Abstract
Iron deficiency is common in individuals with chronic kidney disease and plays a major role in the development of anemia. Oral and intravenous iron agents are both available to replete iron in patients with chronic kidney disease diagnosed with iron deficiency. The choice of which agent to use is most often dictated by goals of therapy, tolerability, convenience, and response to prior therapy. Diminished absorption of iron in the gastrointestinal tract and a high incidence of gastrointestinal adverse effects can reduce the efficacy of oral iron agents, necessitating the use of i.v. iron formulations to treat iron deficiency anemia, particularly in patients requiring kidney replacement therapy. Newer oral agents may help to overcome these limitations and help treat iron deficiency in those not requiring kidney replacement therapy. Recent studies have provided new evidence that more aggressive repletion of iron in patients with chronic kidney disease requiring kidney replacement therapy may provide benefits with respect to anemia management and hard clinical outcomes such as cardiovascular disease and survival.
Keywords: anemia, chronic kidney disease, iron
Iron deficiency is a common complication of kidney disease and plays a central role in the development of anemia of chronic kidney disease (CKD).1 Because of this, treatment of iron deficiency is critical to the successful management of anemia in individuals with CKD, particularly those with kidney failure needing replacement therapy.2,3 Development of novel iron supplements has provided several new tools to effectively address iron deficiency in patients with CKD. This review will overview the current state of iron repletion strategies in patients with CKD with particular emphasis on newer therapeutics that have recently been added for the treatment of iron deficiency as well as new studies that have helped inform best practices for how and when to treat iron deficiency across the spectrum of CKD.
Pathophysiology of Iron Deficiency in CKD: Brief Overview
Before discussing what types of iron supplements are available for the treatment of iron deficiency in CKD, a brief overview of the pathophysiology of iron deficiency in kidney disease is necessary (several excellent, more in-depth reviews have been previously published4, 5, 6). Approximately 1 to 2 mg of iron is typically absorbed daily from the diet to balance the obligatory iron losses from the skin and gastrointestinal tract.7 The proximal small intestine is the site where most iron absorption takes place under tight physiologic regulation. Iron in food can be absorbed by gastrointestinal epithelial cells in its heme or nonheme forms through separate mechanisms.8 Once absorbed, the release of iron into the blood across the basolateral membrane requires ferroportin, expressed in the basolateral membrane of epithelial cells (Figure 1, adapted from Panwar and Gutiérrez6).9,10
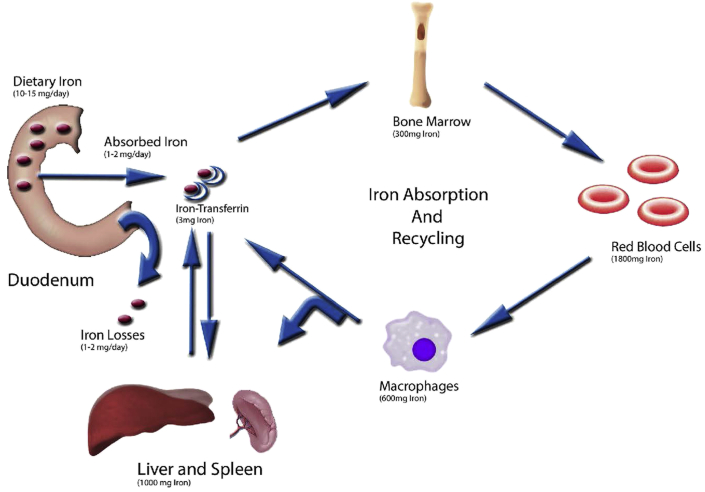
Figure 1.
Systemic iron trafficking. Iron is absorbed across the duodenal enterocytes. Once absorbed, it binds to transferrin in the plasma. Iron bound to transferrin can then be transported and taken up by the bone marrow (for erythropoiesis) or the liver or spleen (for storage). Iron is recycled when macrophages take up senescent red blood cells and release iron back to the plasma pool.
As the only known iron exporter in mammalian cells, ferroportin is critical for facilitating iron efflux across the basolateral membrane of epithelial cells and from the macrophages into the blood plasma (Figure 2, adapted from Panwar and Gutiérrez6).10,11 Hepcidin, a 25-amino acid peptide primarily synthesized and secreted by hepatocytes into the blood,12 is the primary hormonal regulator of iron handling through its effects on ferroportin.13 Hepcidin binds to ferroportin on the basolateral membrane of the enterocytes and on macrophages promoting degradation and endocytosis of ferroportin.13 This reduces the presence of ferroportin on cell membranes, effectively limiting the flux of iron into the blood from gut epithelial cells or from macrophages and hepatocellular cells that form the main storage for iron.
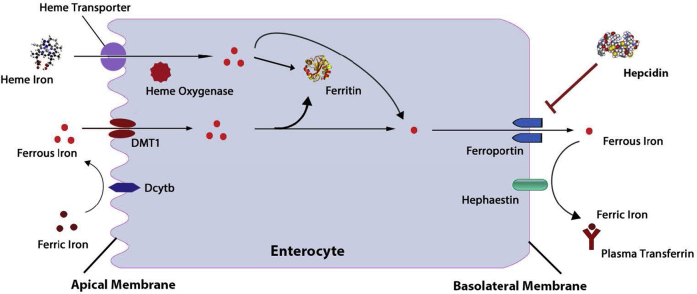
Figure 2.
Mechanisms of intestinal iron absorption. Diet iron is imported across the apical membrane of the duodenal enterocytes using several different mechanisms. Imported iron can be stored as ferritin or transported to the basolateral membrane, where it can exit using the iron exporter ferroportin. Iron is then transported to target tissues via the iron transporter transferrin. Hepcidin blocks iron absorption by reducing the basolateral membrane abundance of ferroportin.
Hepcidin plays a central role in the etiology of iron deficiency in CKD. Iron deficiency in CKD can be broadly classified as absolute iron deficiency, marked by low iron stores and low circulating iron concentrations, and as functional iron deficiency, marked by low circulating iron concentrations in the setting of normal iron stores. Hepcidin concentrations are commonly elevated in individuals with CKD, likely due to a combination of decreased kidney clearance of circulating hepcidin and enhanced levels of systemic inflammation that stimulate hepcidin expression. Increased hepcidin concentrations block intestinal iron absorption and iron release from iron storage sites (macrophages, hepatocytes) in CKD, reducing the availability of iron for erythropoiesis and contributing to the development of anemia.14, 15, 16
The hepcidin-induced blockade of iron absorption in the gut explains the reduced efficacy of oral iron replacement in patients with CKD, often necessitating iron repletion therapies that bypass the gastrointestinal tract in patients with CKD. This has also led to interest in developing novel therapies that target factors stimulating hepcidin secretion and/or ferroportin, the topic of which is covered in several excellent reviews and other reports.17, 18, 19, 20, 21 In addition, new hypoxia-inducible factor 1α prolyl hydroxylase inhibitors may target this pathway by reducing hepcidin concentrations, resulting in improved gastrointestinal iron absorption and reduced sequestration of iron in reticuloendothelial stores, both of which enhance iron availability for erythropoiesis.22
Oral Iron Formulations
Multiple oral supplements are available for the treatment of iron deficiency. Many multivitamins contain iron, typically providing ~18 mg of elemental iron per unit dose. Iron-only supplements usually consist of ferrous salts. The one most commonly used in patients with CKD patients is ferrous sulfate,23 which contains 20% elemental iron per tablet. Other ferrous salts include ferrous gluconate (12% elemental iron), ferrous fumarate (33% elemental iron), ferrous succinate (35% elemental iron), and iron polymaltose (28% elemental iron).24 Table 124, 25, 26 summarizes dosage forms and approximate elemental iron content of the main oral iron products available on the market. Oral iron supplements frequently cause gastrointestinal adverse effects in 35% to 60% of patients,27,28 particularly with administration of ≥ 45 mg elemental iron per day, limiting the ability to replete iron using high doses of oral formulations alone.
Table 1.
| Supplement | Elemental iron per dosage unit | Frequency |
|---|---|---|
| Ferrous sulfate | 65 mg/tableta | 1 tablet, 1–3 times per day |
| Ferrous gluconate | 38 mg/tablet a | 1 tablet, 1–3 times per day |
| Ferrous fumarate | 106 mg/tablet a | 1 tablet, 1–3 times per day |
| Ferric maltol | 30 mg/tablet | 1 tablet, twice per day |
| Ferric citrate | 210 mg/tablet | 1-2 tablets, 3 times per day |
| Liposomal iron | 30 mg/tablet | 1 tablet per day |
For 325-mg tablets.
In addition to ferrous salts, there are several ferric salt formulations for oral iron supplementation. The best studied to date is ferric citrate, which is the only oral iron supplement that is approved by the United States Food and Drug Administration for the treatment of iron deficiency anemia in individuals with CKD. Ferric citrate provides ~210 mg of elemental iron per 1000-mg tablet. Other ferric salts include ferric maltol, which consists of a complex of ferric iron with maltol in a 3:1 ratio providing 30 mg of elemental iron with each capsule,25 and sucrosomial iron, in which ferric polyphosphate is enveloped by a phospholipid bilayer with a sucrester matrix that promotes gastrointestinal absorption.25 One potential advantage of sucrosomial iron is that it does not require a prescription. Ferric maltol is approved for the treatment of iron deficiency anemia in individuals with inflammatory bowel disease and has been studied in individuals with CKD not requiring kidney replacement therapy (NCT02968368).25
I.V. Iron Formulations
As discussed above, chronically elevated circulating concentrations of hepcidin limit gastrointestinal absorption of iron, hampering the efficacy of oral iron supplements in patients with CKD. Because of this, i.v. iron infusion is a mainstay in the treatment of iron deficiency in CKD, particularly in individuals with kidney failure needing replacement therapy, who almost exclusively receive i.v. iron.
Several i.v. formulations are in use (Table 2),29,30 all of which consist of colloids made up of elemental iron surrounded by a carbohydrate shell.31 The carbohydrate shell slows the release of iron after uptake by the reticuloendothelial system, thus reducing the occurrence of severe toxic reactions related to the immediate release of bioactive free iron.29,31,32 For the most part, the efficacy of the formulations does not seem to appreciably differ; instead, cost, number of doses required, and adverse effect profile are often the most important factors that dictate when one product is used over another. For example, iron formulations that require only 1 (iron isomaltoside) or 2 infusions that can be administered over a relatively short period of time (e.g., ferumoyxtol or ferric carboxymaltose) may be particularly convenient in the management of iron deficiency in individuals with non–dialysis-dependent CKD. As another example, infusion of ferric carboxymaltose can cause acute and sometimes severe hypophosphatemia through a mechanism that involves stimulation of fibroblast growth factor 23 (FGF23),33 potentially limiting its efficacy in situations in which long-term infusions are required.
Table 2.
| Formulation | Dosage | Frequency |
|---|---|---|
| Iron sucrose | 200 mg | 5 doses over 2 weeks |
| Ferumoxytol | 510 mg | 2 doses, 3-8 days apart |
| Ferric gluconate in sucrose complex | 250 mg | 4 doses weekly |
| Ferric carboxymaltose | 750 mg | 2 doses, 1 week apart |
| Iron isomaltoside | 1000 mg | 1 dose |
| Iron dextran (low molecular weight) | 500 to 1000 mg | Variable |
In addition to i.v. infusion of iron, iron supplementation of the bicarbonate component of the dialysate fluid (ferric pyrophosphate citrate) has emerged a novel approach to deliver iron in individuals receiving kidney replacement therapy.34 Although approved by the Food and Drug Administration in 2015, use of ferric pyrophosphate citrate remains fairly limited.
Iron Replacement in Patients With CKD Not Requiring Kidney Replacement Therapy
Current Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines recommend checking for iron deficiency in individuals with CKD who have anemia.35 Because of the impractical nature of obtaining bone marrow iron stores as the gold standard for assessing iron status, transferrin saturation and ferritin remain the major laboratory tests used to diagnose iron deficiency. The thresholds for defining iron sufficiency in patients with CKD not requiring kidney replacement therapy are controversial and not backed by robust clinical trial data (Table 3).
Table 3.
Treatment targets for therapy
| Variable | Nondialysis-dependent chronic kidney disease | End-stage kidney disease |
|---|---|---|
| Iron deficiency unconditionala | TSAT ≤ 20% and/or serum ferritin ≤ 100 ng/ml | TSAT ≤ 20% and/or serum ferritin ≤ 200 ng/ml |
| Iron deficiency conditionalb | TSAT ≤ 30% and/or serum ferritin ≤ 500 ng/ml | TSAT ≤ 30% and/or serum ferritin ≤ 500 ng/ml |
Threshold for therapy in iron deficiency anemia under any circumstance.
Threshold for therapy in individuals with iron deficiency anemia who are not receiving iron therapy or who require erythropoiesis-stimulating agents if the goal is to raise hemoglobin, reduce the dose of the erythropoiesis-stimulating agent, or reduce the need for blood transfusions.
There is general agreement that the criteria for absolute iron deficiency anemia in CKD prompting treatment in any circumstance should include a transferrin saturation ≤ 20% and a serum ferritin concentration ≤ 100 ng/ml.5 In individuals with anemia not on iron therapy or who require erythropoiesis-stimulating agents, KDIGO guidelines suggest a transferrin saturation ≤ 30% and serum ferritin ≤ 500 ng/ml as triggers for administering iron if the goal is to raise hemoglobin, reduce the delivered dose of erythropoiesis-stimulating agents, or reduce the need for blood transfusions. This is generally in line with other major society recommendations, although some have endorsed a higher ferritin ceiling of 800 ng/ml.36, 37, 38 This is partly due to the recognition that functional iron deficiency—in which total body iron stores are not depleted but instead sequestered in the reticuloendothelial system, blocking participation in erythropoiesis—is characterized by transferrin saturation ≤ 20% but elevated ferritin concentrations up to 800 ng/ml that may still be responsive to iron supplementation.
The choice of whether to use oral or i.v. iron for treatment of iron deficiency is usually a prudential one, individualized to the unique circumstances of each patient. Oral iron remains the most common first option because it is readily available without a prescription, inexpensive, and avoids the need for i.v. access, which can injure blood vessels that may be needed for vascular access.39
As mentioned above, ferrous sulfate is the most common formulation in use in clinical practice. Although often assumed to be ineffective because of diminished gut iron absorption, data from several large randomized controlled trials suggest that treatment with ferrous sulfate increases circulating iron stores and hemoglobin in patients with CKD not requiring dialysis. In an international clinical trial of 626 patients with CKD not requiring dialysis randomized to ferric carboxymaltose targeting a higher or lower ferritin concentration or oral ferrous sulfate (100 mg by mouth twice a day) for 52 weeks, participants who received ferrous sulfate (n = 308) had significant increases in hemoglobin (1.0 [SE, 0.1] g/dl), ferritin (137 [SE, 8] μg/l) and transferrin saturation (14% [SE, 1%]) after 12 months of therapy.40 Similarly, in a study of patients with stage 3 or 4 CKD with iron deficiency anemia randomized to oral ferrous sulfate (325 mg by mouth, 3 times daily) or iron sucrose for 8 weeks, participants who received ferrous sulfate had a significant increase in hemoglobin (0.61 g/dl) and transferrin saturation (0.03), but not ferritin, at 3 months.41 Thus, depending on the goal of therapy, the use of ferrous sulfate to treat iron deficiency may be sufficient if the gastrointestinal adverse effects are tolerable and the choice is consistent with other aspects of shared decision making, such as a convenience of administration.
More recently, oral ferric citrate has been found to be very effective in increasing both hemoglobin and iron indexes in individuals with CKD not requiring dialysis.42,43 A phase 3 double-blind clinical trial randomized individuals with an estimated glomerular filtration rate < 60 ml/min per 1.73 m2 not receiving kidney replacement therapy and iron deficiency (transferrin saturation ≤ 25% and ferritin ≤ 200 ng/ml) to receive ferric citrate (1 g by mouth, 3 times daily; n = 117) or matching placebo (n = 115) for 16 weeks, with titration at weeks 4, 8, and 12 by an additional 3 tablets per day to achieve an increase in hemoglobin of > 1 g/dl above baseline.43 Participants randomized to ferric citrate were significantly more likely to achieve the primary end point of a ≥ 1 g/dL increase from baseline than those randomized to placebo (52% vs. 19%, P < 0.001). Similarly, the mean change in hemoglobin, transferrin saturation, and ferritin over 16 weeks was significantly greater in those randomized to ferric citrate vs. placebo.
In a subsequent randomized clinical trial comparing the efficacy of ferric citrate vs. ferrous sulfate in individuals with stage 3 and 4 CKD over 12 weeks, as compared with participants randomized to ferrous sulfate (325 mg by mouth, 3 times daily, n = 30), participants randomized to a fixed dose of ferric citrate (2 g by mouth, 3 times daily, n = 30) had a greater mean increase in transferrin saturation (between-group difference in mean change, 8%; 95% CI, 1%–15%; P = 0.02) and ferritin (between-group difference in mean change, 37 ng/ml; 95% CI, 10–64 ng/ml; P = 0.009).44 Further, hemoglobin significantly increased after 12 weeks in those who received ferric citrate (0.3 g/dl; 95% CI, 0.1–0.5 g/dl) but not those who received ferrous sulfate (0.3 g/dl; 95% CI, −0.1 to 0.2 g/dl). Importantly, there were no significant differences in the frequency and severity of adverse effects by study arm.
In aggregate, these studies suggest that ferric citrate is effective in treating iron deficiency anemia in patients with CKD not requiring dialysis and may also be more efficacious than the current standard of care (ferrous sulfate). Whether this efficacy translates to meaningful clinical or patient-focused outcomes, such as death, cardiovascular disease, or quality of life, has yet to be adequately tested in a randomized controlled trial. Nonetheless, a trial of 203 patients with advanced CKD (estimated glomerular filtration rate ≤ 20 ml/min per 1.73 m2) randomized to a fixed dose of ferric citrate (1 g, 3 times daily) versus usual care for 9 months showed that those who received ferric citrate had fewer annualized hospital admissions and lower incidence of the composite end point of death, dialysis, or transplantation (all exploratory end points).45 These data provide provocative evidence that treatment with iron improves outcomes in advanced CKD irrespective of the presence or absence of anemia, a hypothesis that will need to be formally tested in an adequately powered study.
I.V. iron administration is another option for iron-deficient patients with CKD not requiring dialysis who are unable to tolerate oral therapies or for whom oral therapies are not effective. Numerous studies have shown that i.v. iron is effective in increasing hemoglobin and iron stores in these patients.46 Moreover, the plurality of studies have shown that i.v. iron is more effective in treating iron deficiency anemia than oral therapies (consisting mostly of ferrous sulfate).46,47 The magnitude of the advantage is most pronounced for ferritin, with an end-of-study mean difference comparing i.v. iron to oral iron of 213 ng/ml (95% CI, 124–303 ng/ml) and transferrin saturation (mean difference, 5%; 95% CI, 3%–8%), and more modest with respect to the hemoglobin response (mean difference, 0.41 g/dl; 95% CI, 0.28–0.55 g/dl).47 This advantage in efficacy was somewhat counterbalanced by a 3.5-fold higher relative risk of allergic reactions or hypotension in those receiving i.v. iron versus oral iron, although i.v. iron was also associated with a better gastrointestinal adverse effect profile compared with oral iron (relative risk, 0.47; 95% CI, 0.33–0.66).47 Differences in the safety profile of oral versus i.v. iron therapies with respect to infection, oxidative stress, cardiovascular disease, kidney function decline, and iron overload have been inconsistent, with the bulk of evidence showing no major differences.
In summary, oral and i.v. iron formulations are both safe and effective in treating iron deficiency in patients with CKD not yet requiring kidney replacement therapy. The choice of which one to start with is often dictated by goals of therapy, response to prior therapy, concomitant use of erythropoiesis-stimulating agents, patient preference, and other practical considerations, such as ease of access to an infusion center. In addition, there is some evidence that administration of oral iron every other day instead of daily may enhance iron absorption by reducing the stimulatory effect of daily oral iron on hepcidin secretion.48 As such, it is possible that alternative-day dosing strategies may help enhance the utility of oral iron in patients with CKD given their constitutively elevated hepcidin concentrations. Nonetheless, no clinical trials to date have studied this in CKD patients, and so whether this strategy can improve the efficacy or oral iron in CKD not requiring dialysis is entirely unclear.
Iron Replacement in Patients With CKD Requiring Hemodialysis
Current thresholds for treatment of iron deficiency in patients with CKD requiring kidney replacement therapy are similar to those who are not dependent on dialysis (Table 3). However, there is more controversy about the upper ceiling of ferritin that should prompt withholding or discontinuing iron replacement, with some advocating iron supplementation even when ferritin concentrations are > 800 ng/ml.49 There is not much debate about whether to use oral or i.v. iron in patients with CKD requiring kidney replacement therapy given the plethora of data showing the far superiority of i.v. iron formulations over oral iron for treating iron deficiency anemia and the ease of administration of iron using the existing vascular access in those receiving hemodialysis.46,50 Instead, the relevant clinical questions are the frequency and amount of iron to deliver to these patients. Although studied in multiple clinical trials, several trials have been most influential in addressing this question in clinical practice.
The Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study randomized patients with anemia who were receiving hemodialysis and had a serum ferritin between 500 and 1200 ng/ml and transferrin saturation ≤ 25% to receive 1 g of ferric gluconate or no iron for 6 weeks.51 Participants randomized to receiving ferric gluconate had a higher increase in iron parameters and hemoglobin without any major differences in adverse events compared with participants randomized to no iron. This study challenged the notion that an upper ferritin threshold of 800 ng/ml should be adopted when deciding to restrict i.v. iron administration in patients receiving hemodialysis. A follow-up study by the same group (DRIVE II) showed that participants from the original DRIVE study who received ferric gluconate and were monitored for an additional 6 weeks after the intervention maintained higher hemoglobin and ferritin concentrations, required significantly less epoetin dosage, and experienced fewer serious adverse events than the control group.52
More recently, the Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) trial randomized 2141 patients receiving maintenance hemodialysis to receive iron sucrose in a proactive approach (400 mg monthly, unless the ferritin was > 700 ng/ml or transferrin saturation was ≥ 40%) or a reactive approach (administration of iron sucrose only when ferritin was < 200 ng/ml or transferrin saturation was < 20%).53 Importantly, unlike prior studies, the trial was specifically powered to detect a difference in the composite outcome of nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or death. After a median of 2.1 years of follow-up, participants randomized to the proactive approach had higher serum ferritin and transferrin concentrations, more rapid increases in hemoglobin from baseline despite lower cumulative doses of erythropoiesis-stimulating agents, and lower risk of the primary composite outcome (hazard ratio, 0.85; 95% CI, 0.73–1.00; P = 0.04 for superiority) without any major differences in the adverse effect profile. In the aggregate, these data support using a higher safety threshold for ferritin to restrict further i.v. iron infusion in hemodialysis patients if the goal is to improve anemia and hard clinical outcomes. However, what the upper bound of ferritin should be for safety remains unclear.
As mentioned above, iron replacement via the dialysate fluid is another option for treating iron deficiency in individuals receiving hemodialysis. Results from 2 pivotal phase III clinical trials in individuals on maintenance hemodialysis showed that use of ferric pyrophosphate citrate was more effective in increasing hemoglobin compared with placebo, without any differences in side effect profile.54,55
Iron Replacement in CKD Patients: Other Scenarios
The literature with respect to iron therapy in those receiving peritoneal dialysis is less abundant but generally supports the notion that i.v. iron is more effective in increasing hemoglobin than oral iron in adults on maintenance peritoneal dialysis.56, 57, 58, 59, 60, 61, 62 Given that vascular access is not readily available in patients on peritoneal dialysis, newer oral agents, such as ferric citrate, may provide a more convenient method for treating iron deficiency anemia in these patients.63,64 The optimal approach to treating iron deficiency is also not well established in individuals after kidney transplant, with a few studies demonstrating that i.v. infusions are effective in increasing iron stores and raising hemoglobin and are reasonably well tolerated by stable kidney transplant recipients.65, 66, 67
I.V. iron supplementation has been shown to improve clinical outcomes in individuals with moderate to severe heart failure accompanied by reduced ejection fraction.68 The reasons for this are not clear, because the benefits of iron supplementation are independent of any concomitant rise in hemoglobin, suggesting that iron replacement alone may be important for enhancing cardiac structure and function, perhaps by improving mitochondrial dysfunction.68 Given the common coexistence of heart failure and CKD (often referred to as cardiorenal syndrome), it is reasonable to speculate that iron therapy may also improve outcomes in individuals with CKD and heart failure. Although no clinical trials have formally tested this hypothesis, a prior study of individuals with CKD receiving ferric citrate for treatment of iron deficiency anemia showed that ferric citrate was just as effective in improving iron stores and hemoglobin in patients with heart failure as those without heart failure.69 These data support the use of i.v. iron infusions or ferric citrate in a clinical trial testing the efficacy of iron repletion in improving clinical outcomes in individuals with CKD and heart failure.
Iron Repletion and Adverse Events
Among the most controversial aspects of iron therapy in CKD is the question of whether i.v. iron supplementation results in adverse events that potentially outweigh any benefits from raising hemoglobin. This is partly related to adverse reactions to high-molecular-weight iron dextran—one of the first i.v. iron preparations available in clinical practice—characterized by anaphylactoid reactions, including respiratory arrest in its most severe form.31 Although relatively rare, the potential for severe hypersensitivity reactions resulted in a black box warning and the requirement of a test dose to ensure safety.
With the introduction of safer i.v. iron preparations, iron dextran has largely been supplanted by second- and third-generation iron products. Nonetheless, less severe hypersensitivity reactions, such as dizziness and hypotension, can still occur with current iron agents, and there remains real concern that the release of free iron with i.v. infusion may cause tissue damage via oxidative stress or increase susceptibility to infection.31,70,71 Studies have both supported and refuted these concerns, with the balance of evidence suggesting that exposure to i.v. iron does not result in any greater risk of severe adverse reactions compared with oral iron or placebo.70
Summary
Iron deficiency is common in individuals with CKD and plays a critical role in the development of anemia. The constitutively elevated circulating concentration of hepcidin makes treatment of iron deficiency with oral agents challenging in patients with CKD who do not require kidney replacement therapy and virtually impossible in individuals who require kidney replacement therapy, making i.v. iron an essential tool in the management of iron deficiency anemia. Newer oral agents, such as ferric citrate, provide some promise that treatment of iron deficiency with oral agents alone may be more tenable. Recent data suggesting that more aggressive treatment of iron deficiency in hemodialysis patients redounds to their benefit with respect to hard clinical outcomes should prompt further clinical trials investigating whether treatment of iron deficiency should be a key goal in all individuals with kidney disease whether or not they have anemia or require kidney replacement therapy.
Disclosure
OMG has received grant funding and honoraria from Akebia, grant funding and honoraria from Amgen, grant funding from GSK, honoraria from AstraZeneca, Reata, and Ardelyx, and serves on a data monitoring committee for QED Therapeutics.
Acknowledgments
OMG is supported by grant K24DK116180 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
References
- 1.Fishbane S. Iron supplementation in renal anemia. Semin Nephrol. 2006;26:319–324. doi: 10.1016/j.semnephrol.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Eschbach J.W., Egrie J.C., Downing M.R., Browne J.K., Adamson J.W. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 3.Adamson J.W., Eschbach J.W. Erythropoietin for end-stage renal disease. N Engl J Med. 1998;339:625–627. doi: 10.1056/NEJM199808273390910. [DOI] [PubMed] [Google Scholar]
- 4.Lopez A., Cacoub P., Macdougall I.C., Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–916. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- 5.Batchelor E.K., Kapitsinou P., Pergola P.E., Kovesdy C.P., Jalal D.I. Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. 2020;31:456–468. doi: 10.1681/ASN.2019020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panwar B., Gutiérrez O.M. Disorders of iron metabolism and anemia in chronic kidney disease. Semin Nephrol. 2016;36:252–261. doi: 10.1016/j.semnephrol.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Green R., Charlton R., Seftel H. Body iron excretion in man: a collaborative study. Am J Med. 1968;45:336–353. doi: 10.1016/0002-9343(68)90069-7. [DOI] [PubMed] [Google Scholar]
- 8.Chung J., Wessling-Resnick M. Molecular mechanisms and regulation of iron transport. Crit Rev Clin Lab Sci. 2003;40:151–182. doi: 10.1080/713609332. [DOI] [PubMed] [Google Scholar]
- 9.Abboud S., Haile D.J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 10.Donovan A., Lima C.A., Pinkus J.L. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Knutson M.D., Oukka M., Koss L.M., Aydemir F., Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park C.H., Valore E.V., Waring A.J., Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth E., Tuttle M.S., Powelson J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Sci Signal. 2004;306:2090. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth E. Anti-hepcidin therapy for iron-restricted anemias. Blood. 2013;122:2929–2931. doi: 10.1182/blood-2013-08-522466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooke K.S., Hinkle B., Salimi-Moosavi H. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013;122:3054–3061. doi: 10.1182/blood-2013-06-505792. [DOI] [PubMed] [Google Scholar]
- 16.Sasu B.J., Cooke K.S., Arvedson T.L. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 17.Wang C.Y., Babitt J.L. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol. 2016;23:189–197. doi: 10.1097/MOH.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y., Alfaro-Magallanes V.M., Babitt J.L. Physiological and pathophysiological mechanisms of hepcidin regulation: clinical implications for iron disorders. Br J Haematol. 2021;193:882–893. doi: 10.1111/bjh.17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Eijk L.T., John A.S., Schwoebel F. Effect of the antihepcidin Spiegelmer lexaptepid on inflammation-induced decrease in serum iron in humans. Blood. 2014;124:2643–2646. doi: 10.1182/blood-2014-03-559484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohlbaum A.M., Gille H., Trentmann S. Sustained plasma hepcidin suppression and iron elevation by Anticalin-derived hepcidin antagonist in cynomolgus monkey. Br J Pharmacol. 2018;175:1054–1065. doi: 10.1111/bph.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheetz M., Barrington P., Callies S. Targeting the hepcidin-ferroportin pathway in anaemia of chronic kidney disease. Br J Clin Pharmacol. 2019;85:935–948. doi: 10.1111/bcp.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugahara M., Tanaka T., Nangaku M. Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int. 2017;92:306–312. doi: 10.1016/j.kint.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 23.Witmer C.M. Hematologic manifestations of systemic disease (including iron deficiency, anemia of inflammation and DIC) Pediatr Clin North Am. 2013;60:1337–1348. doi: 10.1016/j.pcl.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Manoguerra A.S., Erdman A.R., Booze L.L. Iron ingestion: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2005;43:553–570. doi: 10.1081/clt-200068842. [DOI] [PubMed] [Google Scholar]
- 25.Pergola P.E., Fishbane S., Ganz T. Novel oral iron therapies for iron deficiency anemia in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:272–291. doi: 10.1053/j.ackd.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Murray-Kolb L., Beard J. In: Encyclopedia of Dietary Supplements. 2nd ed. Coates P., Betz J., Blackman M., editors. CRC Press; 2015. Iron; pp. 432–437. [Google Scholar]
- 27.Barton J.C., Barton E.H., Bertoli L.F., Gothard C.H., Sherrer J.S. Intravenous iron dextran therapy in patients with iron deficiency and normal renal function who failed to respond to or did not tolerate oral iron supplementation. Am J Med. 2000;109:27–32. doi: 10.1016/s0002-9343(00)00396-x. [DOI] [PubMed] [Google Scholar]
- 28.Henry D.H., Dahl N.V., Auerbach M., Tchekmedyian S., Laufman L.R. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist. 2007;12:231–242. doi: 10.1634/theoncologist.12-2-231. [DOI] [PubMed] [Google Scholar]
- 29.Cançado R.D., Muñoz M. Intravenous iron therapy: how far have we come? Rev Bras Hematol Hemoter. 2011;33:461–469. doi: 10.5581/1516-8484.20110123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kassianides X., Hazara A.M., Bhandari S. Improving the safety of intravenous iron treatments for patients with chronic kidney disease. Expert Opin Drug Saf. 2021;20:23–35. doi: 10.1080/14740338.2021.1853098. [DOI] [PubMed] [Google Scholar]
- 31.Auerbach M., Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematology Am Soc Hematol Educ Program. 2010;2010:338–347. doi: 10.1182/asheducation-2010.1.338. [DOI] [PubMed] [Google Scholar]
- 32.Shah S.V., Rajapurkar M.M., Baliga R. The role of catalytic iron in acute kidney injury. Clin J Am Soc Nephrol. 2011;6:2329–2331. doi: 10.2215/CJN.08340811. [DOI] [PubMed] [Google Scholar]
- 33.Wolf M., Rubin J., Achebe M. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323:432–443. doi: 10.1001/jama.2019.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albright T., Al-Makki A., Kalakeche R., Shepler B. A review of ferric pyrophosphate citrate (Triferic) use in hemodialysis patients. Clin Ther. 2016;38:2318–2323. doi: 10.1016/j.clinthera.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Chapter 1: diagnosis and evaluation of anemia in CKD. Kidney Int Suppl. 2012;2:288–291. doi: 10.1038/kisup.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Collaborating Centre for Chronic Conditions (UK) Royal College of Physicians; 2006. Anaemia Management in Chronic Kidney Disease: National Clinical Guideline for Management in Adults and Children. NICE Clinical Guidelines, No. 39. [PubMed] [Google Scholar]
- 37.Mikhail A., Brown C., Williams J.A. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 2017;18:345. doi: 10.1186/s12882-017-0688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Locatelli F., Bárány P., Covic A. Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant. 2013;28:1346–1359. doi: 10.1093/ndt/gft033. [DOI] [PubMed] [Google Scholar]
- 39.Bhandari S., Naudeer S. Improving efficiency and value in health care. Intravenous iron management for anaemia associated with chronic kidney disease: linking treatment to an outpatient clinic, optimizing service provision and patient choice. J Eval Clin Pract. 2008;14:996–1001. doi: 10.1111/j.1365-2753.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- 40.Macdougall I.C., Bock A.H., Carrera F. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant. 2014;29:2075–2084. doi: 10.1093/ndt/gfu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal R., Kusek J.W., Pappas M.K. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015;88:905–914. doi: 10.1038/ki.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Block G.A., Fishbane S., Rodriguez M. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3-5. Am J Kidney Dis. 2015;65:728–736. doi: 10.1053/j.ajkd.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Fishbane S., Block G.A., Loram L. Effects of ferric citrate in patients with nondialysis-dependent CKD and iron deficiency anemia. J Am Soc Nephrol. 2017;28:1851–1858. doi: 10.1681/ASN.2016101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Womack R., Berru F., Panwar B., Gutiérrez O.M. Effect of ferric citrate versus ferrous sulfate on iron and phosphate parameters in patients with iron deficiency and CKD: a randomized trial. Clin J Am Soc Nephrol. 2020;15:1251–1258. doi: 10.2215/CJN.15291219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Block G.A., Block M.S., Smits G. A pilot randomized trial of ferric citrate coordination complex for the treatment of advanced CKD. J Am Soc Nephrol. 2019;30:1495–1504. doi: 10.1681/ASN.2018101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shepshelovich D., Rozen-Zvi B., Avni T., Gafter U., Gafter-Gvili A. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis. 2016;68:677–690. doi: 10.1053/j.ajkd.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 47.O’Lone E.L., Hodson E.M., Nistor I., Bolignano D., Webster A.C., Craig J.C. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2019;2:CD007857. doi: 10.1002/14651858.CD007857.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoffel N.U., Cercamondi C.I., Brittenham G. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533. doi: 10.1016/S2352-3026(17)30182-5. [DOI] [PubMed] [Google Scholar]
- 49.Fishbane S., Coyne D.W. How I treat renal anemia. Blood. 2020;136:783–789. doi: 10.1182/blood.2019004330. [DOI] [PubMed] [Google Scholar]
- 50.Rozen-Zvi B., Gafter-Gvili A., Paul M., Leibovici L., Shpilberg O., Gafter U. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: systematic review and meta-analysis. Am J Kidney Dis. 2008;52:897–906. doi: 10.1053/j.ajkd.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 51.Coyne D.W., Kapoian T., Suki W. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol. 2007;18:975–984. doi: 10.1681/ASN.2006091034. [DOI] [PubMed] [Google Scholar]
- 52.Kapoian T., O’Mara N.B., Singh A.K. Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol. 2008;19:372–379. doi: 10.1681/ASN.2007050606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macdougall I.C., White C., Anker S.D. Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. N Engl J Med. 2019;380:447–458. doi: 10.1056/NEJMoa1810742. [DOI] [PubMed] [Google Scholar]
- 54.Gupta A., Lin V., Guss C., Pratt R., Alp Ikizler T., Besarab A. Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis-stimulating agent use and maintains hemoglobin in hemodialysis patients. Kidney Int. 2015;88:1187–1194. doi: 10.1038/ki.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fishbane S.N., Singh A.K., Cournoyer S.H. Ferric pyrophosphate citrate (Triferic™) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Transplant. 2015;30:2019–2026. doi: 10.1093/ndt/gfv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H., Wang S.-X. Intravenous iron sucrose in peritoneal dialysis patients with renal anemia. Perit Dials Int. 2008;28:149–154. [PubMed] [Google Scholar]
- 57.Besarab A., Chernyavskaya E., Motylev I. Roxadustat (FG-4592): correction of Anemia in Incident Dialysis Patients. J Am Soc Nephrol. 2016;27:1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ficheux M., Cuny P., Lecouf A., Ryckelynck J.P., Hurault de Ligny B., Lobbedez T. Treatment of iron deficiency by a bolus intravenous iron dextran in peritoneal dialysis. Article in French. Nephrol Ther. 2011;7:558–561. doi: 10.1016/j.nephro.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Zeidan A., Bhandari S. Anemia in peritoneal dialysis patients; iron repletion, current and future therapies. Perit Dial Int. 2017;37:6–13. doi: 10.3747/pdi.2016.00193. [DOI] [PubMed] [Google Scholar]
- 60.Ahsan N. Intravenous infusion of total dose iron is superior to oral iron in treatment of anemia in peritoneal dialysis patients: a single center comparative study. J Am Soc Nephrol. 1998;9:664–668. doi: 10.1681/ASN.V94664. [DOI] [PubMed] [Google Scholar]
- 61.Johnson D.W., Herzig K.A., Gissane R., Campbell S.B., Hawley C.M., Isbel N.M. A prospective crossover trial comparing intermittent intravenous and continuous oral iron supplements in peritoneal dialysis patients. Nephrol Dial Transplant. 2001;16:1879–1884. doi: 10.1093/ndt/16.9.1879. [DOI] [PubMed] [Google Scholar]
- 62.Singh H., Reed J., Noble S., Cangiano J.L., Van Wyck D.B. Effect of intravenous iron sucrose in peritoneal dialysis patients who receive erythropoiesis-stimulating agents for anemia: a randomized, controlled trial. Clin J Am Soc Nephrol. 2006;1:475–482. doi: 10.2215/CJN.01541005. [DOI] [PubMed] [Google Scholar]
- 63.Umanath K., Jalal D.I., Greco B.A. Ferric citrate reduces intravenous iron and erythropoiesis-stimulating agent use in ESRD. J Am Soc Nephrol. 2015;26:2578–2587. doi: 10.1681/ASN.2014080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yokoyama K., Akiba T., Fukagawa M., Nakayama M., Hirakata H. JTT-751 for treatment of patients with hyperphosphatemia on peritoneal dialysis. Nephron Clin Pract. 2014;128:135–140. doi: 10.1159/000366482. [DOI] [PubMed] [Google Scholar]
- 65.Grimmelt A.C., Cohen C.D., Fehr T., Serra A.L., Wuethrich R.P. Safety and tolerability of ferric carboxymaltose (FCM) for treatment of iron deficiency in patients with chronic kidney disease and in kidney transplant recipients. Clin Nephrol. 2009;71:125–129. doi: 10.5414/cnp71125. [DOI] [PubMed] [Google Scholar]
- 66.Mudge D.W., Tan K.S., Miles R. A randomized controlled trial of intravenous or oral iron for posttransplant anemia in kidney transplantation. Transplantation. 2012;93:822–826. doi: 10.1097/TP.0b013e318248375a. [DOI] [PubMed] [Google Scholar]
- 67.Rozen-Zvi B., Gafter-Gvili A., Zingerman B. Intravenous iron supplementation after kidney transplantation. Clin Transplant. 2012;26:608–614. doi: 10.1111/j.1399-0012.2012.01602.x. [DOI] [PubMed] [Google Scholar]
- 68.McDonagh T., Macdougall I.C. Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur J Heart Fail. 2015;17:248–262. doi: 10.1002/ejhf.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCullough P.A., Uhlig K., Neylan J.F., Pergola P.E., Fishbane S. Usefulness of oral ferric citrate in patients with iron-deficiency anemia and chronic kidney disease with or without heart failure. Am J Cardiol. 2018;122:683–688. doi: 10.1016/j.amjcard.2018.04.062. [DOI] [PubMed] [Google Scholar]
- 70.Nuhu F., Seymour A.M., Bhandari S. Impact of intravenous iron on oxidative stress and mitochondrial function in experimental chronic kidney disease. Antioxidants (Basel) 2019;8:498. doi: 10.3390/antiox8100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macdougall I.C., Bhandari S., White C. Intravenous iron dosing and infection risk in patients on hemodialysis: a prespecified secondary analysis of the PIVOTAL Trial. J Am Soc Nephrol. 2020;31:1118–1127. doi: 10.1681/ASN.2019090972. [DOI] [PMC free article] [PubMed] [Google Scholar]