Abstract
Background
Coronavirus disease 2019 (COVID-19) is a viral infectious disease caused by the severe acute respiratory syndrome coronavirus 2 virus that is affecting the entire world population. The objective of this study was to analyze the repercussion of the disease in a group of patients at risk such as heart transplant recipients.
Methods
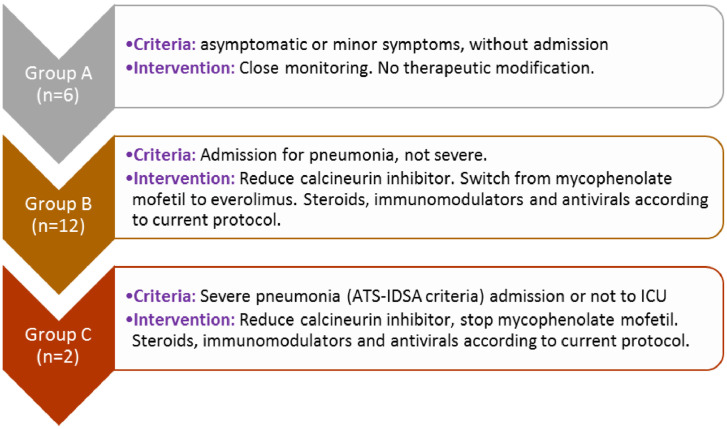
From February 2020 to February 2021, heart transplant recipients diagnosed with COVID-19 were consecutively included. The total number of transplant recipients in outpatient follow-up at that time was 381. Three levels of infection were determined: group A: asymptomatic patients or with trivial symptoms without the need for hospital admission (6 patients); group B: patients admitted to the hospital for respiratory symptoms (12 patients); and group C: patients with severe symptoms and need for admission to the critical care unit (2 patients). At each risk level, medical performance was different: group A: close control, no therapeutic modification; group B: reduction of calcineurin inhibitor and substitution of mycophenolate mofetil for everolimus; group C: reduction of calcineurin inhibitor and withdrawal of mycophenolate mofetil.
Results
The prevalence of infection in the series was 5.2%. Most patients admitted had a pathologic chest x-ray with fever, cough, dyspnea, or vomiting. The change in immunosuppression performed in patients in group 2 was well tolerated and there was no graft rejection. Antiviral treatment was little used. However, boluses of steroids and some antibiotics were used frequently. The need for supplemental oxygen was 50% in group 2 and 100% in group 3.
Conclusions
A significant number of transplant recipients will be affected by COVID-19 (5.3%). Management of the infection will depend on the severity of the infection and must be based on a balance between reduction and adjustment of immunosuppression, strict control of the cardiologic situation, and treatment of the infection.
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by a newly discovered virus. Most people who become ill with COVID-19 experience mild to moderate symptoms and recover without special treatment. However, some subgroups of patients, such as heart transplant recipients, may experience more severe infection and higher mortality because of their immunosuppressed condition. Thus, heart transplant recipients constitute a complex group vulnerable to COVID-19 because of the challenge of simultaneously managing infection and immunosuppression to avoid rejection.
The objective of this study was to present the series of cases of heart transplant recipients with COVID-19 in a third-level transplant hospital and the approach taken according to the patient's risk.
Materials and Methods
Heart transplant recipients diagnosed with COVID-19 were consecutively recruited from February 2020 to February 2021. The diagnosis could be accidental or due to infectious symptoms with positive polymerase chain reaction test. The total number of transplant recipients in outpatient follow-up at that time was 381. At the beginning of the pandemic, and considering the possibility and risk of infection in heart transplant recipients, the center's heart transplant team drew up a protocol for therapeutic action according to the patient's infectious situation. This protocol was carried out in all infected patients. Three levels of infection were determined: group A; asymptomatic patients or with trivial symptoms without the need for hospital admission (6 patients); group B: patients admitted to the hospital ward for respiratory symptoms (12 patients); group C; patients with severe symptoms and need for admission to the critical care unit (2 patients). At each level of risk, medical performance was different: group A: close control, no therapeutic modification; group B: reduction of calcineurin inhibitor (CNI) and substitution of mycophenolate mofetil (MMF) for everolimus (EVE); group C: reduction of CNI and withdrawal of MMF. In addition, patients were administered antiviral and general treatment corresponding to their clinical situation. Figure 1 shows the 3 established groups.
Table 1.
Clinical Profile and Therapeutic Approach According to Risk Group
| Group A (n = 6) | Group B (n = 12) | Group C (n = 2) | |
|---|---|---|---|
| Age (y) | 51.2 ± 17.5 | 59.9 ± 13.2 | 64.0 ± 14.6 |
| Male sex, n (%) | 5 (83) | 11 (92) | 2 (100) |
| Time since HTx (d) | 2897 ± 2780 | 3777 ± 2462 | 1901 ± 2480 |
| Reason for admission, n (%) Fever (with cough, dyspnea, or vomiting) Dyspnea Diarrhea |
0 (0) 0 (0) 0 (0) |
11 (92) 2 (17) 2 (17) |
2 (100) 2 (100) 0 (0) |
| Chest x-ray, n (%) Normal Pneumonia |
6 (100) 0 (0) |
2 (17) 10 (83) |
0 (0) 2 (100) |
| QT prolongation, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Ventricular arrhythmias, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Treatment during infection, n (%) Tacrolimus Cyclosporine MMF Everolimus Deflazacort |
4 (66) 2 (33) 5 (83) 1 (17) 6 (100) |
5 (42) 7 (58) 0 (0) 12 (100) 12 (100) |
1 (50) 1 (50) 0 (0) 0 (0) 2 (100) |
| Antiviral treatment, n (%) Baricitinib Remdesivir |
0 (0) 0 (0) |
1 (8) 1 (8) |
1 (50) 0 (0) |
| Other treatments, n (%) Lopinavir + ritonavir Chloroquine Azithromycin Steroid bowling Other antibiotics |
0 (0) 0 (0) 0 (0) 0 (0) 0 (0) |
1 (8) 4 (33) 6 (50) 9 (75) 9 (75) |
0 (0) 0 (0) 0 (0) 2 (100) 2 (100) |
| Supplemental O2, n (%) Nasal cannulas Venturi mask OI |
0 (0) 0 (0) 0 (0) |
4 (33) 2 (17) 0 (0) |
0 (0) 0 (0) 2 (100) |
| Days of admission | 0 (0) | 18.2 ± 23.8 | 19.0 ± 20.1 |
| Exitus, n (%) | 0 (0) | 0 (0) | 2 (100) |
Data expressed as mean ± standard deviation or as otherwise noted. Two patients in group B died at the first and second month after infection owing to extra coronavirus disease causes (neoplasm of the colon and acute coronary syndrome).
HTx, heart transplant; MMF, mycophenolate mofetil; O2, oxygen; OI, orotracheal intubation.
Fig 1.
Risk levels and guidelines for action in transplant patients with coronavirus disease 2019. ATS-IDSA, American Thoracic Society-Infectious Diseases Society of America; ICU, intensive care unit.
Results
The prevalence of infection in the series was 5.2%. Most patients in the 3 groups were male. Those in higher risk groups were older. Most patients were admitted because of fever associated with cough, dyspnea, or vomiting. These symptoms, together with a pathologic chest x-ray, motivated the hospital admission. Electrocardiographic or arrhythmic abnormalities were observed in none of the 20 patients in the series (Table 1).
The change in immunosuppression performed in patients in group 2 was well tolerated and there was no rejection. Antiviral treatment was little used. However, boluses of steroids and some antibiotics were used frequently. The need for supplemental oxygen was close to 50% in group 2 and 100% in group 3.
The mean hospital stay was just over 2 weeks for hospitalized patients and almost 3 weeks for those admitted to critical care. The need for intubation was identified in a subgroup with a mortality of 100%, although there were only 2 cases.
Discussion
Since the coronavirus pandemic began in Wuhan, China, this infection has spread throughout the worlds. The entire world population is susceptible to being infected, but there are some groups at higher risk owing to their immunosuppressive situation, including heart transplant recipients, in whom prevention of rejection during an infection continues to be a challenge. It is advisable to modify the immunosuppressive treatment according to the severity of the infection to try to protect heart transplant recipients. In this series, 3 risk groups were considered and it was found that patients with few symptoms have a good evolution; when respiratory symptoms (pneumonia) are present it is possible to control the infection with changes in immunosuppression. However, when the patient requires orotracheal intubation, the chances of survival are slim.
In this series, the prevalence of COVID-19 infection in heart transplant recipients was 5.2%. This coincides with the figures in the literature, although the prevalence is variable [1,2] in a country where the prevalence of infection in the general population is around 8% [3]. Most patients are men between the ages of 50 and 64 years, a profile similar to that described in other series of severe acute respiratory syndrome coronavirus 2–infected heart transplant recipients [2]. None of the cases occurred early after transplantation. The reason for consulting these patients was the same as in the general population and published series of transplants [2]; that is, the presentation of fever, dyspnea, cough, and/or vomiting. Thirty percent of patients (6) did not require hospital admission, 60% (12) required hospital admission without the need for critical care, and only 2 patients (10%) required admission to the critical care unit—a distribution similar to that observed in previous studies [1].
Immunosuppression is common in hear transplant recipients [4], with treatment including CNIs, MMF, and corticosteroids. The usual strategy followed in transplant recipients infected with COVID-19 is to reduce immunosuppression [2,5]. In this series, immunosuppression was not reduced in patients with a mild infection and the usual pattern was maintained, adjusting the CNI dose to the detected plasma levels. In group B, on the other hand, the CNI dose was reduced, as in other reported series [2], with the substitution of MMF for the mammalian target of rapamycin inhibitor EVE. This change was made because EVE reduces the risk of infection by other viruses, such as cytomegalovirus, by regulating factors involved in several cellular functions crucial for cytomegalovirus replication [6]. In contrast, MMF has traditionally been associated with viral infections and reactivations [7]. The change in immunosuppression was well tolerated. Only 2 of the 20 patients had poor evolution from the respiratory point of view, requiring orotracheal intubation. In this case, we opted for the suspension of MMF without substitution by EVE, reducing the dose of CNI, and maintaining or increasing if corticosteroid therapy was required. However, these 2 patients died. The mortality of the series was therefore 10%, lower than that reported in other registries [1,2], although the mortality in group 3 was 100% (2 patients).
Conclusions
A significant number of transplant recipients will be affected by COVID-19. Management of the infection will depend on the severity of the infection and should be based on a balance between reduction and adjustment of immunosuppression, strict control of the cardiologic situation, and treatment of the infection.
References
- 1.García-Cosío M, Flores Hernán M, Caravaca Pérez P, López-Medrano F, Arribas F, Delgado Jiménez J. Heart transplantation during the coronavirus disease 2019 pandemic: follow-up organization and characteristics of infected patients. Rev Esp Cardiol (Engl Ed) 2020;73:1077–1080. doi: 10.1016/j.rec.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottio T, Bagozzi L, Fiocco A, Nadali M, Caraffa R, Bifulco O. COVID-19 in heart transplant recipients: A Multicenter Analysis of the Northern Italian Outbreak. JACC Heart Fail. 2021;9:52–61. doi: 10.1016/j.jchf.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonio L, Borraz M, Giménez M, Carrera P, González E, Ortíz C, et al. [Prevalence of SARS-CoV-2 coronavirus infection in patients and professional staff at a medium or long-stay hospital in Spain] Rev Esp Geriatr Gerontol. 2021;56:75–80. doi: 10.1016/j.regg.2020.10.005. [in Spanish] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González-Vílchez F, Almenar Bonet L, Crespo-Leiro MG, Gómez-Bueno M, González-Costello J, Pérez-Villa F, et al. Spanish Heart Transplant Registry. 31th Official Report of the Heart Failure Association of the Spanish Society of Cardiology. Rev Esp Cardiol (Engl Ed) 2020;74:954–962. doi: 10.1016/j.rec.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Sa MA, Ferna C, Defilippis EM, Farr MA, Givertz MM. Challenges in heart transplantation in the era of COVID-19. Circulation. 2020;73:2048–2051. doi: 10.1161/CIRCULATIONAHA.120.047096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan L, Sato N, Shiraki A, Yanagita M, Yoshida Y, Takemura Y, et al. Everolimus delayed and suppressed cytomegalovirus DNA synthesis, spread of the infection, and alleviated cytomegalovirus infection. Antiviral Res. 2019;162:30–38. doi: 10.1016/j.antiviral.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Song AT, Abdala E, Bonazzi PR, Bacchella T, Machado MC. Does mycophenolate mofetil increase the risk of cytomegalovirus infection in solid organ transplant recipients? A mini-review. Braz J Infect Dis. 2006;10:132–138. doi: 10.1590/s1413-86702006000200011. [DOI] [PubMed] [Google Scholar]