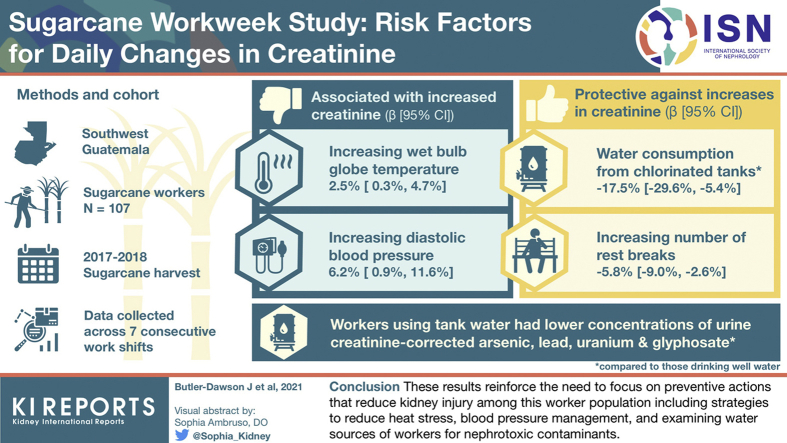
Abstract
Introduction
Agricultural workers laboring in thermally stressful environments are at increased risk for kidney injury and chronic kidney disease of unknown origin (CKDu), and their environmental and occupational exposures have been considered to be important risk factors. This study examined the effects of repeated kidney stress from the simultaneous strain of work and other factors experienced by workers in Guatemala during a typical workweek.
Methods
We collected data from 107 sugarcane workers across 7 consecutive work shifts. Data included information on daily occupational, meteorological, environmental, and lifestyle factors. We used multivariable linear mixed models to evaluate associations of these factors with percent change in creatinine.
Results
We observed that increasing wet bulb globe temperature (β = 2.5%, 95% confidence interval [CI] = 0.3%, 4.7%) and increasing diastolic blood pressure (β = 6.2%, 95% CI = 0.9%, 11.6%) were associated with increases in creatinine across the shift, whereas consumption of water from chlorinated dormitory tanks as compared to artesian well water (β = −17.5%, 95% CI = −29.6%, −5.4%) and increasing number of rest breaks (β = −5.8%, 95% CI = −9.0%, −2.6%) were found to be protective against increases in creatinine. Workers reporting drinking tank water had lower concentrations of urine creatinine−corrected arsenic, lead, uranium, and glyphosate compared to workers reporting the use of well water or municipal water.
Conclusion
These results reinforce the need to focus on preventive actions that reduce kidney injury among this worker population, including strategies to reduce heat stress, managing blood pressure, and examining water sources of workers for nephrotoxic contaminants.
Keywords: agriculture, heat stress, kidney injury, worker health
Graphical abstract
An epidemic of chronic kidney disease continues to affect agricultural communities and workers, mainly in hot regions around the globe, including Latin America, Sri Lanka, India, and Egypt.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 The etiology of the epidemic, coined “chronic kidney disease of unknown origin” (CKDu), remains unidentified, as it is not linked to traditional risk factors such as hypertension and diabetes.1
Recurrent episodes of kidney injury may cumulatively impair kidney function and contribute to chronic renal decline and CKDu.1,17,18 A high burden of acute kidney injury has been reported among agricultural workers laboring in hot climates.1,19, 20, 21, 22 Agricultural workers, specifically sugarcane workers, are exposed to conditions that precipitate kidney injury, including inadequate fluid intake leading to volume depletion, exercise- or heat-induced muscle damage, and the use of medications such as nonsteroidal anti-inflammatory drugs (NSAIDs).1,23,24 During intense labor and exposure to heat stress, renal blood flow decreases, which can contribute to increased stress on the kidneys.25 Although heat stress and dehydration most likely play a role, interventions that improve hydration, rest, and shade have provided only partial relief.19,26,27 Sugarcane workers, as well as other populations at risk for CKDu, might concurrently be exposed to other nephrotoxins, including environmental pollutants, in conjunction with heat stress and/or dehydration.1,28 The combined effects of these stressors and exposures may compromise kidney function and increase risk of daily kidney injury during an entire harvest season.
Prior work from our team demonstrated a high incidence of acute kidney injury among sugarcane workers in Guatemala, and higher urinary specific gravity, lower electrolyte solution intake, and NSAID use were observed risk factors for injury.19 In another study on sugarcane workers in Guatemala (the same worker population as in this current study), we identified a subgroup of workers who experienced severe fluctuations in creatinine across repeated work shifts.18 This subgroup of workers experienced a greater reduction in kidney function across the 6-month harvest season. These data provide evidence that cross-shift changes in creatinine, an acute form of injury that has traditionally been thought of as transient, may contribute to observed cross-season declines in kidney function.
Our goal with this study was to investigate the factors that influence the severity of cross-shift creatinine changes during 7 consecutive work shifts among sugarcane workers. An additional goal was to explore workers’ environmental exposures during their work shifts. It is the first known study to investigate how occupational, environmental, and lifestyle risk factors relate to day-to-day variation in markers of kidney function in a population at-risk for CKDu. Through this study, we hope to elucidate the role of multifactorial risk factors contributing to recurrent kidney injury at a time when the injury might be reversible, thus potentially preventing the development of CKDu.
Materials and Methods
Study Population
The study was conducted during the 2017 to 2018 sugarcane harvest. All eligible study participants were male, ≥18 years of age, sugarcane cutters, and employed by a large agribusiness in southwestern Guatemala. Cutters harvest the sugarcane manually with a machete. Participants provided informed consent in November 2017, the start of the 6-month harvest, at which time participants also completed the pre-employment hiring eligibility process including assessment of kidney function. The company enforces an early season acclimatization period, during which workers labor fewer hours and cut less sugarcane, typically lasting the first 2 weeks of the harvest in November. The participants comprised of male sugarcane cutters from 2 work groups using stratified random sampling stratified by residence (local residents vs. highland residents). Workers in the local work group lived in the communities adjacent to the sugarcane fields (i.e., Santa Barbara and Escuintla) and commuted daily to work. Workers in the highland group were from the mountainous regions of Guatemala (i.e., San Miguel Uspantan and Cubulco) and lived in dormitories at the sugarcane plantation for the entire 6-month season. The work setting has previously been described.19 The study was approved by the Colorado Multiple Institutional Review Board (COMIRB) and the Comité de Ética Independiente ZUGUEME in Guatemala.
Data Collection
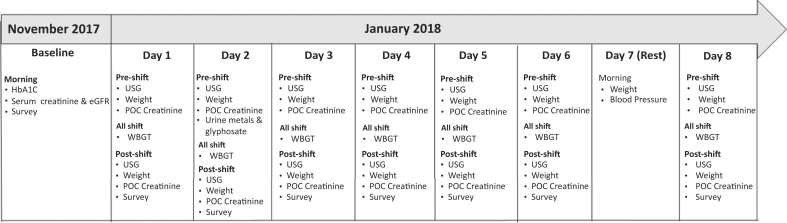
Clinical, biomarker, and survey data were collected from study participants at the start of the season (November) and the middle of the harvest (January) (Figure 1). In November, venous blood samples were collected for baseline measurements of hemoglobin A1c (HbA1c) and serum creatinine. In January, the work groups were followed for 16 consecutive days of data collection: 8 consecutive days for the highland group and the subsequent 8 days for the local group. Data were collected before and after work shifts on 6 uninterrupted days coinciding with the 6-day work week (days 1−6) and the first day of next 6-day work week (day 8) after 1 rest day. We also collected clinical data in the morning of the rest day (day 7). For the study workdays (days 1−6 and 8), upon arrival to the field via bus, participants provided a urine sample and a fingerprick blood sample, and were weighed prior to the start of work shift. We measured urine specific gravity (USG) immediately in the field using a digital refractometer (ATAGO PAL-10S digital refractometer, Tokyo, Japan). Bodyweight was measured using a digital scale (Seca 874 DR, Seca Corporation, Chino, CA) that was calibrated prior to each data collection session. Workers were weighed in work clothing only (pants and a long-sleeved shirt); shin guards and boots were removed. Participants repeated the process immediately after finishing the work shift, along with an interviewer-administered survey. The survey inquired about the study participant’s behaviors in the past 24 hours, as previously described.19 On the workers’ rest day (day 7), clinical data were collected in the morning, including blood pressure and weight. Blood pressure was collected after 3 minutes of sitting.
Figure 1.
Summary of data collection over the 2017 to 2018 sugarcane harvest used in the present study. BP, blood pressure; eGFR, estimated glomerular filtration rate; POC Creatinine, point-of-care capillary creatinine; USG, urinary specific gravity; WBGT, Wet Bulb Globe Temperature.
Kidney Function
In November, serum creatinine was collected by venipuncture and sent to an independent, licensed clinical laboratory (Herrera Llerandi laboratory, Guatemala City, Guatemala). Creatinine values were performed in duplicate using the Creatinine Jaffe Generation 2 method. Serum creatinine values were used to calculate the estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for all participants, setting race to “non-Black.”29
The primary outcome of this study was the daily magnitude of change in creatinine, measured by calculating the percent change in creatinine from pre-shift to post-shift for each study workday (days 1−6 and 8) in January ([post-shift creatinine – pre-shift creatinine]/pre-shift creatinine × 100). Capillary blood was collected by finger prick, and creatinine was read instantly in the field using a point-of-care (POC) meter (Nova Statscan, Nova Biomedical Corporation, Waltham, MA). We applied a correction factor of 0.7775 to all the post-shift capillary POC creatinine values based on previous comparisons between venous and capillary samples.30 In other analyses, among similar cohorts, we have observed good agreement between venous and capillary samples of pre-shift creatinine measurements, thus justifying an adjustment factor of 1 (i.e., no adjustment) to relate pre-shift capillary creatinine values to venous creatinine values.31
Fluid Intake
Hydration status was assessed using 3 indicators: pre- and post-shift USG, percent change in body weight from pre-shift to post-shift, and self-reported electrolyte packet use. The workers were distributed electrolyte powder packets that contained 2.6 g NaCl, 2 g KCl, 13.5 g carbohydrates (glucose), and 40 kcal per liter. Workers were encouraged to mix packets with 3 liters of water. Self-reported free water intake and mixed electrolyte solution intake during the work shift were also included in the questionnaire. Because workers used the same 5-L container for both free water and electrolyte solution, we found that these amounts were difficult to differentiate using the questionnaire responses (Supplementary Table S1). Because of this, we decided to include only the 3 hydration indicators listed above.
We also assessed self-reported sugar-sweetened beverage intake during the day and the number of alcoholic beverages consumed the previous night. We independently asked about the amount of juice, energy drinks, soda, and “atol” consumed during the day. Atol is a traditional drink that is served warm in Central America and is often made with corn, plantain, or another starch, and sugar. We combined these variables to create a new variable, “number of sugar-sweetened beverages.”
Work Intensity and Heat Exposure
We evaluated work intensity by collecting information on the number of rest breaks taken during a shift, working the day prior, and the standardized number of tons of sugarcane that an individual cut during the study day work shift relative to an individual’s daily average tons cut during the entire season. We examined standardized productivity rather than raw tonnage to account for individual variability in production rates.
The average and maximum wet bulb globe temperature (WBGT) measurements were collected for each study workday from 2 local weather stations within 6 km of the fields, 1 weather station for the local work group fields, and 1 station for the highland group fields (see map, Supplementary Figure S1). We intended to use field WBGT measures; however, measures during the work shift were missing for 5 of the study days; therefore, weather station measures were used for this analysis. We examined correlations between study days with complete field measures and station measures (n = 11 study days).
Lifestyle Factors and Drinking Water Source
The survey included several questions on lifestyle factors and was administered on each of the 7 workdays (days 1−6 and 8). We asked about the number of cigarettes smoked since waking up and use of oral and parenteral medications, vitamins, and supplements in the last 24 hours. Similar to a previous published study,19 we showed participants pictures of locally available medications, vitamins, and supplements as visual prompts and identified NSAIDs. We also asked participants their source of drinking water used to fill up their 5-L container before coming to work. Responses included their residence, a dormitory tank, or a mobile field tank. For workers reporting “residence,” we further asked if the source was an artesian well in or near their home or a public distribution system (municipal water source). The artesian wells found in the homes were observed to be generally of low depth and not chlorinated. Municipal water is treated; however, the processes vary across systems and can range from rainwater catchment to treated surface or ground water. The water in the dormitory and mobile field tanks was chlorinated and from a deep well. This question was used to explore differences in drinking water sources, as water used for consumption is thought to be a major source of contaminants that are known nephrotoxicants.32
Urinary Biomarkers of Exposure
To assess potential environmental exposures, we measured several metals, glyphosate, and cotinine concentrations with the urinary samples collected on the second day of the 6-day work week (day 2 only). Metals that were measured included cadmium, arsenic, nickel, lead, and uranium. We compared levels in this study with those measured in a representative national sample of Mexican Americans participating in the U.S. National Health and Nutrition Examination Survey (NHANES).33
Spot morning urine samples were collected and transported on ice to an on-site clinic, where the urine was aliquoted into Fisherbrand sterile polypropylene tubes without preservatives and was frozen at −20°C. The urine aliquots for metal analyses were shipped to the Columbia University (Trace Metals Core Laboratory), and the urine aliquot for glyphosate was sent to Colorado State University (Center for Environmental Medicine Analytical Laboratory). Urine samples were analyzed for metals using a PerkinElmer NexION 350S Inductively Coupled Plasma Mass Spectrometry. The Inductively Coupled Plasma Mass Spectrometry with dynamic reaction cell method for metals in urine was developed according to published procedures,34,35 the Centers for Disease Control and Prevention (CDC) method,36 and with modifications suggested by the PerkinElmer application laboratory. Levels below the limit of detection were entered as the limit of detection divided by the square root of 2 for each biomarker.37 Urinary metal and glyphosate concentrations were adjusted for urinary creatinine to take into consideration urine concentration.38
Statistical Analysis
To assess the association between daily percent change in POC creatinine and daily characteristics, we used linear mixed models with random intercept for individual to account for the repeated measurements in our data. We ran univariate models for each of the characteristics described above. We then ran a multivariable model that included each of the covariates from the univariate analysis that had a P value of <0.05. Given the high correlation between water source and home of residence, we ran the final model with the inclusion of water source and not home of residence. This decision was made because of previous findings observing kidney function differences between local workers and highland workers, with highland workers having better kidney function,22,39,40 and because of the hypothesis that water source may play a role in the observed differences between home of residence.
We did not include urinary biomarkers of exposure in the regression models, as they were measured only on day 2 of the study. With the urinary biomarkers of exposure, first the Spearman correlation coefficients were calculated to compare correlations between the exposure concentrations. Second, the relationships between water source and urine biomarkers of exposure were examined using analysis of variance. Third, the relationships between urinary metal concentrations and mild hypertension were examined using analysis of variance based on the literature reporting that toxic metals such as cadmium, arsenic, and lead can have cardiovascular effects.41, 42, 43 Mild hypertension was defined as systolic blood pressure between 130 and 139 mm Hg and/or diastolic blood pressure between 80 and 89 mm Hg.44 All analyses were done in R version 3.4.345 using the “lme4” package.46
Results
Characteristics of the Study Population
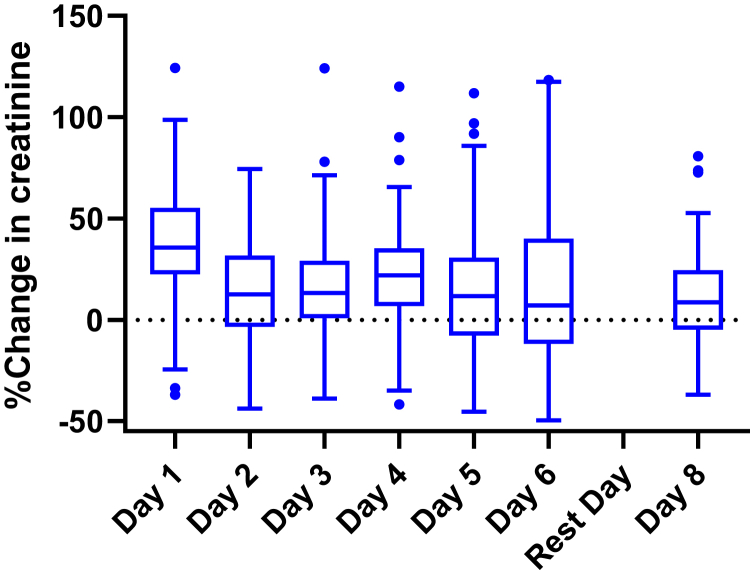
There were 107 male sugarcane cutters who were present at least 1 of the 7 study workdays in January. An overview of baseline characteristics is presented in Table 1. As shown in Figure 2 and Supplementary Table S1, we observed daily increases in creatinine on all 7 workdays, ranging from an average of 11% to 38% increase from pre- to post-shift. Supplementary Table S1 presents the participant characteristics across the 7 workdays (days 1−6 and 8). Workers reported taking an average of 3 rest breaks during the work shift. A low percentage of workers (<1%) reported smoking cigarettes and taking NSAIDs (2%−13%), with 13% on day 5. Slightly more than one-half of the workers (52%−57%) reported using dormitory tanks to fill their 5-L container before heading to the field (96% were highland workers), whereas one-third reported using a municipal drinking water source (30%−35%) and the rest reported using well water (10%−13%).
Table 1.
Baseline characteristics for study participants, collected in November 2017
| Characteristics | Overall (N = 107, 100%) |
Local (n = 52, 49%) |
Highland (n = 55, 51%) |
|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Age, yr | 28 (9) | 29 (10) | 26 (8) |
| HbA1c, % | 5.4 (0.3) | 5.4 (0.3) | 5.4 (0.3) |
| Systolic blood pressure, mm Hga | 114 (18) | 120 (15) | 108 (15) |
| Diastolic blood pressure, mm Hga | 68 (11) | 69 (10) | 63 (11) |
| BMI, kg/m2a | 22.2 (2.2) | 21.9 (2.1) | 22.4 (2.3) |
| Pre-harvest eGFR, ml/min per 1.73 m2 | 118 (21) | 115 (19) | 118 (18) |
| Number of previous harvests worked | 7 (6) | 8 (8) | 6 (7) |
| N (%) | N (%) | N (%) | |
|---|---|---|---|
| Self-reported current smoker | 26 (28%) | 19 (42%) | 7 (14%) |
BMI, body mass index; eGFR, estimated glomerular filtration rate.
Collected on workers’ rest day (day 7) in January.
Figure 2.
Distribution of percent change in creatinine across study workdays. A positive percent change indicates a decline in kidney function from pre- to post-shift (N = 107 sugarcane cutters).
Daily average WBGT measures ranged from 19°C to 25°C, and maximum WBGT measures ranged from 21°C to 28°C. We observed a significant correlation (r = 0.62) between weather station measures (n = 2 stations) and field measures (n = 14 fields) on the days with complete measures for both (n = 11 days).
Factors Associated With Daily Percent Change in Creatinine
Table 2 and Table 3 present the factors associated with daily percent change in creatinine in the univariate and multivariable models. In the multivariable models, while controlling for age and pre-shift creatinine, we observed that increasing average WBGT (β = 2.5%, 95% confidence interval [CI] = 0.3%, 4.7%) and increasing diastolic blood pressure (β = 6.2%, 95% CI = 0.9%, 11.6%) were associated with increases in creatinine across the shift. The association between increasing post-shift USG and increases in creatinine approached significance (β = 2.8%, 95% CI = −0.3%, 6.0%). Consumption of dormitory tank water as compared to well water (β = −17.5%, 95% CI = −29.6%, −5.4%) and increasing the number of rest breaks (β = −5.8%, 95% CI = −9.0%, −2.6%) were found to be protective against cross-shift increases in creatinine.
Table 2.
Univariate regression analysis of factors with percent change in creatinine from pre- to post-shift over 7 workdays
| Characteristic | Estimate (95% CI), %a | P value |
|---|---|---|
| Demographics | ||
| Age (per yr) | 0.45 (0.04, 0.85) | 0.03b |
| Local home of residence (ref: Highland) | 21.61 (17.39, 25.84) | <0.01b |
| Systolic blood pressure (per 10 mm Hg)c | 4.03 (1.5, 6.55) | <0.01b |
| Diastolic blood pressure (per 10 mm Hg)c | 5.79 (2.61, 8.96) | <0.01b |
| Body mass index (per kg/m2)c | –0.55 (–2.1, 1.0) | 0.49 |
| Pre-shift creatinine (per 0.1 mg/dl) | –11.46 (–12.64, –10.22) | <0.01b |
| Hydration and fluid intake | ||
| Weight change across shift (per 1%) | 0.11 (–0.91, 1.12) | 0.84 |
| Pre-shift urine specific gravity (per 0.01) | –2.99 (–7.05, 1.03) | 0.13 |
| Post-shift urine specific gravity (per 0.01) | 5.54 (1.84, 9.25) | <0.01b |
| Number of electrolyte packets (per 1 packet) | 11.87 (7.90, 15.78) | <0.01b |
| Number of sugar-sweetened beverages (per 1 beverage) | 0.83 (–0.17, 1.83) | 0.09 |
| Work intensity and heat exposure | ||
| Number of rest breaks (per 1 break) | –8.67 (–12.54, –4.8) | <0.01b |
| Worked the prior day (ref: no) | –8.98 (–13.58, –4.38) | <0.01b |
| Standardized amount of sugarcane harvested on study day | –0.28 (–2.64, 2.09) | 0.81 |
| Average Wet Bulb Globe Temperature (per 1°C) | 4.45 (3.38, 5.50) | <0.01b |
| Maximum wet bulb globe temperature (per 1°C) | 3.30 (2.34, 4.23) | <0.01b |
| Lifestyle factors | ||
| Current smoker (ref: former/never smoker)d | 5.23 (–1.75, 12.21) | 0.15 |
| Number of cigarettes smoked (per 1 cigarette) | 21.3 (–3.86, 46.57) | 0.10 |
| NSAID usee (ref: no) | 3.23 (–6.5, 13.05) | 0.51 |
| Drinking water source for first 5-L water container fill-up | ||
| Municipal (ref: well water) | 5.76 (–1.45, 12.99) | 0.12 |
| Dormitory tank (ref: well water) | –16.95 (–23.79, –10.08) | <0.01b |
NSAID, nonsteroidal anti-inflammatory drug; ref, reference.
Coefficient is expressed as a percentage (100 × β estimate). Interpreted as a percent change in creatinine from pre- to post-shift.
Indicates significance at a P value of <0.05.
Collected only on the worker’s rest day (day 7).
Collected at baseline.
Types of NSAIDs included ibuprofen, aspirin, diclofenac, naproxen, or local brands that were identified as NSAIDs.
Table 3.
Multivariable associations between factors and percent change in creatinine from pre- to post-shift over 7 workdays
| Characteristic | Estimate (95% CI), %a | P value |
|---|---|---|
| Drinking water source | ||
| Municipal (ref: well water) | 0.16 (–8.42, 8.66) | 0.97 |
| Dormitory tanks (ref: well water) | –17.47(–29.6, –5.39) | <0.01b |
| Average WBGT (per 1°C) | 2.54 (0.28, 4.70) | 0.02b |
| Number of rest breaks | –5.84 (–9.04, –2.57) | <0.01b |
| Diastolic blood pressure (per 10 mmHg)c | 6.23 (0.87, 11.58) | 0.03b |
| Systolic blood pressure (per 10 mmHg)c | –3.31 (–7.55, 0.95) | 0.14 |
| Post-shift specific gravity (per 0.01) | 2.78 (–0.34, 5.97) | 0.09 |
| Worked the prior day (ref: no) | –3.47 (–7.53, 0.47) | 0.09 |
| Number of electrolyte packets | 1.61 (–2.62, 5.89) | 0.46 |
ref, reference; WBGT, wet bulb globe temperature.
Model controled for pre-shift creatinine and age.
Coefficient is expressed as a percentage (100 × β estimate). Interpreted as a percent change in creatinine from pre- to post-shift.
Indicates significance at a P value of <0.05.
Collected only on the worker’s rest day (Day 7).
Biomarkers of Exposure
The urinary biomarkers of exposure are shown in Table 4.47, 48, 49, 50, 51, 52 Cadmium, arsenic, and nickel were detected in more than 90% of the urine samples and lead was detected in 78% of the samples. In this study population, 23% of the urine samples were above the 90th percentile level for total arsenic among the Mexican Americans participating in NHANES, 30% of the samples were above the 90th percentile level for lead, and 27% of the samples for uranium. We observed weak to strong positive correlations between the metals and glyphosate (Supplementary Table S2), with the strongest correlations between uranium and glyphosate (r = 0.73) and lead and glyphosate (r = 0.52). Urinary cotinine was weakly correlated with lead (r = 0.22, P = 0.04) and was not correlated with urinary cadmium or arsenic (r = 0.12, P = 0.29 and r = 0.14, P = 0.21, respectively).
Table 4.
Urine biomarkers of exposure, measured on day 2 only, with NHANES 90th percentile levels presented for comparison
| Urine exposures | n | LODa | % Detected | Mean (SD) | NHANES, 90th percentile (95% CI) | Number (%) above NHANES 90th percentile |
|---|---|---|---|---|---|---|
| Cadmium (μg/g creatinine) | 82 | 0.01 | 77 (94%) | 0.16 (0.07) | 0.39 (0.33, 0.42) | 0 |
| Arsenic (μg/g creatinine) | 82 | 0.07 | 82 (100%) | 14.83 (13.19) | 16.9 (13.8, 20.2) | 19 (23%) |
| Nickel (μg/g creatinine) | 82 | 0.04 | 82 (100%) | 3.04 (1.29) | N/Ab | — |
| Lead (μg/g creatinine) | 82 | 0.10 | 64 (78%) | 0.74 (0.74) | 0.87 (0.74, 1.02) | 25 (30%) |
| Uranium (μg/g creatinine) | 82 | 0.01 | 11 (34%) | 0.02 (0.02) | 0.018 (0.013, 0.028) | 22 (27%) |
| Glyphosate (ng/g creatinine) | 82 | 0.075 | 38 (48%) | 2.32 (3.35) | N/Ab | — |
| Cotinine (ng/ml) | 96 | 5.0 | 34 (42%) | 343 (1057.5) | 50c | 25 (26%) |
| Urine creatinine (mg/dl) | 97 | — | 100% | 84.27 (55.40) | N/Ab | — |
CI, confidence interval; LOD, limit of detection; N/A, not available; NHANES, National Health and Nutrition Examination Survey.
Percentage of frequency of detection (%) for each compound measured above the LOD. Levels <LOD are imputed with LOD/sqrt (2).
NHANES biomonitoring data on nickel, glyphosate, cotinine, and creatinine are not available.
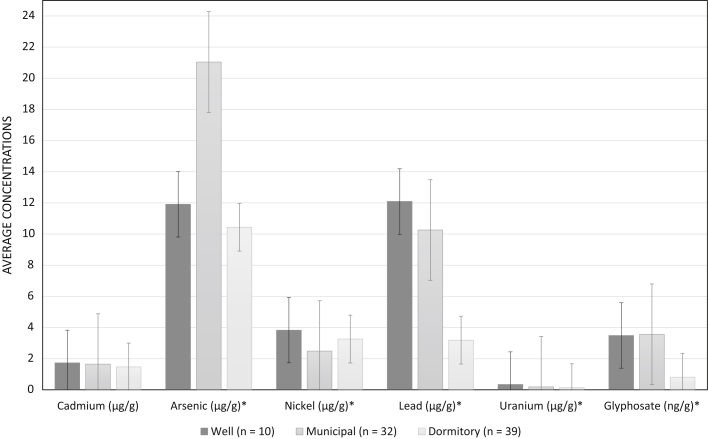
We examined whether urinary concentrations of metals and glyphosate were different between water sources (Supplementary Table S3). As shown in Figure 3, workers reporting using dormitory tanks had lower concentrations of urine creatinine−corrected arsenic, lead, uranium, and glyphosate compared to workers reporting the use of well or municipal water. Participants with mild hypertension had higher but nonsignificant urinary metal concentrations of arsenic, cadmium, uranium, and lead compared to participants with no mild hypertension. In a post hoc analysis, we examined the relationship between baseline eGFR and water source, and found that workers who used well water as their drinking water source had significantly lower eGFR (106 ml/min per 1.73 m2) compared to those who used municipal water (eGFR: 112 ml/min per 1.73 m2) and dormitory water (119 ml/min per 1.73 m2) (Supplementary Table S4).
Figure 3.
Day 2 urinary metals and glyphosate concentrations (corrected for urine creatinine) by morning drinking water source. ∗Significance at p < 0.05 based on analysis of variance.
Discussion
We examined the impact of occupational, meteorological, environmental, and lifestyle factors on daily, recurrent cross-shift changes in creatinine. We observed 3 important findings. First, we found that heat exposure contributes to increases in creatinine across the work shift as well as protective factors in which individual workers can protect themselves under hot conditions. Second, we observed that workers under these conditions who consumed artesian well water experienced more severe increases in creatinine across the work shift. Third, this study supports earlier reports that workers who have even mild hypertension, when exposed to these work conditions, are at greater risk for greater increases in creatinine.22 Importantly we have demonstrated in a separate analysis that among these same workers, those who experience the greatest cross-shift changes in creatinine are at greater risk for cross-season declines in kidney function.18 These cross-shift and cross-season declines in markers of kidney function may indicate early damage that will lead to the development of CKDu. These findings reinforce the need to focus on preventive actions that reduce acute kidney injury. Preventive strategies should include not only promoting hydration and rest among these workers but also addressing personal risk factors such as hypertension and examining potential nephrotoxic contaminants of workers’ drinking water sources.
Our study provides evidence that increases in creatinine, a marker of kidney injury, are occurring repetitively over 7 consecutive work shifts. We have every reason to believe that this recurrent injury is commonly occurring throughout the rest of the 6-month harvest. Although kidney stress induced during the work shift may seem transient considering that the average pre-shift creatinine remained stable, Dally et al. (2020) used data from this same study population and found that participants who experienced repeated severe fluctuations in creatinine across the work shift (n = 30) had higher daily pre-shift creatinine values and experienced a greater reduction in eGFR across the season compared to workers with moderate fluctuations (n = 73).18 Other previous findings show that leukocyturia is commonly detected in sugarcane workers’ urine, along with evidence of reduced blood flow, suggesting that such workers experience a degree of acute interstitial nephritis that is not solely transient kidney stress.28 The reduction of renal blood flow can cause a transient kidney injury, which may resolve; however, when linked with heat stress, it may lead to more severe kidney damage and manifest as acute fluctuations in creatinine. We did not observe associations with work intensity (i.e., productivity), and in the past we have not observed a relationship between creatine kinase, a marker of muscle damage, and increases in creatinine.28 Thus, we do not believe the observed cross-shift increases in creatinine are solely due to muscle damage.
As temperatures rise, it is necessary to understand the roles of heat stress, rest, and adequate fluid intake in the mitigation of renal stress. We observed that heat exposure plays a role in the daily increase in creatinine. Studies have shown biochemical evidence of heat-induced kidney injury.23,24,27, 59 which can be induced with increases in serum and urine uric acid and extracellular volume depletion.53 In addition, rest breaks were found to be protective against severe increases in creatinine and a nonsignificant trend that increasing USG was associated with increases in creatinine. These findings are consistent with our previous research as well as others’ and reinforce the protective value of rest and hydration.19,26,54 We have previously reported that supplemental electrolytes had a protective effect.19 In the interval between the previous study and present study, the employer changed its electrolyte practices right before the start of the present study, which seems to have resulted in higher electrolyte consumption by workers in this study. In the current study, we found no association between electrolyte consumption and cross-shift increase in creatinine, possibly because the higher level of use has already maximized the benefit provided by supplementation.
The present study showed a possible connection between drinking water source and daily change in creatinine as well as baseline kidney function. Workers who reported using artesian well water for their first 5-L container fill-up had greater increases in creatinine across the work shift compared to workers who reported using water from the dormitories. Workers who reported using artesian well water or municipal water most likely have the same drinking water source year-around, since almost all of them were local residents. Workers reporting using dormitory tank water used this water source only for 6 months during the harvest, as almost all of these workers were highland migrant workers. In addition, we determined that workers who reported using well water had higher urinary levels of all metals and glyphosate compared to workers who reported using chlorinated water from the dormitory tank. The metals could be coming from natural occurrence (soil and volcanic) and anthropogenic activities (industry and agricultural),55 and glyphosate is one of the most widely used herbicides in the endemic areas of CKDu. Exposures may also be coming from contaminants in the air, soil, and ash due to sugarcane burning practices.56 Interestingly, a previous study conducted within a population of first-year sugarcane workers in the same region observed that workers who reported using a well water source at home had 6.1 times the odds of being in the subgroup that had lower kidney function at the start of their first year and had greater declines in kidney function over the harvest compared to all other sources of home water.22 A few other studies have observed renal impairment among agricultural workers who are chronically exposed to glyphosate and paraquat.57,58 Daily reductions in blood flow during intense labor in the heat could cause ischemic damage and nephrotoxicity in the renal tubules and may leave the tubules vulnerable to other nephrotoxicants. It has also been suggested that glyphosate-metal complexes could form in the water and could lead to oxidative stress and necrosis of the renal tissue.57
Higher diastolic blood pressure was also associated with daily increases in creatinine. Although stage 2 hypertension has not been linked with CKDu, compared to traditional CKD, mildly elevated blood pressure has been shown to be a clinical predictor of declines in kidney function.22 In addition, several studies have reported significant associations between chronic arsenic exposure and increased systolic and diastolic blood pressure60; however, this relationship and the mechanism of arsenic on blood pressure remains unclear. It is important to recognize that even mild hypertension may be an added risk factor or contributor to kidney decline in this setting.
Despite important prevention and research implications, this study has some limitations. First, this is a repeated cross-sectional study, and causality cannot be ensured. Second, there may be intra-individual variability with creatinine due to factors such as protein intake, fluid intake, and exercise.61 However, we used serial measurements of the outcome across 7 workdays to help address this issue. Third, reductions in renal blood flow with exercise could lead to elevations in creatinine, which may be solely transient increases. In addition, all of the temperature data were acquired from meteorological stations. Although these stations were within 6 km of the study fields and WBGT measures were correlated with field measures, it would have been more accurate to measure field WBGT or core temperature at an individual level. Some of the risk factors were self-reported, including smoking status, rest break, NSAID use, and electrolyte intake. In a preliminary analysis, smoking status has been shown to be underreported based on urinary cotinine concentrations (data not shown). Use of NSAIDs may be underreported, as the agribusiness has discouraged their use. It is possible that that kidney function for our study population is better than that in individuals not included in our study based on the healthy worker effect; the most ill workers may have dropped out of the workforce or were not hired due to poor kidney function. In addition, there is a possibility that the difference in POC correction factors between pre- and post-shift creatinine measurements may be due to random variation; however, correction factors have been validated in multiple cohorts across several years. We do not know exactly why we are observing a difference in POC correction factors, although a likely mechanism of discrepancy is due to hemoconcentration following physical exertion, as the pre-shift POC capillary values would presumably not be affected. Of note, if the same correction factors were used across both shifts, the observed pre-to-post shift differences would have been even larger. Finally, information on water source rather than actual drinking water metal and glyphosate concentrations was used. Water source was highly correlated with residence, and the findings surrounding water source could be due to other unmeasured factors related to residence. Ideally, exposure data would have been measured multiple times, and we would have speciated arsenic. However, we believe that the total arsenic measurement is not a reflection of arsenic ingestion from consumption of seafood based on previous nutrition surveys in these communities.62
It has been observed that sugarcane cutters may be susceptible to daily, recurrent kidney injury during the harvest season. Based on these findings, preventive strategies designed to reduce the risk of kidney injury should be implemented among this worker population, as well as blood pressure monitoring and management. The results have important implications for worker protection and risk mitigation for agricultural populations who are laboring in hot climates.
Disclosure
The University of Colorado has a memorandum of agreement with Pantaleon, a Guatemala-based agribusiness. Pantaleon provides partial financial support for research through a contract with the university and has provided access to the employees who volunteered to participate in this research project. The University of Colorado employed appropriate research methods in keeping with academic freedom, based conclusions on critical analysis of the evidence and reported findings fully and objectively. The terms of this arrangement have been reviewed and approved by the University of Colorado in accordance with its conflict of interest policies.
Acknowledgments
We wish to thank all our collaborators including Stephen Brindley, Nicholas Smith, and all the workers who have made this work possible. This study was supported by Centers for Disease Control and Prevention (CDC) (U19 OH011227) and National Institutes of Health (NIH) (R21 ES028826), and in part by Pantaleon and the Chancellor, University of Colorado, CU Anschutz Campus. Funders had no role in data analysis, interpretation of data, writing the manuscript, or the decision to submit the findings for publication.
Footnotes
Figure S1. Map of study field locations by work group and date and location of weather stations.
Table S1. Distribution of markers of kidney function and daily risk factors; mean (SD) or n (%).
Table S2. Spearman correlation coefficients for biomarkers of exposure A (day 2 only).
Table S3. Urinary biomarkers of exposure distributions by reported water source for first 5-L water container fill-up.
Table S4. Baseline kidney function by reported drinking water source for first 5-L water container fill-up.
Supplementary Material
Figure S1. Map of study field locations by work group and date and location of weather stations.
Table S1. Distribution of markers of kidney function and daily risk factors; mean (SD) or n (%).
Table S2. Spearman correlation coefficients for biomarkers of exposure A (day 2 only).
Table S3. Urinary biomarkers of exposure distributions by reported water source for first 5-L water container fill-up.
Table S4. Baseline kidney function by reported drinking water source for first 5-L water container fill-up.
References
- 1.Johnson R.J., Wesseling C., Newman L.S. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med. 2019;380:1843–1852. doi: 10.1056/NEJMra1813869. [DOI] [PubMed] [Google Scholar]
- 2.Glaser J., Lemery J., Rajagopalan B. Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. Clin J Am Soc Nephrol. 2016;11:1472–1483. doi: 10.2215/CJN.13841215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Trabanino R., Jarquín E., Wesseling C. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador─a cross-shift study of workers at risk of Mesoamerican nephropathy. Envir Res. 2015;142:746–755. doi: 10.1016/j.envres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Herrera R., Orantes C.M., Almaguer M. Clinical characteristics of chronic kidney disease of nontraditional causes in Salvadoran farming communities. MEDICC Rev. 2014;16:39–48. doi: 10.37757/MR2014.V16.N2.7. [DOI] [PubMed] [Google Scholar]
- 5.Nanayakkara S., Komiya T., Ratnatunga N. Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Envir Health Prev Med. 2012;17:213. doi: 10.1007/s12199-011-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham G., Varughese S., Thandavan T. Chronic kidney disease hotspots in developing countries in South Asia. Clin Kidney J. 2016;9:135–141. doi: 10.1093/ckj/sfv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanigasuriya K. Update on uncertain etiology of chronic kidney disease in Sri Lanka’s north-central dry zone. MEDICC Rev. 2014;16:61–65. doi: 10.37757/MR2014.V16.N2.10. [DOI] [PubMed] [Google Scholar]
- 8.Gifford F.J., Gifford R.M., Eddleston M. Endemic nephropathy around the world. Kidney Int Rep. 2017;2:282–292. doi: 10.1016/j.ekir.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orantes C.M., Herrera R., Almaguer M. Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa Study, 2009. MEDICC Rev. 2011;13:14–22. doi: 10.37757/MR2011V13.N4.5. [DOI] [PubMed] [Google Scholar]
- 10.Orantes C.M., Herrera R., Almaguer M. Epidemiology of chronic kidney disease in adults of Salvadoran agricultural communities. MEDICC Rev. 2014;16:23–30. doi: 10.37757/MR2014.V16.N2.5. [DOI] [PubMed] [Google Scholar]
- 11.Raines N., Gonzalez M., Wyatt C. Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by Mesoamerican nephropathy. MEDICC Rev. 2014;16:16–22. doi: 10.37757/MR2014.V16.N2.4. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell J.K., Tobey M., Weiner D.E. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011;26:2798–2805. doi: 10.1093/ndt/gfq385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peraza S., Wesseling C., Aragon A. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis. 2012;59:531–540. doi: 10.1053/j.ajkd.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 14.Wesseling C., de Joode BvW, Crowe J. Mesoamerican nephropathy: geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup Envir Med. 2015;72:714–721. doi: 10.1136/oemed-2014-102799. [DOI] [PubMed] [Google Scholar]
- 15.Jayatilake N., Mendis S., Maheepala P. Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 2013:14. doi: 10.1186/1471-2369-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy D.V., Gunasekar A. Chronic kidney disease in two coastal districts of Andhra Pradesh, India: role of drinking water. Envir Geochem Health. 2013;35:439–454. doi: 10.1007/s10653-012-9506-7. [DOI] [PubMed] [Google Scholar]
- 17.Fischer R.S.B., Vangala C., Mandayam S. Clinical markers to predict progression from acute to chronic kidney disease in Mesoamerican nephropathy. Kidney Int. 2018;94:1205–1216. doi: 10.1016/j.kint.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dally M., Butler-Dawson J., Johnson R.J. Creatinine fluctuations forecast cross-harvest kidney function decline among sugarcane workers. Kidney Int Rep. 2020;5:1558–1566. doi: 10.1016/j.ekir.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler-Dawson J., Krisher L., Yoder H. Evaluation of heat stress and cumulative incidence of acute kidney injury in sugarcane workers in Guatemala. Int Arch Occup Envir Health. 2019;92:977–990. doi: 10.1007/s00420-019-01426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesseling C., Aragón A., González M. Kidney function in sugarcane cutters in Nicaragua─a longitudinal study of workers at risk of Mesoamerican nephropathy. Envir Res. 2016;147:125–132. doi: 10.1016/j.envres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Wesseling C., Aragon A., Gonzalez M. Heat stress, hydration and uric acid: a cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dally M., Butler-Dawson J., Cruz A. Longitudinal trends in renal function among first time sugarcane harvesters in Guatemala. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansson E., Glaser J., Jakobsson K. Pathophysiological mechanisms by which heat stress potentially induces kidney inflammation and chronic kidney disease in sugarcane workers. Nutrients. 2020;12 doi: 10.3390/nu12061639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roncal-Jimenez C., Lanaspa M.A., Jensen T. Mechanisms by which dehydration may lead to chronic kidney disease. Ann Nutr Metab. 2015;66:10–13. doi: 10.1159/000381239. [DOI] [PubMed] [Google Scholar]
- 25.Schlader Z.J., Hostler D., Parker M.D. The potential for renal injury elicited by physical work in the heat. Nutrients. 2019;11 doi: 10.3390/nu11092087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodin T., Garcia-Trabanino R., Weiss I. Intervention to reduce heat stress and improve efficiency among sugarcane workers in El Salvador: phase 1. Occup Envir Med. 2016;73:409–416. doi: 10.1136/oemed-2016-103555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansson E., Glaser J., Weiss I. Workload and cross-harvest kidney injury in a Nicaraguan sugarcane worker cohort. Occup Envir Med. 2019;76:818–826. doi: 10.1136/oemed-2019-105986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorensen C.J., Butler-Dawson J., Dally M. Risk factors and mechanisms underlying cross-shift decline in kidney function in Guatemalan sugarcane workers. J Occup Environ Med. 2019;61:239–250. doi: 10.1097/JOM.0000000000001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey A.S., Stevens L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin B.R., Butler-Dawson J., Dally M. Unadjusted point of care creatinine results overestimate acute kidney injury incidence during field testing in Guatemala. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler-Dawson J. Re: Unadjusted point of care creatinine results overestimate acute kidney injury incidence during field testing in Guatemala [Comment]. 2019. https://journals.plos.org/plosone/article/comment?id=10.1371/annotation/35061b06-beee-49a8-881a-d13d5f28546d Available at: [DOI] [PMC free article] [PubMed]
- 32.Kulathunga M., Wijayawardena M.A.A., Naidu R. Chronic kidney disease of unknown aetiology in Sri Lanka and the exposure to environmental chemicals: a review of literature. Envir Geochem Health. 2019;41:2329–2338. doi: 10.1007/s10653-019-00264-z. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention . Volume One. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2019. (Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, January 2019). [Google Scholar]
- 34.Pruszkowski E., Neubauer K., Thomas R. An overview of clinical applications by inductively coupled plasma mass spectrometry. Atom Spectrosc. 1998;19:111–115. [Google Scholar]
- 35.Nixon DES, Butz J, Burrit M, et al. Determination of arsenic, lead, cadmium, and mercury in whole blood and urine using dynamic reaction cell ICP-MS. Application Notes, Perkin-Elmer SCIEX Instruments.
- 36.Jarrett JM CK, Jones RL. Urine multi-element ICP-DRC-MS: antimony, arsenic, barium, beryllium, cadmium, cesium, cobalt, lead, manganese, molybdenum, platinum, strontium, thallium, tin, tungsten, and uranium. CDC─Division of Laboratory Sciences, Laboratory Protocol; Adopted October 1, 1994; Updated September 15 2014.
- 37.Hornung R., Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Envir Hygiene. 1990:46–51. [Google Scholar]
- 38.Barr D.B., Wilder L.C., Caudill S.P. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler-Dawson J., Krisher L., Asensio C. Risk factors for declines in kidney function in sugarcane workers in Guatemala. J Occup Envir Med. 2018;60:548–558. doi: 10.1097/JOM.0000000000001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dally M., Butler-Dawson J., Krisher L. The impact of heat and impaired kidney function on productivity of Guatemalan sugarcane workers. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi P., Jing H.M., Xi S.H. Urinary metal/metalloid levels in relation to hypertension among occupationally exposed workers. Chemosphere. 2019;234:640–647. doi: 10.1016/j.chemosphere.2019.06.099. [DOI] [PubMed] [Google Scholar]
- 42.Navas-Acien A., Guallar E., Silbergeld E.K. Lead exposure and cardiovascular disease─a systematic review. Envir Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tellez-Plaza M., Navas-Acien A., Crainiceanu C.M. Cadmium exposure and hypertension in the 1999-2004 National Health and Nutrition Examination Survey (NHANES) Envir Health Perspect. 2008;116:51–56. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whelton P.K., Carey R.M., Aronow W.S. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults : a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e13–e115. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 45.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2017. R: A language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- 46.Bates D., Mächler M., Bolker B. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015;1:1. [Google Scholar]
- 47.Valladolid-Lopez Maria del Carmen, Barrientos-Gutierrez Tonatiuh, Reynales-Shigematsu Luz Myriam, Thrasher James, Pelaez-Ballestas Ingres. Evaluating the validity of self-reported smoking in Mexican adolescents. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-007485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong S.L., Shields M., Leatherdale S., Malaison E., Hammond D. Assessment of validity of self-reported smoking status. Health Rep. 2012;23:47–53. [PubMed] [Google Scholar]
- 49.Hwang J.H., Kim J.Y., Lee D.H., Jung H.G., Park S.W. Underestimation of self-Reported smoking prevalence in Korean adolescents: evidence from Gold Standard by combined method. Int J Environ Res Public Health. 2018;15 doi: 10.3390/ijerph15040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim B.J., Seo D.C., Kim B.S., Kang J.H. Relationship between cotinine-verified smoking status and incidence of hypertension in 74,743 Korean adults. Circ J. 2018;82:1659–1665. doi: 10.1253/circj.CJ-17-1188. [DOI] [PubMed] [Google Scholar]
- 51.SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 52.Riboli E., Preston-Martin S., Saracci R., Haley N.J., Trichopoulos D., Becher H. Exposure of nonsmoking women to environmental tobacco smoke: a 10-country collaborative study. Cancer Causes Control. 1990;1:243–252. doi: 10.1007/BF00117476. [DOI] [PubMed] [Google Scholar]
- 53.Sato Y., Roncal-Jimenez C.A., Andres-Hernando A. Increase of core temperature affected the progression of kidney injury by repeated heat stress exposure. Am J Physiol Renal Physiol. 2019;317:F1111–F1121. doi: 10.1152/ajprenal.00259.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wegman D.H., Apelqvist J., Bottai M. Intervention to diminish dehydration and kidney damage among sugarcane workers. Scand J Work Environ Health. 2018;44:16–24. doi: 10.5271/sjweh.3659. [DOI] [PubMed] [Google Scholar]
- 55.Sagastume R. ETD Collection for University of Texas, El Paso; 1996. Water quality at Lago de Izabal, Guatemala: geochemical characterization and assessment of trophic status.https://scholarworks.utep.edu/dissertations/AAI9718114 Available at: [Google Scholar]
- 56.Schaeffer J., Adgate J., Reynolds S. A pilot study to assess inhalation exposures among sugarcane workers in Guatemala: implications for chronic kidney disease of unknown origin. Int J Environ Res Public Health. 2020;17:5708. doi: 10.3390/ijerph17165708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jayasumana C., Gunatilake S., Siribaddana S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 2015;16 doi: 10.1186/s12882-015-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee K., Park E.K., Stoecklin-Marois M. Occupational paraquat exposure of agricultural workers in large Costa Rican farms. Int Arch Occup Envir Health. 2009;82:455–462. doi: 10.1007/s00420-008-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roncal-Jimenez C., Garcia-Trabanino R., Barregard L. Heat stress nephropathy from exercise-induced uric acid crystalluria: a perspective on Mesoamerican nephropathy. Am J Kidney Dis. 2016;67:20–30. doi: 10.1053/j.ajkd.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Abhyankar L.N., Jones M.R., Guallar E. Arsenic exposure and hypertension: a systematic review. Envir Health Perspect. 2012;120:494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stevens L.A., Coresh J., Greene T., Levey A.S. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 62.Krisher L, Butler-Dawson J, Asensio C, et al. A Total Worker Health approach to assessing kidney health in sugarcane workers in Guatemala: an opportunity for nutrition intervention. Poster presented at the Third International Workshop on Chronic Kidney Diseases of Uncertain/Non-Traditional Etiology in Mesoamerica and Other Regions, Costa Rica, March 2019, PerkinElmer, Inc; Shelton, CT.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.