Abstract
Objective
Vaccination is one of the most important measures that the world is relying on to end the COVID-19 pandemic. A number of vaccines have been authorized; however, there are several differences in the available vaccines which may lead to differences in public hesitancy levels toward each vaccine. Therefore, the aim of this study was to assess the young Jordanian population's acceptance of the COVID-19 vaccine, their knowledge, and attitudes toward different vaccine types, and to explore the variables that could influence their preferences.
Material and methods
An online questionnaire was distributed via Jordanian multipurpose Facebook groups. COVID-19 knowledge, and practice scores were calculated, in addition to general and specific COVID-19 vaccine knowledge scores. Repeated measures analysis was conducted to investigate the association between the participants’ knowledge about each vaccine and their willingness to take it. Quantile regressions were conducted to determine the predictors of the participants’ willingness to take each vaccine.
Results
A total of 1897 participants completed the survey. One fifth of the participants (19.9%) were willing to take the COVID-19 vaccine. The acceptance of Pfizer/BioNTech vaccine and the knowledge about it were significantly different from all the other vaccines. Predictors of acceptance of the different vaccines were sex, estimation of the severity of the disease, COVID-19 knowledge score, practice score, and specific vaccine knowledge score.
Conclusion
The young Jordanian adults had limited acceptance of the COVID-19 vaccine. Differences in the participants’ acceptance of different vaccines were observed and specific vaccine knowledge was a significant predictor of acceptance of the vaccine.
Keywords: COVID-19, Vaccine, Acceptance, Types, Jordan
Abstract
Objetivo
La vacunación es una de las medidas más importantes en la que el mundo se basa para acabar con la pandemia de la COVID-19. Varias vacunas han sido autorizadas para ser usadas; sin embargo, existen diferencias en las vacunas disponibles que pueden dar lugar a diferencias en los niveles de vacilación del público para ponerse cada una. Por lo tanto, este estudio tiene como objetivo evaluar la aceptación de la población joven jordana de la vacuna COVID-19, su conocimiento y actitudes hacia los diferentes tipos de vacunas, y variables que pueden influir sus preferencias.
Material y métodos
En enero de 2021 se distribuyó un cuestionario en línea por vía de grupos jordanos usuarios de Facebook. Se calculó la puntuación de conocimiento de COVID-19, la puntuación de práctica y las puntuaciones de conocimiento de vacunas específicas. Se realizaron repetidos análisis de medidas para investigar las diferencias entre el conocimiento de los participantes acerca de cada vacuna y la voluntad para ponerse cada vacuna. Se realizaron regresiones cuantílicas para determinar los predictores de la disposición de los participantes a recibir la vacuna.
Resultados
Completaron la encuesta 1.897 participantes. El 19,9% estuvo dispuesto a recibir la vacuna frente a COVID-19. La aceptación de la vacuna Pfizer/BioNTech y el conocimiento acerca de ella estaban significativamente diferenciados de todas las otras vacunas. Los factores predictivos de la aceptación de las diferentes vacunas fueron el sexo, la estimación de la gravedad de la enfermedad, la puntuación sobre el conocimiento de COVID-19, la puntuación sobre la práctica y la puntuación sobre el conocimiento de las vacunas específicas.
Conclusión
Los jóvenes adultos jordanos tuvieron aceptación limitada de la vacuna de COVID-19. Se observaron diferencias en los participantes en cuanto a la aceptación de diferentes vacunas, y el conocimiento de las vacunas específicas fue un factor predictivo significativo en cuanto a su aceptación.
Palabras clave: COVID-19, Vacuna, Aceptación, Tipos, Jordania
Introduction
Coronavirus Disease 2019 (COVID-19) is a novel disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 It has high transmissibility which facilitates its quick spread around the world.2 The disease cases reached more than 100 million with over 2 million deaths by the end of January 2021,3 and it continues to be a major global challenge that has adverse impacts on all life aspects. Several precautionary measures have been followed by the governments to decrease COVID-19 spread and mitigate its effects. Vaccination is one of the most important precautionary measures as its role in reducing viral diseases spreading has been established for decades.4
Different COVID-19 vaccines had been developed and approved for emergency use across the world starting from December 2020.5 Moreover, over 50 vaccine candidates are under development.6 Global efforts are intensified to distribute the vaccines efficiently, however, hesitancy and refusal of COVID-19 vaccines were observed among different populations. In a global survey that covers participants from 19 countries, vaccine acceptance rates ranged between less than 55% to over 90% and overall acceptance was 71.5%.7
At the time that this study was conducted (January 2021), the vaccination rate in the Middle East was limited as only two countries including United Arab Emirates and Bahrain had relatively high vaccination rate (33 and 11.56 per 100 people respectively), while the remaining countries had vaccination rates of less than 2 per 100 people.8 In Jordan, the vaccination rate was less than 0.5%.9 Furthermore, vaccines acceptance rates in the Middle East are lower than the global ones. For example, studies conducted to assess vaccination acceptance rates among the Middle East populations reported acceptance rates of 23.6% in Kuwait,10 and 64.7% in Saudi Arabia.11 In Jordan, vaccination acceptance rate did not exceed 40%.12 Nevertheless, these rates were reported before the availability of the vaccines; therefore, the acceptance rates may differ now as more information about the vaccines had been revealed. Besides, the available COVID-19 vaccines differ considerably in mechanisms of action, countries of origin, storage recommendations, doses regimens, efficacy rates, and availability of information which may perhaps affect the populations’ acceptance rates of different COVID-19 vaccines.
Jordanian population is a young population with more than 63% under the age of 30.13 Therefore, it is crucial to evaluate the attitudes toward COVID-19 vaccination, of those who are eligible for the vaccination, from this young group as they represent a large portion of the community.
Aim of the study
The study aim was to evaluate the acceptance of different COVID-19 vaccines and its’ associated factors among young Jordanian population.
Method
Study design and participants
This is a cross sectional web-based survey that was distributed in January 2021. Since the targeted population in this study was the young adults, internet based survey is a suitable method to recruit this age group as they constitute the highest percentage of Internet users.14 The participants were Jordan residents in the age group between 18 and 30 years. The survey was formulated on Google forms and circulated via all-purpose Facebook groups and pages that include members from Jordan. To ensure that all the participants met the inclusion criteria, the survey included questions about age, country of residence, and if the respondents did not meet the inclusion criteria, based on their answers, the respondent was automatically directed to submit the survey. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by Al Zaytoonah University ethics committee. Informed consent was given by all the participants through the online form of the questionnaire.
Sampling type and sample size
Simple random sampling method was used in this study. To calculate the required sample size, Kish formula was used at a 95% significance level and a 3-percentage-point margin of error. The estimated sample size was 1014, however, 1897 participants were included in the study.
Instrument validity and reliability
The survey was developed in Arabic language after literature review. Arabic is the main spoken language for 98% of the Jordanian citizens.15 The survey was originally formulated in English, translated to Arabic, and then back translated to English by different translator and the two English versions were found to be comparable. The content validity of the developed survey was assessed by an expert panel. To confirm face validity, the questionnaire was sent to 30 individuals and changes were implemented based on their comments. The data of these participants was not included in the final study. The reliability of generated questionnaire scores including general knowledge about COVID-19 vaccines, knowledge scores for each vaccine type included in the study and practice toward COVID-19 were evaluated by computing Cronbach's alpha. The acceptable Cronbach's alpha range was determined to be above 0.5,16 as lower Cronbach's alpha are expected in binary data17 and low number of questions.18 The computed Cronbach's alpha were all above 0.5 (0.51–0.80).
Study instrument
The first part of the survey collected the participants’ socio-demographics information including sex, marital status, pregnancy status, and if the participant has children or not, in addition to smoking habits, weight status, health status, education level, average household monthly income (measured in Jordanian dinars (JOD)), and if the participant study or work in a medical field. The second section collected information about the participants’ vaccination history (Influenza and COVID-19 vaccines). If the participant had already been vaccinated against COVID-19, he/she would be automatically directed to submit the survey. The third section gathered information about participants’ experience with COVID-19 and their estimation of disease seriousness in a scale from 1 (not serious) to 10 (extremely serious). The fourth section evaluated the participants’ knowledge, attitudes, and practices toward COVID-19 and their acceptance of COVID-19 vaccine. If the participants answered “No” for the COVID-19 vaccine question, he/she was automatically directed to submit the survey. The fifth section evaluated the participants’ specific knowledge, attitudes, and acceptance of different types of COVID-19 vaccines. Lastly, the sixth section assessed the factors that affect the participants’ acceptance of different vaccine types.
The participants were classified into three groups based on their COVID-19 risk degree which was based on Central of Disease Control and Prevention (CDC) risk factors.19 High-risk group included the participants who suffer from Type 2 diabetes mellitus/Chronic Obstructive Pulmonary Disease (COPD)/Cancer/Kidney Failure/Heart diseases/Organ transplantation/Sickle Cell Anemia and the participants who were smokers, pregnant, or obese. The medium risk group included the participants who did not fit for the high-risk group but were overweight or had one of the following: Type 1 diabetes mellitus/Hypertension/Bone marrow transplant/Cerebrovascular diseases or stroke/Cystic Fibrosis/Asthma/Taking steroids or immunosuppressant drugs/Hepatic diseases/Thalassemia/Lung fibrosis. The low-risk group included all other participants that do not fit the previously mentioned criteria.
Seven scores were calculated, first score is the COVID-19 knowledge score, which was calculated based on the participants’ knowledge of COVID-19 symptoms and the measures that could prevent the infection as one point was granted if the participant chose “Yes” for the correct statements and one point was granted if the participant chose “No” for the incorrect statements (Table 3). Practice score was calculated as the sum of the practice statements which represent the participants’ adherence to protective measures with answers ranged from “Never” (1 point) to “All the time” (5 points) (Table 3). The remaining scores were specific vaccine knowledge scores which evaluated the participants’ knowledge about each vaccine type as one point was added for each correct answer with a maximum possible score of 19 (Online Resource). The information about each vaccine type was gathered from CDC,20, 21 United Kingdom government website,22 Sputnik vaccine website,23 and Sinopharm vaccine website.24
Table 3.
The participants’ knowledge, attitudes, and practice toward COVID-19 and COVID-19 vaccine.
| Frequency (%) or mean (±SD) | |
|---|---|
| Knowledge statements | |
| What are the symptoms of COVID-19? (If the participant chose “Yes”) | |
| Fevera | 1813 (97.1) |
| Chillsa | 1311 (70.2) |
| Diarrheaa | 1267 (67.9) |
| Cougha | 1617 (86.6) |
| Otitis mediab | 427 (22.9) |
| Loss of smell and taste sensesa | 1829 (98.0) |
| No symptomsa | 1656 (88.7) |
| What procedure do you think that may prevent COVID-19 infection? | |
| Wearing face masksa | 1760 (94.3) |
| Using detergentsa | 1789 (95.8) |
| Social distancinga | 1811 (97.0) |
| Consume vitamin Cb | 1516 (81.2) |
| Avoid eating meatb | 143 (7.7) |
| Get COVID-19 vaccinea | 1131 (60.6) |
| Practice statements | |
| What is the degree of your adherence to COVID-19 protective measures? | |
| Wearing face masks | 4.06 (±0.89) |
| Washing hands with regular soap | 4.38 (±0.74) |
| Using detergents | 3.92 (±0.98) |
| Social distancing | 3.80 (±1.00) |
| Avoid touching face/mouth/nose/eyes | 3.45 (±1.13) |
| Attitude toward COVID-19 and COVID-19 vaccine | |
| Have you ever been infected with COVID-19? | |
| No | 1014 (88.6) |
| Maybe | 88 (7.7) |
| Yes | 42 (3.7) |
| In your opinion, what is the degree of COVID-19 seriousness? | |
| The opinion of the participants who were infected or suspected their infection | 6.14 (±2.17) |
| The opinion of the participants who were not infected | 6.43 (±2.04) |
| In your opinion, what is the likelihood that you will be infected with Coronavirus during the next six months | |
| I think I will be infected and my symptoms will be severe | 75 (6.6) |
| I think I will be infected and my symptoms will be mild | 566 (49.7) |
| I do not think that I will be infected | 498 (43.7) |
| Are you willing to take COVID-19 vaccine? | |
| No | 959 (51.4) |
| Not sure | 536 (28.7) |
| Yes | 372 (19.9) |
Correct information.
Incorrect information.
Statistical analysis
All data analysis was conducted using SPSS version 25. Categorical variables were presented as frequencies and percentages (%) and continuous variables were presented as means and standard deviations (SD). Normality of the scores of willingness to be vaccinated and knowledge for each vaccine was assessed using Kolmogorov–Smirnov test, as the test indicated that the scores were not normally distributed, nonparametric test were conducted to analyze the results. Friedman test with pairwise comparisons were conducted to determine if there are significant differences between the participants’ knowledge about each vaccine type and between their willingness to take each vaccine. Quantile regressions at median points were conducted to predict the variables associated with the participants’ willingness to take each vaccine.
Results
A total of 1897 participants completed the survey. As shown in Table 1 , the participants’ age mean was 21.80 (±2.97) years. The majority of the participants were females (76.7%), not married (93.5%) had no children (96.7%) and smokers (78.8%). About half of the participants (58.6%) were university students and 50.2% were working/studying in a medical field. The participants were distributed almost equally between the two-household average monthly income groups (47.6% less than 1000 JOD vs. 52.4% 1000 JOD or more).
Table 1.
Sample characteristics.
| Sample characteristic | Frequency (%) or mean (±SD) (n = 1897) |
|---|---|
| Sex | |
| Female | 1455 (76.7) |
| Male | 442 (23.3) |
| Age | 21.80 (±2.97) |
| Marital status | |
| Not married | 1774 (93.5) |
| Married | 123 (6.5) |
| Are you pregnant? | |
| No | 1876 (98.9) |
| Yes | 21 (1.1) |
| Do you have children? | |
| No | 1826 (96.3) |
| Yes | 71 (3.7) |
| Are you a smoker? | |
| No | 1494 (78.8) |
| Ex-smoker | 41 (2.2) |
| Yes | 362 (19.1) |
| Weight status | |
| Underweight | 531 (28) |
| Normal weight | 859 (45.3) |
| Overweight | 463 (24.4) |
| Obese | 44 (2.3) |
| Education level | |
| High school or less | 118 (6.2) |
| Diploma | 98 (5.2) |
| University student | 1112 (58.6) |
| Bachelor's degree | 491 (25.9) |
| Postgrad | 78 (4.1) |
| Household average monthly income | |
| Less than 1000 JOD | 903 (47.6) |
| 1000 JOD or more | 994 (52.4) |
| Are you working/studying in a medical filed? | |
| No | 945 (49.8) |
| Yes | 952 (50.2) |
As shown in Table 2 , thirty participants were already vaccinated against COVID-19, and they were directed to submit the survey. According to risk classification criteria for COVID-19, 22.7% of the participants were in the high-risk group and 20.1% were in the medium risk group.
Table 2.
Study sample health status.
| Items | Frequency (%) |
|---|---|
| Did you take influenza vaccine last year? | |
| No | 1733 (91.4) |
| Yes | 164 (8.6) |
| Did you take COVID-19 vaccine? | |
| No | 1867 (98.4) |
| Yes | 30 (1.6) |
| Do you have any chronic diseases? | |
| No | 1768 (94.7) |
| Yes | 99 (5.3) |
| Do you have any of the following disease (Type 2 diabetes mellitus/Chronic Obstructive Pulmonary Disease (COPD)/Cancer/Kidney Failure/Heart diseases/Organ transplantation/Sickle Cell Anemia)? | |
| No | 1474 (77.7) |
| Yes | 423 (22.3) |
| Do you have any of the following diseases? (Type 1 diabetes mellitus/Hypertension/Bone marrow transplant/Cerebrovascular diseases or stroke/Cystic Fibrosis/Asthma/Taking steroids or immunosuppressant drugs/Hepatic diseases/Thalassemia/Lung fibrosis) | |
| No | 1515 (79.9) |
| Yes | 382 (20.1) |
| Risk degree | |
| High risk | 423 (22.7) |
| Medium risk | 382 (20.5) |
| Low risk | 1062 (56.8) |
The participants’ knowledge, attitudes, and practices toward COVID-19 and their acceptance of COVID-19 vaccine are presented in Table 3 . The most known symptom of COVID-19 was the loss of smell and taste senses (98.0%) followed by fever (97.1%). The majority of the participants knew that wearing face masks (94.3%), using detergents (95.8%), and social distancing (97.0%) can prevent COVID-19 infection. 7.7% of the participants were confirmed cases of COVID-19 and 3.7% suspected their infection. Only 19.9% of the participants were willing to take COVID-19 vaccine, while 28.7% were not sure, and 51.4% were not willing.
As indicated by repeated measures, the highest vaccine knowledge median score was for Pfizer/BioNTech score (median = 3.75) and it was significantly different from all other vaccines scores (p-values < 0.01). Sputnik-V vaccine had the lowest median (2.63) and it was significantly lower than Oxford/AstraZeneca, Sinopharm, and Pfizer/BioNTech vaccines scores (p-values < 0.01). The participants’ willingness to take each vaccine differs significantly (p-value < 0.01) with the highest willingness was for Pfizer/BioNTech vaccine (median = 3.89) and the lowest was for Sputnik-V vaccine (median = 2.50). The only insignificant differences were between Oxford/AstraZeneca vaccine and Sinopharm and Sputnik-V vaccines.
The results of quantile regression of the factors associated with participants’ willingness to take each vaccine are shown in Table 4 . Sex was significantly associated with the participants’ willingness to take all vaccines except of Sputnik-V vaccine as females were significantly less willing to take the vaccines. The higher participants’ estimation of disease seriousness, the higher their willingness to take all the vaccines. Significant positive associations were found between COVID-19 knowledge score and specific vaccine knowledge score and the participants’ willingness to take all studied vaccines except Sputnik-V vaccine. The only significant association between household monthly average income and the participants willingness to take a vaccine was found with Pfizer/BioNTech vaccine as those with low income were significantly less willing to take the vaccine compared to those with high income (B = −0.62, p-value = 0.02).
Table 4.
Quantile regression of the factors associated with participants’ acceptance of each vaccine type.
| Variable/vaccine type | Pfizer/BioNTech |
Moderna |
Sinopharm |
Sputnik-V |
Oxford/AstraZeneca |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | p-Value | B | p-Value | B | p-Value | B | p-Value | B | p-Value | |
| Sex (females compared to males) | −1.78 | <0.01** | −0.86 | 0.03* | −1.17 | <0.01** | −0.56 | 0.15 | −1.37 | <0.01** |
| Age | 0.01 | 0.78 | −0.04 | 0.50 | −0.07 | 0.14 | −0.06 | 0.24 | −0.01 | 0.80 |
| Marital status (not married compared to married) | 0.75 | 0.20 | 0.37 | 0.61 | 0.15 | 0.83 | −1.09 | 0.13 | −0.69 | 0.32 |
| Household monthly average income (low income compared to high income) | −0.62 | 0.02* | 0.05 | 0.87 | −0.44 | 0.16 | −0.03 | 0.93 | 0.24 | 0.45 |
| Working/Studying in medical field (not in medical field compared to in medical field) | −0.23 | 0.39 | −0.21 | 0.53 | 0.31 | 0.32 | 0.30 | 0.37 | 0.05 | 0.87 |
| Moderate risk for COVID-19 complications | −0.46 | 0.17 | −0.01 | 0.97 | −0.09 | 0.82 | −0.23 | 0.58 | −0.04 | 0.92 |
| High risk for COVID-19 complications | −0.31 | 0.37 | −0.33 | 0.45 | −1.12 | <0.01* | −0.77 | 0.08 | −0.19 | 0.65 |
| Estimation of disease seriousness | 0.19 | <0.01** | 0.28 | <0.01** | 0.28 | <0.01** | 0.28 | <0.01** | 0.34 | <0.01** |
| COVID-19 knowledge score | 0.33 | <0.01** | 0.36 | 0.01* | 0.34 | <0.01** | 0.19 | 0.18 | 0.54 | <0.01** |
| Practice score | 0.20 | <0.01** | 0.16 | <0.01** | 0.13 | <0.01** | 0.10 | 0.05* | 0.09 | 0.06 |
| Specific vaccine knowledge score | 0.21 | <0.01** | 0.13 | 0.02* | 0.19 | <0.01** | 0.03 | 0.61 | 0.11 | 0.03* |
Significant at p-value < 0.05.
Significant at p-value < 0.01.
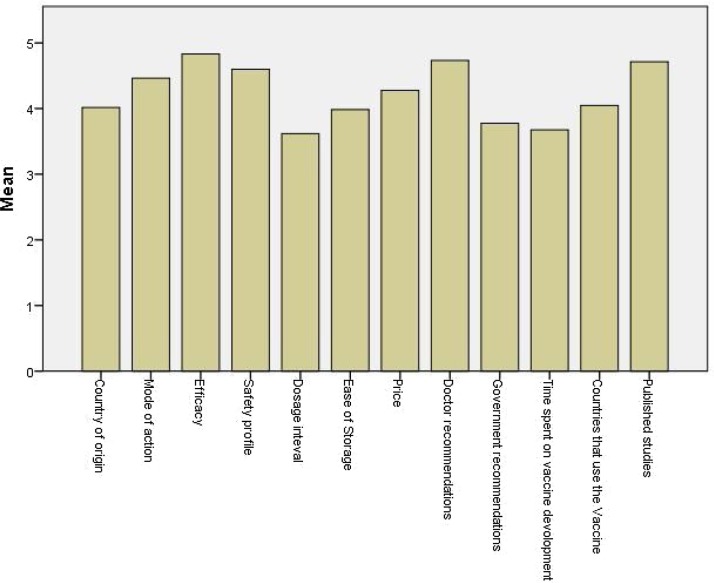
The influences that affect the participants’ preferences of one vaccine over another are shown in Fig. 1 . Vaccine's efficacy, the published studies, and doctors’ recommendations had the highest importance to the participants. On the other hand, dosage intervals and time spent on vaccine development had the lowest importance.
Fig. 1.
The mean of the participants’ estimation of the importance of influences that affect their preferences of one vaccine over another.
Discussion
Despite hundreds of clinical trials conducted to develop an effective drug against COVID-19 infection, no drug has been approved to treat the infection. Therefore, the COVID-19 vaccination is the only available approach to decrease the disease spread and help the world to recover from the pandemic devastating impacts. In order for vaccination to be an effective approach, herd immunity should be achieved, which means that 50–90% of the population should become immune either naturally or by vaccination.25 However, studies which evaluated the Jordanian population attitudes toward COVID-19 vaccine before it became available in Jordan reported low vaccination acceptance among the population and the need for more information was one of the significant factors that lead to vaccination refusal or hesitancy.12 Therefore, this study aimed to evaluate the Jordanian population acceptance of COVID-19 vaccine as its distribution has started and the information about the vaccines became widely available in Jordan. Because different vaccines have been approved to be used and these vaccines vary in many aspects, this study aimed to evaluate the acceptance for different vaccine types and the factors associated with it among young Jordanian population.
Vaccination intention
Low vaccine acceptance was observed among the current study participants as only 19.9% of the participants were willing to take the vaccine and 28.7% were not sure. Studies which have been conducted before the vaccine became available in October 202012 and December 202026 reported higher percentages of vaccine acceptance (36.8% and 28.4%) respectively in Jordan. This variance could be justified by the fact that the current study only included participants who aged from 18 to 29 years who might consider themselves not at risk of COVID-19 complications. Furthermore, unlike earlier studies which were conducted during the first wave of COVID-19 disease; the current study was conducted during the declining phase of the number of the new cases of COVID-19 which might make the population feel that the disease is over and therefore vaccination is no longer necessary.
Participants’ knowledge and acceptance of each vaccine type
The participants’ knowledge about vaccines varied significantly as the median of the Pfizer/BioNTech knowledge score was significantly higher than the scores of other vaccines. This result is reasonable as Pfizer/BioNTech vaccine was the first approved vaccine to be used against COVID-19 virus and the information about this vaccine are widely available.27 On the other hand, the published studies about Sputnik-V vaccine are limited, which may explain the present study results as the participants’ knowledge and willingness to be vaccinated scores for Sputnik-V vaccine was the lowest among the studied vaccines. Similar to the differences in the participants’ knowledge about the vaccines, their willingness to take each vaccine type also varied with the highest willingness was for Pfizer/BioNTech and the lowest was for Sputnik vaccine. Several factors contributed to the participants’ willingness to take the vaccines. One of these factors is sex as females were significantly more reluctant to take all vaccine types except for Sputnik vaccine. The association between the female sex and the vaccine refusal in general was reported in many studies including those conducted in China28 and Europe.29
Estimation of disease seriousness was a significant predictor for the participants’ willingness to take all the studied vaccines which is in accordance to the results of different studies that evaluated the vaccinations intentions in general.28, 30 Based on these findings, increasing the populations’ perceptions of the negative health impacts of the COVID-19 disease can significantly improve their vaccination intentions.
The participants’ knowledge about the COVID-19 disease itself and about the vaccine types (except for Sputnik-V vaccine) were also significant predictors of the participants’ willingness to take the different types of vaccines. Therefore, the specific information about each vaccine type should be available to the general population in a simple language to increase their confidence in the different types of COVID-19 vaccines.
Factors that influence the participants’ preferences of one vaccine over another
Doctors’ recommendation was one of the most important factors that influence the participants’ preferences of different COVID-19 vaccine types. Therefore, doctors should address the populations’ concerns and correct the myths about the vaccines, in order to improve the general population perception and intention for vaccination.
Making the published studies about the different COVID-19 vaccine types accessible and summarizing their results by experts in an easy-to-understand manner can improve the general population knowledge and therefore their intention for vaccination. The importance of this measure can also be emphasized by the results of this study, as the most preferred vaccine was Pfizer/BioNTech vaccine which has the most publicized studies.
Vaccines’ mechanism of action, country of origin, countries that use each vaccine type were important influencers on the participants’ vaccines’ types’ preferences, however, these factors are, in fact, irrelevant to vaccines efficacy or safety. Therefore, its public health authorities’ responsibility to clarify that all approved vaccines are effective and safe, and any available vaccine should be taken.
Study limitations and strengths
One of the study limitations that some of the information about the vaccines was taken from the manufacturers’ websites, which may affect the accuracy of the knowledge related questions in the current study survey as manufacturers are unlikely to be impartial. The study was based on an online survey which may result in recall and selection biases. However, web-based studies have been proven to recruit a representative sample and provide a private environment that allows the respondents to complete the survey accurately and honestly.31, 32 Another limitation of this study that it only included young adults (aged between 18 and 29), therefore the attitudes of older age groups were not evaluated. Moreover, half of the participants were working/studying in a medical field which may limit the generalizability of the study results. However, the attitudes of the medical staff are particularly important as they are in the frontlines and consistently exposed to the virus making them at high risk of infection. Moreover, as reported in the current study, medical staff recommendations can influence the attitudes of the general population. One last limitation of the current study is the high percentage of females and non-smokers among the participants which do not reflect their percentages in the Jordanian young population. Nevertheless, the study included a large sample size which can decrease the influences of aforementioned limitations.
Conclusion
The acceptance of COVID-19 vaccines among the young Jordanian adults is limited. Significant differences in the participants’ knowledge and acceptability of the different COVID-19 vaccine types were observed. Estimation of disease seriousness and knowledge about each vaccine were significant predictors of the vaccine acceptability. Therefore, it is necessary to increase the population familiarity with different COVID-19 vaccine types and improve population attitudes toward vaccines by implementing vaccines’ awareness campaigns that aim to improve the knowledge about all available vaccines. The current study findings should be considered by the healthcare policy makers when developing strategies to improve COVID-19 vaccination acceptance.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available in the Mendeley repository,33 https://doi.org/10.17632/vpm6m3fkcp.1.
Funding
This work was supported by Al-Zaytoonah University of Jordan, grant number 22/23/2019-2020.
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vacun.2021.07.008.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.What is coronavirus? | Johns Hopkins Medicine.
- 2.Silveira A., Pereira A. Estimation and monitoring of COVID-19's transmissibility from publicly available data. Front Appl Math Stat. 2020;6:565336. doi: 10.3389/fams2020565336. [DOI] [Google Scholar]
- 3.Qingqing C., Hui Z., Juecheng Z. Global coronavirus cases hit 100m, can humanity really learn from the apocalypse-like pandemic? Global Times. 2021 [Google Scholar]
- 4.14 Diseases You Almost Forgot About (Thanks to Vaccines) | CDC.
- 5.Different COVID-19 Vaccines | CDC.
- 6.COVID-19 vaccines WHO.
- 7.Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2020 doi: 10.1038/s41591-020-1124-9. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rate of COVID-19 vaccination by country 2021 | Statista.
- 9.Coronavirus (COVID-19) vaccinations – statistics and research – our world in data.
- 10.Sallam M. COVID-19 vaccine hesitancy worldwide: a systematic review of vaccine acceptance rates. medRxiv. 2021 doi: 10.1101/2020.12.28.20248950. 2020.12.28.20248950. Published online January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Mohaithef M., Padhi B.K. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: a web-based national survey. J Multidiscip Healthc. 2020;13:1657–1663. doi: 10.2147/jmdh.s276771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Qerem W.A., Jarab A.S. COVID-19 vaccination acceptance and its associated factors among a Middle Eastern population. Front Public Heal. 2021;9:34. doi: 10.3389/fpubh.2021.632914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youth | UNICEF Jordan.
- 14.Internet users by age worldwide | Statista.
- 15.CIA. CIA Factbook – Jordan; 2020.
- 16.Streiner D.L., Norman G.R., Cairney J. 5th ed. Oxford University Press; 2015. Health measurement scales: a practical guide to their development and use. [DOI] [Google Scholar]
- 17.Cortina J.M. What is coefficient alpha? An examination of theory and applications. J Appl Psychol. 1993;78:98–104. doi: 10.1037/0021-9010.78.1.98. [DOI] [Google Scholar]
- 18.Sharma B. A focus on reliability in developmental research through Cronbach's Alpha among medical, dental and paramedical professionals. Asian Pac J Heal Sci. 2016;3:271–278. doi: 10.21276/apjhs.2016.3.4. [DOI] [Google Scholar]
- 19.Certain Medical Conditions and Risk for Severe COVID-19 Illness | CDC.
- 20.Information about the Pfizer-BioNTech COVID-19 Vaccine | CDC.
- 21.Information about the Moderna COVID-19 Vaccine | CDC.
- 22.Information for UK recipients on COVID 19 Vaccine AstraZeneca – GOV.UK.
- 23.Sputnik V. the first registered vaccine against COVID-19. Official website vaccine against coronavirus Sputnik V.
- 24.Chinese Covid-19 Vaccine Efficacy Better than Expected Interview with Mr. Liu Jingzhen, Chairman of Sinopharm. Sinopharm http://www.sinopharm.com/en/s/1395-4173-38923.html.
- 25.D'Souza G, Dowdy D. What is herd immunity and how can we achieve it with COVID-19? – COVID-19 – Johns Hopkins Bloomberg School of Public Health.
- 26.Sallam M., Dababseh D., Eid H., Al-Mahzoum K., Al-Haidar A., Taim D., et al. High Rates of COVID-19 Vaccine Hesitancy and Its Association with Conspiracy Beliefs: A Study in Jordan and Kuwait among Other Arab Countries. Vaccines. 2021;9:42. doi: 10.3390/vaccines9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfizer-BioNTech COVID-19 Vaccine | FDA.
- 28.Wang J., Jing R., Lai X., Zhang H., Lyu Y., Knoll M.D., et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines. 2020;8:482. doi: 10.3390/VACCINES8030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neumann-Böhme S., Varghese N.E., Sabat I., Barros P.P., Brouwer W., van Exel J., et al. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur J Heal Econ. 2020;21:977–982. doi: 10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward J.K., Alleaume C., Peretti-Watel P. The French public's attitudes to a future COVID-19 vaccine: the politicization of a public health issue. Soc Sci Med. 2020 doi: 10.31235/osf.io/xphe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenner Y., Garland S.M., Moore E.E., Jayasinghe Y., Fletcher A., Tabrizi S.N., et al. Web-based recruiting for health research using a social networking site: an exploratory study. J Med Internet Res. 2012;14:e20. doi: 10.2196/jmir.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantrell M.A., Lupinacci P. Methodological issues in online data collection. J Adv Nurs. 2007;60:544–549. doi: 10.1111/j.1365-2648.2007.04448.x. [DOI] [PubMed] [Google Scholar]
- 33.Al-Qerem W., Jarab A., Qarqaz R., Al Hayek M. 2021. Attitudes of a sample of middle eastern young adults toward different available COVID-19 vaccines. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available in the Mendeley repository,33 https://doi.org/10.17632/vpm6m3fkcp.1.