Abstract
Due to the development of novel functionalities, distinct SARS-CoV-2 variants such as B.1.1.7 fuel the current pandemic. B.1.1.7 is not only more transmissible, but may also cause an increased mortality compared to previous SARS-CoV-2 variants. Human tissue analysis of the SARS-CoV-2 lineage B.1.1.7 is urgently needed, and we here present autopsy data from 7 consecutive SARS-CoV-2 B.1.1.7 cases. The initial RT-qPCR analyses from nasopharyngeal swabs taken post mortem included typing assays for B.1.1.7. We quantitated SARS-CoV-2 B.1.1.7 viral load in autopsy tissue of multiple organs. Highest levels of SARS-CoV-2 B.1.1.7 copies normalized to ß-globin were detected in the respiratory system (lung and pharynx), followed by the liver and heart. Importantly, SARS-CoV-2 lineage B.1.1.7 was found in 100% of cases in the lungs and in 85.7% in pharynx tissue. Detection also in the kidney and brain highlighting a pronounced organ tropism. Comparison of the given results to a former cohort of SARS-CoV-2 deaths during the first wave in spring 2020 showed resembling organ tropism. Our results indicate that also SARS-CoV-2 B.1.1.7 has a relevant organ tropism beyond the respiratory tract. We speculate that B.1.1.7 spike protein’s affinity to human ACE2 facilitates transmission, organ tropism, and ultimately morbidity and mortality. Further studies and larger cohorts are obligatory to proof this link.
Keywords: Autopsy, SARS-CoV-2, Variants of concern, Organ tropism, B.1.1.7
Due to the development of novel functionalities, distinct SARS-CoV-2 variants such as B.1.1.7 fuel the current pandemic. SARS-CoV-2 lineage B.1.1.7 was first identified in the UK and spread in multiple regions worldwide [1]. B.1.1.7 is not only more transmissible, but may also cause an increased mortality compared to previous SARS-CoV-2 variants [2]. We have previously reported a multiorgan tropism of the initial SARS-CoV-2 lineage [3, 4] that can also associate with organ outcome [5]. Organ tropism thereby correlated with the presence of comorbidities including chronic kidney disease and diabetes [5]. The first case report on autopsy results of a B.1.1.7 fatality was published recently in Int J Legal Med [6].
Human tissue analysis of the SARS-CoV-2 lineage B.1.1.7 is urgently needed, and we here present autopsy data from 7 consecutive SARS-CoV-2 B.1.1.7 cases (clinical data in Table 1). These individuals died out-of-hospital (n = 3) and in-hospital (n = 4). The cohort was of high age (median 75 years, interquartile range 52–78 years) with a sex ratio of 4:3 (male:female). The initial RT-qPCR analyses from nasopharyngeal swabs taken post mortem included typing assays for B.1.1.7 in the form of N501Y and del HV69//70 as recently established [7] in combination with screening for E484K and P681H (TIB Molbiol, Berlin, Germany). All out-of-hospital deceased were first tested positive post mortem. Full autopsies were done in all cases following guidelines on the handling of COVID-19 deaths [8]. Organ samples were processed and analyzed for the E gene of SARS-CoV-2.
Table 1.
Summary of case characteristics including sex, age, post-mortem interval (PMI), number of pre-existing medical conditions and places of death (OH out of hospital, NW normal ward, ICU intensive care unit). Forensic aspects of no. 1 have been published as case report [6]
| No | Sex | Age | PMI | Place of death | Comorbidities (n) | Airway (n) | Cardiovascular (n) | Kidney (n) | Brain (n) | Metabolism (n) | Other (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | f | 70 | 6 | OH | 4 | 1 | 2 | 1 | 0 | 0 | 0 |
| 2 | m | 76 | 2 | NW | 4 | 0 | 3 | 0 | 0 | 0 | 0 |
| 3 | f | 78 | 6 | OH | 4 | 1 | 2 | 0 | 0 | 1 | 0 |
| 4 | m | 85 | 4 | ICU | 4 | 0 | 3 | 0 | 0 | 0 | 1 |
| 5 | m | 45 | 2 | ICU | 2 | 0 | 1 | 0 | 0 | 0 | 1 |
| 6 | m | 75 | 12 | ICU | 5 | 1 | 2 | 1 | 0 | 1 | 0 |
| 7 | f | 52 | 8 | OH | 3 | 0 | 2 | 0 | 0 | 1 | 0 |
We quantitated SARS-CoV-2 B.1.1.7 viral load in autopsy tissue of multiple organs. Highest levels of SARS-CoV-2 B.1.1.7 copies normalized to ß-globin were detected in the respiratory system (lung and pharynx), followed by the liver and heart (Fig. 1). Importantly, SARS-CoV-2 lineage B.1.1.7 was found in 100% of cases in the lungs (7/7), in 85.7% in pharynx tissue (6/7), in 57.1% in the liver and heart (4/7 each), and in 42.9% in the kidney and brain (3/7) highlighting a pronounced organ tropism (Fig. 1). Comparison of the given results to a former cohort of 27 SARS-CoV-2 deaths during the first wave in spring 2020 [3] showed resembling organ tropism (Fig. 1).
Fig. 1.
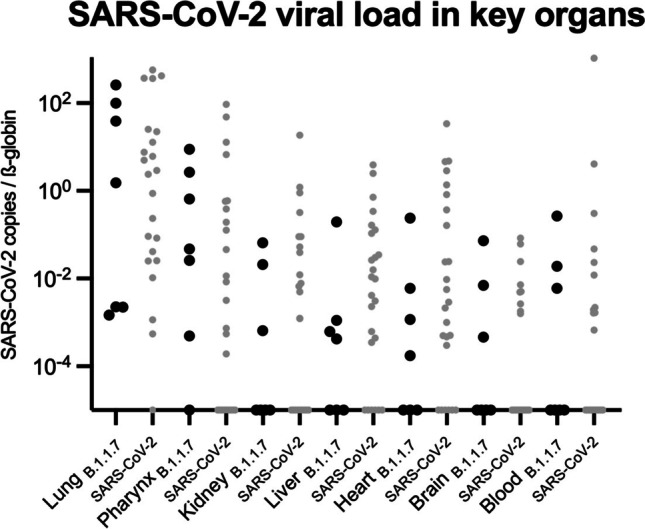
Multi-organ tropism of the SARS-CoV-2 B.1.1.7 lineage. SARS-CoV-2 viral load in key organs, documenting organotropism of B.1.1.7 (virus copies normalized to ß-globin). For comparative reasons, values from Puelles et al. [3] were opposed in grey dots as SARS-CoV-2 original lineage values and were also normalized to ß-globin
Our results indicate that also SARS-CoV-2 B.1.1.7 has a relevant organ tropism beyond the respiratory tract. We speculate that B.1.1.7 spike protein’s affinity to human ACE2 facilitates transmission, organ tropism, and ultimately morbidity and mortality. Further studies and larger cohorts are obligatory to proof this link and our forensic discipline is again one of the key players for this task.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors were funded by the German Federal Ministry of Education and Research (BMBF: 01KX2021) as part of the DEFEAT PANDEMIcs project.
TBH was supported by the German Research Foundation (DFG: CRC/1192, HU 1016/8–2, HU 1016/11–1, HU 1016/12–1), the BMBF (STOP-FSGS-01GM1518C), and DEFEAT PANDEMIcs (01KX2021).
Declarations
Informed consent
Informed consent was obtained from the relatives of the deceased. We want to offer condolences to the families and friends of all COVID-19 deceased.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Benjamin Ondruschka and Fabian Heinrich are first authors and contributed equally to this work.
Marc Lütgehetmann and Tobias B. Huber are shared senior authors contributing equally to this work.
Change history
2/19/2022
OA funding note shall be added to the article to fulfill the contractual requirement of the Compact agreement.
Contributor Information
Benjamin Ondruschka, Email: b.ondruschka@uke.de.
Tobias B. Huber, Email: t.huber@uke.de
References
- 1.Plante JA, Mitchll BM, Plante KS, Debbink K, Weaver SC, Menachery VD. The variant gambit: COVID-19’s next move. Cell Host Microbe. 2021;29:508–515. doi: 10.1016/j.chom.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies NG, Jarvis CI, CMMID COVID-19 Working Group, Edmunds WJ, Jewell NP, Diaz-Ordaz K, Keogh RH Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593:270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JT, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Püschel K, Aepfelbacher M, Huber TB. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, Klein A, Langenwalder F, Lütgehetmann M, Meißner K, Püschel K, Schäder J, Steurer S, Mushumba H, Sperhake JP. Dying with SARS-CoV-2 infection – an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, Nörz D, Heinrich F, Meißner K, Wichmann D, Kluge S, Gross O, Püschel K, Schröder AS, Edler C, Aepfelbacher M, Puelles VG, Huber TB. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020;396:597–598. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinrich F, Romich C, Zimmermann T, Kniep I, FItzek A, Steurer S, Glatzel M, Nöz D, Günther T, Czech-Sioli M, Fischer N, Grundhoff A, Lütgehetmann M, Ondruschka B, Dying of VOC-202012/01 – multimodal investigations in a death case of the SARS-CoV-2 variant. Int J Legal Med. 2021 doi: 10.1007/s00414-021-02618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nörz D, Grunwald M, Olearo F, Fischer N, Aepfelbacher M, Pfefferle S, Lütgehetmann M (2021) Evaluation of a fully automated high-throughput SARS-CoV-2 multiplex qPCR assay with build-in screening functionality to DelHV69/70- and N501Y variants such as B.1.1.7. J Clin Virol 141:104894 [DOI] [PMC free article] [PubMed]
- 8.Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020;73:239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]


