise CD1c combined with these lipids. This is supported by our data showing that CD1c loaded with self-lipids, sulfatide or GD3, detected T cells that failed to bind CD1c-endo tetramers, suggesting that self-lipids can contribute to epitope formation. Thus, interpretation of studies and enumeration of CD1c-restricted autoreactive T cell frequencies must consider the role of the bound self lipids, and considering the diverse pool of lipids that can bind CD1c(21), the total autoreactive T cell pool may be much larger than our experiments in this paper suggest. It is important to note that while loading of CD1c tetramers with GD3 and sulfatide was validated by reciprocal staining patterns between different donors, the PC- and LPC-loaded tetramers typically looked similar to CD1c-endo tetramers. We therefore cannot rule out the potential that PC and LPC-reactivity was explained by poor loading-efficiency, however given CD1c-endo is known to be enriched for these lipids(17), it is not surprising that CD1c-PC and -LPC tetramers give similar staining profiles to CD1c-endo.
Using CD1c-endo tetramers with CD36 blockade, we have further characterised ‘CD1c-endo-reactive’ cells. The αβTCR repertoire of these cells was enriched for the TRBV4–1 gene, in line with previous observations(35), but also utilised TRAV17, a gene enriched in the CD1b-GMM-reactive TCR repertoire(34), as well as TRAV38–1. We also showed that in some donors, a large proportion of CD1c-endo-reactive αβ T cells utilise Vδ1 and are thus ‘δ/αβ’ T cells(36). This supports the notion that Vδ1 may have a particular predilection for CD1c(20), as it does for CD1d(36–39), regardless of whether it is part of an αβ or γδ TCR. Along with the recent discovery of CD1b-reactive Vδ1+ γδ T cells(48), it now appears that at least 3 members of the CD1 family can be recognised by Vδ1+ T cells. These observations add to a growing number of TCR genes that are similarly enriched in multiple CD1-restricted T cell populations(33), suggesting a possible co-evolution between CD1 molecules and certain TCR genes.
CD1c-restricted αβ T cells, while moderately enriched for CD4+ and DN T cells, were phenotypically diverse, expressing limited amounts of NK markers CD56 and CD161 and varied memory marker expression, suggesting differential levels of prior antigen-exposure. Thus CD1c-endo-reactive T cells appear to be phenotypically similar to CD1a and CD1b-restricted T cells(16, 40), exhibiting substantial phenotypic heterogeneity relative to MAIT and NKT cells whose innate-like phenotype is thought to be programmed through their selection by CD1d- and MR1-expressing CD4+CD8+ double-positive (DP) thymocytes(49) and driven by the transcription factor PLZF. The lack of PLZF expression by CD1c-restricted T cells and more diverse and adaptive T cell-like features suggest that their development may align more closely with conventional MHC-restricted T cells selected on thymic epithelial cells(49).
In summary, we have discovered an interaction between CD36 family members and the CD1 family of antigen-presenting molecules. In addition we showed that CD36-blockade could be a means to limit CD1 tetramer staining to TCR-reactivity will facilitate further studies on these T cell populations, helping to unlock key aspects of their fundamental biology, their roles in disease and homeostasis, and to aid in the development of therapeutics harnessing this knowledge. This finding furthermore paves the way for studies on the biological role of the CD36-CD1 axis.
Materials and Methods:
Study Design
This study was designed to identify non-TCR ligands for CD1 molecules present on human blood cells. We aimed to isolate immortalised cell lines that bind CD1c tetramers and used this in whole-genome CRISPR/Cas9 knockout screens to identify the surface proteins mediating CD1c binding. After we identified CD36 family members as the CD1-binding partners, and discovered that this could be abrogated with anti-CD36 mAb, we validated this approach and characterised the phenotype, TCR-repertoire and lipid-antigen descrimination by CD1c-restricted T cells.
Human samples
Buffy coats from healthy donors were obtained, with written informed consent, from the Australian Red Cross Blood Service after approval from the University of Melbourne Human Ethics Committee (1035100). Buffy coats were processed by standard density gradient using Ficoll-paque Plus (GE Healthcare) and cryopreserved in liquid nitrogen.
Tetramers
Human CD1a, CD1b, CD1c and CD1d tetramers were produced as previously described(23, 50). In brief, 293S.GnTI− cells were co-transfected using PEI-transfection reagent with pHLSec expression vectors encoding the truncated ectodomain of CD1b, CD1c or CD1d together with a separate pHLSec vector encoding full-length human β2-microglobulin (β2m). For chimeric CD1c/b proteins, constructs were based on a previous publication(28) which linked β2m to the truncated CD1 ectodomain via a glycine-serine linker (GGGGSGGSGSGGGSS) and fused the CD1c α1 and α2 domains (A1-V186) to the CD1b α3 domain (K186-P280), followed by C-terminal AVI-tag and 6xHIS tag. Recombinant CD1 proteins were purified using Ni-NTA agarose, biotinylated with BirA enzyme and further purified by size-exclusion chromatography. MR1–5-OP-RU tetramers were produced as previously described(51). In brief, truncated ectodomains of human MR1.C262S were expressed as inclusion bodies in Escherichia coli (strain BL21) along with human β2-microglobulin (β2m). MR1 and β2m inclusion bodies were refolded in vitro in the presence of 5-A-RU (Fairlie laboratory, University of Queensland) and methylglyoxal (Sigma) using oxidative refolding, prior to dialysis and subsequent purification using Ni-NTA agarose (ThermoFisher). Human MR1–5-OP-RU was biotinylated and purified as per CD1 proteins. All monomers were tetramerised using streptavidin-PE, -PE-Cy7 or -BV421 (BD Pharmingen).
Flow cytometry and cell sorting
All flow cytometry samples were acquired using an LSRFortessa (BD Biosciences) and analysed using FlowJo v10 software (Treestar). Sorting was performed using a FACSAriaIII cell sorter (BD Biosciences). For PBMC analysis, LIVE/DEAD Near-IR+ cells were removed, followed by doublet exclusion. Platelets were gated as FSC-A/SSC-A low, CD42b+ cells. Monocytes were gated as FSC-A/SSC-A high, CD14+ cells, and lymphocytes gated as CD45+ FSC-A/SSC-A intermediate cells. For T cell analysis, CD14+ and CD19+ cells were removed from analysis. More specific details on staining are listed below in the subsequent protocols.
Ex vivo staining of human PBMCs
PBMCs were incubated in DNAse for 30 min at 37°C prior to being filtered. For experiments examining CD1c-restricted T cells, cells were washed in PBS and incubated in PBS + 50 nM dasatinib (Stem Cell Technologies) + DNAse at 37°C for 30 min. Cells were then blocked with human Fc-block (Miltenyi) in the presence or absence of purified anti-CD36 antibody (Clone 5–271; Biolegend) at 10 μg/ml for a further 15 min at 4°C. CD1c tetramers were then added directly to blocking cocktail at a final concentration of 1 μg/ml and stained for 30 min at room temperature. Cells were washed once before being stained with surface mAb (Table S1) and viability dye LIVE/DEAD Fixable Near-IR (Thermo-Fisher Scientific, USA) for 30 min at 4°C. Cells were then washed twice, fixed in 2% PFA, washed once more and filtered prior to immediate FACS acquisition. For dual tetramer labelling experiments, cells were stained with mAb cocktail containing the first tetramer, followed by avidin and biotin blocking (Dako) before staining with the second tetramer. For staining of δ/αβ T cells, enriched cells were stained with anti-Vδ1 clone A13 supernatant (provided by L. Moretta, University of Genova) for 30 min at 4°C, washed three times, then stained with anti-mouse IgG, washed three times, blocked with 5% normal mouse serum, and then stained with other surface mAb. For intracellular staining, cells were first enriched as below, and surface stained as above, and subsequently subject to permeabilisiation using a transcription factor fix/perm kit (eBioscience). In brief, cells were incubated in fix/perm for 30 min at 4°C. Cells were then washed twice in permwash, before being resuspended in permwash containing 2% normal mouse serum plus intracellular antibodies and incubated for 30 min at 4°C. Cells were then washed twice in permwash and resuspended in FACS buffer, filtered with 100 μm mesh and acquired by flow cytometry.
Magnetic enrichment
Human PBMCs were isolated as above. Cells were then treated with dasatinib for 1 hr at 37°C prior to blocking with Fc-block (BD Bioscience), 5% normal mouse serum, and purified anti-CD36 for 20 min at room temperature (RT). Cells were then stained with CD1c-endo tetramer-PE for 30 min at RT, washed twice and then incubated with anti-PE magnetic microbeads (Miltenyi) for 20 min at 4°C. Cells were washed twice more, followed by magnetic enrichment using LS MACS enrichment columns (Miltenyi). Enriched cells were then stained with surface mAb as above and subsequently FACS-sort purified or FACS analysed.
Staining of cell lines
Cell lines were maintained in RF10 complete media consisting of RPMI-1640 supplemented with 10% FBS (JRH Biosciences), Penicillin (100 U/mL), Streptomycin (100 μg/mL), Glutamax (2 mM), sodium pyruvate (1 mM), nonessential amino acids (0.1 mM), HEPES buffer (15 mM), pH7.2–7.5 (Invitrogen, Life Technologies) and 2-mercaptoethanol (50 μM, Sigma). Cells were harvested, washed with PBS and stained for 30 min at RT with staining cocktails including LIVE/DEAD Near-IR, tetramers and/or mAbs (see table 1). Cells were then washed and filtered through 100 μm mesh prior to immediate acquisition by flow cytometry.
In vitro T cell expansion
Sorted cells were stimulated with platebound anti-CD3 (10 μg/ml; clone OKT3; BD Biosciences) and anti-CD28 (2 μg/ml; clone CD28.2; BD Bioscience) in the presence of rhuIL-2 (200 U/ml; Peprotech), rhuIL-7 (50 ng/ml; eBiosciences) and rhuIL-15 (5ng/ml; eBiosciences). After 48 hrs, cells were removed from stimulus and allowed to expand for approximately 10–14 d in cytokine media. Cells were assessed directly or cryopreserved for subsequent analysis. T cell culture media consisted of a base of 1:1 RPMI-1640 and AIM-V media (Invitrogen, Life Technologies), supplemented as per RF10 above with the addition of 2% human AB serum (Sigma).
Single cell TCR sequencing
For sequencing of TCRs, magnetically-enriched CD1c-endo tetramer+ cells were FACS-purified and expanded in vitro prior to restaining with CD1c-endo tetramers and single-cell sorting. Human TCR genes were sequenced as previously described(52). In brief, single CD1c tetramer+ T cells were sorted into 96-well PCR plates. cDNA was generated using SuperScript VILO cDNA synthesis kit (ThermoFisher). cDNA was then subjected to 2 rounds of semi-nested PCR using multiplexed primer sets to specifically amplify TCR chains. Amplified DNA was sequenced using Sanger Sequencing, and sequences were analysed using the IMGT/V-QUEST online tool. For sequencing of TCRs in figure 5, cells were single-cell sorted directly ex vivo and PCRs performed as above.
Lipids
Lipids were dissolved by sonication in TBS (10 mM Tris pH 8.0, 150 mM NaCl) containing 0.05 % (v/v) tyloxapol (Sigma) and stored at −20°C prior to use. Prior to loading, lipids were thawed and re-sonicated for 30 min. All loading was performed overnight at RT. For staining in figure 1, CD1d was loaded with α-GalCer (KRN7000) at a 3:1 molar ratio (lipid:CD1). For Figure 6, CD1d was loaded with PBS44 (provided by Paul Savage, Brigham Young University) at 6:1, LPC (C18:1) or sulfatide (C24:1), both at 12:1. CD1c was loaded with LPC (C18:1), sulfatide (C24:1), PC (C18:0-C18–1) or GD3 at 12:1. KRN7000, LPC, PC, sulfatide and GD3 were purchased from Avanti Polar Lipids. CD1b was loaded with GMM (Moody laboratory, Brigham and Women’s Hospital, Boston) at 6:1.
Transient transfections
Full-length CD36, SCARB1 and SCARB2 genes, as well as TCRα- and β-chain genes linked by a p2A-linker, were cloned into the pMIGII expression vector. Vectors were used to transiently transfect 293T or 293T.SCARB1−/− cells using FUGENE (Promega). TCR transductions also included a pMIG vector encoding CD3ε, δ, γ and ζ, Cells. Cells were harvested after 48 hr, washed once in PBS + 2% FBS, then stained for 30 mins at RT with CD1 tetramers. Cells were then washed twice, fixed in 2% PFA for 15 min at RT before a final wash step.
Jurkat cell lines
Stable TCR-expressing cell lines were generated by retroviral transduction of parental Jurkat76 cells using pMIGII plasmids above, as previously described(38, 53).
CRISPR/Cas9-mediated
Methods for CRISPR-based experiments can be found in supplementary information.
Recombinant mAb production
DNA constructs encoding anti-LIMP-2 antibody heavy and light chain variable domains (clone JL2(54)) were synthesized (ThermoFisher) and cloned into mammalian expression vectors containing a mouse IGHV signal peptide and IgG1 constant regions. Antibodies were expressed by transient transfection of mammalian Expi293F cells and purified using Protein G column chromatography (GE; Fig. S8).
Statistical Analysis:
Statistics were generated using Graphpad Prism software. Statistical tests in Figure 4D and Figure S6C–F were Wilcoxon matched-pairs signed-rank test and in 4E were Mann-Whitney tests. * indicates p < 0.05, ** indicates p < 0.005, ** indicates p < 0.0005 and **** indicates < 0.00005
Supplementary Material
Table S1: List of mAbs
Table S3: Raw data file
Figure S1: CD1-endo tetramer staining of lymphoid subsets
Figure S2: In vitro culture reduces non-TCR-mediated CD1c-endo tetramer staining on human T cells
Figure S3: CD1b/c chimeric tetramers stain CD1c-restricted T cells, but exhibit non-TCR mediated PBMC staining.
Figure S4: CRISPR GeCKO screen supplementary data
Figure S5: Control 293T transient transfections with CD36 family members
Figure S6: Validation of CD36-blockade for the isolation of CD1c-restricted T cells
Figure S7: Enrichment of CD1c-endo reactive T cells from human PBMCs
Figure S8: JL2 antibody production
Table S2: CRISPR Screen raw data
Acknowledgements:
We are grateful to Dr. Paul Savage (Brigham Young University, UT, USA) for providing α-galactosylceramide analogue PBS44 used for production of CD1d-α-GalCer tetramers, Prof. David Fairlie and Jeffrey Mak (University of Queensland, Australia) for providing 5-A-RU used for production of MR1–5-OP-RU tetramers, Prof. Lorenzo Moretta (Bambino Gesu Children’s Hospital, Rome, Italy) for anti-Vδ1 clone A13 supernatant and Dr. Adam Wheatley (Peter Doherty Institute, University of Melbourne, Australia) for plasmids used for JL2 mAb production. We thank staff from the flow cytometry facilities at the Department of Microbiology and Immunology at the Peter Doherty Institute and the Melbourne Brain Centre at the University of Melbourne. This work was supported by the Australian Research Council (ARC; CE140100011, DP120102471) and the National Health and Medical Research Council, Australia (NHMRC; 1113293, 1145373) and the National Institutes of Health, USA (NIH; R01 AR048632). DIG was supported by an NHMRC Senior Principal Research Fellowship (1117766), NAG and HEGM were supported by ARC Discovery Early career Researcher Awards (DECRA; DE210100705 and DE170100575 respectively), DGP was supported by a CSL centenary fellowship and JAV was supported by an NHMRC Senior Research Fellowship (1058193).
Footnotes
Competing interests:
D.I.G., N.A.G. and C.A. are listed as inventors on patent applications PCT/AU2020/051432 and PCT/AU2020/051433, pertaining to the interaction between CD1 and CD36. D.I.G. has also served as a paid member of an advisory committee, and shareholder, for Avalia Immunotherapies.
Data availability:
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. The raw CRIPSR screen data can be found in supplemental table S3. TCR sequence data has been desposited in Genbank repository under accession numbers MZ190477-MZ190530.
References and Notes:
- 1.Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J, T cell antigen receptor recognition of antigen-presenting molecules. Annual review of immunology 33, 169–200 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB, The burgeoning family of unconventional T cells. Nature immunology 16, 1114–1123 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB, Lipid and small-molecule display by CD1 and MR1. Nature reviews. Immunology 15, 643–654 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotton RN, Shahine A, Rossjohn J, Moody DB, Lipids hide or step aside for CD1-autoreactive T cell receptors. Current opinion in immunology 52, 93–99 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brigl M, Brenner MB, CD1: antigen presentation and T cell function. Annual review of immunology 22, 817–890 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Crosby CM, Kronenberg M, Tissue-specific functions of invariant natural killer T cells. Nature reviews. Immunology, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawano T, CD1d-Restricted and TCR-Mediated Activation of V14 NKT Cells by Glycosylceramides. Science 278, 1626–1629 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J, CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448, 44–49 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang C-R, Koezuka Y, Kronenberg M, Tracking the Response of Natural Killer T Cells to a Glycolipid Antigen Using Cd1d Tetramers. The Journal of experimental medicine 192, 741–754 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A, In Vivo Identification of Glycolipid Antigen–Specific T Cells Using Fluorescent Cd1d Tetramers. The Journal of experimental medicine 191, 1895–1904 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Rhijn I, Ly D, Moody DB, CD1a, CD1b, and CD1c in Immunity Against Mycobacteria. Advances in experimental medicine and biology 783, 181–197 (2013). [DOI] [PubMed] [Google Scholar]
- 12.de Jong A, Cheng TY, Huang S, Gras S, Birkinshaw RW, Kasmar AG, Van Rhijn I, Pena-Cruz V, Ruan DT, Altman JD, Rossjohn J, Moody DB, CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nature immunology, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Rhijn I, Iwany SK, Fodran P, Cheng TY, Gapin L, Minnaard AJ, Moody DB, CD1b-mycolic acid tetramers demonstrate T-cell fine specificity for mycobacterial lipid tails. European journal of immunology 47, 1525–1534 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasmar AG, van Rhijn I, Cheng TY, Turner M, Seshadri C, Schiefner A, Kalathur RC, Annand JW, de Jong A, Shires J, Leon L, Brenner M, Wilson IA, Altman JD, Moody DB, CD1b tetramers bind alphabeta T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. The Journal of experimental medicine 208, 1741–1747 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ly D, Kasmar AG, Cheng TY, de Jong A, Huang S, Roy S, Bhatt A, van Summeren RP, Altman JD, Jacobs WR Jr., Adams EJ, Minnaard AJ, Porcelli SA, Moody DB, CD1c tetramers detect ex vivo T cell responses to processed phosphomycoketide antigens. The Journal of experimental medicine, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lalla C, Lepore M, Piccolo FM, Rinaldi A, Scelfo A, Garavaglia C, Mori L, De Libero G, Dellabona P, Casorati G, High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. European journal of immunology 41, 602–610 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Wun KS, Reijneveld JF, Cheng TY, Ladell K, Uldrich AP, Le Nours J, Miners KL, McLaren JE, Grant EJ, Haigh OL, Watkins TS, Suliman S, Iwany S, Jimenez J, Calderon R, Tamara KL, Leon SR, Murray MB, Mayfield JA, Altman JD, Purcell AW, Miles JJ, Godfrey DI, Gras S, Price DA, Van Rhijn I, Moody DB, Rossjohn J, T cell autoreactivity directed toward CD1c itself rather than toward carried self lipids. Nature immunology 19, 397–406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepore M, de Lalla C, Gundimeda SR, Gsellinger H, Consonni M, Garavaglia C, Sansano S, Piccolo F, Scelfo A, Haussinger D, Montagna D, Locatelli F, Bonini C, Bondanza A, Forcina A, Li Z, Ni G, Ciceri F, Jeno P, Xia C, Mori L, Dellabona P, Casorati G, De Libero G, A novel self-lipid antigen targets human T cells against CD1c+ leukemias. The Journal of experimental medicine 211, 1363–1377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB, Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med 191, 937–948 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy S, Ly D, Castro CD, Li NS, Hawk AJ, Altman JD, Meredith SC, Piccirilli JA, Moody DB, Adams EJ, Molecular Analysis of Lipid-Reactive V 1 T Cells Identified by CD1c Tetramers. J Immunol 196, 1933–1942 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, Cheng TY, Young DC, Layre E, Madigan CA, Shires J, Cerundolo V, Altman JD, Moody DB, Discovery of deoxyceramides and diacylglycerols as CD1b scaffold lipids among diverse groove-blocking lipids of the human CD1 system. Proceedings of the National Academy of Sciences of the United States of America 108, 19335–19340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, Eckle SB, Uldrich AP, Birkinshaw RW, Patel O, Kostenko L, Meehan B, Kedzierska K, Liu L, Fairlie DP, Hansen TH, Godfrey DI, Rossjohn J, McCluskey J, Kjer-Nielsen L, Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. The Journal of experimental medicine 210, 2305–2320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gherardin NA, Souter MN, Koay HF, Mangas KM, Seemann T, Stinear TP, Eckle SB, Berzins SP, d’Udekem Y, Konstantinov IE, Fairlie DP, Ritchie DS, Neeson PJ, Pellicci DG, Uldrich AP, McCluskey J, Godfrey DI, Human blood MAIT cell subsets defined using MR1 tetramers. Immunology and cell biology 96, 507–525 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Rhijn I, van Berlo T, Hilmenyuk T, Cheng TY, Wolf BJ, Tatituri RV, Uldrich AP, Napolitani G, Cerundolo V, Altman JD, Willemsen P, Huang S, Rossjohn J, Besra GS, Brenner MB, Godfrey DI, Moody DB, Human autoreactive T cells recognize CD1b and phospholipids. Proceedings of the National Academy of Sciences of the United States of America, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinink P, Souter MNT, Cheng TY, van Gorkom T, Lenz S, Kubler-Kielb J, Strle K, Kremer K, Thijsen SFT, Steere AC, Godfrey DI, Pellicci DG, Moody DB, Van Rhijn I, CD1b presents self and Borrelia burgdorferi diacylglycerols to human T cells. European journal of immunology 49, 737–746 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahine A, Reinink P, Reijneveld JF, Gras S, Holzheimer M, Cheng TY, Minnaard AJ, Altman JD, Lenz S, Prandi J, Kubler-Kielb J, Moody DB, Rossjohn J, Van Rhijn I, A T-cell receptor escape channel allows broad T-cell response to CD1b and membrane phospholipids. Nature communications 10, 56 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiroishi M, Kuroki K, Rasubala L, Tsumoto K, Kumagai I, Kurimoto E, Kato K, Kohda D, Maenaka K, Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d). Proceedings of the National Academy of Sciences of the United States of America 103, 16412–16417 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharf L, Li NS, Hawk AJ, Garzon D, Zhang T, Fox LM, Kazen AR, Shah S, Haddadian EJ, Gumperz JE, Saghatelian A, Faraldo-Gomez JD, Meredith SC, Piccirilli JA, Adams EJ, The 2.5 a structure of CD1c in complex with a mycobacterial lipid reveals an open groove ideally suited for diverse antigen presentation. Immunity 33, 853–862 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McWilliam HEG, Mak JYW, Awad W, Zorkau M, Cruz-Gomez S, Lim HJ, Yan Y, Wormald S, Dagley LF, Eckle SBG, Corbett AJ, Liu H, Li S, Reddiex SJJ, Mintern JD, Liu L, McCluskey J, Rossjohn J, Fairlie DP, Villadangos JA, Endoplasmic reticulum chaperones stabilize ligand-receptive MR1 molecules for efficient presentation of metabolite antigens. Proceedings of the National Academy of Sciences of the United States of America 117, 24974–24985 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itskovich SS, Gurunathan A, Clark J, Burwinkel M, Wunderlich M, Berger MR, Kulkarni A, Chetal K, Venkatasubramanian M, Salomonis N, Kumar AR, Lee LH, MBNL1 regulates essential alternative RNA splicing patterns in MLL-rearranged leukemia. Nature communications 11, 2369 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canton J, Neculai D, Grinstein S, Scavenger receptors in homeostasis and immunity. Nature reviews. Immunology 13, 621–634 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Pepino MY, Kuda O, Samovski D, Abumrad NA, Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr 34, 281–303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinink P, Shahine A, Gras S, Cheng TY, Farquhar R, Lopez K, Suliman SA, Reijneveld JF, Le Nours J, Tan LL, Leon SR, Jimenez J, Calderon R, Lecca L, Murray MB, Rossjohn J, Moody DB, Van Rhijn I, A TCR beta-Chain Motif Biases toward Recognition of Human CD1 Proteins. J Immunol 203, 3395–3406 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Rhijn I, Gherardin NA, Kasmar A, de Jager W, Pellicci DG, Kostenko L, Tan LL, Bhati M, Gras S, Godfrey DI, Rossjohn J, Moody DB, TCR bias and affinity define two compartments of the CD1b-glycolipid-specific T Cell repertoire. J Immunol 192, 4054–4060 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo T, Koo MY, Kagoya Y, Anczurowski M, Wang CH, Saso K, Butler MO, Hirano N, A Subset of Human Autoreactive CD1c-Restricted T Cells Preferentially Expresses TRBV4–1(+) TCRs. J Immunol 200, 500–511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellicci DG, Uldrich AP, Le Nours J, Ross F, Chabrol E, Eckle SB, de Boer R, Lim RT, McPherson K, Besra G, Howell AR, Moretta L, McCluskey J, Heemskerk MH, Gras S, Rossjohn J, Godfrey DI, The molecular bases of delta/alphabeta T cell-mediated antigen recognition. The Journal of experimental medicine 211, 2599–2615 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai L, Picard D, Anderson B, Chaudhary V, Luoma A, Jabri B, Adams EJ, Savage PB, Bendelac A, The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur J Immunol 42, 2505–2510 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, Patel O, Beddoe T, Gras S, Rossjohn J, Godfrey DI, CD1d-lipid antigen recognition by the gammadelta TCR. Nat Immunol 14, 1137–1145 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, Anderson B, Scharf L, Kung JE, Sibener LV, Savage PB, Jabri B, Bendelac A, Adams EJ, Crystal Structure of Vdelta1 T Cell Receptor in Complex with CD1d-Sulfatide Shows MHC-like Recognition of a Self-Lipid by Human gammadelta T Cells. Immunity 39, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, de Vries N, Godfrey DI, Altman JD, de Jager W, Rossjohn J, Moody DB, A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nature immunology 14, 706–713 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhodapkar MV, Kumar V, Type II NKT Cells and Their Emerging Role in Health and Disease. J Immunol 198, 1015–1021 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freigang S, Landais E, Zadorozhny V, Kain L, Yoshida K, Liu Y, Deng S, Palinski W, Savage PB, Bendelac A, Teyton L, Scavenger receptors target glycolipids for natural killer T cell activation. J Clin Invest 122, 3943–3954 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, Besra GS, Kent SC, Moody DB, Brenner MB, Apolipoprotein-mediated pathways of lipid antigen presentation. Nature 437, 906–910 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Moody DB, Porcelli SA, Intracellular pathways of CD1 antigen presentation. Nature reviews. Immunology 3, 11–22 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Roy S, Ly D, Li NS, Altman JD, Piccirilli JA, Moody DB, Adams EJ, Molecular basis of mycobacterial lipid antigen presentation by CD1c and its recognition by alphabeta T cells. Proceedings of the National Academy of Sciences of the United States of America, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McFarland DC, Zhang C, Thomas HC, T LR, Confounding effects of platelets on flow cytometric analysis and cell-sorting experiments using blood-derived cells. Cytometry. Part A : the journal of the International Society for Analytical Cytology 69, 86–94 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Hui H, Fuller K, Erber WN, Linden MD, Measurement of monocyte-platelet aggregates by imaging flow cytometry. Cytometry. Part A : the journal of the International Society for Analytical Cytology 87, 273–278 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Reijneveld JF, Ocampo TA, Shahine A, Gully BS, Vantourout P, Hayday AC, Rossjohn J, Moody DB, Van Rhijn I, Human gammadelta T cells recognize CD1b by two distinct mechanisms. Proceedings of the National Academy of Sciences of the United States of America 117, 22944–22952 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pellicci DG, Koay HF, Berzins SP, Thymic development of unconventional T cells: how NKT cells, MAIT cells and gammadelta T cells emerge. Nature reviews. Immunology, (2020). [DOI] [PubMed] [Google Scholar]
- 50.Birkinshaw RW, Pellicci DG, Cheng TY, Keller AN, Sandoval-Romero M, Gras S, de Jong A, Uldrich AP, Moody DB, Godfrey DI, Rossjohn J, alphabeta T cell antigen receptor recognition of CD1a presenting self lipid ligands. Nature immunology, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koay HF, Gherardin NA, Xu C, Seneviratna R, Zhao Z, Chen Z, Fairlie DP, McCluskey J, Pellicci DG, Uldrich AP, Godfrey DI, Diverse MR1-restricted T cells in mice and humans. Nature communications 10, 2243 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang GC, Dash P, McCullers JA, Doherty PC, Thomas PG, T cell receptor alphabeta diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Science translational medicine 4, 128ra142 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DA, Generation of T-cell receptor retrogenic mice. Nature protocols 1, 406–417 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Yang P, Wang N, Zhang J, Li J, Guo H, Yin X, Rao Z, Wang X, Zhang L, The binding of a monoclonal antibody to the apical region of SCARB2 blocks EV71 infection. Protein Cell 8, 590–600 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: List of mAbs
Table S3: Raw data file
Figure S1: CD1-endo tetramer staining of lymphoid subsets
Figure S2: In vitro culture reduces non-TCR-mediated CD1c-endo tetramer staining on human T cells
Figure S3: CD1b/c chimeric tetramers stain CD1c-restricted T cells, but exhibit non-TCR mediated PBMC staining.
Figure S4: CRISPR GeCKO screen supplementary data
Figure S5: Control 293T transient transfections with CD36 family members
Figure S6: Validation of CD36-blockade for the isolation of CD1c-restricted T cells
Figure S7: Enrichment of CD1c-endo reactive T cells from human PBMCs
Figure S8: JL2 antibody production
Table S2: CRISPR Screen raw data
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. The raw CRIPSR screen data can be found in supplemental table S3. TCR sequence data has been desposited in Genbank repository under accession numbers MZ190477-MZ190530.