Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has especially affected kidney transplant (KT) recipients, who are more vulnerable than the general population because of their immunosuppressive status and added comorbidities. The purpose of this study was to determine risk factors related to infection and mortality from COVID-19 in KT recipients.
Methods
The study included 113 stable KT recipients who had polymerase chain reaction–confirmed COVID-19 infection between March 2020 and February 2021, from a total of 2150 KT recipients. Outcomes related to patient survival were analyzed.
Results
The mean (standard deviation) age of the patients was 56 (14) years; 62% (n = 70) were men. The median time between KT and infection was 88 months (interquartile range, 39-155 months); 90% (n = 102) were on tacrolimus therapy and 81% (n = 92) on mycophenolate mofetil. The clinical presentation was pneumonia (n = 57; 51%), fever (n = 61; 54%), cough (n = 62; 55%), dyspnea (n = 43; 38%), lymphopenia (n = 57; 50%), and gastrointestinal symptoms (n = 28; 25%). A total of 21% (n = 24) required intubation and intensive care unit admission, and 27 patients (25%) were asymptomatic. A total of 9% (n = 10) received hydroxychloroquine therapy plus azithromycin, 11% (n = 12) tocilizumab, 3.7% (n = 4) lopinavir/ritonavir, 49% (n = 55) steroids, 0.9% (n = 1) remdesivir, and 9.3% (n = 11) convalescent plasma. Immunosuppression was reduced in all symptomatic patients. Nineteen patients (17%) died. Cox univariate analysis showed that the factors significantly associated with death were patient age, presence of pneumonia or lymphopenia, and elevated C-reactive protein on admission.
Conclusions
Mortality in KT recipients with COVID-19 is very high, more than for the general population. Risk factors are patient age, presence of pneumonia or lymphopenia, and a higher C-reactive protein level at the time of diagnosis.
Immunosuppression is a known risk factor for a worse evolution of coronavirus disease 2019 (COVID-19). Patients who have undergone kidney transplant (KT) have been especially hit by the COVID-19 pandemic, partly because of their added comorbidities, such as advanced age, hypertension, diabetes, chronic kidney disease, or cardiovascular disease [1]. In Spain, the COVID-19 pandemic in KT has evolved differently depending on the various waves experienced [2].
Materials and Methods
This observational, cross-sectional study included 113 stable KT recipients who had polymerase chain reaction–COVID-19 infection between March 2020 and February 2021, from a total of 2150 KT recipients. Outcomes related to patient survival were analyzed.
Statistical Analysis
Descriptive results are expressed as mean (standard deviation) for continuous variables. Comparisons of continuous variables across the study groups were made by univariate analysis of variance. Categorical variables were compared by χ2 test. Patient survival was calculated using the Kaplan-Meier method. Survival rates of the different groups were compared using the log-rank test. Cox univariate and bivariate analyses were made to determine the factors associated with patient death. Receiver operating characteristic curves were made to determine the value of age and the C-reactive protein (CRP) levels on admission in relation to death.
Results
The mean (standard deviation) age of the patients was 56 (14) years; 62% (n = 70) were men. The median time from transplant to infection was 88 months (interquartile range, 39-155 months). One patient became infected 31 days after transplant. A total of 86% (n = 97) of the patients had hypertension, 35% (n = 40) had diabetes, 14% (n = 16) had ischemic cardiomyopathy, and 70% (n = 79) were receiving treatment with angiotensin II receptor antagonist. A total of 9% (n = 10) were infected in the first wave (March-June 2020), 49% (n = 55) in the second wave (July-November 2020), and 42% (n = 47) in the third wave (December 2020-February 2021).
The presenting symptoms were pneumonia (n = 57; 51%), fever (n = 61; 54%), cough (n = 62; 55%), dyspnea (n = 43; 38%), lymphopenia (n = 57; 50%), and gastrointestinal symptoms (n = 28; 25%). A total of 58.4% (n = 66) required admission, and 14% (n = 16) required intubation and admission to the intensive care unit; 27 patients (25%) were asymptomatic.
Nine percent of the patients received hydroxychloroquine therapy plus azithromycin, 11% (n = 12) tocilizumab, 3.7% (n = 4) lopinavir/ritonavir, 49% (n = 55) steroids, 0.9% (n = 1) remdesivir, and 9.3%(n = 11) convalescent plasma. Immunosuppression was reduced in 55% (n = 62) of the symptomatic patients and was withdrawn in 45% (n = 50), increasing the steroid dose. No patient experienced acute rejection; 19 patients (17%) died. Table 1 compares the characteristics of the patients who survived with those who died.
Table 1.
Clinical and Laboratory Characteristics of the Patients Who Died Compared With Those Who Survived
| Characteristics | Survived (n = 94) | Died (n = 19) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 55 (15) | 67 (6) | .01 |
| Sex, % male(n) | 60 (56) | 68 (13) | .6 |
| Time after transplant, mean (SD), mo | 117 (111) | 113 (96) | .08 |
| Creatinine, mean (SD), mg/dL | 1.7 (0.7) | 2 (1.3) | .3 |
| BMI | 28 (6) | 27 (3) | .7 |
| Hypertension, %(n) | 76 (71) | 100 (19) | .06 |
| Diabetes Mellitus, %(n) | 34 (32) | 42 (8) | .5 |
| ACEIs/ARAII, %(n) | 67 (63) | 78 (15) | .4 |
| Tacrolimus, %(n) | 89 (84) | 90 (17) | .9 |
| Mycophenolate mofetil, %(n) | 82 (77) | 78 (15) | .8 |
| mTOR inhibitors, %(n) | 9 (8) | 26 (5) | .06 |
| Pneumonia, %(n) | 40 (38) | 100 (19) | < .001 |
| Gastrointestinal, %(n) | 24 (22) | 31 (6) | .5 |
| Respiratory, %(n) | 60 (56) | 73 (14) | .4 |
| Fever, %(n) | 51 (48) | 68 (13) | .1 |
| Lymphopenia, %(n) | 43 (40) | 73 (14) | .01 |
| Baseline creatinine, mean (SD), mg/dL | 1.7 (0.7) | 2 (1.3) | .2 |
| Ferritin, mean (SD), ng/mL | 858 (577) | 1113 (752) | .4 |
| C-reactive protein, mean (SD), mg/L | 55 (45) | 105 (62) | .01 |
| D-dimer, mean (SD), ng/mL | 1572 (2500) | 55,009 (270,799) | .06 |
| Tocilizumab, %(n) | 6 (6) | 31 (6) | .06 |
| Lopinavir/ritonavir, %(n) | 1 (1) | 15 (3) | .01 |
| Steroids, %(n) | 39 (37) | 94 (18) | < .001 |
| Hydroxychloroquine+azathioprine, %(n) | 7 (7) | 21 (4) | .07 |
| Hyperimmune plasma, %(n) | 10 (9) | 5 (1) | .9 |
| Remdesivir, %(n) | 0 (0) | 5 (1) | .1 |
ACEI, angiotensin-converting enzyme inhibitor; ARAII, angiotensin II receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); mTOR, mammalian target of rapamycin; SD, standard deviation.
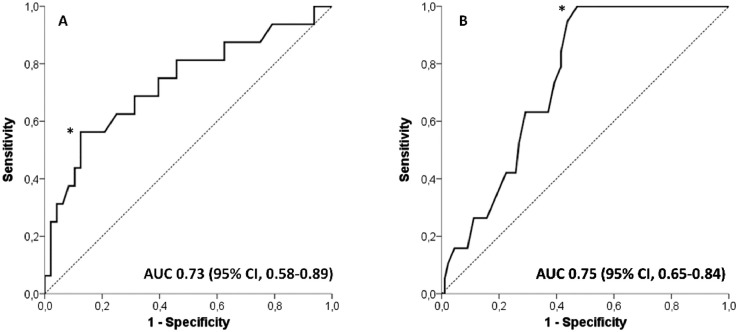
Cox univariate analysis showed that the factors significantly associated with death were the presence of pneumonia, lymphopenia, elevated CRP on admission, and patient age (Table 2 ). The Cox bivariate analysis showed that the CRP level on admission was the factor most associated with the death of the patient (Table 3 ). The receiver operating characteristic curves associated with patient death determined the cut point for age as older than 57 years and for CRP >108 mg/dL (Fig 1 ).
Table 2.
Risk Factors Associated With Death; Cox Univariate and Bivariate Analysis
| Univariate Analysis | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Recipient age | 3.21 (1.23-8.31) | .001 |
| Pneumonia | 68.1 (1.64-2.67) | .02 |
| Lymphopenia | 2.7 (1.23-7.25) | .04 |
| CRP on admission | 1.01 (1.01-1.02) | .001 |
CI, confidence interval; CRP, C-reactive protein.
Table 3.
Relationship between C-reactive protein level and overall mortality by Cox bivariate regression analysis
| Adjusted For | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age | 1.011 (1.003-1.02) | .008 |
| Lymphopenia | 1.014 (1.006-1.022) | < .001 |
CI, confidence interval.
Fig 1.
Receiver operator characteristic (ROC) curve for the C-reactive protein value on admission as a predictor of patient death. The optimal predictor cutoff value (*) was that of the highest sensitivity together with the lowest number of false positives (specificity). This value corresponds to C-reactive protein 108 mg/L (A). ROC curve for the value recipient age as a predictor of patient death. The optimal predictor cutoff value (*) was that of the highest sensitivity together with the lowest number of false positives (specificity). This value corresponds to 57 years (B). AUC, area under the curve; CI, confidence interval.
Discussion
Mortality associated with COVID-19 in KT patients is higher than that reported in the general population, with advanced age being one of the main risk factors associated with this mortality [3]. In our series, risk factors for mortality were patient age older than 57 years, presence of pneumonia and lymphopenia, and CRP levels >108 mg/L at the time of diagnosis. We experienced more infections in the second wave. In addition, we now have a new approach in the management of immunosuppression, with the use of corticosteroids increasing compared with other therapies according to the evolution of the pandemic.
Footnotes
Funded by grants from the Instituto de Salud Carlos III, Madrid, Spain (ICI 14/0016; PI17/02043; CM19-00210; REDinREN Network, RD16/0009/0006), FONDOS FEDER.
References
- 1.Kates OS, Haydel BM, Florman SS, et al. Coronavirus disease 2019 in solid organ transplant: A multicenter cohort study [e-pub ahead of print]. Clin Infect Dis doi:10.1093/cid/ciaa1097, accessed June 1, 2021. [DOI] [PMC free article] [PubMed]
- 2.Villanego F, Mazuecos A, Pérez-Flores IM, et al. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish Registry. Am J Transplant. 2021;21:2573–2582. doi: 10.1111/ajt.`16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crespo M, Pérez-Sáez MJ, Redondo-Pachón D, et al. COVID-19 in elderly kidney transplant recipients. Am J Transplant. 2020;20:2883–2889. doi: 10.1111/ajt.16096. [DOI] [PMC free article] [PubMed] [Google Scholar]