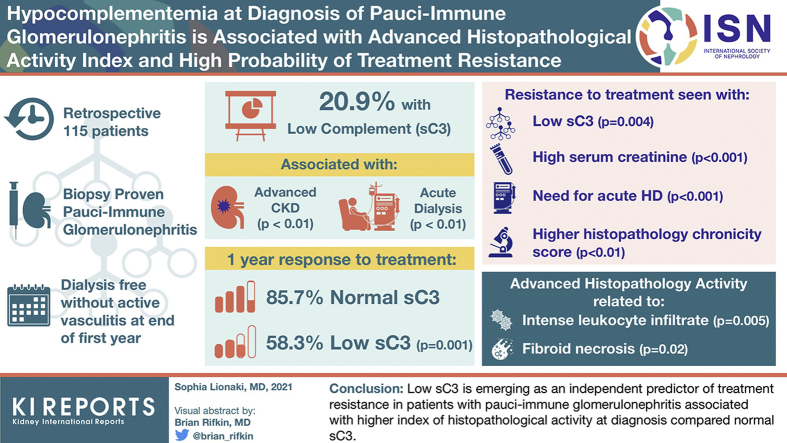
Abstract
Introduction
Recent evidence suggests that complement activation is important in the pathogenesis of pauci-immune (PI) vasculitis. This is a retrospective investigation of the frequency of hypocomplementemia at pauci-immune glomerulonephritis (PIGN) diagnosis, in relation to vasculitic manifestations, renal histopathology, and treatment outcomes.
Methods
A total of 115 patients with biopsy-proven PIGN were categorized based on their serum complement C3 (sC3). Histopathology evaluation included activity and chronicity indexes. The primary outcome of interest was treatment resistance, defined as a progressive decline in kidney function, with persistently active urine sediment, leading to dialysis dependency or vasculitis-related death.
Results
In all, 20.9% of patients had low sC3 levels associated with more advanced renal impairment (P < 0.01), requiring acute dialysis (P < 0.01) more frequently compared to patients with normal sC3. Within 1 year, 85.7% of patients with normal sC3 responded to therapy, versus 58.3% of those with low sC3 (P = 0.001). The probability of treatment resistance was strongly associated with low sC3 (P = 0.004), high serum creatinine (P < 0.001), acute dialysis requirement (P < 0.001), and high histopathological score of chronicity (P < 0.01). Advanced histopathological activity was related to more intense interstitial leukocyte infiltration (P = 0.005) and higher likelihood of fibrinoid necrosis documentation in a vessel wall (P = 0.02). The probability of treatment resistance was higher in patients with low sC3 (odds ratio [OR] = 6.47, 95% confidence interval [CI] 1.47−28.35, P = 0.013), oliguria (OR = 29.57, 95% CI = 4.74−184, P < 0.0001), and high chronicity score (OR = 1.77, 95% CI = 1.23−2.54, P = 0.002).
Conclusion
Low sC3 is emerging as an independent predictor of treatment resistance in patients with PIGN associated with higher index of histopathological activity at diagnosis compared to normal sC3.
Keywords: complement, glomerulonephritis, histopathology, outcome, pauci-immune
Graphical abstract
Evidence from animal models and histopathological findings suggest that complement plays a critical role in the pathogenesis of pauci-immune (PI) vasculitis and glomerulonephritis (GN).1 Pauci-immune vasculitis is most often characterized by acute inflammatory lesions that are extremely destructive, with influx of neutrophils, leukocytoclasia, and necrosis.2,3 Observations in human beings and in experimental animal models of PIGNs clearly implicate activation of the alternative complement pathway, with the presence of hypocomplementemia at the onset and increased levels of factors Bb, C3a, and C5a.4,5 In an anti-myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA) vasculitis murine model, C5a and its receptor C5aR/CD88 were proved to be critical actors in the development of PIGN.6, 7, 8 Wu et al.8 showed that complement activation occurs in both MPO-ANCA and PR3-ANCA vasculitis, but the profile of activation seems to differ between active disease versus disease in remission. Typically, lesions of PI vasculitis are characterized by a paucity of immune deposits, but low-intensity deposition of complement has been reported to be common, especially at focal sites of inflammation and necrosis.9 From a clinical viewpoint, hypocomplementemia in PIGN has been associated with more advanced renal involvement and significantly worst prognosis.10, 11, 12
The aim of this study was to investigate what proportion of patients with PIGN have low serum complements at diagnosis, if any, and whether they have different clinical and/or histopathological features or experience different treatment outcomes compared to patients with normal serum complement levels.
Materials and Methods
Study Population and Definitions
Patients with PIGN who were recruited by nephrologists or referred from rheumatologists to nephrologists in Laiko Hospital (Athens, Greece) between 2006 and 2019 were studied retrospectively. All patients were identified at or near the initial diagnosis and were eligible to be included in this study if they had the following prerequisites: diagnosis of PIGN in a native kidney biopsy sample with the combined use of light microscopy and immunofluorescence parameters, serum complement measurements at diagnosis (prior to initiation of immunosuppressive therapy), age 16 years or more, and signed informed consent for review of medical charts. For the purpose of histopathological analysis, the included patients were required to have a minimum of 10 glomeruli in the diagnostic kidney biopsy sample. Exclusions were eosinophilic granulomatosis with polyangiitis, overlap syndromes with other diseases (i.e., anti−glomerular basement membrane or lupus), non-adherence issues, and inadequate follow-up time. All patients were tested for anti-neutrophil−associated autoantibodies (ANCA) by immunofluorescence or enzyme-linked immunosorbent assay13 or both. Clinical phenotypes of PI vasculitis were assigned according to the Chapel Hill Vasculitides Nomenclature Consensus Conference.14 Thus, a diagnosis of GPA was defined by the presence of necrotizing granulomatous inflammation in any tissue by histology, and/or imaging showing pulmonary nodules or cavities (noninfectious) and/or bony erosions, and/or subglottic stenosis in the upper respiratory tract. Eosinophilic granulomatosis with polyangiitis was defined by the presence of asthma, eosinophilia, and necrotizing granulomatous inflammation. Microscopic polyangiitis was defined by systemic necrotizing small vessel vasculitis without evidence of granulomatous inflammation or asthma. Organ involvement was defined based on previously described criteria.15
The primary outcome of interest was treatment resistance, which was assessed by the end of the first year, and was determined after a minimum of 3 months of therapy with immunosuppressants. It was defined as a progressive decline in kidney function, with persistently active urine sediment leading to dialysis dependency (with or without new or persistent extrarenal vasculitic manifestations) or vasculitis-related death.
Secondary outcomes of interest included treatment response, remission, relapse, end-stage kidney disease (ESKD) in the long term, and death. Treatment response, which was assessed by the end of the first year after diagnosis and initiation of immunosuppressive treatment, was defined as avoidance of death due to vasculitis, along with dialysis independency (i.e., glomerular filtration rate >15 ml/min per 1.73 m2) with no clinical signs of active systemic vasculitis.16 Remission, following response to immunosuppressive treatment, was defined as the stabilization or improvement of kidney function, as measured by serum creatinine levels, with resolution of hematuria or other manifestations of systemic vasculitis for more than 1 month. Persistent proteinuria with bland urine sediment was not considered indicative of active renal vasculitis. Relapse could be recorded only among patients who had achieved remission, and was characterized by recurrent or new signs and symptoms of active vasculitis in any organ.17,18 End-stage kidney disease was characterized by the initiation of chronic dialysis.
Data Collection
Clinical, laboratory, and serological variables, which were recorded at diagnosis of PIGN, included the following: demographics, clinical phenotype, organ involvement, serum complements C3 and C4 (sC3 and sC4), serum creatinine, peak serum creatinine, estimated glomerular filtration rate (eGFR), oliguria, acute dialysis requirement, and ANCA type. The eGFR was determined using the 4-variable Modification of Diet in Renal Disease equation,19 and disease activity was estimated using the Birmingham Vasculitis Score (BVAS).20
Histopathological Evaluation
Histopathological evaluation was performed by an experienced renal pathologist (GL), by review of the data in diagnostic pathology reports, following a detailed scoring system according to the concept presented by Lee et al.16 Specifically, the number of normal glomeruli was presented as a percentage of the total (grade 4 if <10%, 3 if ≥10 <25%, 2 if ≥25% to <50%, and 1 if ≥50%). Glomerular and tubulointerstitial lesions were scored separately and as part of the activity index and chronicity index scores (Table 1). The activity index score represented the sum of scores from 6 categories: glomerular necrosis, cellular crescents, interstitial leukocyte infiltration, fibrinoid necrosis in vessel walls, red blood cell (RBC) casts, and circumferential crescents. Each of the first 3 categories was scored semi quantitatively from 1 (none/mild) to 3 (severe), and the other 3 categories were scored as 1 if the characteristic was present in the specimen or as 0 if it was absent, for a maximum of 12 points. The feature of cellular crescents was defined as the percentage of the involved glomeruli out of the total number of glomeruli in the biopsy sample (grading: 1 for 1%−25%, 2 for 26%−50%, and 3 for >50%). Glomerular necrosis was defined as the percentage of the involved glomeruli out of the total number of glomeruli in the biopsy sample (grading: 1 for 1%−25%, 2 for 26%−50%, and 3 for >50%). Interstitial leucocyte infiltration was defined as the percentage of the inflamed interstitium out of the total (grading: 1 for 1%−25%, 2 for 26%−50%, and 3 for >50%). Red blood cell casts were noted as 0 for absent and 1 for present). Circumferential crescents were noted as 0 for absent and 1 for present. Necrosis in the wall of arterioles, interlobular or arcuate arteries were noted as 0 for absent and 1 for present. The chronicity index score represented the sum of scores from 4 categories, namely, global glomerulosclerosis, interstitial fibrosis, tubular atrophy and fibrotic crescents/segmental sclerosis, which were scored semiquantitatively from 1 to 3 (grade 1 for 0%−25%, grade 2 for 26%−50%, and grade 3 for >50%) for a maximum of 12 points. Fibrotic crescents/segmental sclerosis was defined as the sum of fibrotic crescents (crescents containing >50% fibrosis) and segmental glomerular sclerosis (unspecified sclerosis, probably due to a repair process after necrosis or crescent formation but without cellular remnants), reflected as a percentage of the total number of the glomeruli (grading from 1 to 3). Immunofluorescence examination on frozen tissue was applied in all cases for immunoglobulins (IgG, IgA, IgM), complement components (C3 and C1q), fibrinogen, and kappa and lambda light chains (DAKO FITC, polyclonal rabbit 1/50 dilution). Traces were defined as stain intensity of <1+, and all others stain intensities were recorded on a 4-grade scale (0−3).
Table 1.
Comparison of demographics, disease-related characteristics, and histopathological parameters of patients with pauci-immune glomerulonephritis with low or normal serum C3 complement at diagnosis
| Characteristic | Normal C3 n = 91 | Low C3 n = 24 | P value |
|---|---|---|---|
| Patients | 91 (79.1%) | 24 (20.1%) | |
| Sex, male | 46 (50.5) | 14 (58.3) | 0.49 |
| Race, Caucasian | 88 (96.7) | 23 (95.8) | 1 |
| Age, yr | 56.8 (17.5) | 57.6 (17.5) | 0.82 |
| ANCA type | |||
| Negative | 5 (5.5) | 2 (8.3) | 0.69 |
| C/ PR3-ANCA | 30 (30) | 9 (37.5) | |
| P/MPO-ANCA | 56 (61.5) | 13 (54.2) | |
| Clinical phenotype | |||
| Renal limited disease | 29 (31.8) | 11 (45.8) | 0.46 |
| Granulomatosis with polyangiitis | 22(24.2) | 4(16.7) | |
| Microscopic polyangiitis | 40 (44) | 9 (37.5) | |
| Oliguria | 35 (38.5) | 12 (50) | 0.30 |
| Acute dialysis requirement | 20 (22) | 13 (54.2) | < 0.01 |
| Serum creatinine, mg/dl | 3.5 (2.2) | 5.3 (3.4) | <0.01 |
| Estimated GFR, ml/min/1.73 m2 | 24.7 (22.8) | 18.3(15.0) | 0.11 |
| Peak serum creatinine, mg/dl | 4.5 (2.7) | 6.4 (3.5) | 0.02 |
| BVAS score | 17.4 (5.7) | 17.5 (6.2) | 0.83 |
| Immunosuppressive therapy (initial) | |||
| Cyclophosphamide + glucocorticoids | 91(100) | 24 (100) | |
| Plasma exchange | 21 (29.6) | 9 (37.5) | 0.45 |
| Rituximab | 18 (19.7) | 7 (29.2) | 0.25 |
| Histopathological parameters | |||
| Normal glomeruli (%) | N=85 | N=24 | 0.51 |
| Grade 1, >50% | 12 (14.1) | 3 (12.5) | |
| Grade 2, 25%–50% | 21 (24.7) | 9 (37.5) | |
| Grade 3, 10%–24% | 22 (25.8) | 9 (37.5) | |
| Grade 4 <10% | 30 (35.3) | 8 (33.3) | |
| Normal glomeruli (>10%) | 55 (64.7) | 16 (66.7) | 0.8 |
| EUVAS categories | 0.5 | ||
| Focal class | 21(24.7) | 4(16.7) | |
| Crescentic class | 21(24.7) | 6(25) | |
| Mixed class | 32(37.7) | 8(33.3) | |
| Sclerotic class | 11(12.9) | 6(25) | |
| Global glomerulosclerosis | 0.8 | ||
| Mild (0%–25%) | 52 (61.2) | 15 (62.5) | |
| Moderate (25%–50%) | 18 (21.2) | 4 (16.6) | |
| Severe (>50%) | 15 (17.6) | 5 (20.9) | |
| Fibrotic crescents/segmental sclerosis | 0.8 | ||
| None/mild (0%–25%) | 59 (70.2) | 19 (79.2) | |
| Moderate (25%–50%) | 14 (16.7) | 3 (12.5) | |
| Severe (>50%) | 12 (14.1) | 2 (8.3) | |
| Tubular atrophy | 0.08 | ||
| None/mild (0%–25%) | 42 (49.4) | 16 (66.7) | |
| Moderate (25%–50%) | 39 (45.9) | 6 (25) | |
| Severe (>50%) | 4 (4.7) | 2 (8.3) | |
| Interstitial fibrosis | 0.65 | ||
| None/mild (0%–25%) | 37 (43. 5) | 13 (54.2) | |
| Moderate (25%–50%) | 42 (49.4) | 10 (41.7) | |
| Severe (>50%) | 6 (7.1) | 1 (4.1) | |
| Chronicity (1–12) | 5.5 (2.4) | 5.2 (2.5) | 0.58 |
| Cellular crescents | 0.17 | ||
| Grade 1, 0%–25% | 42 (49.4) | 7 (29.2) | |
| Grade 2, 25%–50% | 21 (24.7) | 10 (41.6) | |
| Grade 3, >50% | 22 (25.9) | 7 (29.2) | |
| Glomerular necrosis | 0.21 | ||
| None/Mild (0%–25%) | 63 (74.1) | 15 (62.5) | |
| Moderate (25%–50%) | 17 (20) | 5 (20.8) | |
| Severe (>50%) | 5 (5.9) | 4 (16.7) | |
| Interstitial leucocyte infiltration | 0.005 | ||
| None/Mild (0%–25%) | 67 (78.8) | 14 (58.3) | 0.04 |
| Moderate (25%–50%) | 18 (21.2) | 7 (29.2) | 0.4 |
| Severe (>50%) | 0 | 3 (12.5) | 0.0007 |
| Circumferential crescents | 29(34.5) | 13(54.2) | 0.08 |
| Red blood cell casts | 26 (31) | 12(50) | 0.08 |
| Fibrinoid necrosis in the vessel wall | 5 (6) | 5(20.8) | 0.02 |
| Activity score (1–12) | 4.98 (1.9) | 6.33 (2.5) | <0.01 |
| Arteriosclerosis | 0.82 | ||
| None/mild | 32 (37.6) | 8 (33.3) | |
| Moderate | 37 (43.5) | 12 (50) | |
| Severe | 16 (18.9) | 4 (16.7) | |
| Immunofluorescence findings | n = 85 | = 24 | |
| C3 0 | 40 (44) | 11 (45.8) | |
| C3 any staining | 25 (27.4) | 3 (12.5) | |
| C3 1+ | 10 (11) | 5 (20.8) | |
| C3 2+ | 15 (17.6) | 5 (20.8) |
Data are n (%) or mean (SD).
EUVAS, European Vasculitis Society; GFR, glomerular filtration rate.
Statistical Analyses
Continuous variables were expressed as the mean and standard deviation, whereas categorical variables as frequencies and percentages on the basis of the serum C3 level at the time of diagnosis of PIGN. To investigate the differences between clinical and histopathological variables between patients with normal and low C3 levels, the t test and Mann−Whitney U test for independent samples for continuous variables and the χ2 and Fisher exact test for categorical variables were applied. Univariate logistic regression analyses were performed for different outcomes (remission, response to immunosuppressive therapy, ESKD, death) and time periods (at the first year after diagnosis, from diagnosis to end of follow-up), and Cox regression analysis for investigating the association between the renal survival time of the patients and their clinical and histopathological characteristics. Variables that were found to be significant in the univariate analyses were included in the multivariate models. The estimated odds ratios (ORs) and hazard ratios (HRs) of both the univariate and multivariate models, as well as the 95% confidence intervals (CIs) of the ORs/HRs and the related P values, are presented. Given the short time period after diagnosis (12 months) that was set for estimations of the first outcome, only a small number of deaths were reported. For this reason, when implementing models of logistic regression analysis for the estimation of a possible relation between the measured variables and the outcomes in the first year, those 4 patients were included in the group of patients with an “adverse outcome,” which included patients who developed ESKD or died during the same period of time. Time to ESKD (in the long-term) was calculated using the Kaplan–Meier estimates, and categories were compared using the log-rank test. The low sC3 variable, albeit not significant from the univariate analysis, was included in the multivariate model for the first-year outcome, as it was the primary explanatory variable for this study. Data were analyzed using Stata 13.0 software (Stata Corporation, College Station, TX), and significance was set at α = 0.05. All tests proceeded as 2 tailed.
Results
Baseline Characteristics of the Cohort
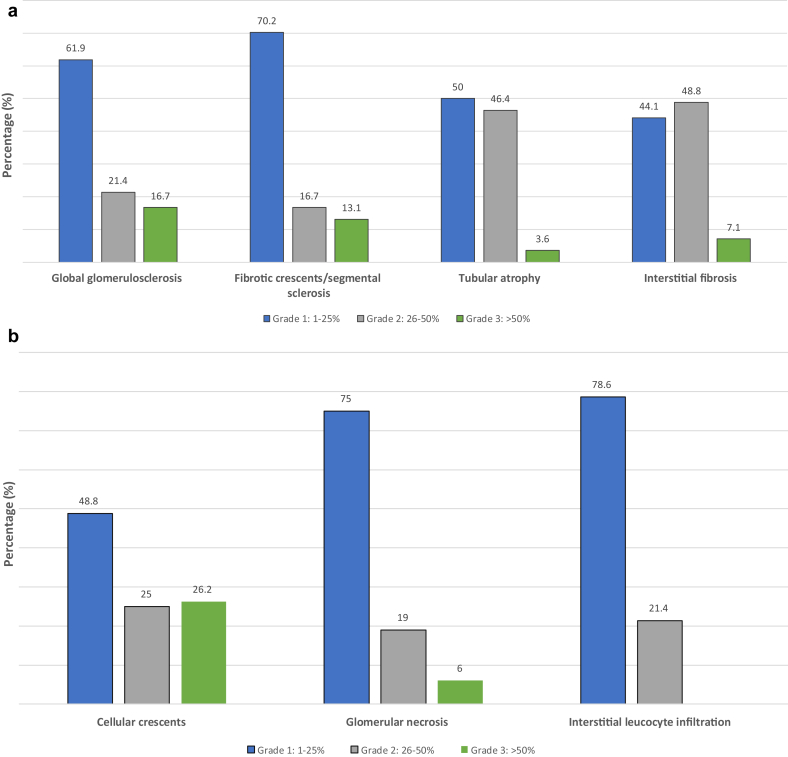
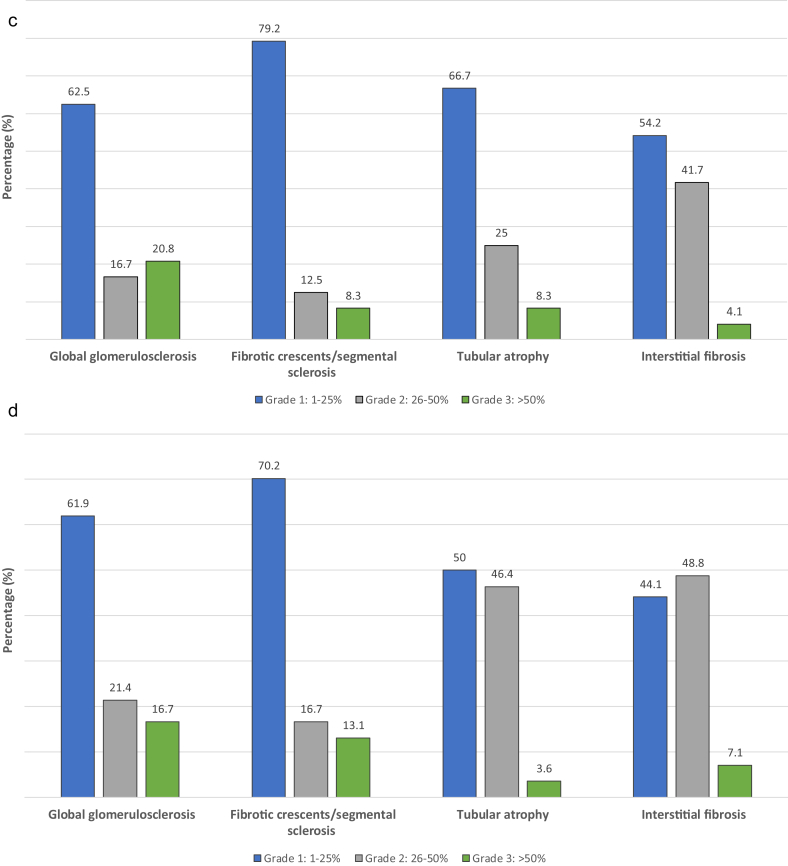
A total of 115 patients with biopsy-proven PIGN were included in the study, 24 (20.9%) of whom had sC3 values below the lower limit of normal range (i.e., <90 mg/dl). One patient (0.8%) had sC4 below the lower normal limit and 1 patient had sC4 above the upper normal limit. Patients were categorized according to the measurement of sC3 into 2 groups, 1 group with low sC3 and 1 group with normal sC3. No differences were identified in terms of demographics (Table 1 and Supplementary Table S1) and disease-related characteristics. Patients with low sC3 had more severe renal involvement, that is, higher serum creatinine and lower eGFR, and they were more likely to experience oliguria and to require acute dialysis around the initial diagnosis (P < 0.01). Data from 109 renal biopsy samples were reviewed. The pattern of glomerular injury according to the European Vasculitis Society (EUVAS) classification schema21 was similar between groups. The mean histopathological activity score, assessed in the diagnostic biopsy sample, was significantly higher in patients with low sC3 than in patients with normal sC3 (6.33 vs. 4.98, P = 0.01) (Table 1). Advanced histopathological activity was related to more intense interstitial leukocyte infiltration (P = 0.005) in the renal tissue and higher likelihood of documented fibrinoid necrosis in a vessel wall in a certain specimen (P = 0.02) (Figure 1a, b). Histopathological parameters related to chronic renal damage were similar between groups, as were immunofluorescence findings (Figure 1c, d). All patients were treated with a combination of cyclophosphamide (intravenous or oral) and glucocorticoids, and similar proportions of patients in both groups also received plasma exchange or rituximab as part of the initial treatment (Table 1).
Figure 1.
Histopathological parameters in the diagnostic kidney biopsy samples of patients with pauci-immune glomerulonephritis. (a) Histopathological parameters of activity in patients with low serum C3. (b) Histopathological parameters of activity in patients with normal serum C3. (c) Histopathological parameters of chronicity in patients with low serum C3. (d) Histopathological parameters of activity in patients with normal serum C3.
Incidence and Predictors of Treatment Failure by the End of the First Year
By univariate analysis, the composite outcome of ESKD or death (Supplementary Table S2) was significantly associated with a low sC3; that is, patients with low sC3 at diagnosis were 4 times more likely to experience a worst outcome (OR = 4.28, 95% CI = 1.57−1.67, P = 0.004). A higher baseline serum creatinine value was associated with higher probability of ESKD or vasculitis-related death, and patients with oliguria at presentation were found to have a 10.85 times higher likelihood of ESKD or vasculitis-related death. In terms of histopathology, patients with <10% normal glomeruli in the diagnostic biopsy, severe arteriosclerosis, moderate or severe global glomerulosclerosis, and cellular crescents in >50% of the glomeruli in the biopsy sample were more likely to develop ESKD or death. An activity score of ≥8 in the diagnostic biopsy sample was associated with a 4-fold higher probability of ESKD or death at the end of the first year, compared with an activity score of <8 (Supplementary Table S2). There was a strong correlation between the chronicity score and the probability of a poor outcome, as patients with a chronicity score of ≥8 were shown to be 4 times more likely to experience a poor outcome compared to patients with a lower chronicity score (P = 0.004).
After adjustments, multivariable analysis showed that low sC3 at diagnosis was independently associated with a worse first-year outcome (i.e., ESKD or death), as well as oliguria, moderate or severe global glomerulosclerosis, and cellular crescents in more than 50% of the glomeruli examined. Moreover, low sC3 at diagnosis conferred a 6.47-fold likelihood of ESKD or death within the first 12 months of diagnosis (compared to normal sC3) (OR = 6.47, 95% CI = 1.47−28.35, P = 0.013); significant acute renal dysfunction with oliguria conferred an almost 30-fold higher likelihood of ESKD or vasculitis-related death (compared to absence of oliguria) (OR = 29.57, 95% CI = 4.74-184, P < 0.0001), and a chronicity score of ≥8 conferred a 1.77-fold higher likelihood of a worse outcome (compared to lower chronicity scores) (OR = 1.77, 95% CI = 1.23−2.54, P = 0.002) (Table 2). After adjusting for covariates, a sC3 value below normal at diagnosis conferred a 81% higher probability of treatment resistance, compared to normal sC3 value (OR = 0.19, 95% CI = 0.03−0.96, P = 0.04) (Supplementary Table S3).
Table 2.
Risk factors associated with the composite outcome of ESKD or vasculitis-related death at the end of the first year after pauci-immune glomerulonephritis diagnosis
| Low serum C3, mg/dl | 6.47 (1.47–28.35) | 0.013 |
|---|---|---|
| Oliguria, present versus absent | 29.57 (4.74–184) | <0.0001 |
| Chronicity score | 1.77 (1.23–2.54) | 0.002 |
| Serum creatinine, mg/dl | 0.82 (0.57–1.19) | 0.3 |
| Estimated GFR >30 ml/min per 1.73 m2 | 0.08 (0.0001–52.1) | 0.44 |
| Normal glomeruli >10% | 1.22 (0.13–3.13) | 0.59 |
| Activity score | 1.37 (0.85–2.24) | 0.19 |
| Acute dialysis requirement | 0.85 (0.12–5.81) | 0.87 |
Data are hazard ratio (95% confidence interval) and are based on a multivariate model for first-year outcome.
ESKD, end-stage kidney disease; GFR, glomerular filtration rate.
Incidence and Predictors of Treatment Response
Treatment response was more common in patients with normal sC3 at diagnosis (87.9% vs. 58.3%, P = 0.001). At the end of first year, dialysis independency was significantly more frequent in patients with normal sC3 compared to those with normal sC3 values (41.7% vs. 9.8%, P = 0.002). During the first year, 4 deaths occurred, all in the normal sC3 group, with 2 of them attributed to treatment-resistant vasculitis, 1 attributed to a cardiovascular event, and 1 to sepsis. By univariate analysis, treatment response was associated with normal sC3 (OR = 0.19, 95% CI = 0.06−0.53, P = 0.002), lower serum creatinine, absence of oliguria, no need of acute dialysis near diagnosis, percentage of normal glomeruli >10%, EUVAS focal class (vs. sclerotic class), and chronicity score per unit increase (Supplementary Table S4). Each unit of increase in the chronicity score was associated with a 66% reduction in the probability of responding to treatment (P = 0.006) (Table 3). Specifically, patients with higher chronicity scores (per 1 unit) had a 63% lower probability of responding to initial treatment (P = 0.001) (Table 3). No correlation was observed between sC3 and the intensity of C3 in the renal tissue by immunofluorescence, between patients with normal and low sC3, whereas differences were noted with respect to immunoglobulins IgA, IgM, and IgG.
Table 3.
Predictors of response to initial treatment in patients with pauci-immune glomerulonephritis
| Characteristic | Odds ratio (95% CI) | P value |
|---|---|---|
| Low serum C3 | 0.19 (0.03–0.96) | 0.04 |
| Estimated GFR >30 vs. <30 ml/min/1.73m2 | 4.78 (0.53–663) | 0.53 |
| Oliguria | 0.09 (0.01–0.95) | 0.04 |
| Acute dialysis requirement | 0.35 (0.05– 2.28) | 0.27 |
| Normal glomeruli >10%, versus ≤10% | 1.10 (0.23–5.16) | 0.89 |
| Chronicity score per unit increase | 0.54 (0.35–0.83) | 0.006 |
| Arteriosclerosis (severe versus mild/moderate) | 1.30 (0.24–6.99) | 0.75 |
Data are based on multivariable model.
CI, confidence interval; GFR, glomerular filtration rate.
As shown by multivariate analysis, the probability of treatment response was significantly decreased in patients with low sC3 (OR = 0.19, 95% CI = 0.03−0.96, P = 0.04), as well as in patients with oliguria (OR = 0.09, 95% CI = 0.01−0.95, P = 0.04), and higher chronicity score (OR = 0.54, 95% CI = 0.35−0.83, P = 0.006). Each unit of increase in the chronicity score was associated with a 66% reduction in the probability of responding to treatment (P = 0.006) (Table 3). No correlation was observed between sC3 and the intensity of C3 in the renal tissue by immunofluorescence or between patients with normal and low sC3 (P = 0.31), whereas differences were noted with respect to immunoglobulins IgA, IgM, and IgG.
Incidence and Predictors of ESKD Among Responders
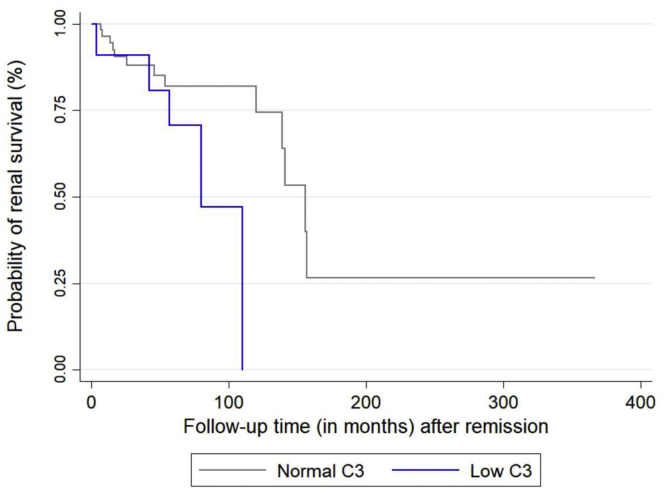
Among patients who responded to initial treatment and achieved remission by the end of the first year, the probability of ESKD in the long term (median follow-up of 57 [45 + 12] months) was higher for patients with low sC3 levels at diagnosis (16.7% vs. 35.7%, P = 0.1) (Figure 2). Moreover, the median renal survival time after remission was significantly shorter for patients who had low sC3 (6.5 years vs. 11.5 years, P = 0.04). Relapse rates among patients who responded to therapy and achieved remission were similar between groups, although there were 7 deaths in the normal sC3 group and 1 death in the low sC3 group during the total observation period.
Figure 2.
Renal survival of patients with pauci-immune glomerulonephritis (PIGN), who responded to initial therapy and achieved remission, stratified by serum C3 at diagnosis.
By multivariate analysis, C3 hypocomplementemia at diagnosis (HR = 1.99, 95% CI = 0.51−7.70, P = 0.31), was not associated with the probability of requiring chronic dialysis (Table 4). Lower levels of serum creatinine, EUVAS focal class (vs. sclerotic), and the presence of RBC casts in the diagnostic biopsy sample were the factors that were associated with lower probability of requiring chronic dialysis as shown by the multivariate analysis.
Table 4.
Risk factors for ESKD in the long term among patients with pauci-immune glomerulonephritis who responded to initial immunosuppressive therapy and achieved remission by the end of the first year
| Variable (at diagnosis) | Hazard ratio (95% CI) | P value |
|---|---|---|
| Multivariable model | ||
| Low serum C3 | 1.99 (0.51–7.70) | 0.31 |
| Serum creatinine | 1.31 (1.03–1.66) | 0.02 |
| Normal glomeruli >10% (versus ≤10%) | 0.61 (0.13–2.85) | 0.53 |
| EUVAS category | ||
| Focal class versus sclerotic class | 0.06 (0.005–0.75) | 0.03 |
| Crescentic class versus sclerotic class | 0.44 (0.08–2.39) | 0.34 |
| Mixed class versus sclerotic class | 0.62 (0.11–3.40) | 0.6 |
| Interstitial fibrosis (moderate or severe versus none/mild) | 1.06 (0.28–4.01) | 0.92 |
| RBC casts present in specimen | 0.11 (0.01–0.66) | 0.01 |
CI, confidence interval; ESKD, end-stage kidney disease; GFR, glomerular filtration rate; RBC, red blood cell; EUVAS, European Vasculitis Society.
Discussion
In this retrospective study, we compared outcomes of patients with biopsy-proven PIGN who were grouped based on the level of sC3 measurements at diagnosis.
Patients with low sC3 at baseline represented one-fifth of the total cohort, had more severe renal impairment at presentation, and required acute dialysis more frequently. Low sC3 at diagnosis of PI vasculitis has been previously described,8, 9, 10, 11, 12 with Fukui et al.10 reporting a rate similar to the one found in this study. More importantly, low sC3 at presentation was shown from our study to be an independent predictor of treatment resistance, as it conferred a 6.47-fold likelihood of ESKD or disease-related death within the first 12 months. Patients with low sC3 were found to have a higher histopathological activity index compared to patients with normal sC3, as reflected in more severe interstitial leukocyte infiltration and advanced cellular crescent formation. Overall, treatment resistance was associated with a lower percentage of normal glomeruli, an advanced chronicity score (especially a chronicity score ≥8), crescentic class by the EUVAS categorization,21 and severe arteriosclerosis. Importantly, low sC3 at diagnosis was also associated with a higher probability of requiring chronic dialysis in the long term.
Treatment-resistant PI vasculitis has been reported in randomized clinical trials22, 23, 24, 25, 26, 27 to occur in 10% to 40% of enrolled patients, as well as in clinical practice.28,29 Certain risk factors for treatment resistance were previously recognized, including older age,28,29 severe kidney disease, female sex, African American race, and the presence of MPO-ANCA. Low baseline sC3 is emerging as an independent predictor of poor prognosis from this study and others.12,30, 31, 32, 33 Our findings agree with those reported by Villacorta et al.,12 in whose study 111 patients with biopsy-proven ANCA glomerulonephritis had been grouped by the level of sC3 at diagnosis; the authors concluded that lower levels of sC3 were associated with a higher need for acute dialysis and lower rates of response to treatment. Another study from Italy, which included 46 patients with ANCA glomerulonephritis of whom 30 had a renal biopsy performed, found that 27% of them had histologic signs of thrombotic microangiopathy associated with low serum C3.31 Augusto et al.32 studied retrospectively a cohort of 45 patients with PIGN, of whom 34 had a renal biopsy, which was used to categorize the patients by the Berden histologic classification.21 The investigators found that patients who were classified in the crescentic/mixed categories had lower levels of sC3, although long-term renal survival was significantly greater in the high-sC3 levels group.32 Altogether, the group of patients with low sC3 at diagnosis seems to follow a different course in terms of response to therapy. Does this mean that patients who do not respond to therapy might be those with significant and/or persistent activation of the alternative complement pathway? A highly activated alternative complement pathway might be a due to prolonged disease, delayed diagnosis, or interference by other factors including genetic involvement33 and epigenetic modifications of genes.34 If so, recognition of these patients and addition of some kind of targeted therapy might be extremely beneficial to bypass treatment resistance in a disease that often presents in a life-threatening setting. There is long-standing evidence that glucocorticoids quickly reduce complement activation,35, 36, 37 but this type of intervention is already used as intravenous pulses of methylprednisolone in patients with PIGN. More recently, avacopan was shown to be effective in inhibiting C5aR, and can be used instead of oral glucocorticoids to block C5a, which is an important inflammatory mediator in PI vasculitis.38
In this cohort, the higher histopathological activity score in patients with low sC3 was associated with severe interstitial leukocyte infiltration, a finding that might be related to enhanced activation of the alternative complement pathway. First, the complement system can be engaged by activation of the classic, lectin, and/or alternative pathway, leading to C3 to divide into C3a and C3b. C3b allows for the formation of C5 convertase that cleaves C5 into C5a and C5b and the assembly of the membrane attack complex C5b-9.39 Importantly, in a murine model of PIGN, the knockout of C5 or factor B but not the C4 or C6 abrogated PIGN formation, which indicates that the alternative complement pathway and not the classic one or membrane attack complex C5b-9 is implicated in renal injury.1,6,7,40 Subsequently, C5a derived from complement activation was shown to cause priming of neutrophils,40 resulting in the release of small amounts of ANCA antigens, which interact with ANCA41 with further neutrophil activation. Properdin secretion by activated neutrophils enhances the activation of the alternative pathway of the complement system,40 whereas activated neutrophils and monocytes activate complement, generating C5a.42, 43, 44 C5a functions as a chemoattractant to neutrophils and monocytes.45, 46, 47, 48, 49, 50 Xiao et al.6 showed that knocking out C5 resulted in strong attenuation of glomerulonephritis, whereas knocking out C4 had no effect in the disease phenotype. We did not find any correlation between sC3 and the intensity of C3 in the renal tissue by immunofluorescence, between patients with normal and low sC3. In contrast, a study including 85 patients with PIGN in which renal histopathology was studied using the Berden categorization21 found that C3d-positive staining, which was observed in 49.4% of the biopsy samples, was associated with the severity of renal impairment and a lower rate of response to treatment.33
The main limitation of this study is its retrospective design. However, it has also important strengths, including the fact that all patients had biopsy-proven disease, and that histopathological evaluation followed a comprehensive scoring system for all kidney biopsy specimens for assessment of activity and chronicity, similar to the report by Lee et al.,16 with excellent correlations with clinical outcomes, and especially response to initial therapy.
In conclusion, in this study one-fifth of patients with PIGN had C3 hypocomplementemia at diagnosis. These patients had advanced activity indexes by histopathology and were more likely to experience treatment resistance compared with patients with normal sC3. If hypocomplementemia was a result of activation of the alternative complement pathway in these patients, and considering that increased activity was associated with enlarged interstitial leukocyte infiltration and significant crescent formation, one might speculate that the intensity and/or duration of complement activation correlates with the severity of the clinical picture and response to treatment. Identification of patients with PIGN with significant complement activation reflected in low sC3 at diagnosis might be helpful in terms of planning the type of immunosuppressive regimen, which might include agents targeting the complement system at the first place at one or multiple sites, to attenuate stimulation of neutrophils, to control inflammation, and to avoid treatment resistance.51
Disclosure
All the authors declared no competing interests.
Footnotes
Table S1. Demographics and baselines characteristics.
Table S2. Univariate analysis for risk factors associated with the composite outcome of ESKD or death at the end of the 1st year after PIGN.
Table S3. Risk factors for ESKD in long-term in patients with PIGN who responded to initial therapy and achieved remission.
Table S4. Univariate analysis for determination of predictors for response to initial immunosuppressive therapy in patients with PIGN.
Supplementary Material
Table S1. Demographics and baselines characteristics.
Table S2. Univariate analysis for risk factors associated with the composite outcome of ESKD or death at the end of the 1st year after PIGN.
Table S3. Risk factors for ESKD in long-term in patients with PIGN who responded to initial therapy and achieved remission.
Table S4. Univariate analysis for determination of predictors for response to initial immunosuppressive therapy in patients with PIGN.
References
- 1.Xiao H., Dairaghi D.J., Powers J.P. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol. 2014;25:225–231. doi: 10.1681/ASN.2013020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennette J.C., Xiao H., Falk R. Experimental models of vasculitis and glomerulonephritis induced by antineutrophil cytoplasmic autoantibodies. Contrib Nephrol. 2011;169:211–220. doi: 10.1159/000314776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennette J.C., Xiao H., Hu P. Complement in ANCA-associated vasculitis. Semin Nephrol. 2013;33:557–564. doi: 10.1016/j.semnephrol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gou S.J., Yuan J., Chen M. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int. 2013;83:129–137. doi: 10.1038/ki.2012.313. [DOI] [PubMed] [Google Scholar]
- 5.Gou S.J., Yuan J., Wang C. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol. 2013;8:1884–1891. doi: 10.2215/CJN.02790313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao H., Schreiber A., Heeringa P. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huugen D., van Esch A., Xiao H. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 2007;71:646–654. doi: 10.1038/sj.ki.5002103. [DOI] [PubMed] [Google Scholar]
- 8.Wu E.Y., McInnis E.A., Boyer-Suavet S. Measuring circulating complement activation products in myeloperoxidase- and proteinase 3-antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2019;71:1894–1903. doi: 10.1002/art.41011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing G.Q., Chen M., Liu G. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol. 2009;29:282–291. doi: 10.1007/s10875-008-9268-2. [DOI] [PubMed] [Google Scholar]
- 10.Fukui S., Iwamoto N., Umeda M. Antineutrophilic cytoplasmic antibody-associated vasculitis with hypocomplementemia has a higher incidence of serious organ damage and a poor prognosis. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshayes S., Aouba A., Khoy K. Hypocomplementemia is associated with worse renal survival in ANCA-positive granulomatosis with polyangiitis and microscopic polyangiitis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villacorta J., Diaz-Crespo F., Acevedo M. Circulating C3 levels predict renal and global outcome in patients with renal vasculitis. Clin Rheumatol. 2016;35:2733–2740. doi: 10.1007/s10067-016-3384-9. [DOI] [PubMed] [Google Scholar]
- 13.Hagen E.C., Daha M.R., Hermans J. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int. 1998;53:743–753. doi: 10.1046/j.1523-1755.1998.00807.x. [DOI] [PubMed] [Google Scholar]
- 14.Jennette J.C., Falk R.J., Bacon P.A. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 15.Lionaki S., Blyth E.R., Hogan S.L. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64:3452–3462. doi: 10.1002/art.34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee T., Gasim A., Derebail V.K. Predictors of treatment outcomes in ANCA-associated vasculitis with severe kidney failure. Clin J Am Soc Nephrol. 2014;9:905–913. doi: 10.2215/CJN.08290813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nachman P.H., Hogan S.L., Jennette J.C., Falk R.J. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:33–39. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 18.Hogan S.L., Nachman P.H., Wilkman A.S. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:23–32. doi: 10.1681/ASN.V7123. [DOI] [PubMed] [Google Scholar]
- 19.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Luqmani R.A., Bacon P.A., Moots R.J. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Q J Med. 1994;87:671–678. [PubMed] [Google Scholar]
- 21.Berden A.E., Ferrario F., Hagen E.C. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 22.Stone J.H., Merkel P.A., Spiera R. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones R.B., Tervaert J.W., Hauser T. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 24.Jayne D., Rasmussen N., Andrassy K. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 25.de Groot K., Harper L., Jayne D.R. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–680. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 26.Guillevin L., Pagnoux C., Karras A. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 27.Walsh M., Merkel P.A., Peh C.A. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382:622–631. doi: 10.1056/NEJMoa1803537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogan S.L., Falk R.J., Chin H. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–631. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 29.Pagnoux C., Hogan S.L., Chin H. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum. 2008;58:2908–2918. doi: 10.1002/art.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molad Y., Tovar A., Ofer-Shiber S. Association of low serum complement C3 with reduced patient and renal survival in antimyeloperoxidase-associated small-vessel vasculitis. Nephron Clin Pract. 2014;126:67–74. doi: 10.1159/000357154. [DOI] [PubMed] [Google Scholar]
- 31.Manenti L., Vaglio A., Gnappi E. Association of serum C3 concentration and histologic signs of thrombotic microangiopathy with outcomes among patients with ANCA-associated renal vasculitis. Clin J Am Soc Nephrol. 2015;10:2143–2151. doi: 10.2215/CJN.00120115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Augusto J.F., Langs V., Demiselle J. Low serum complement C3 levels at diagnosis of renal ANCA-associated vasculitis is associated with poor prognosis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villacorta J., Diaz-Crespo F., Acevedo M. Glomerular C3d as a novel prognostic marker for renal vasculitis. Hum Pathol. 2016;56:31–39. doi: 10.1016/j.humpath.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Lyons P.A., Rayner T.F., Trivedi S. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciavatta D.J., Yang J., Preston G.A. Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis. J Clin Invest. 2010;120:3209–3219. doi: 10.1172/JCI40034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandslund I., Peters N.D., Ejstrup L. Steroids reduce complement activation in rheumatoid arthritis. Int J Tissue React. 1985;7:161–165. [PubMed] [Google Scholar]
- 37.Packard B.D., Weiler J.M. Steroids inhibit activation of the alternative-amplification pathway of complement. Infect Immun. 1983;40:1011–1014. doi: 10.1128/iai.40.3.1011-1014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jayne D.R.W., Bruchfeld A.N., Harper L. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28:2756–2767. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathern D.R., Heeger P.S. Molecules great and small: the complement system. Clin J Am Soc Nephrol. 2015;10:1636–1650. doi: 10.2215/CJN.06230614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreiber A., Xiao H., Jennette J.C. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20:289–298. doi: 10.1681/ASN.2008050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilde B., van Paassen P., Witzke O., Tervaert J.W.C. New pathophysiological insights and treatment of ANCA-associated vasculitis. Kidney Int. 2011;79:599–612. doi: 10.1038/ki.2010.472. [DOI] [PubMed] [Google Scholar]
- 42.Hourcade D.E. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J Biol Chem. 2006;281:2128–2132. doi: 10.1074/jbc.M508928200. [DOI] [PubMed] [Google Scholar]
- 43.Shingu M., Nonaka S., Nishimukai H. Activation of complement in normal serum by hydrogen peroxide and hydrogen peroxide-related oxygen radicals produced by activated neutrophils. Clin Exp Immunol. 1992;90:72–78. doi: 10.1111/j.1365-2249.1992.tb05834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogt W. Complement activation by myeloperoxidase products released from stimulated human polymorphonuclear leukocytes. Immunobiology. 1996;195:334–346. doi: 10.1016/S0171-2985(96)80050-7. [DOI] [PubMed] [Google Scholar]
- 45.O'Flynn J., Dixon K.O., Faber Krol M.C. Myeloperoxidase directs properdin-mediated complement activation. J Innate Immun. 2014;6:417–425. doi: 10.1159/000356980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marder S.R., Chenoweth D.E., Goldstein I.M., Perez H.D. Chemotactic responses of human peripheral blood monocytes to the complement-derived peptides C5a and C5a des Arg. J Immunol. 1985;134:3325–3331. [PubMed] [Google Scholar]
- 47.Hartung H.P., Hadding U. Complement components in relation to macrophage function. Agents Actions. 1983;13:415–428. doi: 10.1007/BF02176404. [DOI] [PubMed] [Google Scholar]
- 48.Schwaeble W., Huemer H.P., Möst J. Expression of properdin in human monocytes. Eur J Biochem. 1994;219:759–764. doi: 10.1111/j.1432-1033.1994.tb18555.x. [DOI] [PubMed] [Google Scholar]
- 49.Camous L., Roumenina L., Bigot S. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood. 2011;117:1340–1349. doi: 10.1182/blood-2010-05-283564. [DOI] [PubMed] [Google Scholar]
- 50.Hilhorst M., van Paassen P., van Rie H. Complement in ANCA-associated glomerulonephritis. Nephrol Dial Transplant. 2017;32:1302–1313. doi: 10.1093/ndt/gfv288. [DOI] [PubMed] [Google Scholar]
- 51.Chen M., Jayne D., Zhao M.H. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat Rev Nephrol. 2017;13:359–367. doi: 10.1038/nrneph.2017.37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.