Membranous nephropathy (MN) is one of the most common causes of nephrotic syndrome in nondiabetic adults.1 It is estimated that even with proper treatment, 10% to 20% of patients with MN will progress to end-stage kidney disease.1,2 Disease pathogenesis involves the production of autoantibodies against podocyte antigens, including the M-type phospholipase A2 receptor, that deposit in the subepithelial space of the glomeruli and activate complement, leading to podocyte injury. Although the exact pathogenic mechanism responsible for autoantibody production is unknown, evidence has been generated showing that patients with active MN have reduced levels of regulatory T cells (TREG), a cell subset responsible for peripheral immune tolerance. Importantly, treatment-induced remission of MN has been associated with increased peripheral TREG levels.3
Adrenocorticotropic hormone (ACTH) has been proposed as a novel therapy for MN, after the incidental finding by Berg et al.4 that this hormone had antiproteinuric effects in 14 patients with MN. ACTH protective effects on podocytes are thought to be mediated by its activation of melanocortin receptors, resulting in a reduction in cellular oxidative stress, apoptosis, injury, and loss.5
Based on the evidence that MN disease activity is associated with reduced TREG, we hypothesized that the antiproteinuric effects of ACTH in MN are also mediated by the promotion of TREG. To test this hypothesis, we designed a mechanistic prospective, open-label study to evaluate the effects of repository corticotropin injection (RCI; Acthar Gel, Mallinckrodt Pharmaceuticals, Staines-upon-Thames, UK) on T- and B-cell subsets (including TREG) in patients with MN. This in vivo study was supplemented by in vitro mechanistic experiments that altogether converge to support the idea that ACTH promotes MN disease remission by inducing TREG.
Results
Patients and Outcomes
Between March 2018 and June 2019, we enrolled 5 eligible patients with MN. All patients had nephrotic range proteinuria before starting Acthar Gel despite having taken an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker for ≥90 days. Three patients had been treated in the past with either steroids, alkylating agents, or rituximab, but were off immunosuppression for ≥6 months and serum albumin levels were stably below the normal range. All patients were negative for anti–phospholipase A2 receptor antibodies (Supplementary Table S1).
Patients were enrolled to receive Acthar Gel subcutaneously starting at the dose of 80 units in the first week of treatment, and then 80 units twice weekly for 6 months. One of the 5 patients discontinued the study drug after 2 months of treatment because of insomnia and was lost to follow-up. Baseline characteristics of the patients are available in Supplementary Table S1.
Clinical Outcomes
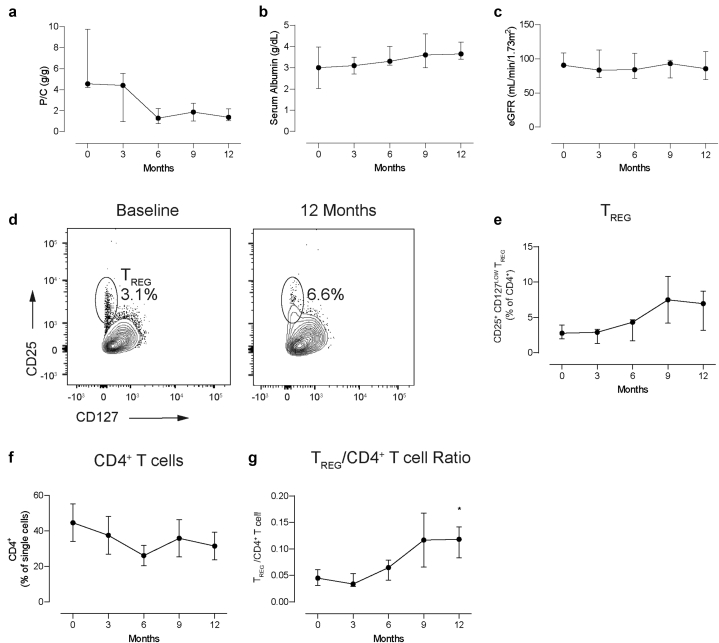
The clinical endpoint of the study was the change in proteinuria and estimated glomerular filtration rate from enrollment to month 12 after initiation of Acthar Gel therapy. All the included patients reached partial remission at 12 months. Overall, the median urine protein/creatinine ratio declined (4.56 [4.41–6.32] vs. 1.36 [1.1–1.78] g/g pretreatment vs. month 12 posttreatment, respectively, P = 0.078) (Figure 1a and Supplementary Figure S1A). Serum albumin trended to increase, while estimated glomerular filtration rate remained stable (Figure 1b–c and Supplementary Figure S1B–C).
Figure 1.
(a) Urinary protein/creatinine ratio, (b) serum albumin, and (c) estimated glomerular filtration rate at baseline and throughout study course. (d) Representative plots for CD4+CD25+CD127LOW T regulatory cells (TREG) at baseline and at 12 months posttreatment; (e) quantification of circulating TREG; (f) total CD4+ T cells, and (g) TREG/CD4+ T cell ratio at baseline and throughout study. Data are represented as median ± interquartile range. P/C, urinary protein/creatinine ration; TREG, regulatory T cells. ∗P < 0.05 versus month 0.
Immune Phenotyping
Our extensive immune phenotyping showed no significant changes in the percentages of any of the major T- and B-cell subsets (Supplementary Figures S1D–E, S2, and S3), except for CD4+CD25+CD127low TREG/CD4+ T cells that significantly increased over time (Figure 1d–g and Supplementary Figure S1F).
Effects of RCI on In Vitro TREG Induction
The increased TREG levels in patients with MN who were treated with Acthar Gel formed the basis to test the hypothesis that RCI promotes the conversion of naïve CD4+ T cells into TREG.
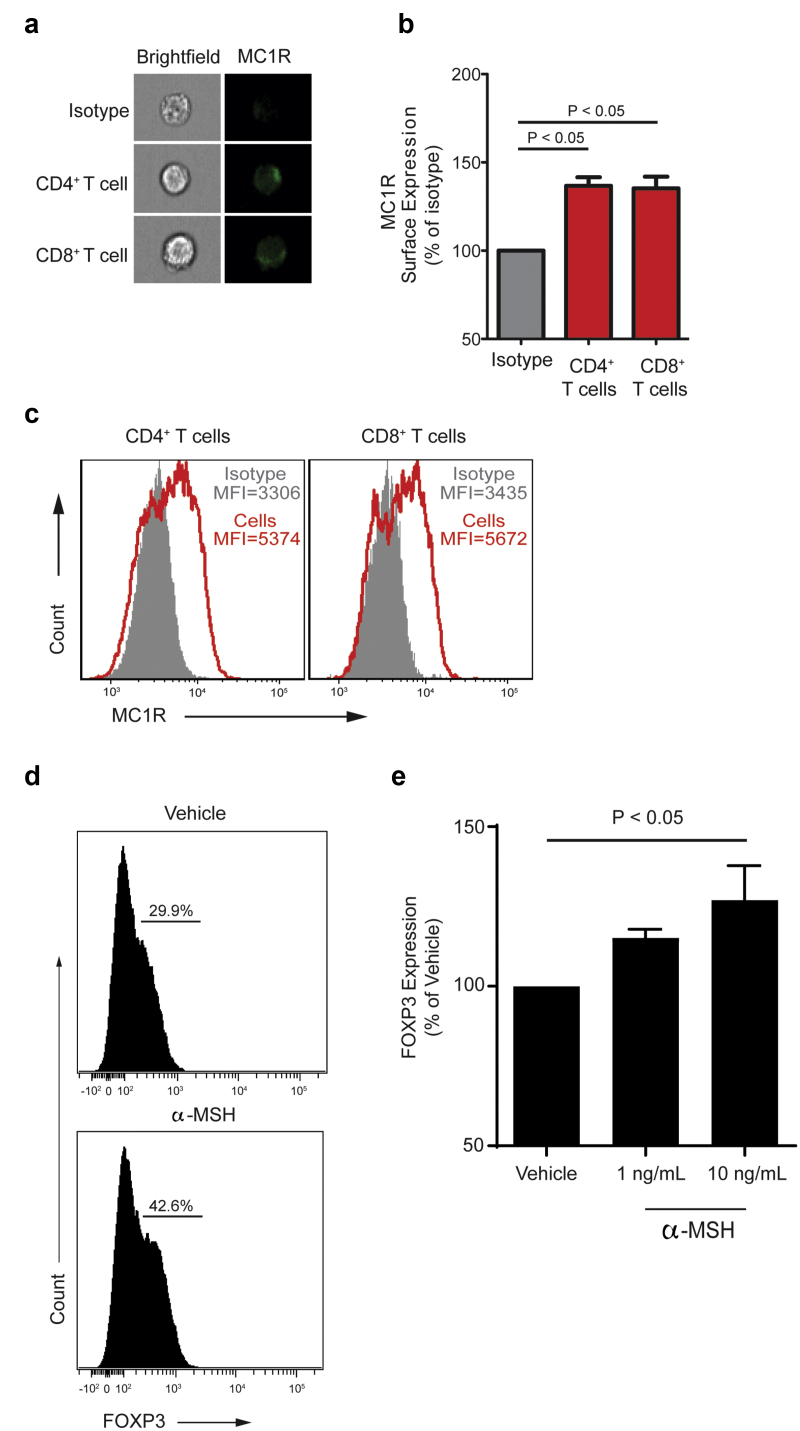
Melanocortin 1 receptor (MC1R), 1 of the 5 known melanocortin receptors (MC1R–MC5R), has been implicated in mediating the antiproteinuric effects of Acthar Gel on podocytes. We documented that it is also expressed by T and B cells using imaging flow cytometry (Figure 2a-c). Next, we cultured naïve human CD4+ T cells under TREG-polarizing conditions in the presence or absence of α-melanocyte-stimulating hormone (α-MSH), a nonselective agonist of melanocortin receptors that is similar to Acthar Gel. We found a significant, dose-dependent increase in FOXP3+ TREG (Figure 2d-e) at 5 days in cells cultured in the presence of α-MSH. Because no antigen-presenting cells were present in the TREG induction culture, the results indicate a direct effect of α-MSH on CD4+ T cells. Altogether, these data support the concept that expansion of peripheral TREG detected in patients with MN who were treated with Acthar Gel might be due, at least in part, to TREG induction.
Figure 2.
(a) Representative imaging flow single-cell images depicting bright-field (left column) and melanocortin 1 receptor (MC1R) expression in CD4+ and CD8+ T cells (right column). (b) Representative plots quantifying membrane MC1R expression on nonpermeabilized CD4+ and CD8+ T cells compared with isotype staining (negative control), and (c) membrane MC1R quantification as mean florescence intensity (MFI). Data are mean + standard error of the mean (SEM) of 2 independent experiments with 2–4 donors each. (d) Representative plots of FOXP3+ TREG at the end of 5 day-induction assay in the presence of α-melanocyte-stimulating hormone (α-MSH) (bottom) or vehicle control (top). Naïve human CD4+CD45RA+CD45RO− T cells were cultured with anti-CD3/anti-CD28 mAb (1 μg/ml), interleukin-2 (100 IU/ml), and transforming growth factor-β (10 ng/ml) ± α-MSH. (e) Quantification of FOXP3 expression measured by flow cytometry 5 days later. Data are represented as mean + SEM of 3 different experiments with 3 different donors.
Discussion
ACTH is produced by the anterior pituitary gland and induces cortisol production by the adrenal glands. Its antiproteinuric effects, however, seem not to be mediated by the increased cortisol synthesis, because steroid therapy alone has been proven ineffective in inducing remission or delaying end-stage kidney disease in patients with MN.6 Conversely, Acthar Gel has been shown to have direct podocyte-protective effects through the activation of melanocortin receptors. These effects could at least partially account for the reduction of proteinuria previously observed in a wide range of glomerular diseases, including, aside from MN, IgA nephropathy, focal segmental glomerulosclerosis, lupus nephritis, and minimal change disease. Intriguingly, previous in vitroS1 and in vivoS2 studies found an inverse relationship between cortisol levels and TREG, suggesting that increased TREG in patients treated with ACTH is likely not caused by increased cortisol but rather by a direct effect on T cells, as supported by our in vitro data.
With the limitation of the small sample size, our results support the use of Acthar Gel as a therapeutic option for patients with MN who have failed to respond to more conventional therapies, such as anti-CD20 antibodies, calcineurin inhibitors, or alkylating agents. Going along with other published reports, we observed reduced proteinuria from baseline in all of the enrolled patients. In a multicenter retrospective series of 11 patients with primary MN, Acthar Gel treatment was associated with a reduction of proteinuria of >50% in 7 patients and 2 patients showed complete remission. Only 2 patients failed to show any response.7
Our mechanistic data show that the antiproteinuric efficacy of Acthar Gel may be due also to its anti-inflammatory effects.8 We showed that human T cells express MC1R and that RCI promotes TREG induction both in vivo and in vitro, although further studies are needed to decipher the underlying molecular mechanism. Our results align with previous findings showing that increased TREGS identify patients with MN who respond to treatment. A phenotypic analysis of lymphocyte subpopulations on 25 patients with severe MN suggested that TREG counts may help predict early responses to rituximab.3 This study found that patients who responded to rituximab had lower TREGS at baseline and significantly increased percentages of these cells at day 8 posttreatment when compared with nonresponders.3 Another study on 17 patients with MN showed a significant increase (up to 10-fold) in TREGS at 12 months posttreatment in individuals who underwent disease remission.9
There are limitations to this study. Since the cohort of patients was small, our findings can be taken for now just as proof-of-concept. Second, the uncontrolled design does not allow us to conclude that clinical remission in our patients was achieved as an effect of the medication rather than the natural history of the disease, because a significant number of patients with MN may undergo spontaneous remission. However, spontaneous remissions would have been unlikely for our patients, given the extended monitoring period pre-enrollment off immunosuppressive therapy. Despite these caveats, our data identify TREG induction as a mechanism of action of Acthar Gel, which could be responsible for its therapeutic effects in MN and, possibly, in other autoimmune diseases.
Conclusion
Our proof-of-concept study shows that Acthar Gel promotes disease remission in patients with MN and increases TREG induction. These cells, on turn, may inhibit autoreactive B cell–producing autoantibodies. Further studies are needed to validate these findings and assess the clinical efficacy, immune effects, and safety of Acthar Gel for the treatment of refractory MN.
Disclosure
KNC reports consulting fees from Mallinckrodt, Travere, Calliditas and Goldfinch Bio. All the other authors declared no competing interests.
Acknowledgments
The study was supported by an investigator-initiated research award from Mallinckrodt Pharmaceuticals. The company had no role in evaluating the clinical data or writing the manuscript.
Footnotes
Table S1. Clinical characteristics at baseline in four patients who completed treatment with Acthar Gel.
Figure S1. (A) Urinary protein/creatinine ratio (P/C), (B) serum albumin, and (C) eGFR. Percentages of circulating (D) CD25+CD27LOWTREG, (E) total CD4+ T cells and (F) TREG/CD4+ T cell ratio. Data are represented as single values for each patient, at baseline and throughout study course.
Figure S2. Changes in total lymphocytes and T cell subsets. Data are represented as mean ± SEM. No subsets differed significantly between time points.
Figure S3. Changes in total lymphocytes and B cell subsets. Data are represented as mean ± SEM. No subsets differed significantly between time points.
Supplementary Methods.
Supplementary References.
Contributor Information
Kirk N. Campbell, Email: kirk.campbell@mssm.edu.
Paolo Cravedi, Email: paolo.cravedi@mssm.edu.
Supplementary Material
Table S1. Clinical characteristics at baseline in four patients who completed treatment with Acthar Gel.
Figure S1. (A) Urinary protein/creatinine ratio (P/C), (B) serum albumin, and (C) eGFR. Percentages of circulating (D) CD25+CD27LOWTREG, (E) total CD4+ T cells and (F) TREG/CD4+ T cell ratio. Data are represented as single values for each patient, at baseline and throughout study course.
Figure S2. Changes in total lymphocytes and T cell subsets. Data are represented as mean ± SEM. No subsets differed significantly between time points.
Figure S3. Changes in total lymphocytes and B cell subsets. Data are represented as mean ± SEM. No subsets differed significantly between time points.
Supplementary Methods.
Supplementary References.
References
- 1.Couser W.G. Primary membranous nephropathy. Clin J Am Soc Nephrol CJASN. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maisonneuve P., Agodoa L., Gellert R. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. Am J Kidney Dis. 2000;35:157–165. doi: 10.1016/S0272-6386(00)70316-7. [DOI] [PubMed] [Google Scholar]
- 3.Rosenzwajg M., Languille E., Debiec H. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int. 2017;92:227–237. doi: 10.1016/j.kint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Berg A.L., Nilsson-Ehle P., Arnadottir M. Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int. 1999;56:1534–1543. doi: 10.1046/j.1523-1755.1999.00675.x. [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith C.J., Hammad S. A review of the re-emergence of adrenocorticotrophic hormone therapy in glomerular disease, more than a drug of last resort? Clin Kidney J. 2015;8:430–432. doi: 10.1093/ckj/sfv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cattran D.C., Delmore T., Roscoe J. A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. N Engl J Med. 1989;320:210–215. doi: 10.1056/NEJM198901263200403. [DOI] [PubMed] [Google Scholar]
- 7.Madan A., Mijovic-Das S., Stankovic A., Teehan G., Milward A.S., Khastgir A. Acthar gel in the treatment of nephrotic syndrome: a multicenter retrospective case series. BMC Nephrol. 2016;17:37. doi: 10.1186/s12882-016-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkovich R., Agius M.A. Mechanisms of action of ACTH in the management of relapsing forms of multiple sclerosis. Ther Adv Neurol Disord. 2014;7:83–96. doi: 10.1177/1756285613518599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roccatello D., Sciascia S., Di Simone D. New insights into immune mechanisms underlying response to Rituximab in patients with membranous nephropathy: a prospective study and a review of the literature. Autoimmun Rev. 2016;15:529–538. doi: 10.1016/j.autrev.2016.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.