Abstract
Background
SARS-CoV-2 mRNA vaccines have proven high efficacy, however, limited data exists on the duration of immune responses and their relation to age and side effects.
Methods
We studied the antibody and memory T cell responses after the two-dose BNT162b2 vaccine in 122 volunteers up to 6 months and correlated the findings with age and side effects.
Findings
We found a robust antibody response to Spike protein after the second dose. However, the antibody levels declined at 12 weeks and 6 months post-vaccination, indicating a waning of the immune response over time. At 6 months after the second dose, the Spike antibody levels were similar to the levels in persons vaccinated with one dose or in COVID-19 convalescent individuals. The antibodies efficiently blocked ACE2 receptor binding to SARS-CoV-2 Spike protein of five variants of concern at one week but this was decreased at three months. 87% of individuals developed Spike-specific memory T cell responses, which were lower in individuals with increased proportions of immunosenescent CD8+ TEMRA cells. We found antibody response to correlate negatively with age and positively with the total score of vaccination side effects.
Interpretation
The mRNA vaccine induces a strong antibody response to SARS-CoV-2 and five VOCs at 1 week post-vaccination that decreases thereafter. T cell responses, although detectable in the majority, were lower in individuals with higher T cell immunosenescence. The deterioration of vaccine response suggests the need to monitor for the potential booster vaccination.
Keywords: SARS-CoV-2 mRNA vaccine, dynamics of the immune response, age, adverse effects
Research in context.
Evidence before this study
The first studies addressing the immune responses in individuals after the administration of SARS-CoV-2 mRNA vaccines have been published. To date, many mRNA vaccine response studies have not been peer-reviewed, and data on the dynamics of antibody response, the role of age, and side effects on SARS-CoV-2-mRNA vaccines in real vaccination situations is limited. Studies on the anti-Spike protein antibody levels after the vaccination have been performed in a relatively short period, within weeks or few months after the full vaccination, but little longer-term evidence exists on the post-vaccination antibody persistence.
Added value of this study
In this study, we assessed the dynamics of antibody response up to six months after the full vaccination with two doses of Pfizer-BioNTech BNT162b2 mRNA vaccine in 122 individuals. Our findings show strong Spike RBD antibody responses one week after the second dose with the capacity to block ACE2-Spike protein interaction of five current variants of concern (Alpha, Beta, Gamma, Delta and Kappa). However, the antibody levels were significantly declined at 3 and 6 months after the second dose. At three months 87% of vaccinated individuals developed either CD4+ or CD8+ T cell responses. In addition, CD4+ T cell response was decreased among vaccinated individuals with elevated levels of senescent CD8+ TEMRA cells. We found a weaker antibody response in older vaccinated individuals, which correlated with fewer side effects at the time of vaccinations.
Implications of all the available evidence
Our results show that two doses of Pfizer-BioNTech BNT162b2 mRNA vaccine induce a strong antibody and T cell responses to the Spike RBD region but the antibody levels are declined at 6 months after the second dose. This decline is somewhat expected as all vaccine-induced short-lived plasmablasts do not necessarily differentiate into long-lived plasma cells. At 6 months after the second dose, the Spike RBD antibody levels were comparable to those after the first dose or the SARS-CoV-2 natural infection. Our findings point to the need to monitor the vaccination response and to consider individualized vaccination protocols, in particular for older people.
Alt-text: Unlabelled box
1. Introduction
New mRNA vaccines have shown high efficacy in clinical trials and are applied worldwide to millions of people. The first two-dose COVID-19 mRNA vaccine, Pfizer-BioNTech BNT162b2 (Comirnaty), accepted for emergency use, was found safe and demonstrated 95% efficacy in phase 3 trials. However, little data exists about the extent and duration of the antibody and T cell responses after the two-dose mRNA vaccination, as well as about the factors influencing the efficacy and side effects in real vaccination situations.
The short-term studies with Pfizer-BioNTech mRNA vaccines have reported weaker immune responses and a higher number of non-responders among older people after the two-dose vaccination with Comirnaty vaccine [1], [2], [3], [4]. Nevertheless, one study failed to show a significant correlation between age and antibody response after the second vaccination but found a lower magnitude of memory B cell responses with increased age [5] highlighting a need for further studies to understand the age-related responses to mRNA vaccination and to monitor for longer periods than less than one month. Also, limited information is available about the side effects and their correlation with vaccination outcomes. For example, one study found no significant association between the antibody levels and severity of adverse events among vaccinees [5]. Furthermore, few preprint studies have reported sex differences in response to COVID-19 vaccination [1,6], although widely described with several other vaccines [7]. The emerging VOCs (variant of concern) escaping the vaccine-induced immunity raise great concern [8], and neutralizing antibodies can provide an important measure of immune protection against VOCs [9]. The conventional virus neutralization test for determining neutralizing antibodies requires a specialized biosafety level 3 laboratory, however, pseudovirus-based assays or competition ELISA (enzyme-linked immunosorbent assay) tests have been found to accurately detect neutralizing antibodies [10,11]. Here we addressed the dynamics of anti-S-RBD IgG and CD4+ and CD8+ T cell responses at three months after two doses of the Comirnaty vaccine in healthy volunteers and assessed its correlation with the age and severity of side effects. We corroborated our findings of anti-S-RBD IgG dynamics by measuring the neutralizing capacity of antibodies against wild type (wt) SARS-CoV-2 and five VOCs.
2. Material and methods
2.1. Recruitment, sample, and data collection
SYNLAB Estonia employees volunteering to be vaccinated with COVID-19 mRNA Comirnaty (Pfizer-BioNTech) vaccine were invited to participate in the study. Participants signed an informed consent form agreeing with sampling and usage of their clinical data. The blood samplings were performed by trained medical personnel at SYNLAB Estonia. Two doses were given three weeks apart, and the samples were taken before the first dose of vaccine (B1D), before the second dose (B2D), one week after the second dose (1wA2D), six weeks after the second dose (6wA2D), 12 weeks after the second dose (12wA2D), and 6 months after the second dose (6mA2D). The study participants filled in a questionnaire about the presence of side-effects after the second dose and rated their side-effect severity with scoring from zero to three (Supplementary Table 1). All samples and volunteers’ data (age, sex, side effects) were stored in a pseudonymized manner. As controls, we used samples from uninfected, non-vaccinated, and healthy donors collected before COVID-19 pandemic (negative control, n=50) and PCR-positive mild COVID-19 (n=97) patients collected and described previously [12].
The study has been approved by the Research Ethics Committee of the University of Tartu on February 15, 2021 (No 335/T-21). Participants signed informed consent before recruitment into the study. The study was performed in accordance with Helsinki Declaration and followed Good Laboratory Practice.
2.2. Antibody testing
Serum samples were analysed for the IgG antibodies to SARS-CoV-2 Spike protein receptor-binding domain (S-RBD) IgG using quantitative Abbott SARS-CoV-2 IgG QN (all time points) and anti-Spike IgM using Abbott SARS-CoV-2 IgM (B1D, B2D, 1wA2D). In both analyses, chemiluminescent micro-particle immunoassay (CLIA) on ARCHITECT i2000SR analyser (Abbott Laboratories) was applied. The cut-off and the upper detection limit of the IgG test were 50 and 80,000 AU/mL, respectively. The sensitivity and specificity of Abbott S-RBD IgG test were 99·37% and 99·55% according to the manufacturer's data. The results of S-RBD IgM were interpreted as positive or negative by the analyser.
2.3. ACE2-Spike interaction blocking assay
The serum capacity to block the angiotensin-converting enzyme 2 (ACE2) receptor interaction with SARS-CoV-2 trimeric S protein receptor-binding domain (RBD) was tested using IVD-CE SARS-CoV-2 Neutralizing Antibody ELISA kit (Icosagen). In brief, the ELISA plates covered with SARS-CoV-2 trimeric S proteins of wild-type (wt, Wuhan), Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Kappa (B.1.617.1) VOCs (Icosagen) were incubated with serum samples in a 1/100 dilution and probed with biotinylated ACE2-hFc protein (Icosagen). Streptavidin-Horseradish Peroxidase was used for colorimetric detection and the light absorbance was measured at 450 nm as optical density (OD) values. The OD values of the measured samples were divided by the mean value of the three repeated samples without serum to obtain relative OD values. The samples with relative OD values of <0·75 (i.e. ≥0·75 was determined as the limit of detection) were considered sufficient in blocking ACE2 binding.
2.4. SARS-CoV-2 Spike-specific CD4+ and CD8+ memory T cell responses
For CD4+ and CD8+ T cell response analysis, freshly isolated PBMCs (2×106 cells) were stimulated with overlapping SARS-CoV-2 S peptide pool (1ug/ml, Miltenyi Biotec, 15-mer sequences with 11 amino acid overlap), and with anti-CD28 and anti-CD49d for 20 hours. CEFX peptides (JPT Peptides) were used as a positive control. After the stimulation T cells were stained for CD3 Brilliant Violet 650, CD4 Alexa Fluor 700, CD8 Brilliant Violet 605, CCR7 Alexa Fluor 488, CD45RA APC, CD69 Brilliant Violet 510, OX40 PE-Dazzle (all from Biolegend), and CD137 PE (from Miltenyi Biotech) (Supplementary Fig. 1). Antigen-specific cells were gated according to the upregulation of activation-induced markers (AIM) CD137 and CD69 in memory CD8+ T cells and CD137, OX40, and CD69 in memory CD4+ T cells (percentage calculated from total CD8+ or CD4+ cells respectively). The percentage of AIM positive cells in the negative control sample (diluent with costimulatory antibodies) was subtracted from the value of the stimulated sample CD8+ TEMRA cells gated as CD3+ CD8+ CD45RA+ CCR7- T cells. The cut-off level for Spike-specific T cell positivity was drawn to 0·02% according to the data from six unvaccinated individuals. 7-AAD was used for the discrimination of dead cells. Flow cytometry was performed using LSRFortessa (BD Biosciences) and the results were analyzed with FCS Express 7 (DeNovo Software).
2.5. Statistics
GraphPad version 9 was used for statistical analyses and generation of box and whiskers plots and correlation plots. Variables of data (S-RBD IgG, T cell results, and ACE2-Spike interaction inhibition values, age, and the score of side effects) were considered non-normally distributed and are reported as medians and interquartile range (IQR). The gender of the participants and IgM values are reported as frequency and percent. Mann-Whitney test was used to analyze the data of continuous variables of T cell data with two study groups, and Kruskal-Wallis test with subsequent Dunn´s multiple comparison testing was used to analyse more than two groups of S-RBD IgG data and ACE2-Spike interaction inhibition assay results. The correlations between S-RBD IgG values and age or number of side effects were analysed using Spearman's correlation with confidence intervals of 95%. For statistical analyses p-values <0·05 were considered to be statistically significant and p-values >0·0001 are reported as exact numbers.
3. Results
We studied 122 individuals (21 (17%) males and 101 (83%) females) who received their first and second COVID-19 Pfizer-BioNTech vaccine doses and gave corresponding pre- and post-vaccination blood samples. From these, 90 completed the questionnaire on post-vaccination side effects. The number of participants in each analysis is presented in Table 1. The age of the vaccinated volunteers ranged from 21 to 69 years (median 34 years; IQR 27 – 45). There was no statistical difference between the age of males (36 years; 28 – 50) and females (34 years; 26 – 45; p=0·36). All participants belonged to the white race of European ethnicity and were without serious comorbidities. None of the participants had been diagnosed with COVID-19 before the study.
Table 1.
Summary statistics of each analysis, giving the median, IQR, and number of studied individuals. For S-RBD IgM antibodies, the percentages of positive individuals are shown.
| B1D | B2D | 1wA2D | 6wA2D | 12wA2D | 6mA2D | |
|---|---|---|---|---|---|---|
| Antibodies to S-RBD | ||||||
| IgG (AU/mL) median/IQR (n) |
1·25/0·3-2·5 (88) |
1246/666-2582 (111) |
24534/13985-36616 (106) |
12752/8225-17348 (89) |
5226/3097-6924 (90) |
1383/893-2463 (84) |
| IgM % (n) | 0% (88) | 52% (104) | 82% (95) | |||
| Inhibition of Spike-ACE2 interaction (relative OD) | ||||||
| SARS-CoV-2 (wt) median/IQR (n) |
0·97/0·95-0·99 (49) |
0·33/0·13-0·46 (49) |
0·76/0·64-0·83 (49) |
|||
| Alpha (B.1.1.7) median/IQR (n) |
0·98/0·95-1·00 (9) |
0·40/0·22-0·53 (49) |
0·78/0·65-0·82 (49) |
|||
| Beta (B.1.351) median/IQR (n) |
1·00/0·97-1·01 (9) |
0·64/0·50-0·71 (49) |
0·86/0·79-0·91 (49) |
|||
| Gamma (P.1) median/IQR (n) Delta (B.1.617.2) Median/IQR (n) Kappa (B.1.617.1) Median/IQR (n) |
1·02/0·99-1·05 (49) 0·99/0·97-1·02 (49) 0·99/0·96-1·02 (49) |
0·64/0·42-0·70 (49) 0·46/0·29-0·58 (49) 0·46/0·32-0·58 (49) |
0·89/=0·81-0·92 (49) 0·80/0·68-0·84 (49) 0·79/0·68-0·84 (49) |
|||
| T cells | ||||||
| Spike-specific CD8+ T cells (% from CD8+, Median/IQR (n)) | 0·070/ 0·008-0·153 (n=79) |
|||||
| Spike-specific CD4+ T cells (% from CD4+, Median/IQR (n) | 0·245/ 0·008-0·510 (n=78) |
|||||
| CD8+ TEMRA (% from CD8+, Median/IQR (n)) | 25·0/ 18·3-36·4 (n=79) |
|||||
IQR - interquartile range; B1D - before the first dose of vaccine; B2D - before the second dose; 1wA2D - one week after the second dose; 6wA2D - six weeks after the second dose; 12wA2D - 12 weeks after the second dose; 6mA2D – 6 months after the second dose. AIM - activation-induced markers. TEMRA - T effector memory cell re-expressing CD45RA.
3.1. Antibody dynamics
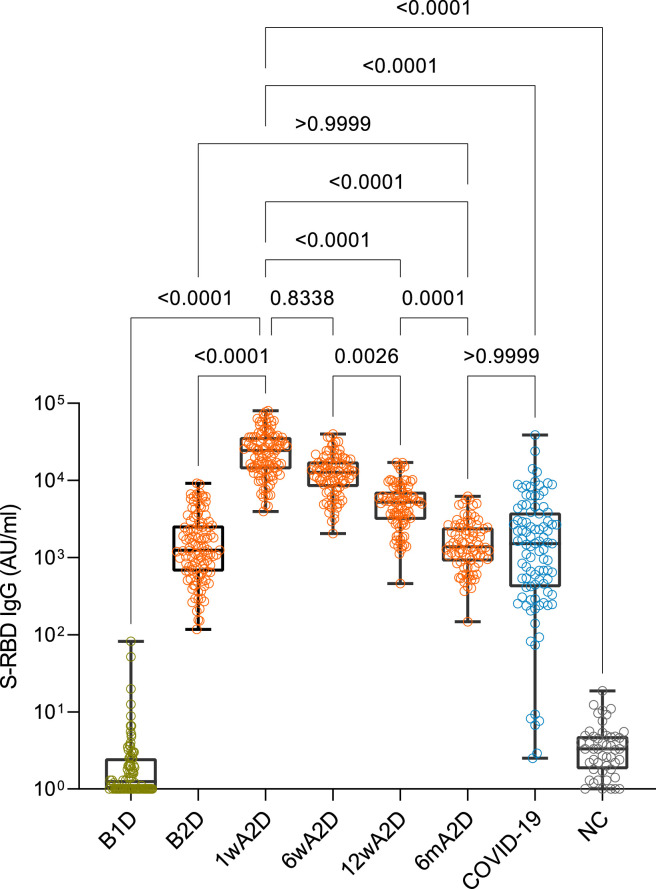
Three weeks after the first vaccine dose, we found elevated S-RBD IgG levels in vaccinated serum samples (Fig. 1), measured by the Abbot Laboratories CLIA method, with median IgG levels of 1246 AU/mL (IQR 666 – 2583; Table 1). Importantly, these S-RBD IgG levels increased significantly after the second vaccination dose – to 24534 AU/mL (IQR 13985 – 36616) and 12752 AU/mL (IQR 8225 – 17348) at 1 and 6 weeks after the second dose, respectively, as compared to the first dose (both p<0·0001). However, we found that the S-RBD IgG levels were decreased to 5226 AU/mL (IQR 3097 – 6924) at 12 weeks (p<0·0001) and to 1383 AU/mL (IQR 893 – 2463) at 6 months (p<0·0001) after the second dose as compared to their peak levels at 1 week after the second dose (Fig. 1). The dynamics of declining antibody levels between one and six weeks after the second dose was present in most of the vaccinees, and on average S-RBD IgG levels decreased 45% between these two time-points (Supplementary Fig. 2). In contrast, we found increased S-RBD IgG levels at six weeks after the second dose in only 4% of individuals. The further decline of the S-RBD IgG levels was present in all participants, and at six months, the S-RBD IgG levels were only from 2 to 25% (median 7%) of their peak levels, detected at one week after the second dose.
Fig. 1.
Antibody responses in individuals vaccinated with Pfizer-BioNTech Comirnaty vaccine. S-RBD IgG levels before vaccination (B1D, n=88), after the single (B2D, n=111) and two-dose immunizations (1 week (1wA2D, n=106); 6 weeks (6wA2D, n=89), 12 weeks (12wA2D, n=90), and 6 months (6mA2D; n=84) in vaccinated individuals compared with post-infection levels in patients recovered from COVID-19 (COVID-19, n=97) and pre-COVID-19 negative controls (NC, n=50). The box plot comparisons were performed with the Kruskall-Wallis test and Dunn's multiple testing correction; p-values >0·0001 are reported as exact numbers.
One individual had slightly elevated S-RBD IgG before vaccination, but negative anti-Nucleocapsid IgG and anti-Spike IgM, also the post-vaccination S-RBD IgG was close to average. Although COVID-19 was never diagnosed in this person we could not exclude possible earlier exposure to SARS-CoV-2.
IgM was negative in all tested pre-vaccination samples, positive in 52% and 82% of samples before and 1 week after the second dose, respectively (Table 1).
We also compared the post-vaccination results with S-RBD antibodies in COVID-19 recovered patients (Fig. 1). The post-infection IgG levels (median 1532; IQR 418 – 4110) were similar to the vaccinated persons who received the first dose and fully vaccinated persons six months after the second dose (COVID-19 vs B2D or 6mA2D; both comparisons p>0.9) but were significantly lower than in those who received two doses of the vaccine tested one to 12 weeks after the second dose (COVID-19 vs 1wA2D or 12wA2D, both comparisons p<0·0001) (Table 1).
Thus, at 6 months after the second vaccination dose, the Spike RBD antibody levels were comparable to the levels after the first vaccine dose or after the SARS-CoV-2 natural infection.
3.2. Inhibition of ACE2-trimeric Spike interaction by vaccine-induced antibodies
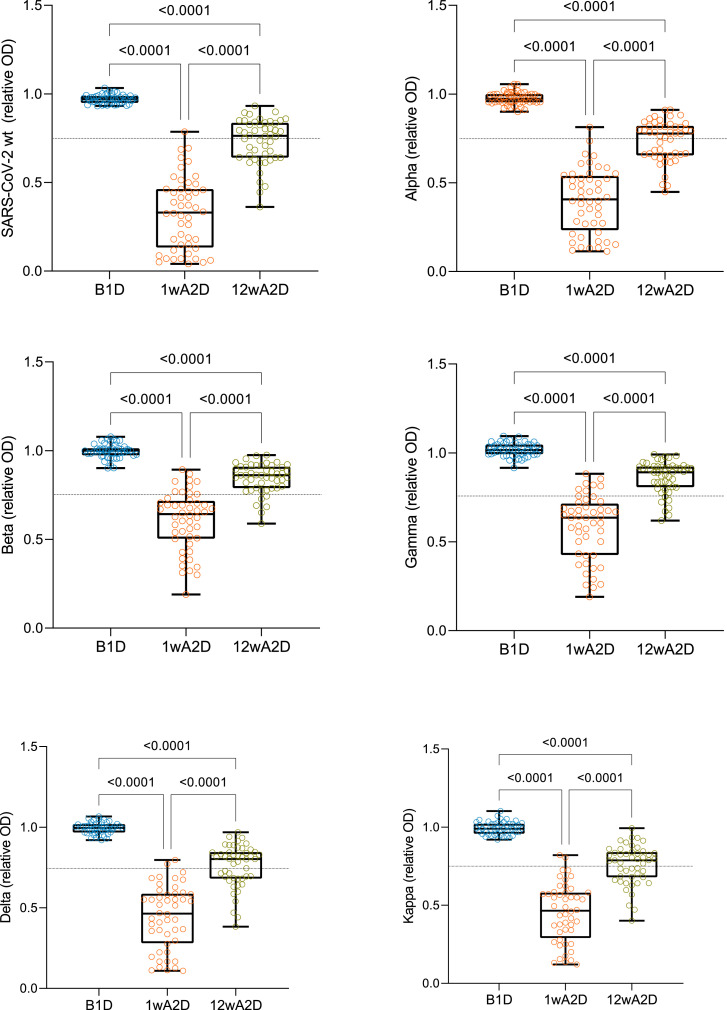
We next tested the inhibition of ACE2 interaction with trimeric S protein from SARS-CoV-2 wt, Alpha, Beta, Gamma, Delta, and Kappa VOCs by the sera of vaccinated participants. The serum samples collected before the vaccination did not block ACE2 binding to trimeric S protein of any of the VOCs analysed (Fig. 2). In contrast, most of the serum samples collected one week after the second dose (1wA2D) were able to block this interaction with median relative OD values ≤0·64 (Table 1), which is significantly lower compared to the median relative OD values (>0·97) before vaccination time point (p<0·0001, Fig. 2). However, 12 weeks after the administration of the second dose (12wA2D), we found diminished values of the median relative OD ranging above the set threshold of 0·75 (Table 1, Fig. 2). The inhibition of ACE2-Spike interaction was significantly weaker with Beta and Gamma VOCs (p<0·0001) and only slightly weaker with Delta (p=0·0471) and Kappa (p=0·0366) VOCs compared to the wt at 1wA2D time point (Supplementary Fig. 3A), whereas there was no difference between wt and Alpha VOC. At the 12wA2D time point, the inhibition was less pronounced (p<0·0001) only with Beta and Gamma VOCs in comparison with wt (Supplementary Fig. 3B). Following S-RBD IgG increase after the vaccination, we found strong correlations between ACE2-trimeric S blocking capacity, with all isolates, and S-RBD IgG levels in 1wA2D and 12wA2D groups (p<0·0001; r= from -0·77 to -0·96; Supplementary Fig. 4). The results show that the blocking antibodies peak at one week after the second dose but then decline, as seen at 12 weeks after the second dose. Our findings also indicate a strong correlation between S-RBD interacting and RBD inhibiting IgG levels.
Fig. 2.
Inhibition of ACE2-trimeric Spike interaction by vaccine-induced antibodies. Serum antibody capacities to block the interaction of ACE2 receptor and Spike protein with the modifications of wild type (wt, Wuhan, n=49) and five VOCs of Alpha (B.1.1.7, n=49), Beta (B.1.351, n=49), Gamma (P.1, n=49), Delta (B.1.617.2, n=48), and Kappa (B.1.617.1, n=48) were analyzed before the vaccination (B1D), one (1wA2D) and 12 (12wA2D) weeks after the second dose. The dotted line indicates the relative OD value of 0·75, which is a threshold for sufficient blocking of ACE2 binding. The box plot comparisons were performed with the Kruskal-Wallis test with Dunn's multiple testing correction; p-values >0·0001 are reported as exact numbers.
3.3. T cell responses
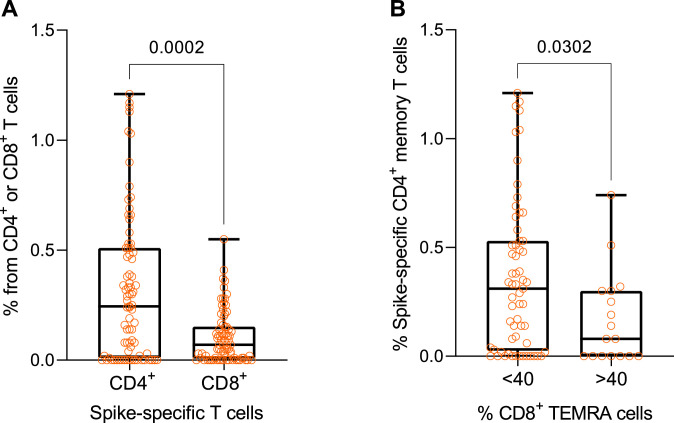
We found 74% and 73% of vaccinated individuals to have CD4+ and CD8+ memory responses, respectively, to Spike peptide pools measured by upregulation of CD69, OX40, and CD137 activation markers, indicating that the majority of vaccinated individuals developed SARS-CoV-2 -specific memory T cell responses 12 weeks after the second dose. Collectively, 87% of vaccinated individuals developed either CD4+ or CD8+ T cell responses to the vaccine. The frequency of S-specific CD4+ T cells was higher than corresponding CD8+ T cells (p=0·0002, Fig. 3A). There was no significant correlation between S-RGD IgG and T cell responses (Supplementary Fig. 5A, B). We next analysed the proportions of T cell subsets, in particular immunosenescence-associated CD8+ TEMRA population, as measured by CCR7 and CD45RA markers in vaccinated individuals. Although there was no significant correlation between TEMRA and S-specific memory responses (Supplementary Fig. 5C, D), we noted from the correlation plot that individuals with TEMRA percentages over 40 tended to have a lower proportion of S-specific CD4+ T cells. Indeed, individuals with a higher frequency of CD8+ TEMRA cells developed lower CD4+ T cell responses to immunized Spike protein (p=0·03, Fig. 3B), suggesting that T cell-related immunosenescence could be negatively associated with the development of the cell-mediated response to the SARS-CoV-2 virus.
Fig. 3.
Spike-specific T cell responses in vaccinated individuals 12 weeks after the second dose. (A) Post-vaccination frequency of S-specific CD4+ (n=79) and CD8+ (n=78) T cells and (B) the percentage of Spike-specific CD4+ T cells in individuals with lower (<40%, n=61) and higher (>40%, n=17) proportions of CD8+ TEMRA cells in their peripheral blood. The data were analyzed with the Mann-Whitney test, two-tailed p-values are given as exact numbers.
3.4. Factors influencing the vaccination response
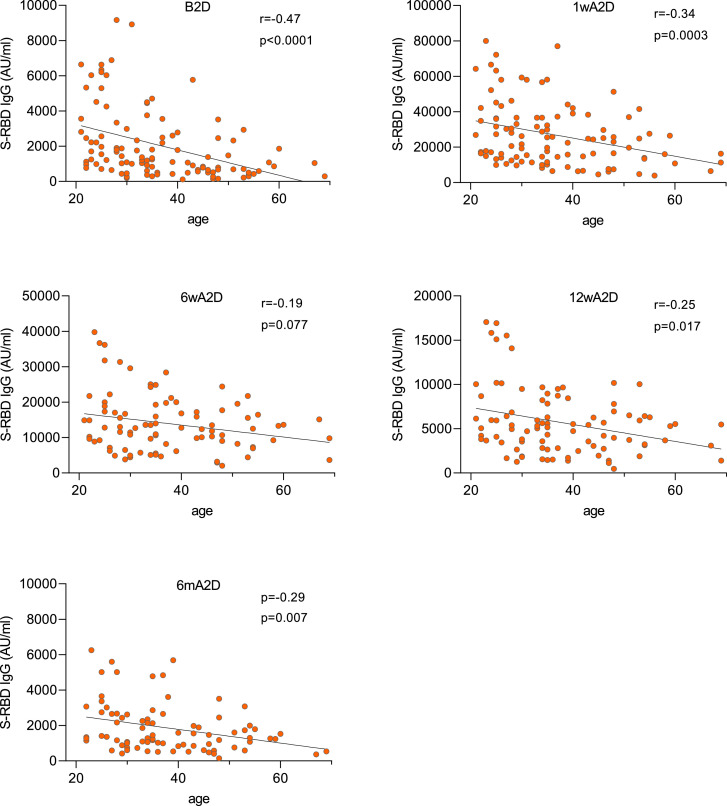
The age of vaccinated individuals had a significant negative correlation with S-RBD IgG response. This was the strongest at B2D timepoint (r= -0·47, p<0·0001) and 1wA2D (r= -0·34, p<0·0003), but weaker at 6wA2D (r= -0·19, p=0·077), 12wA2D (r= -0·25, p=0·017), and 6mA2D (r= -0·29, p=0·007) (Fig. 4).
Fig. 4.
Post-vaccination antibody responses correlate negatively with age. Spearman correlation analysis between age and S-RBD IgG levels before the second dose (B2D, n=111), 1 week (1wA2D, n=106), 6 weeks (6wA2D, n=89), 12 weeks (12wA2D, n=90), and 6 months (6mA2D, n=84) after the second dose. Spearman correlation coefficient and exact p-values are given.
3.5. Side effects of mRNA vaccination
Vaccination side-effects merit investigation as they are common reasons for vaccine hesitancy. Altogether 93% of participants reported some type of adverse effects. The most common side effects were reported pain or swelling (in 84%) at the injection site, fatigue (64%), malaise (50%), headache (42%), chills (41%), fever, and myalgia (both 34%). The majority of the side effects were present as mild to moderate. However, 20 (22%) persons reported one or several symptoms to significantly disturb daily life activities, and lasting for several days and/or causing absence from work. The total score of side effects (sum of all self-rated side effect scores per patient) ranged between zero and 27 (median 6; IQR 2 – 12). The detailed data on individuals’ side effects are presented in Supplementary Table 1.
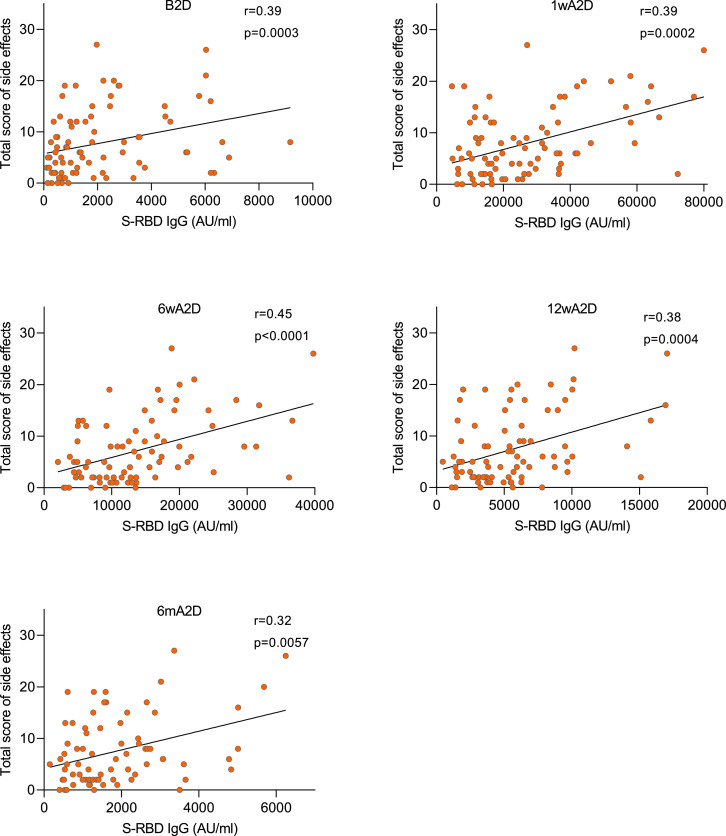
We found several side effects to positively correlate with the antibody response to S-RBD. This was seen with the total score of adverse effects, which significantly associated with the S-RBD IgG levels at all time points (Fig. 5) i.e. B2D (r= 0·39, p=0·0003), 1wA2D (r= 0·39, p=0·0002), 6wA2D (r= 0·45, p<0·0001), 12wA2D (r= 0·38, p=0·0004), and 6mA2D (r= 0·32, p=0·006). An even stronger correlation was present with fever at all time-points: r= 0·40 (p=0·0002), r= 0·40 (p=0·0001), r= 0·50 (p<0·0001), r= 0·42 (p<0·0001), r= 0·44 (p<0·0001), and also other adverse symptoms such as headache, fatigue, malaise, chills, and nausea correlated positively with the vaccine response (Supplementary Table 2).
Fig. 5.
Post-vaccination antibody responses correlate positively with the total score of side effects. Spearman correlation analysis between total score of side effects and S-RBD IgG levels before the second dose (B2D, n=84), 1 week (1wA2D, n=85), 6 weeks (6wA2D, n=82), 12 weeks (12wA2D, n=82), and 6 months (6mA2D; n=75) after the second dose. Spearman correlation coefficient and exact p-values are given.
The age of vaccinated individuals negatively correlated with the total score of side effects (r= -0·38, p=0·0002) as well as with several specific side effects (Supplementary Table 2).
4. Discussion
We report S-RBD IgG responses after COVID-19 mRNA vaccination showing a significant initial increase in antibody levels after the second dose. However, at six months post-vaccination these levels were decreased on average to 7% of their peak level that was comparable to S-RBD antibody levels in patients recovered from COVID-19. This decline is expected as not all vaccine-induced plasmablasts commit or are maintained as long-lived memory plasma cells [13], [14], [15]. A recent study on vaccinated individuals showed high frequencies of Spike-binding germinal centre B cells and plasmablasts in draining lymph nodes at twelve weeks after the second immunization [16]. Further longitudinal studies are needed to identify whether the antibodies will continue to decline or plateau at a lower level.
The vaccinated sera robustly inhibited the ACE2-Spike protein-protein interaction suggesting efficient induction of neutralizing antibodies by mRNA vaccination. The new SARS-CoV-2 variants with mutated structural properties of the Spike glycoprotein have posed concern about their transmissibility, virulence, and neutralizing antibody escape [17]. We here show that Comirnaty mRNA vaccine-induced neutralizing antibodies against the SARS-CoV-2 wt virus, as well as against all the currently circulating VOCs: Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Kappa (B.1.617.1), confirming the results demonstrated in a recent report on Comirnaty-elicited neutralization against SARS-CoV-2 Spike of different variants [18]. At three months post-vaccination the neutralization capacity was significantly decreased, in agreement with lower S-RBD antibody levels. In our study, Beta and Gamma variants showed the lowest neutralization effect. Also, a recent Coronavac vaccination study reported decreased neutralization of Gamma variant [19]. Currently, the long-lasting effect of mRNA vaccines to protect against reinfections or severe COVID-19 disease remains unclear, and might not only depend on antibody responses but also T cell immunity.
The majority of the vaccinated individuals developed T cell responses with similar prevalence in both T cell subsets (74% for CD4+ and 73% for CD8+), in agreement with phase I/II clinical trials with mRNA vaccines, which have demonstrated activation of CD4+ and CD8+ T cells [20,21]. Induction of functional T cells occurs after the Comirnaty vaccination but with variable results in aged individuals, for example, Spike-specific IFNγ T cell responses to vaccines were impaired in the over 80 age group [22]. Our findings showed that individuals with an increased number of immunosenescent CD8+ TEMRA cells had lower Spike-specific T cell responses. This suggests that immunosenescence, i.e. impairment of immune response to pathogens and vaccines, could affect the vaccine response to SARS-CoV-2.
We found a negative correlation between antibody responses and the age of vaccinated individuals. Age is an important factor that influences vaccine responses, and elderly people have been reported to be poor responders to influenza, hepatitis A and B, and pneumococcal vaccines by developing lower antibody levels and weaker cell-mediated responses [7]. In addition to diminished post-vaccine responses, older individuals had a more rapid waning of antibodies after the vaccinations. The adverse effect of age on COVID-19 mRNA vaccination has been reported by other studies [1], [2], [3]. We here report weaker mRNA vaccine response with age after the first and second dose time points, confirming the previous results, but also show that age has a less significant effect at later time points i.e. at six and 12 weeks, and 6 months after the second dose. Thus, our results indicate the benefit of the second dose or extended interval between the two doses [23] for older individuals and its effect to level up the short-term vaccination response, although the long-term persistence of post-vaccination antibody levels in older populations remains to be studied.
Common systemic side effects reported for COVID-19 mRNA vaccines are fatigue, headache, muscle pain, chills, and fever. In our study, 93% of vaccinated individuals reported some type of side-effects, which is higher than previously reported 66% of vaccinated seronegative persons [24]. In agreement with our results, the side effects among some groups were seen in 100% of participants of the mRNA vaccine phase 1/2 study [20]. Older participants reported fewer or even no side effects, and the presence and score of side effects correlated with S-RBD IgG responses. Recent reports have shown vaccine recipients with pre-existing immunity to develop systemic side effects more frequently than those without [25]. The mRNA vaccine-induced antibody levels were higher in subjects with more systemic side effects and the severity of vaccination's side effect was proposed to be a surrogate indicator of short-term antibody responses [4]. Antibody levels have been also reported lower in SARS-CoV-2 infected asymptomatic individuals, suggesting more severe symptoms to correlate with stronger antibody responses [12,26,27].
Our study has limitations since the studied cohort included medical personnel with high occupational risk to COVID-19 and whose vaccination was the priority. No specific risk groups such as individuals with age over 70 years or patients with comorbidities were included and the conclusions of our study concern the adults without serious comorbidities. Furthermore, the male population was underrepresented and conclusions on gender differences in vaccine response need further studies.
Taken together we report a robust initial vaccine response after two doses of Pfizer-BioNTech Comirnaty vaccine, which were declined at six months post-vaccination. Among the vaccinated individuals, we found age to correlate with lower responses and fewer side effects. Our study provides early data on vaccination responses and highlights the importance of monitoring the antibody responses in follow-up studies.
Contributors
PN, PP, JMG, KKi: conceptualization, project administration, writing, editing; VJ: investigation, resources; AA: formal analysis, visualization; ES: investigation, validation, writing original draft; LT, KKa, LH, APR, RM, JK, AP, MU: methodology, investigation.
Declaration of Interests
The authors have nothing to disclose.
Acknowledgments
We thank David James (SYNLAB UK) for language corrections. The study was supported by the Centre of Excellence in Translational Genomics (EXCEGEN), and the Estonian Research Council grant PRG377, PRG1117, Icosagen Cell Factory, and SYNLAB Estonia.
Footnotes
Funding. The Estonian Research Council, Icosagen Cell Factory, SYNLAB.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100208.
Contributor Information
Paul Naaber, Email: paul.naaber@synlab.ee.
Liina Tserel, Email: liina.tserel@ut.ee.
Kadri Kangro, Email: kadri.kangro@icosagen.ee.
Epp Sepp, Email: epp.sepp@ut.ee.
Virge Jürjenson, Email: virge.jurjenson@synlab.ee.
Ainika Adamson, Email: ainika.adamson@synlab.ee.
Liis Haljasmägi, Email: liis.haljasmagi@ut.ee.
Anna Pauliina Rumm, Email: pauliina.rumm@gmail.com.
Regina Maruste, Email: regina.maruste@ut.ee.
Jaanika Kärner, Email: jaanika.karner@ut.ee.
Joachim M. Gerhold, Email: joachim.gerhold@icosagen.ee.
Anu Planken, Email: anu.planken@icosagen.ee.
Mart Ustav, Email: mart.ustav@ut.ee.
Kai Kisand, Email: kai.kisand@ut.ee.
Pärt Peterson, Email: part.peterson@ut.ee.
Appendix. Supplementary materials
References
- 1.Shrotri M, Fragaszy E, Geismar C, et al. Spike-antibody responses following first and second doses of ChAdOx1 and BNT162b2 vaccines by age, gender, and clinical factors - a prospective community cohort study (Virus Watch) medRxiv. 2021 2021.05.12.21257102. [Google Scholar]
- 2.Doria-Rose N, Suthar MS, Makowski M, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jalkanen P, Kolehmainen P, Häkkinen HK, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;12(1):3991. doi: 10.1038/s41467-021-24285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goel RR, Apostolidis SA, Painter MM, et al. Longitudinal Analysis Reveals Distinct Antibody and Memory B Cell Responses in SARS-CoV2 Naïve and Recovered Individuals Following mRNA Vaccination. medRxiv. 2021 doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nace DA, Kip KE, Palmer OMP, et al. Antibody Responses in Elderly Residential Care Persons following COVID-19 mRNA Vaccination. medRxiv. 2021 2021.04.07.21254925. [Google Scholar]
- 7.Zimmermann P, Curtis N. Factors That Influence the Immune Response to Vaccination. Clin Microbiol Rev. 2019;32(2) doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021 doi: 10.1038/s41591-021-01413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 10.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 11.Chi X, Yan R, Zhang J, et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020;369(6504):650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naaber P, Hunt K, Pesukova J, et al. Evaluation of SARS-CoV-2 IgG antibody response in PCR positive patients: Comparison of nine tests in relation to clinical data. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0237548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quast I, Tarlinton D. B cell memory: understanding COVID-19. Immunity. 2021;54(2):205–210. doi: 10.1016/j.immuni.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khodadadi L, Cheng Q, Radbruch A, Hiepe F. The Maintenance of Memory Plasma Cells. Front Immunol. 2019;10:721. doi: 10.3389/fimmu.2019.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgarth N, Nikolich-Žugich J, Lee FE, Bhattacharya D. Antibody Responses to SARS-CoV-2: Let's Stick to Known Knowns. J Immunol. 2020;205(9):2342–2350. doi: 10.4049/jimmunol.2000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner JS, O'Halloran JA, Kalaidina E, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021 doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Beltran WF, Lam EC, St Denis K, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184(9):2523. doi: 10.1016/j.cell.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Liu J, Xia H, et al. BNT162b2-Elicited Neutralization against New SARS-CoV-2 Spike Variants. N Engl J Med. 2021 doi: 10.1056/NEJMc2106083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souza WM AM, Sesti-Costa R, et al. Neutralisation of SARS-CoV-2 lineage P.1 by antibodies elicited through natural SARS-CoV-2 infection or vaccination with an inactivated SARS-CoV-2 vaccine: an immunological study. The Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 21.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 22.Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021 doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parry H, Bruton R, Stephens C, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. medRxiv. 2021 doi: 10.1038/s41541-022-00432-w. 2021.05.15.21257017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krammer F, Srivastava K, Simon V. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. medRxiv. 2021 2021.01.29.21250653. [Google Scholar]
- 25.Krammer F, Srivastava K, Alshammary H, et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dufloo J, Grzelak L, Staropoli I, et al. Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies. Cell Rep Med. 2021;2(5) doi: 10.1016/j.xcrm.2021.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2(6):e240–e2e9. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.