Abstract
The overactivation of the mineralocorticoid receptor (MR) in animal models of chronic kidney disease (CKD) increases sodium retention and hypertension and provokes inflammation and fibrosis in the kidneys, blood vessels, and the heart; these processes play an important role in the progression of cardiorenal disease. Accordingly, blockade of the MR is an attractive therapeutic intervention to retard the progression of CKD and improve cardiovascular morbidity and mortality. Finerenone is a novel, nonsteroidal MR antagonist (MRA) with a unique mode of action that is distinct from currently available steroidal MRAs. In animal models of CKD, finerenone has a more favorable benefit/risk ratio as compared with the steroidal MRAs such as spironolactone and eplerenone. In patients with type 2 diabetes and heart and/or kidney disease, phase II trials have revealed that compared with spironolactone, eplerenone, or placebo, finerenone displays benefits that exceed the risks of MR antagonism. In patients with CKD and type 2 diabetes, a large phase III trial has shown that, compared with placebo, finerenone improved kidney failure and cardiovascular outcomes. In the first part of this article, we explore the safety and efficacy of spironolactone and eplerenone in early- and late-stage CKD. In the second part, we describe the mechanism of action of finerenone and discuss the promising role of this nonsteroidal MRA as a novel therapeutic opportunity to improve clinical outcomes in patients with CKD.
Keywords: chronic kidney disease, eplerenone, finerenone, mineralocorticoid receptor, spironolactone
The MR belongs to the subfamily of nuclear hormone receptors and is expressed in several tissues/cell types, such as in the kidney, heart, vasculature, immune cells, and fibroblasts.1 Physiologically, the MR plays a key role in regulating fluid, electrolytes, and blood pressure (BP). However, MR is overactivated in CKD and heart failure (HF). In addition to increased salt and water retention, MR overactivation increases the expression of target genes implicated in inflammatory and fibrotic pathways; this culminates in organ injury.1 Therefore, MR blockade is an attractive pharmacological target for preserving organ function. Preserving organ function is of key importance, particularly in patients with type 2 diabetes mellitus (T2DM). Kidney injury and early-stage CKD in these patients associates with a far greater likelihood of cardiovascular morbidity than progression to end-stage kidney disease (ESRD). Therefore, MR blockade may abrogate the progression of CKD and reduce cardiovascular morbidity and mortality.2
Landmark randomized trials have shown that, among patients who have HF with reduced ejection fraction (HFrEF), the currently available steroidal MRAs (spironolactone and eplerenone) reduce the risk of hospitalization and cardiovascular death.3,4 Although steroidal MRAs have received the strongest endorsement (class IA) in clinical guidelines for the treatment of HFrEF,5 large-scale observational studies have shown that these lifesaving therapies are underused in clinical practice.6 Notably, the underuse of spironolactone and eplerenone occurs more commonly in patients with impaired kidney function, most likely due to the associated risk of both acute kidney injury (AKI) and hyperkalemia.7 Furthermore, spironolactone is recommended by guidelines as optimal fourth-line therapy of resistant hypertension,8,9 but this indication is restricted only to patients with estimated glomerular filtration rate (eGFR) >45 ml/min per 1.73 m2 and serum K ≤ 4.5 mEq/l.9 Despite the fact that the burden of resistant hypertension increases progressively with the decline in eGFR,10,11 the risk of hyperkalemia also increases as eGFR declines.12,13 Once again, hyperkalemia acts as a barrier and limits the use of spironolactone as add-on antihypertensive therapy in advanced CKD.14 The inability to offer complete blockade of the renin-angiotensin system (RAS) affects clinical outcomes. Pharmacoepidemiologic studies have shown that, compared with patients receiving RAS-blockade on maximum doses, patients treated with submaximum doses or those who permanently discontinued RAS-blocker therapy due to hyperkalemia had higher long-term risk for adverse cardiorenal events and mortality.15 Most importantly, unlike the established cardioprotective benefit of steroidal MRAs in patients with HFrEF, whether these agents are also effective in slowing the progression of kidney injury among patients with CKD remains uncertain given that high-quality clinical trial evidence in this high-risk population is limited.7
The critical unmet need to slow the progression of cardiorenal disease with a more favorable side-effect profile sparked considerable efforts towards the discovery and development of nonsteroidal MRAs. Finerenone is a novel MRA with nonsteroidal structure and a unique binding mode that determines its high potency and selectivity for the MR.1,2 Preclinical studies provided preliminary evidence that finerenone modifies tissue remodeling by exerting anti-inflammatory, antifibrotic, and antiproliferative actions on both the heart and the kidney.16, 17, 18 Phase II clinical trials have shown a dose-dependent reduction in albuminuria with finerenone and side-effect profile similar with placebo, as well as smaller treatment-induced increases in serum potassium levels with finerenone compared with spironolactone.19, 20, 21 Furthermore, in a large phase III trial involving patients with CKD and T2DM, finerenone improved long-term kidney and cardiovascular outcomes as compared with placebo.12
In the first part of this article, we explore the safety and efficacy of steroidal MRAs in patients with early- and late-stage CKD. In the second part, we discuss the promising role of finerenone as a novel therapeutic opportunity to slow the progression of kidney injury and improve cardiovascular outcomes in patients with CKD.
Efficacy and Safety of Steroidal MRAs
Early-stage CKD
Pilot randomized trials have explored the hypothesis of whether steroidal MRAs (spironolactone or eplerenone) offer an additive kidney protective benefit in patients who have CKD already treated with an RAS inhibitor (an angiotensin-converting enzyme inhibitor [ACEi] or an angiotensin receptor blocker [ARB]).7,22 As shown in Table 1, 2 Cochrane meta-analyses, published in 2009 and 2014 respectively, have suggested that add-on therapy with steroidal MRAs is associated with significant reductions in albuminuria and in-office BP, but there was no clear benefit on the rate of eGFR decline.23,24 In contrast, both meta-analyses showed that the addition of spironolactone to an ACEi or an ARB was associated with excess risk of side effects, mainly with a two- to three-fold higher risk of hyperkalemia.23,24 Limitations of the included trials included small sample sizes, short follow-up durations, and the absence of kidney failure endpoints; only intermediate endpoints such as albuminuria or BP reductions were reported.
Table 1.
Meta-Analyses of Randomized Controlled Trials Exploring the Safety and Efficacy of Steroidal MRAs Among Patients With CKD
| Author | Year | Studies | N | Patient Characteristics | Intervention | Key Results |
|---|---|---|---|---|---|---|
| Early-stage CKD | ||||||
| Navaneethan et al.24 | 2009 | 10 | 845 | Patients with CKD currently treated with an ACEi and/or ARB | Add-on MRA therapy vs. placebo | Reduction in 24-hour proteinuria (WMD: -0.80 g; 95% CI: -1.23 to -0.38; 7 studies, N = 372). No improvement in eGFR (WMD: -0.70 ml/min per 1.73 m2; 95% CI: -4.73 to 3.34; 5 studies, N = 306). Increased risk of hyperkalemia (RR: 3.06; 95% CI: 1.26 to 7.41; 8 studies; N = 436). |
| Bolignano et al.23 | 2014 | 27 | 1549 | Patients with proteinuric CKD (nephrotic and non-nephrotic range) | MRAs alone or in combination with an ACEi or ARB vs. other antihypertensive strategies or placebo | Reduction in 24-hour proteinuria (SMD: -0.61; 95% CI: -1.08 to -0.13; 11 studies, N = 596). Imprecise effect on eGFR (WMD: -2.55 ml/min per 1.73 m2; 95% CI: -5.67 to 0.51; 9 studies, N = 528). Increased risk of hyperkalemia (RR: 2.00; 95% CI: 1.25 to 3.20; 11 studies, N = 632). Increased risk of gynecomastia (RR: 5.14; 95% CI: 1.14 to 23.23; 4 studies, N = 281). |
| Chung et al.25 | 2020 | 44 | 5745 | Patients with proteinuric CKD (nephrotic and non-nephrotic range) | MRAs in combination with an ACEi or ARB vs. other antihypertensive strategies or placebo | Reduction in 24-hour proteinuria (SMD: -0.51; 95% CI: -0.82 to -0.20; 14 studies, N =1193) and in eGFR (WMD: -3.00 ml/min per 1.73 m2; 95% CI: -5.51 to -0.49; 13 studies, N = 1165). Increased risk of hyperkalemia (RR: 2.17; 95% CI: 1.47 to 3.22; 17 studies, N = 3001) and increased risk of gynecomastia (RR: 5.14; 95% CI: 1.14 to 23.23; 4 studies, N = 281). Uncertain effects on kidney failure (RR: 3.00; 95% CI: 0.33 to 27.65; 2 studies, N = 84), all-cause death (RR: 0.58; 95% CI: 0.10 to 3.50; 3 studies, N = 421) and cardiovascular events (RR: 0.95; 95% CI: 0.26 to 3.56; 3 studies, N = 1067). |
| Late-stage CKD | ||||||
| Quach et al.29 | 2016 | 9 | 829 | ESRD patients on hemodialysis or peritoneal dialysis with or without HF | Spironolactone or eplerenone vs. placebo or standard-of-care | Reduction in the risk of cardiovascular mortality (RR: 0.34; 95% CI: 0.15 to 0.75; 5 studies, N = 655) and all-cause mortality (RR: 0.40; 95% CI: 0.23 to 0.69; 6 studies, N = 721). Increased risk of hyperkalemia (RR: 3.05; 95% CI: 1.21 to 7.70; 7 studies, N = 755) Uncertain benefit/risk ratio due to wide variability in RRs in sensitivity analysis. |
| Hasegawa et al.32 | 2021 | 16 | 1446 | Patients with CKD requiring dialysis | Spironolactone or eplerenone vs. placebo or standard care | Reduction in the risk of cardiovascular mortality (RR: 0.37; 95% CI: 0.22 to 0.64; 6 studies, N = 908) and all-cause mortality (RR: 0.45; 95% CI: 0.30 to 0.67; 9 studies, N = 1119). Increased risk of gynecomastia (RR: 5.95; 95% CI: 1.93 to 18.3; 4 studies, N = 768) and little to no difference in the risk of hyperkalemia (RR: 1.41; 95% CI: 0.72 to 2.78; 9 studies, N = 981). Marginal effect of left ventricular mass (SMD: -0.42; 95% CI: -0.78 to 0.05; 8 studies, N = 633). |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HF, heart failure; MRA, mineralocorticoid receptor antagonist; RR, relative risk; SMD, standardized mean difference; WMD, weighted mean difference.
Table 2.
Phase II Randomized Controlled Trials Testing the Safety and Efficacy of Finerenone
|
Trial |
N | Patient Characteristics | Intervention | Follow-Up, days | Primary Outcome | Key Results |
|---|---|---|---|---|---|---|
| ARTS21 | Part A: 65 Part B: 392 |
HFrEF (NYHA functional class II-IV and LVEF ≤40%) with eGFR of 60 to <90 ml/min per 1.73 m2 in Part A and eGFR of 30 to 60 ml/min per 1.73 m2 in Part B | Part A: finerenone (2.5, 5, or 10 mg once daily) vs. placebo; Part B: finerenone (2.5, 5, or 10 mg daily or 5 mg twice daily) compared with placebo and open-label spironolactone (25 or 50 mg/d) |
28 | Change in serum potassium | Significantly smaller treatment-induced increases in serum potassium with finerenone than with spironolactone (0.04 to 0.30 vs. 0.45 mmol/l, P < 0.0001 to 0.0107). Lower incidence of hyperkalemia with finerenone than with spironolactone (5.3% vs. 12.7%, P = 0.048). Finerenone provoked at least similar reductions in BNP, NT-proBNP, and albuminuria compared with spironolactone. |
| ARTS-HF20 | 1066 | HFrEF requiring hospitalization and intravenous diuretic therapy. Patients also had type 2 DM and/or CKD (eGFR of >30 ml/min/1.73m2 in diabetics and eGFR of 30-60 ml/min/1.73m2 in non-diabetics) | Finerenone (2.5 to 15 mg daily titrated up to 5 to 20 mg daily) vs. eplerenone (25 mg every other day that could be increased to 25 mg/d and then to 50 mg/d) | 90 | Proportion of patients with a decrease of >30% in NT-proBNP from baseline to study end | The proportion of patients with >30% decrease in NT-proBNP did not differ between the finerenone and eplerenone groups. The exploratory endpoint of all-cause death, cardiovascular hospitalization, or emergency visit due to worsening HF occurred numerically less frequently in finerenone-treated than in eplerenone-treated patients. The overall incidence of hyperkalemia was 4.3% and was equally balanced among treatment groups. |
| ARTS-DN19 | 823 | Type 2 DM with albuminuria (UACR ≥30 mg/g) and eGFR of >30 ml/min/1.73m2 under treatment with at least the minimum recommended of a RAS-blocker | Finerenone (1.25, 2.5, 5, 7.5, 10, 15, or 20 mg daily) vs. placebo | 90 | Change in albuminuria | Finerenone provoked a dose-dependent reduction in UACR. Drug discontinuation due to hyperkalemia was not observed with placebo and finerenone 10 mg/d, but occurred in 2.1%, 3.2%, and 1.7% of patients in the finerenone 7.5-, 15- and 20-mg/d groups. |
ARTS, Mineralocorticoid Receptor Antagonist Tolerability Study; ARTS-DN, ARTS-Diabetic Nephropathy study; ARTS-HF, ARTS-Heart Failure study; BNP, B type natriuretic peptide; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NYHA; New York Heart Association; NT-proBNP, N-terminal-pro-B-type natriuretic peptide; RAS, renin-angiotensin system; UACR, urinary albumin-to-creatinine ratio.
The earlier 2014 meta-analysis included 27 trials with 1549 patients23; in comparison, an updated 2020 Cochrane meta-analysis in mild-to-moderate proteinuric CKD expanded these observations to 44 trials involving 5745 patients.25 Compared to placebo or standard-of-care, add-on therapy with a steroidal MRA was associated with improvement in albuminuria (standardized mean difference: -0.51; 95% confidence interval [CI]: -0.82 to -0.20), in office systolic BP (weighted mean difference: -4.98 mm Hg; 95% CI: -8.22 to -1.75), and in eGFR (weighted mean difference: -3.00 ml/min per 1.73 m2; 95% CI: -5.51 to -0.49).25 With respect to the side-effect profile, the addition of spironolactone to an RAS blocker was associated with 2.17-fold higher risk of hyperkalemia (relative risk [RR]: 2.17; 95% CI: 1.47 to 3.22), 2.04-fold higher risk of AKI (RR: 2.04; 95% CI: 1.05 to 3.97) and 5.14-fold higher incidence of gynecomastia (RR: 5.14; 95% CI: 1.14 to 23.23).25 The risk of hyperkalemia and AKI accumulates over time; none of the studies was long-term. Therefore, the RR evaluated was over the short-term. These trials did not include kidney failure endpoints (such as dialysis or kidney transplantation) or major adverse cardiovascular events (such as myocardial infarction or stroke). In comparison to MRAs added to a RAS inhibitor, dual RAS blockade improves albuminuria but fails to protect the heart or the kidney in long-term trials.26, 27, 28 Similarly, in the absence of long-term kidney failure and cardiovascular endpoints, the benefit/risk ratio of steroidal MRAs remains unclear.
Late-Stage CKD
In 2016, a meta-analysis of nine trials (N = 829) aimed to explore the safety and efficacy of steroidal MRAs in ESRD patients with or without HF.29 Compared with control group, add-on therapy with spironolactone or eplerenone reduced by 66% the risk of cardiovascular death (RR: 0.34; 95% CI: 0.15 to 0.75) and by 60% the risk of all-cause death (RR: 0.40; 95% CI: 0.23 to 0.69). However, this cardioprotective benefit was accompanied by a 3.05-fold higher incidence of hyperkalemia in MRA-treated patients than in controls (RR: 3.05; 95% CI: 1.21 to 7.70).29 These risk estimates should be interpreted cautiously because five of the included trials had less than 6 months of follow-up and four trials had sample sizes of less than 30 patients each, indicating lack of high-quality evidence. The benefit/risk ratio of steroidal MRAs among ESRD patients was investigated in two subsequent trials. The safety and cardiovascular (CV) efficacy of spironolactone in dialysis-dependent ESRD showed a dose-dependent increase in the risk of hyperkalemia, particularly when spironolactone was administered at a dosage of 50 mg/d.30 Similarly, in the Mineralocorticoid Receptor Antagonists in End-Stage Renal Disease (MiREnDa) trial,31 moderate hyperkalemia (defined as predialysis serum potassium level of 6.0 to 6.5 mmol/l) occurred more commonly in the spironolactone than in the placebo group. Furthermore, both spironolactone in dialysis-dependent ESRD and MiREnDa failed to show a benefit of spironolactone on left ventricular diastolic function over 36 weeks30 or on left ventricular mass over 40 weeks of follow-up,31 respectively.
In an updated 2021 Cochrane meta-analysis of 16 trials (N = 1446),32 compared with placebo, treatment with spironolactone or eplerenone was associated with 63% reduction in the risk of CV death (RR: 0.37; 95% CI: 0.22 to 0.64) and with 55% reduction in the risk of all-cause death (RR: 0.45; 95% CI: 0.30 to 0.67). Add-on MRA therapy increased the risk of gynecomastia (RR: 5.95; 95% CI: 1.93 to 18.3), but there was little to no difference in the risk of hyperkalemia between active-treatment and control groups (RR: 1.41; 95% CI: 0.72 to 2.78).32 This seemingly more favorable benefit/risk profile of spironolactone or eplerenone in late-stage CKD should be carefully interpreted; most of the trials included in this larger meta-analysis were once again small, short-term, and potentially had a high risk of bias.
More conclusive evidence on the safety and efficacy of steroidal MRAs in ESRD is anticipated by two large ongoing phase III trials.33 In the Aldosterone Antagonist Chronic Hemodialysis Interventional Survival Trial (ALCHEMIST), 825 patients on hemodialysis will be randomized to spironolactone or placebo; the primary outcome of this trial is defined as the time to first occurrence of nonfatal myocardial infarction, acute coronary syndrome, hospitalization for HF, nonfatal stroke, or CV death.33 The even larger Aldosterone Blockade for Health Improvement Evaluation in End-Stage Renal Disease (ACHIEVE) trial is planning to recruit 2750 patients receiving either hemodialysis or peritoneal dialysis aiming to compare the effect of spironolactone versus placebo on the composite outcome of CV death or hospitalization for HF.33 Until firm evidence from these two large ongoing trials becomes available, the use of spironolactone and eplerenone for CV protection in ESRD patients remains uncertain.
Cardiorenal Protection with the Nonsteroidal MRA Finerenone
Mechanism of Action
Finerenone is a third-generation, nonsteroidal molecule with a unique binding mode to the MR that determines potency, selectivity, and nuclear cofactor recruitment.1 Some key differences in the molecular mode of action between steroidal MRAs and finerenone exist and merit attention. Finerenone has higher selectivity for the MR than spironolactone and eplerenone and is at least equally potent compared with spironolactone.34 Unlike steroidal MRAs, molecular modeling studies have shown that finerenone acts as a bulky and passive antagonist to the MR.35,36 This distinct mechanism of action modifies substantially the transcriptional cascade mediated by the MR blockade in a cell-specific manner.18 In contrast to spironolactone and eplerenone which both exert a partial agonistic effect on cofactor recruitment, finerenone acts in vitro as an inverse agonist to the MR.1,18,36,37 Therefore, finerenone suppresses downstream expression of proinflammatory and profibrotic genes regardless of the presence or absence of aldosterone.
Physicochemical and Pharmacokinetic Properties
The duration of action may modify the pharmacodynamic response to MRA therapy.1 For example, eplerenone has a short plasma half-life of 4 to 6 hours and clinical studies have shown that the BP-lowering efficacy of eplerenone is greater when this short-acting MRA is administered at twice-daily as compared with once-daily dosing regimens.38 However, in large outcome trials conducted with patients who have HFrEF, eplerenone effectively reduced the risk of CV mortality and hospitalization for HF even when administered at doses of 25 to 50 mg once daily.4 This differentiation generates the hypothesis that, although the hemodynamic action or the effects on potassium homeostasis may be determined by a prolonged MRA exposure, the anti-inflammatory and antifibrotic actions that mediate end-organ protection may require a shorter MRA exposure.1 Compared with steroidal MRAs, finerenone is a molecule with greater polarity and six- to 10-fold less lipophilicity1; these unique physicochemical characteristics of finerenone determine its high tissue penetration and distribution. Spironolactone and eplerenone exhibit greater accumulation in the kidney than in the heart1,39; in contrast, finerenone has an equal kidney-heart distribution.37 This differentiation may, at least partly, explain the greater impact of steroidal MRAs on renal handling of sodium and potassium balance. Most importantly, the clearance of finerenone is predominantly mediated through nonkidney routes of elimination.40,41 Finerenone is also characterized by a short plasma half-life (2 to 3 hours in patients with CKD) and lack of biologically active metabolites.40,42,43 In sharp contrast, spironolactone is a prodrug with multiple metabolites that have prolonged duration of action.44 This has been shown in a recent randomized trial, in which spironolactone (25 to 50 mg/d) was administered as add-on therapy in patients with resistant hypertension and eGFR of 25 to 45 ml/min per 1.73 m2 for 12 weeks.45 In a prespecified post-trial follow-up visit, 75% of patients had detectable urinary excretion of metabolites (i.e., canrenone and 7α-thiomethyl spironolactone) 2 weeks after the administration of the last dose of spironolactone.45 More than half of the systolic BP reduction provoked by spironolactone over the course of this trial was sustained 2 weeks after drug discontinuation.45 It is therefore likely that restoration of normokalemia may not occur immediately after drug discontinuation due to the accumulation of active metabolites of spironolactone, particularly in patients with moderate-to-advanced CKD.
Preclinical Studies
A growing body of evidence from preclinical studies suggests that finerenone is effective in causing regression of end-organ damage in cardiorenal disease. In animal models, the administration of finerenone prevented the AKI inducible by ischemia/reperfusion as well as the chronic and progressive deterioration in kidney structure and function.16,17,37 These kidney protective effects were mediated through downregulation of expression of proinflammatory and profibrotic markers, reduction in albuminuria, and regression of tubulointerstitial fibrosis.16,17,37 These beneficial actions were observed even when finerenone was administered at dosages not significantly modifying systemic BP,37 indicating a BP-independent mechanism of action of finerenone. Furthermore, in animal models of cardiac hypertrophy, compared with equinatriuretic doses of eplerenone, finerenone reduced more effectively cardiac fibrosis, plasma prohormone levels of brain natriuretic peptide (BNP), and albuminuria.46, 47, 48, 49
Preclinical studies also provided preliminary evidence that inhibition of inflammation and fibrosis in response to therapy with nonsteroidal MRAs may not be counteracted by excess risk of hyperkalemia, suggesting a more favorable benefit/risk ratio of this novel drug class.1 In a rat model of CKD, a nonsteroidal MRA (PF-03882845) was compared with eplerenone and a therapeutic index was determined as the ratio of drug concentration that induced hyperkalemia to the corresponding drug concentration that induced a significant albuminuria-lowering effect.50 The therapeutic index of PF-03882845 against hyperkalemia was 57-fold higher than that of eplerenone.50 In a mouse model of rapidly progressive glomerulonephritis, BR-4628, a precursor to finerenone, was shown to effectively suppress glomerular damage via anti-inflammatory and antifibrotic actions without modifying urinary sodium/potassium excretion and without inducing hyperkalemia.51
Phase II Clinical Trials
The safety and efficacy of finerenone was explored in a large phase II clinical trial program that included >2000 patients with HFrEF, CKD, and/or T2DM or with CKD and T2DM (Table 2).19, 20, 21
The Mineralocorticoid Receptor Antagonist Tolerability Study (ARTS) was a phase IIb trial that was conducted in two parts.21 In part A, the safety and tolerability of finerenone (2.5, 5, or 10 mg once daily) relative to placebo was investigated in 65 patients with HFrEF and mild CKD (eGFR: 60 to <90 ml/min per 1.73 m2). In part B, the effect of finerenone (2.5, 5, or 10 mg once daily, or 5 mg twice daily) on serum potassium levels was compared with that of placebo or active treatment with spironolactone (25 to 50 mg once daily) in 392 patients with HFrEF and moderate CKD (eGFR: 30 to 60 ml/min per 1.73 m2). The mean increase in serum potassium levels over 28 days was significantly smaller in all four finerenone dose groups than in the spironolactone group (0.04 to 0.30 vs. 0.45 mmol/l, respectively).21 The adverse events of hyperkalemia and worsening of kidney function occurred less commonly in patients treated with finerenone than in patients randomly assigned to spironolactone. With respect to the efficacy outcomes, finerenone appeared to be at least equally effective with spironolactone in lowering the levels of BNP, N-terminal proBNP, and albuminuria over the course of this randomized trial.21
The ARTS-Heart Failure (ARTS-HF)20 stukdy included 1066 patients with a recent hospitalization due to worsening HFrEF requiring treatment with intravenous diuretics. According to the inclusion criteria, patients enrolled in ARTS-HF had also T2DM and/or CKD (eGFR of >30 ml/min per 1.73 m2 in those with concomitant T2DM and 30 to 60 ml/min per 1.73 m2 in those without T2DM).20 This phase IIb trial aimed to compare the safety and efficacy of finerenone (2.5 to 15 mg/d titrated up to 5 to 20 mg/d) versus eplerenone (25 mg every other day that could be increased to 25 mg/d on day 30 and then to 50 mg/d on day 60) over 90 days. The proportion of patients with >30% decline in the levels of N-terminal proBNP during follow-up, the primary efficacy outcome, did not significantly differ between treatment groups.20 However, a signal of benefit with finerenone was evident in the composite clinical outcome of all-cause death, CV hospitalization, or emergency presentation due to worsening HF. This key exploratory clinical endpoint occurred less commonly in finerenone-treated than in eplerenone-treated patients; the between-drug difference reached nominal significance in those randomized to the eplerenone 10→20–mg/d group (hazard ratio: 0.56; 95% CI: 0.35 to 0.90; P = 0.02),20 despite the fact that this trial was not originally designed to detect significant differences in this composite clinical outcome. Hyperkalemia (defined as serum potassium elevation >5.6 mmol/l) occurred in 4.3% of the patients, but the incidence of hyperkalemic events was similar in the finerenone and eplerenone groups.20
In the ARTS-Diabetic Nephropathy (ARTS-DN)19 phase IIb trial, 823 patients with T2DM, urinary albumin-to-creatinine ratio (UACR) ≥30 mg/g and eGFR >30 ml/min per 1.73 m2 receiving background treatment with a RAS blocker were randomized to add-on finerenone at progressively increasing doses (1.25 up to 20 mg/d) or matching placebo for 90 days. Compared with placebo, finerenone provoked a dose-dependent reduction in albuminuria; the primary efficacy outcome, the placebo-subtracted mean ratio of the UACR at day 90 versus baseline, was reduced by 21%, 24%, 33%, and 38% in the finerenone 7.5-, 10-, 15- and 20-mg/d groups, respectively.19 Permanent drug discontinuation due to hyperkalemia was not observed in the placebo and finerenone 10-mg/d groups, but occurred in 2.1%, 3.2%, and 1.7% of patients randomly assigned to the finerenone 7.5-, 15-, and 20-mg/d groups, respectively.19 The secondary safety outcome of ≥30% decline in eGFR as well as the incidence of other serious adverse events did not significantly differ between groups.19
Taken together, these phase II trials showed that finerenone exerted beneficial short-term actions on intermediate cardiorenal endpoints (i.e., albuminuria and N-terminal proBNP levels), whereas hyperkalemia or AKI were not acting as barriers against its use.19, 20, 21 These promising results provided the rationale for the design of a large phase III clinical trial program aiming to fully elucidate the benefit/risk ratio of finerenone in patients with CKD and T2DM.
Phase III Clinical Trials
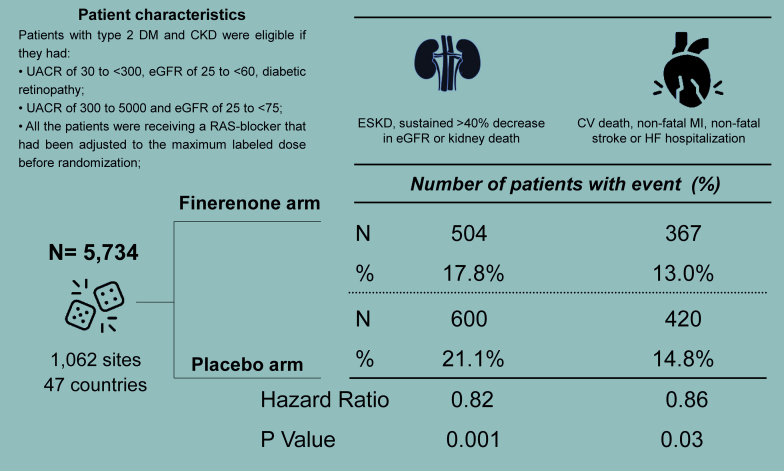
The Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD)12 trial randomized 5734 patients with CKD and T2DM to double-blind therapy with finerenone or placebo over a median follow-up of 2.6 years (Figure 1).12 According to the eligibility criteria, FIDELIO-DKD enrolled patients with high albuminuria (UACR of 30 to <300 mg/g), eGFR of 25 to <60 ml/min per 1.73 m2 and diabetic retinopathy, or patients with very high albuminuria (UACR of 300 to <5000 mg/g) and eGFR of 25 to <75 ml/min per 1.73 m2. The design of the trial prespecified the optimization of background therapy; the administration of an ACEi or an ARB had been adjusted to the maximum tolerated dose in the overall study population before randomization.12 This means that the trial screened patients who still had high or very high albuminuria despite optimized RAS inhibition.
Figure 1.
The design and main results of the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease trial. CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HF, heart failure; MI, myocardial infarction; RAS, renin-angiotensin system; UACR, urinary albumin-to-creatinine ratio.
Compared with placebo, finerenone reduced by 18% the primary composite outcome of kidney failure and sustained >40% decline in eGFR or death from renal causes (hazard ratio: 0.82; 95% CI: 0.73 to 0.93).12 A key secondary outcome was a composite outcome of kidney failure and sustained >57% decline in eGFR or death from renal causes; this is more in line with kidney failure outcomes used in clinical trials of CKD in T2DM. The hazard ratio of this outcome was 0.76 (95% CI: 0.65 to 0.90). This translates to 24% RR reduction in kidney failure outcome; one-third more than the primary outcome. Furthermore, finerenone provoked a placebo-subtracted reduction of 14% in the key secondary composite outcome of CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for HF (hazard ratio: 0.86; 95% CI: 0.75 to 0.99).12 With respect to side-effect profile, finerenone treatment was well tolerated and the distribution of adverse events was similar in the finerenone and placebo arms. However, the expected adverse effect of hyperkalemia was more with finerenone, as discussed in detail below.
The underlying mechanisms mediating the cardiorenal protection afforded by finerenone in the FIDELIO-DKD trial require a closer examination. The change in systolic BP from baseline to month 12 of follow-up was -2.1 mm Hg with finerenone and 0.9 mm Hg with placebo, respectively. Over the first 4 months, finerenone was associated with a 31% higher reduction in the UACR as compared with placebo. The separation of Kaplan-Meier curves for the secondary composite CV outcome was observed within weeks of starting therapy.12 Accordingly, the modest BP-lowering effect as well as the early benefit on albuminuria and CV events suggests that part of the cardiorenal benefit of finerenone might be mediated through natriuretic or hemodynamic mechanisms. The separation of Kaplan-Meier curves for the primary composite kidney failure outcome occurred after 12 months of follow-up; this benefit was persistent until the completion of the trial.12 Therefore, it is likely that long-term tissue remodeling through anti-inflammatory, antifibrotic, and antiproliferative actions, as suggested by several preclinical studies,16,18,37 might be an additional mediator of the sustained cardiorenal protection afforded by finerenone over the course of the FIDELIO-DKD trial.12 The relative contribution of hemodynamic and inflammation/fibrosis may differ by organ, stage of CKD, environmental (such as dietary sodium intake), and genetic factors among patients.
The benefits of finerenone on kidney failure and CV outcomes should be placed in context against the risk of treatment-induced hyperkalemia. In the FIDELIO-DKD study, the incidence of hyperkalemia-related adverse events was two-fold higher in finerenone-treated than in placebo-treated patients (18.3% and 9.0%, respectively).12 However, serious hyperkalemia-related adverse events requiring hospitalization occurred rarely (1.4% and 0.3%, respectively), whereas there was no report of any mortal event directly attributable to hyperkalemia. Permanent drug discontinuation was observed in 64 (2.3%) patients in the finerenone group and 25 (0.9%) patients in the placebo group.12 However, on a closer examination, the tolerability of finerenone over the course of the FIDELIO-DKD trial was substantially higher than that previously reported with spironolactone in the Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease (AMBER) trial.45 In AMBER, 295 patients with resistant hypertension and advanced CKD (eGFR of 25-45 ml/min per 1.73 m2) were randomized to receive either placebo or the potassium-binder patiromer in addition to open-label spironolactone and their baseline BP-lowering medications. Permanent discontinuation of spironolactone due to hyperkalemia occurred in 23.0% of patients in the placebo group.45 This contrasts with the 2.3% discontinuation rate seen with finerenone. Furthermore, the AMBER trial was 12 weeks in duration; the median follow-up in the FIDELIO-DKD trial was 2.6 years. Even in those randomized to the patiromer group, the discontinuation rate of spironolactone due to hyperkalemia was 6.8%.45 Therefore, although the risk of hyperkalemia with finerenone — in contrast to the preclinical studies — is real, this risk is small and manageable. Baseline serum potassium categories did not impair the heart and the kidney protection offered by finerenone. Thus, hyperkalemia is not a factor that limits its kidney and heart protection efficacy in patients T2DM and advanced CKD.
Perspectives
The FIDELIO-DKD study showed that finerenone is effective in retarding the progression of kidney injury and in improving CV outcomes among patients with T2DM and moderate-to-advanced CKD. The 2020 Cochrane meta-analysis of 44 trials of steroidal MRAs included 5745 patients25; the FIDELIO-DKD trial alone included 5734 patients.12 The meta-analysis did not include kidney failure or CV outcomes unlike the FIDELIO-DKD trial. The mean eGFR of patients enrolled in this landmark trial was 44.3±12.6 ml/min per 1.73 m2; more than half of these patients had CKD stage 3b or higher.12 Patients with T2DM with an earlier stage of CKD may benefit even more. Further insight will be provided by the ongoing Finerenone in Reducing CV Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trial.52 This sister study was initiated in September 2015 and randomized 7437 patients with T2DM and less advanced CKD (62% of patients with stage 1 or 2 CKD and a mean eGFR of 67.8±21.7 ml/min per 1.73 m2) aiming to compare the effect of finerenone relative to placebo on a primary composite outcome defined as time to first occurrence of CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for HF.52 Thus, the ongoing FIGARO-DKD is anticipated to answer the question whether MR blockade with finerenone at an earlier stage of diabetic kidney disease confers primarily CV protection in patients with T2DM and CKD. In such patients, the risk of CV morbidity and mortality is much higher than the risk of kidney injury progression to ESRD.53 The results of the FIGARO-DKD trial will be announced in 2021. Future trials are needed to explore whether MR antagonism with finerenone is an effective strategy to mitigate the diabetic kidney disease risk specifically in patients with type 1 diabetes mellitus.
Finerenone is an investigational drug and its approval for use in clinical practice is still pending. The unique mechanism of action and the distinct pharmacodynamic properties of finerenone indicate that this novel agent is not anticipated to replace some established clinical indications of the currently available steroidal MRAs. For example, spironolactone is recommended by guidelines as fourth-line therapy for the management of resistant hypertension,9 but the barrier of hyperkalemia limits its use in patients with eGFR <45 ml/min per 1.73 m2.14 Despite the fact that the risk of hyperkalemia is much less with finerenone than with spironolactone, add-on therapy with spironolactone is probably more effective to control BP in patients with resistant hypertension and moderate-to-advanced CKD. In the FIDELIO-DKD trial,12 the mean difference between the finerenone and placebo groups in office systolic BP was less than 3 mm Hg. This modest BP-lowering effect contrasts with the persistent reduction of 11 to 12 mm Hg in unattended automated office systolic BP that was provoked by spironolactone over the course of the AMBER trial.45 Therefore, enablement of spironolactone use with newer therapies that bind potassium in the gut is another therapeutic opportunity to improve clinical outcomes in patients with moderate-to-advanced CKD that warrants further investigation in future clinical trials.
Disclosure
RA is supported by NIH 5 R01 HL126903 and a grant from VA Merit Review 5I01CX001753.
RA is a member of data safety monitoring committee with Chinook; a member steering committees for randomized trials with Akebia, Bayer, and Reata; a consultant with Akebia, Bayer, Boehringer Ingelheim, Relypsa, Reata, and Diamedica; and a member of the adjudication committee with Bayer.
PIG has nothing to disclose.
References
- 1.Agarwal R., Kolkhof P., Bakris G. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152–161. doi: 10.1093/eurheartj/ehaa736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal R., Anker S.D., Bakris G. Investigating new treatment opportunities for patients with chronic kidney disease in type 2 diabetes: the role of finerenone [Online ahead of print] Nephrol Dial Transplant. 2020 doi: 10.1093/ndt/gfaa294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitt B., Zannad F., Remme W.J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B., Remme W., Zannad F. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 6.Savarese G., Carrero J.J., Pitt B. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11,215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail. 2018;20:1326–1334. doi: 10.1002/ejhf.1182. [DOI] [PubMed] [Google Scholar]
- 7.Georgianos P.I., Agarwal R. Revisiting RAAS blockade in CKD with newer potassium-binding drugs. Kidney Int. 2018;93:325–334. doi: 10.1016/j.kint.2017.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey R.M., Calhoun D.A., Bakris G.L. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–e90. doi: 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams B., Mancia G., Spiering W. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 10.Tanner R.M., Calhoun D.A., Bell E.K. Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol. 2013;8:1583–1590. doi: 10.2215/CJN.00550113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas G., Xie D., Chen H.Y. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report from the Chronic Renal Insufficiency Cohort Study. Hypertension. 2016;67:387–396. doi: 10.1161/HYPERTENSIONAHA.115.06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakris G.L., Agarwal R., Anker S.D. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen R.W., Nicolaisen S.K., Hasvold P. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes-a Danish population-based cohort study. Nephrol Dial Transplant. 2018;33:1610–1620. doi: 10.1093/ndt/gfx312. [DOI] [PubMed] [Google Scholar]
- 14.Georgianos P.I., Agarwal R. Resistant hypertension in chronic kidney disease (CKD): prevalence, treatment particularities, and research agenda. Curr Hypertens Rep. 2020;22:84. doi: 10.1007/s11906-020-01081-x. [DOI] [PubMed] [Google Scholar]
- 15.Epstein M., Reaven N.L., Funk S.E., McGaughey K.J., Oestreicher N., Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21:S212–S220. [PubMed] [Google Scholar]
- 16.Barrera-Chimal J., Estrela G.R., Lechner S.M. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018;93:1344–1355. doi: 10.1016/j.kint.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Blazquez R., Somoza B., Gil-Ortega M. Finerenone attenuates endothelial dysfunction and albuminuria in a chronic kidney disease model by a reduction in oxidative stress. Front Pharmacol. 2018;9:1131. doi: 10.3389/fphar.2018.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grune J., Beyhoff N., Smeir E. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone's antifibrotic activity. Hypertension. 2018;71:599–608. doi: 10.1161/HYPERTENSIONAHA.117.10360. [DOI] [PubMed] [Google Scholar]
- 19.Bakris G.L., Agarwal R., Chan J.C. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884–894. doi: 10.1001/jama.2015.10081. [DOI] [PubMed] [Google Scholar]
- 20.Filippatos G., Anker S.D., Bohm M. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105–2114. doi: 10.1093/eurheartj/ehw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitt B., Kober L., Ponikowski P. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–2463. doi: 10.1093/eurheartj/eht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shavit L., Lifschitz M.D., Epstein M. Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: current concepts and emerging treatment paradigms. Kidney Int. 2012;81:955–968. doi: 10.1038/ki.2011.505. [DOI] [PubMed] [Google Scholar]
- 23.Bolignano D., Palmer S.C., Navaneethan S.D., Strippoli G.F. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;(4):CD007004. doi: 10.1002/14651858.CD007004.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Navaneethan S.D., Nigwekar S.U., Sehgal A.R., Strippoli G.F. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2009;(3):CD007004. doi: 10.1002/14651858.CD007004.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Chung E.Y., Ruospo M., Natale P. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2020;10:CD007004. doi: 10.1002/14651858.CD007004.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried L.F., Emanuele N., Zhang J.H. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–1903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 27.Mann J.F., Schmieder R.E., McQueen M. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 28.Parving H.H., Brenner B.M., McMurray J.J. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 29.Quach K., Lvtvyn L., Baigent C. The safety and efficacy of mineralocorticoid receptor antagonists in patients who require dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68:591–598. doi: 10.1053/j.ajkd.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Charytan D.M., Himmelfarb J., Ikizler T.A. Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney Int. 2019;95:973–982. doi: 10.1016/j.kint.2018.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammer F., Malzahn U., Donhauser J. A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney Int. 2019;95:983–991. doi: 10.1016/j.kint.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa T., Nishiwaki H., Ota E., Levack W.M., Noma H. Aldosterone antagonists for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev. 2021;2:CD013109. doi: 10.1002/14651858.CD013109.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgianos P.I., Vaios V., Eleftheriadis T., Zebekakis P., Liakopoulos V. Mineralocorticoid antagonists in ESRD: an overview of clinical trial evidence. Curr Vasc Pharmacol. 2017;15:599–606. doi: 10.2174/1570161115666170201113817. [DOI] [PubMed] [Google Scholar]
- 34.Kolkhof P., Barfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234:T125–T140. doi: 10.1530/JOE-16-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amazit L., Le B.F., Kolkhof P. finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem. 2015;290:21876–21889. doi: 10.1074/jbc.M115.657957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolkhof P., Borden S.A. Molecular pharmacology of the mineralocorticoid receptor: prospects for novel therapeutics. Mol Cell Endocrinol. 2012;350:310–317. doi: 10.1016/j.mce.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Kolkhof P., Delbeck M., Kretschmer A. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64:69–78. doi: 10.1097/FJC.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 38.Weinberger M.H., Roniker B., Krause S.L., Weiss R.J. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002;15:709–716. doi: 10.1016/s0895-7061(02)02957-6. [DOI] [PubMed] [Google Scholar]
- 39.Platt D., Pauli H. Studies on organ- and subcellular distribution of 3 H-spironolactone in animals. Arzneimittelforschung. 1972;22:1801–1802. [PubMed] [Google Scholar]
- 40.Gerisch M., Heinig R., Engelen A. Biotransformation of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist, in dogs, rats, and humans, in vivo and in vitro. Drug Metab Dispos. 2018;46:1546–1555. doi: 10.1124/dmd.118.083337. [DOI] [PubMed] [Google Scholar]
- 41.Snelder N., Heinig R., Drenth H.J. Population pharmacokinetic and exposure-response analysis of finerenone: insights based on phase IIb data and simulations to support dose selection for pivotal trials in type 2 diabetes with chronic kidney disease. Clin Pharmacokinet. 2020;59:359–370. doi: 10.1007/s40262-019-00820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heinig R., Gerisch M., Engelen A., Nagelschmitz J., Loewen S. Pharmacokinetics of the novel, selective, non-steroidal mineralocorticoid receptor antagonist finerenone in healthy volunteers: results from an absolute bioavailability study and drug-drug interaction studies in vitro and in vivo. Eur J Drug Metab Pharmacokinet. 2018;43:715–727. doi: 10.1007/s13318-018-0483-9. [DOI] [PubMed] [Google Scholar]
- 43.Heinig R., Lambelet M., Nagelschmitz J., Alatrach A., Halabi A. Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94-8862) in individuals with mild or moderate hepatic impairment. Eur J Drug Metab Pharmacokinet. 2019;44:619–628. doi: 10.1007/s13318-019-00547-x. [DOI] [PubMed] [Google Scholar]
- 44.Gardiner P., Schrode K., Quinlan D. Spironolactone metabolism: steady-state serum levels of the sulfur-containing metabolites. J Clin Pharmacol. 1989;29:342–347. doi: 10.1002/j.1552-4604.1989.tb03339.x. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal R., Rossignol P., Garza D. Patiromer to enable spironolactone use in the treatment of patients with resistant hypertension and chronic kidney disease: rationale and design of the AMBER study. Am J Nephrol. 2018;48:172–180. doi: 10.1159/000492622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grune J., Benz V., Brix S. Steroidal and nonsteroidal mineralocorticoid receptor antagonists cause differential cardiac gene expression in pressure overload-induced cardiac hypertrophy. J Cardiovasc Pharmacol. 2016;67:402–411. doi: 10.1097/FJC.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 47.Gueret A., Harouki N., Favre J. Vascular smooth muscle mineralocorticoid receptor contributes to coronary and left ventricular dysfunction after myocardial infarction. Hypertension. 2016;67:717–723. doi: 10.1161/HYPERTENSIONAHA.115.06709. [DOI] [PubMed] [Google Scholar]
- 48.Lavall D., Jacobs N., Mahfoud F., Kolkhof P., Bohm M., Laufs U. The non-steroidal mineralocorticoid receptor antagonist finerenone prevents cardiac fibrotic remodeling. Biochem Pharmacol. 2019;168:173–183. doi: 10.1016/j.bcp.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Martinez E., Buonafine M., Boukhalfa I. Aldosterone target NGAL (neutrophil gelatinase-associated lipocalin) is involved in cardiac remodeling after myocardial infarction through NFkappaB pathway. Hypertension. 2017;70:1148–1156. doi: 10.1161/HYPERTENSIONAHA.117.09791. [DOI] [PubMed] [Google Scholar]
- 50.Orena S., Maurer T.S., She L. PF-03882845, a non-steroidal mineralocorticoid receptor antagonist, prevents renal injury with reduced risk of hyperkalemia in an animal model of nephropathy. Front Pharmacol. 2013;4:115. doi: 10.3389/fphar.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma F.Y., Han Y., Nikolic-Paterson D.J., Kolkhof P., Tesch G.H. Suppression of rapidly progressive mouse glomerulonephritis with the non-steroidal mineralocorticoid receptor antagonist BR-4628. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruilope L.M., Agarwal R., Anker S.D. Design and baseline characteristics of the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am J Nephrol. 2019;50:345–356. doi: 10.1159/000503712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen C.P., Chang C.H., Tsai M.K. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int. 2017;92:388–396. doi: 10.1016/j.kint.2017.01.030. [DOI] [PubMed] [Google Scholar]