Abstract
Background
Patients on dialysis have a high burden of bone-related comorbidities, including fractures. We report a post hoc analysis of the prospective cohort study HDF, Hearts and Heights (3H) to determine the prevalence and risk factors for chronic kidney disease-related bone disease in children on hemodiafiltration (HDF) and conventional hemodialysis (HD).
Methods
The baseline cross-sectional analysis included 144 children, of which 103 (61 HD, 42 HDF) completed 12-month follow-up. Circulating biomarkers of bone formation and resorption, inflammatory markers, fibroblast growth factor-23, and klotho were measured.
Results
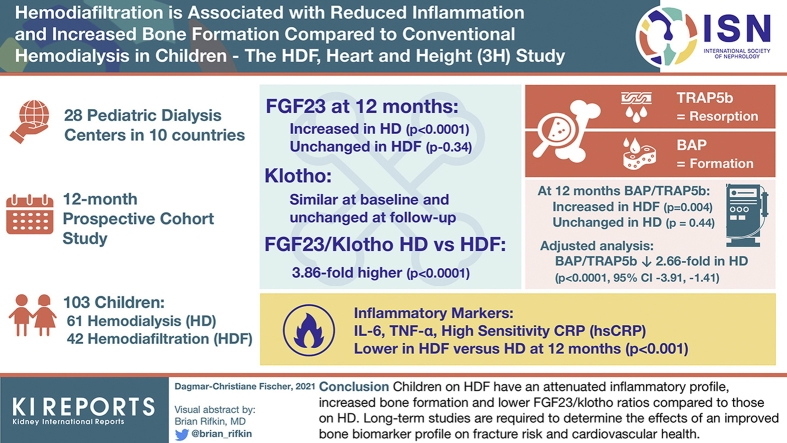
Inflammatory markers interleukin-6, tumor necrosis factor-α, and high-sensitivity C-reactive protein were lower in HDF than in HD cohorts at baseline and at 12 months (P < .001). Concentrations of bone formation (bone-specific alkaline phosphatase) and resorption (tartrate-resistant acid phosphatase 5b) markers were comparable between cohorts at baseline, but after 12-months the bone-specific alkaline phosphatase/tartrate-resistant acid phosphatase 5b ratio increased in HDF (P = .004) and was unchanged in HD (P = .44). On adjusted analysis, the bone-specific alkaline phosphatase/tartrate-resistant acid phosphatase 5b ratio was 2.66-fold lower (95% confidence interval, −3.91 to −1.41; P < .0001) in HD compared with HDF. Fibroblast growth factor-23 was comparable between groups at baseline (P = .52) but increased in HD (P < .0001) and remained unchanged in HDF (P = .34) at 12 months. Klotho levels were similar between groups and unchanged during follow-up. The fibroblast growth factor-23/klotho ratio was 3.86-fold higher (95% confidence interval, 2.15–6.93; P < .0001) after 12 months of HD compared with HDF.
Conclusion
Children on HDF have an attenuated inflammatory profile, increased bone formation, and lower fibroblast growth factor-23/klotho ratios compared with those on HD. Long-term studies are required to determine the effects of an improved bone biomarker profile on fracture risk and cardiovascular health.
Keywords: hemodiafiltration, hemodialysis, inflammation, mineral bone disease, pediatric nephrology
Graphical abstract
Chronic kidney disease (CKD) is associated with nearly universal disturbances in mineral bone disease (MBD),1,2 manifesting as bone pain,3 deformities,3 growth retardation in children,4 fractures,3,5 and vascular calcification.6 Although assessment of bone health is integral to the care of patients with CKD, it remains a major challenge for physicians.7 Histomorphometric analysis of bone biopsy specimens is the gold standard for diagnosis of renal bone disease8 but is invasive and not suited for screening and longitudinal monitoring. On the other hand, radiologic bone assessment, such as conventional X-ray imaging or dual-energy X-ray absorptiometry, although noninvasive and widely available, has limited sensitivity and specificity in predicting changes in bone turnover and mineralization.9,10
Circulating biomarkers, reflecting global skeletal activity, are recognized as important tools11,12 to detect changes in bone homeostasis, enabling the assessment of both bone formation and resorption.13,14 Classical formation markers include bone alkaline phosphatase (BAP), a protein found on the surface of osteoblasts reflecting their activity. Bone resorption markers include tartrate-resistant acid phosphatase 5b (TRAP5b), an enzyme produced by osteoclasts reflecting their number,15, 16, 17, 18 and C-terminal telopeptide of type I collagen. Also studied was sclerostin, a canonical Wnt signaling pathway inhibitor.19 More recently, the effects of chronic inflammation, which is common in patients with advanced CKD, has come into focus. Several conditions characterized by chronic inflammation are associated with excessive bone resorption,20,21 but the effects, if any, of different dialysis modalities on the inflammatory phenotype and CKD-related bone disease have not been studied.
The HDF, Hearts and Height (3H) study, a multicenter, longitudinal study in children receiving hemodiafiltration (HDF) compared with conventional hemodialysis (HD),22 showed that subclinical cardiovascular disease is prevalent in children on dialysis, with attenuated progression of vascular changes in children receiving HDF compared with conventional HD.23 In a cross-over trial when children on conventional HD were switched to HDF, and all other dialysis-related parameters were kept constant, a significant improvement in inflammation, antioxidant capacity, and endothelial risk profile was seen even within a short time (3 months),24 but the effect of these biochemical changes on CKD-related bone disease was not explored. We present a post hoc analysis of the 3H data to determine the changes in bone biomarkers in children on dialysis and the effect of different dialysis modalities on the evolution of MBD over a 1-year follow-up.
Material and Methods
Patients
This is a post hoc analysis of the 3H data set, a registered multicenter, parallel-arm intervention study, performed across 28 pediatric dialysis centers in 10 countries, monitoring children receiving kidney replacement therapy with HD or HDF for 1 year (ClinicalTrials.gov: NCT02063776). The 3H study protocol22 and primary outcomes23 have been previously published.
Briefly, standardized procedures for HDF and HD were provided to all centers, but to keep the study as “real life” as possible, individualized changes to the dialysis prescription were left to the treating physician. Incident and prevalent patients aged between 5 and 20 years undergoing postdilution HDF or HD on a 4 hours per session, 3 times per week schedule were eligible. A minimum follow-up of 12 months was required. Children in whom a living donor kidney transplant was planned and those on predilution HDF were excluded. To ensure good dialysis adequacy, prevalent patients on HD in whom the single pool Kt/V (K, dialyzer clearance of urea; t, dialysis time; V, volume of distribution of urea) was <1.2 in the month preceding recruitment were excluded.
Here we focus on MBD, including biomarkers of CKD-MBD, their evolution over the 12 months of follow-up, and the effect of inflammation, medications, and physical activity on these biomarkers. Because changes in height SD scores are extensively described in the original study,23 this is not addressed here. All patients in whom a serum sample at baseline was available for biomarker analysis were included in the cross-sectional analysis (n = 144; 83 on HD and 61 on HDF) and all those with serum samples at 12 months in the longitudinal follow-up (n = 103; 61 on HD and 42 on HDF). A Consolidated Standards of Reporting Trials figure is shown in Supplementary Figure S1. Data are reported as per Strengthening the Reporting of Observational studies in Epidemiology guidelines.25
Ethics Approval
The study was performed according to the principles of the declaration of Helsinki. It has been approved by the National Research Ethics Service Committee London—Bloomsbury, a Research Ethics Committee established by the Health Research Authority, England. Approval from local Institutional Review Boards was obtained for each participating site. Full written informed consent was obtained from all parents or caregivers, and assent from children, where applicable.
Sampling and Storage of Blood
Nonfasting blood samples were collected before the start of a midweek dialysis session, centrifuged, and stored at −80 °C in the local hospitals before being shipped to a central biorepository for aliquoting and long-term storage.
Measurement of Biomarkers
All analyses were performed in a blinded fashion in central laboratories. Samples were batched for analyses, and reagents from the same enzyme-linked immunosorbent assay kits were used for each batch, following manufacturer instructions. Measures of mineral homeostasis (calcium, phosphate, parathyroid hormone; Immulite 2500 Intact Parathyroid Hormone assay; Siemens Healthcare Diagnostics, Malvern, PA), 25-hydroxyvitamin D (tandem mass spectrometry), β2 microglobulin (β2M; Immulite Immunoassay System, Siemens,) and inflammation, including high-sensitivity C-reactive protein (hs-CRP; Sigma-Aldrich, St Louis, MO), interleukin-6 (IL-6; enzyme-linked immunosorbent assay, Thermo Fisher Scientific, Waltham, MA), and tumor necrosis factor-α (TNF-α; Thermo Fischer Scientific), were performed in the Chemical Pathology Unit at UCL Great Ormond Street Hospital. Enzymatic activities of BAP (MicroVue Quidel; Athens, OH), TRAP5b (MicroVue Quidel), and serum concentrations of intact fibroblast growth factor-23 (FGF23; Kainos Laboratories, Tokyo, Japan), soluble klotho (Immuno Biological Laboratories Co Ltd, Tokyo, Japan), sclerostin (Teco, Sissach, Switzerland), and C-terminal telopeptide of type I collagen (Immunodiagnostic Systems, Boldon, UK) were measured in the Research Unit of the Department of Pediatrics, Rostock University Medical Center, as described previously.13,14,26 The Infinite M200 microplate reader (Tecan, Crailsheim, Germany) with corresponding software (Magellan version 6.6) was used for data acquisition and transformation.
For quantification of fetuin-A, serum samples were adjusted to a protein concentration of 2 mg/ml and analyzed with sodium dodecyl sulfate–polyacrylamide gel electrophoresis with subsequent Western blotting and chemiluminescence detection.27 The GEL Logic 1500 (Kodak, Rochester, NY) was used for acquisition of images, and densitometry was performed with Gelanalyser 2010a software (GelAnalyzer.com) in the “quantity calibration mode.” Per gel, recombinant fetuin-A (15 g/μl to1 ng/μl) and a serum sample with known fetuin-A concentrations (3 ng/μl) served as standards and controls, respectively.
Statistical Methods
Continuous data are reported as median (interquartile range). Within-patient comparisons of biochemical measures on HD and HDF were analyzed using the Wilcoxon signed rank test. Comparisons between the HD and HDF groups were analyzed using the Mann-Whitney U test or χ2 test, as appropriate. Correlations between continuous variables were made using Pearson correlation coefficients if variables were normally distributed or Spearman rank correlation coefficients otherwise. BAP and TRAP5b that are significantly correlated with age and sex were converted to z-scores for comparison across the cohort.13
Linear regression models were used to analyze the evolution of bone disease over a 12-month follow-up. Regression models were built for the BAP/TRAP5b ratio and FGF23/klotho ratio, adjusting for their corresponding baseline values. The FGF23/klotho ratio followed a log-normal distribution, so log-linear regression was performed, and estimates were then back transformed; effect sizes are therefore presented as fold-change.
All potential confounders for the association between dialysis modality and MBD were included in multivariable analyses if P < .2 in univariable analyses. We decided a priori to include dialysis modality (HD vs. HDF), regardless of its level of statistical significance, because this was the primary independent variable of interest. Finally, to gain insight into their potential role as mediators of the effects of HD or HDF treatment, linear regression models were additionally adjusted for a measure of inflammation (12-month TNF-α) and a measure of middle-molecule clearance (12-month β2M) where P < .2 in univariable analyses (adjusted model 2 in Tables 1 and 2). To avoid multicollinearity, parathyroid hormone was excluded from both regression models. Given the risk of center bias, all regression models were adjusted for country as well as baseline values.
Table 1.
Factors associated with the BAP/TRAP5b ratio at 1 yeara
| Factor | Unadjusted |
Adjusted 1b |
Adjusted 2–including mediatorsc |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| Dialysis modality (HD vs HDF) | −2.64 | −3.77 to −1.51 | <.0001 | −2.66 | −3.91 to −1.41 | <.0001 | −1.74 | −4.25 to 0.77 | .18 |
| Dialysis vintage (per year longer) | −0.14 | −0.41 to 0.13 | .31 | ||||||
| Baseline weight-adjusted urine output (per 10 higher) | 0.29 | −0.16 to 0.73 | .21 | ||||||
| Growth hormone medication (yes vs no) | 0.54 | −1.07 to 2.16 | .51 | ||||||
| Physical activity (ref = 3) | .14 | 0.65 | .94 | ||||||
| 1 | −1.74 | −3.48 to 0.01 | −0.52 | −2.23 to 1.20 | −0.48 | −2.50 to 1.53 | |||
| 2 | −0.79 | −2.42 to 0.84 | 0.10 | −1.49 to 1.69 | −0.46 | −2.29 to 1.36 | |||
| Impaired motor development (yes vs no) | −1.17 | −3.22 to 0.88 | .26 | ||||||
| Potential mediators measured at 1 year | |||||||||
| β-2 microglobulin (per 10 higher) | −0.70 | −1.24 to −0.15 | .01 | −0.20 | −0.97 to 0.56 | .60 | |||
| Interleukin-6 (per 100 higher) | −0.07 | −0.16 to 0.15 | .11 | ||||||
| Tumor necrosis factor-α (per 100 higher) | −0.18 | −0.32 to −0.04 | .01 | 0.05 | −0.18 to 0.28 | .67 | |||
| hs-CRP (per 10 higher) | −0.48 | −1.04 to 0.09 | .10 | ||||||
| Calcium (per 1 higher) | 0.00 | −2.87 to 2.87 | 1.00 | ||||||
| Phosphate (per 1 higher) | −1.12 | −2.21 to −0.03 | .05 | −1.67 | −2.95 to −0.39 | .01 | |||
| Vitamin D (per 10 higher) | 0.04 | −0.35 to 0.45 | .82 | ||||||
| Convective volume adjusted for BSA (per 10 higher) | −0.91 | −2.23 to 0.41 | .17 | ||||||
BAP, bone alkaline phosphatase; BSA, body surface area; CI, confidence interval; HD, hemodialysis; HDF, hemodiafiltration; hs-CRP, high-sensitivity C-reactive protein; TRAP5b, tartrate-resistant acid phosphatase 5b.
All independent variables were measured at baseline. Results from linear regression models were additionally adjusted for baseline BAP/TRAP ratio and country.
Adjusted model 1: Adjusted for all potential confounders for the association between dialysis modality and bone disease with P < .2 in univariable analyses (n =104).
Adjusted model 2: Adjusted for all potential confounders, plus potential mediators measured at 12 months with P < .2 in univariable analyses (n = 78).
Table 2.
Factors associated with the FGF/klotho ratio at 1 yeara
| Factor | Unadjusted |
Adjusted 1b |
Adjusted 2–including mediatorsc |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Fold-change | 95% CI | P | Fold-change | 95% CI | P | Fold-change | 95% CI | P | |
| Dialysis modality (HD vs HDF) | 3.86 | 2.15–6.93 | <.0001 | 3.86 | 2.15–6.93 | <.0001 | 3.46 | 1.05–11.52 | .04 |
| Dialysis vintage (per year longer) | 1.07 | 0.94–1.23 | .31 | ||||||
| Weight adjusted urine output (per 10 higher) | 0.87 | 0.69–1.09 | .23 | ||||||
| Growth hormone medication (yes vs no) | 0.64 | 0.27–1.52 | .32 | ||||||
| BAP SD score (per 1 higher) | 0.99 | 0.84–1.16 | .89 | ||||||
| Physical activity (ref=3) | |||||||||
| 1 | 2.16 | ||||||||
| 2 | 1.84 | 0.88–5.32 | .22 | ||||||
| Impaired motor development (yes vs no) | 1.25 | 0.82–4.15 | .68 | ||||||
| Potential mediators measured at 1 year | 0.43–3.69 | ||||||||
| β-2 microglobulin (per 10 higher) | 1.41 | 1.08–1.84 | .01 | 1.08 | 0.75–1.56 | .68 | |||
| Interleukin-6 (per 100 higher) | 1.07 | 1.03–1.12 | .0005 | ||||||
| Tumor necrosis factor-α (per 100 higher) | 1.08 | 1.01–1.16 | .03 | 0.93 | 0.75–1.56 | .20 | |||
| hs-CRP (per 10 higher) | 1.34 | 1.02–1.77 | .04 | ||||||
| Calcium | 0.75 | 0.16–3.54 | .72 | ||||||
| Phosphate | 1.07 | 1.03–1.12 | .0008 | 1.58 | 0.78–3.20 | .20 | |||
| Vitamin D (per 10 higher) | 1.07 | 0.89–1.30 | .47 | ||||||
| Convective volume adjusted for BSA (per 10 higher) | 1.55 | 0.85–2.85 | .16 | ||||||
BAP, bone alkaline phosphatase; BSA, body surface area; CI, confidence interval; FGF, fibroblast growth factor; HD, hemodialysis; HDF, hemodiafiltration; hs-CRP, high-sensitivity C-reactive protein.
All independent variables measured at baseline. Results from linear regression models, additionally adjusted for baseline FGF/klotho ratio and country.
Adjusted model 1: Adjusted for all potential confounders for the association between dialysis modality and bone disease with P < .2 in univariable analyses (n = 144.)
Adjusted model 2: Adjusted for all potential confounders, plus potential mediators measured at 12 months with P < .2 in univariable analyses (n = 71).
Analyses were performed in SAS 9.4 software (SAS Institute Inc., Cary, NC). All analyses were 2-sided, and P < .05 was nominally considered statistically significant.
Results
Study Cohort
Demographics of the study cohort are presented in Supplementary Table S1. The HD and HDF patients were comparable for age, sex, race, underlying kidney disease, comorbidities, proportion of incident and prevalent patients, and the time on dialysis before the start of the 3H study. The number of incident and prevalent dialysis patients and dialysis vintage were comparable between study arms, but more patients on HDF had received a previous transplant.
As with all dialysis studies, incident patients were allowed a period of stability on dialysis before inclusion in the 3H study and had a median dialysis vintage of 1.2 months (interquartile range, 0.22–1.4 months) in the HD group and 1.12 months (interquartile range, 0.18–1.6 months in the HDF group (P = .76). Although there was no significant difference in height SD score at baseline between HD and HDF patients in the original 3H cohort23; in this substudy, children on HD were shorter (Supplementary Table S1), but there was no significant difference in the height SD score at 12 months between HD and HDF patients.
Significantly more children in the HDF cohort received cholecalciferol (62.3% vs. 38.6% on HD; P = .005), but there was no difference in any other medications prescribed between groups. Notably, there was no difference in the routinely measured biomarkers of CKD-MBD, including albumin-corrected calcium, phosphorus, parathyroid hormone, alkaline phosphatase, and 25-hydroxyvitamin D, which were comparable between HD and HDF cohorts at baseline and at 12 months (Supplementary Table S2). The dialysate composition was comparable between groups. Children who could not be included in the final follow-up due to lack of sufficient serum samples for biomarker analyses had comparable demographics as well as baseline concentrations of biomarkers compared with those included in the follow-up analyses.
Inflammation
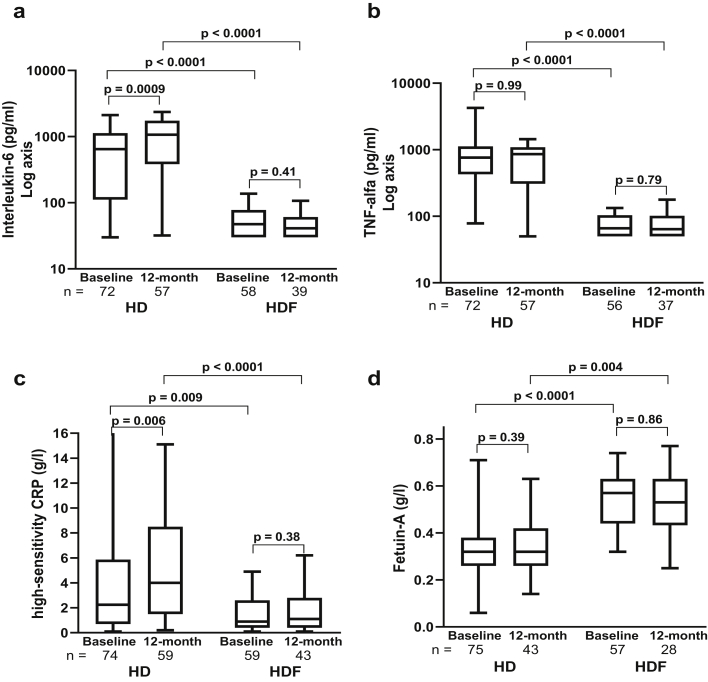
The inflammatory markers IL-6, TNF-α, and hs-CRP were significantly and consistently lower in the HDF compared with HD patients at baseline and at 12 months (Figure 1 and Supplementary Table S3). Given that incident patients received HD or HDF for approximately 1 month before the start of the 3H study, even the baseline levels of inflammation were lower in HDF compared with HD, consistent with previous data.23,24 Notably, IL-6 and hs-CRP increased significantly from baseline to 12 months in HD, but no further reduction was seen in the HDF cohort over the 12-month follow-up. Fetuin-A, which is downregulated in inflammation, was higher in HDF compared with HD at baseline and at 12 months, but with no change from baseline in either group (Figure 1 and Supplementary Table S3). Serum phosphate levels showed a weak inverse correlation with the height SD score at 12 months, but no correlation was seen with the inflammatory markers (Supplementary Table S4).
Figure 1.
Changes in inflammatory markers at baseline and 12 months in the hemodialysis (HD) and hemodiafiltration (HDF) cohorts: (a) interleukin-6, (b) tumor necrosis factor (TNF)-α, (c) high-sensitivity C-reactive protein (CRP), and, (d) fetuin-A. Within-group analyses were performed by Wilcoxon matched-pairs signed rank test and the HD versus HDF cohorts were compared by the Mann-Whitney U test. The horizontal line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively, and the whiskers mark minimum and maximum of all the data.
Biomarkers of Bone Formation and Resorption
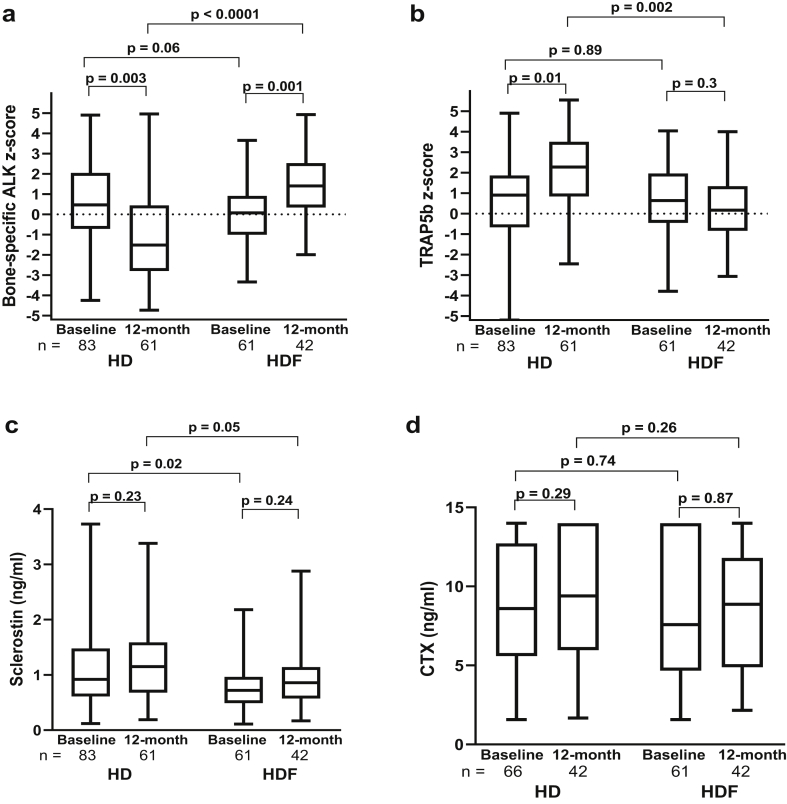
The BAP and TRAP5b z-score and C-terminal telopeptide of type I collagen were comparable between HD and HDF cohorts at baseline (Figure 2 and Supplementary Table S3). The BAP z-score increased in HDF and decreased in HD patients over the 12-month follow-up, with significantly higher levels in HDF compared with HD patients at 12 months (P < .0001). In contrast, TRAP5b z-scores increased in HD over the 12 months but remained unchanged in HDF, with significantly lower levels in HDF compared with HD at 12 months (P = .002). Sclerostin, which is known to be cleared by convective clearance,28 was lower in HDF compared with HD patients even at baseline (P = .02), but no change was seen over the 12-month follow-up in either cohort. There was no difference in C-terminal telopeptide of type I collagen in either group at any time point.
Figure 2.
Changes in bone markers at baseline and at 12 months in the hemodialysis (HD) and hemodiafiltration (HDF) cohorts: (a) bone-specific alkaline phosphatase (ALK) z-score, (b) tartrate-resistant acid phosphatase 5b (TRAP5b) z-score, (c) sclerostin, and (d) C-terminal telopeptide of type I collagen (CTX). Within-group analyses were performed by the Wilcoxon matched-pairs signed-rank test, and HD versus HDF cohorts were compared by the Mann-Whitney U test. The horizontal line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively, and the whiskers mark minimum and maximum of all the data.
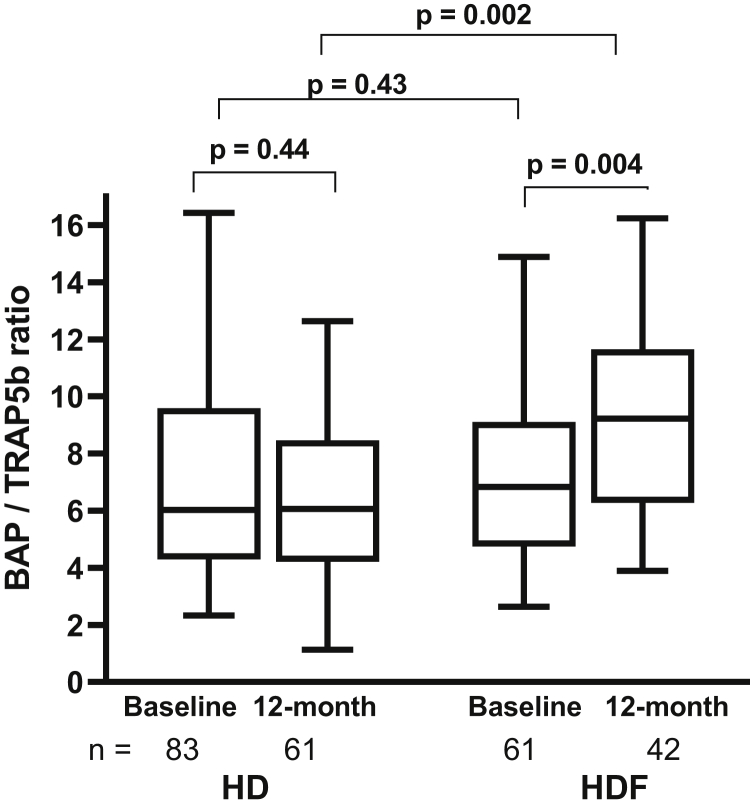
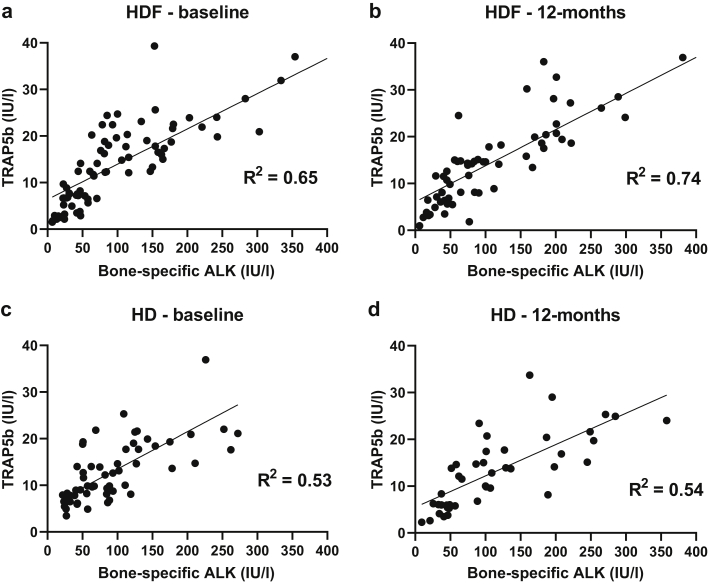
The ratio of the enzymatic activity of BAP/TRAP5b increased in HDF patients (P = .004) but remained unchanged in HD (P = .44) over 12 months, resulting in a significant increase in the HDF compared with the HD cohort at 12 months (P = .002; Figure 3). Unlike healthy children in whom BAP and TRAP5b are strongly correlated with each other,13 BAP and TRAP5b were modestly correlated in both HD and HDF patients (Figure 4), implying a relative uncoupling of bone formation and resorption activity. However, while HDF patients showed an improvement of the BAP/TRAP5b ratio (R2 = 0.65 to 0.74 from baseline to 12 months), there was no change in HD patients (R2 = 0.53 to 0.54) during the same period.
Figure 3.
Ratio of the enzymatic activity of bone alkaline phosphatase (BAP)/tartrate-resistant acid phosphatase 5b (TRAP5b) at baseline and 12 months in the hemodialysis (HD) and hemodiafiltration (HDF) cohorts. Within-group analyses were performed by the Wilcoxon matched-pairs signed rank test, and HD versus HDF cohorts were compared by the Mann-Whitney U test. The horizontal line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively, and the whiskers mark minimum and maximum of all the data.
Figure 4.
Correlation of bone-specific alkaline phosphatase (ALK) and tartrate-resistant acid phosphatase 5b (TRAP5b) in the hemodiafiltration (HDF) cohort at (a) baseline and (b) 12 months and in the hemodialysis (HD) cohort at (c) baseline and (d) 12 months.
On univariable analysis, significant predictors of the BAP/TRAP5b ratio at 12 months were the dialysis modality (HD vs. HDF) and 12-month serum concentrations of β2M, TNF-α, and phosphate (Table 1). After adjustment for potential confounders, dialysis modality remained associated with BAP/TRAP5b: children receiving HD had an average 2.66 lower BAP/TRAP5b ratio (95% confidence interval, −3.91 to −1.41; P < .0001). Additional adjustment for the inflammatory markers attenuated the difference between the HD and HDF groups to −1.74 but did not completely remove the association, suggesting that a reduction in the inflammatory milieu may contribute to an improvement in the BAP/TRAP5b ratio in the HDF cohort. There was no difference in the BAP/TRAP5b ratio in patients who received or did not receive growth hormone treatment.
FGF23 and Klotho
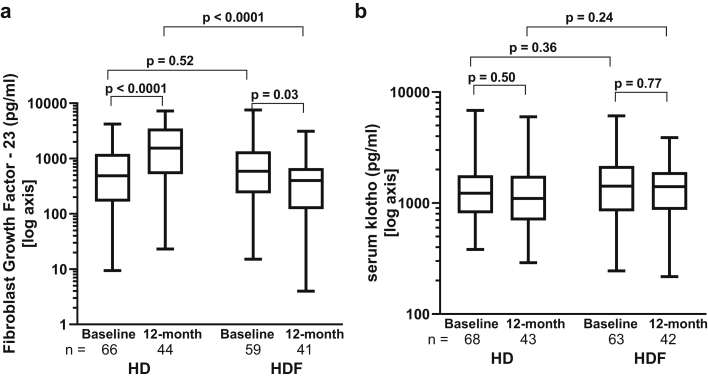
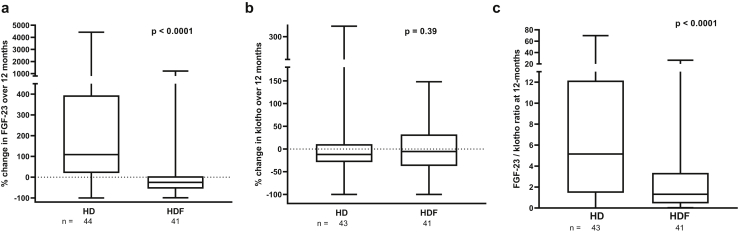
Intact FGF23, a secreted protein with a molecular weight of ~31 kDa, is cleared by HDF.29 Consequently, FGF23 levels decreased by 25% (−55% to 4.6%) in HDF but increased by 109% (20% to 394%) in HD, resulting in a significant difference between groups at 12 months (P < .0001; Figures 5a and 6a and Supplementary Table S3). Serum klotho levels were comparable between HD and HDF cohorts and did not show any change over 12 months (Figures 5b and 6b and Supplementary Table S3).
Figure 5.
(a) Changes in fibroblast growth factor 23 (FGF-23) and (b) serum klotho from baseline to 12 months in the hemodialysis (HD) and hemodiafiltration (HDF) cohorts. (c) FGF-23/klotho ratio (based on their molecular weights) at 12 months in the HD and HDF cohorts. HD versus HDF cohorts were compared by the Mann-Whitney U test. The horizontal line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively, and the whiskers mark minimum and maximum of all the data.
Figure 6.
Percentage changes in (a) fibroblast growth factor 23 (FGF-23) and (b) serum klotho during 12 months of hemodialysis (HD) and hemodiafiltration (HDF) treatment and (c) in the FGF23/klotho ratio (based on their molecular weights) at 12 months in the HD and HDF cohorts. HD versus HDF cohorts were compared by the Mann-Whitney U test. The horizontal line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively, and the whiskers mark minimum and maximum of all the data.
The FGF23/klotho ratio (based on their molecular weights) was significantly higher in HD compared with HDF (P < 0.0001; Figure 6c). On univariable analysis, the significant predictors of a higher FGF23/klotho ratio were the dialysis modality and the 12-month β2M and phosphate levels as well as inflammatory markers IL-6, TNF-α, and hs-CRP (Table 2). As no potential confounders, including dialysis vintage, residual kidney function (expressed as urine output in ml/kg per day from a 24-hour midweek urine collection), medications, and physical activity markers were found to be associated with the FGF23/klotho ratio, results of multivariable analysis were similar: those receiving HD had a 3.86-times higher FGF23/klotho ratio than those on HDF (95% confidence interval, 2.15–6.93; P < .0001). Additional adjustment for inflammatory markers at 12 months showed a significant but attenuated association with dialysis modality (HD patients had a 3.46-fold higher FGF23/klotho ratio [95% confidence interval, 1.05–11.52; P = .04]), suggesting that although inflammatory markers could modulate the association between dialysis modality and the FGF23/klotho ratio, they did not explain the observed differences. Exclusion of dialysis modality from the latter regression model reduced the observed R2 value from 33.9% to 29.7%.
Discussion
Dialysis is known to cause a proinflammatory milieu and is associated with profound dysregulation of mineral bone metabolism, but the impact of different dialysis modalities on CKD-MBD in adults and children remains largely undescribed. This prospective observational study, a post hoc analysis of bone-related outcomes in children on maintenance dialysis, has shown that children on HDF have an attenuated inflammatory profile, increased bone formation, stable sclerostin concentrations, and lower FGF23/klotho ratios compared with those on conventional HD. The ~25% reduction in FGF23 and a lower FGF23/klotho ratio in patients on HDF may explain the lower left ventricular mass in the 3H cohort23 and reduced cardiovascular mortality in adult randomized studies comparing HDF with conventional HD.30 HDF may achieve these effects through an improved clearance of the large middle-sized molecules, including proinflammatory cytokines and other “uremic toxins,” as well as reduced production of these molecules in the more biocompatible milieu in HDF. There was no difference in residual renal function in HD and HDF cohorts, and this did not influence markers of mineral metabolism.
While bone turnover is best described on bone histology from tetracycline-labeled bone biopsy specimens, circulating biomarkers are useful surrogate measures: serum BAP levels are reflective of bone formation and TRAP5b of bone resorption.7 Unlike other bone biomarkers, BAP and TRAP5b are both unaffected by CKD stage.31 A large prospective observational study measuring a range of biomarkers in children with CKD stages 3 to 5 showed a high bone turnover, with a clear increase in the BAP z-score and a small but significant increase of TRAP5b z-scoress.14 We showed that the BAP-to-TRAP5b activity remained almost constant in children on HD, whereas children on HDF had an increase in the BAP/TRAP ratio that was comparable to that seen in healthy children.13 Although we did not see an association with growth hormone treatment, a previous study showed that BAP was higher and TRAP5b was lower in children receiving recombinant growth hormone than in untreated controls,14 further strengthening the case for an increased BAP/TRAP5b ratio reflecting osteoanabolism in the HDF cohort.
We showed that the BAP/TRAP5b ratio inversely correlated with the inflammatory markers on univariable analysis. However, on multivariable analysis, adjustment for TNF-α attenuated but did not completely remove the difference between HD and HDF groups, suggesting that a reduction in the inflammatory milieu may contribute to but is not the sole cause of the observed improvement in the BAP/TRAP5b ratio in the HDF cohort. A similar inverse association of BAP and TRAP5b with CRP has been shown in children with CKD 3 to 5, implying that the effect of inflammation on reduced bone turnover is independent of the underlying renal disease or the effect of dialysis per se.14
A recent cross-sectional study in 63 children with CKD stages 2 to 5 showed that TNF-α correlated with biomarkers of CKD-MBD and showed an inverse association with the height z-score, suggesting that inflammation may contribute to growth impairment in pediatric CKD.32 This study, however, showed an unexpected positive correlation between TNF-α and both bone formation markers alkaline phosphatase and BAP, making the inhibitory effect of inflammatory cytokines on bone turnover difficult to reconcile.32
Chronic inflammation is a multisystem disorder, and there are several potential mechanisms by which inflammatory cytokines can aggravate bone disease in CKD. TNF-α inhibits the expression of RUNX2, a major transcription factor that blocks osteoblast differentiation33 and promotes osteoclastogenesis.34 Receptor activator of nuclear factor κB ligand (RANKL; also known as TNF ligand superfamily, member 11) is an essential cytokine in osteoclastogenesis but may play a paradoxical role in bone homeostasis by also increasing the expression of bone morphogenic protein 2 and stimulating the Wnt pathway.35 Binding of RANKL to the RANK receptor leads to activation of TNF receptor-associated factors and upregulation of osteoclast target genes.36
TNF-α inhibitors are used in chronic inflammatory disorders of childhood, such as inflammatory bowel disease and juvenile idiopathic arthritis, wherein children treated with TNF-α inhibitors showed improved trabecular bone mineral density and cortical structure,37 reduced bone loss,38 and greater statural growth.39,40 Our data suggest that high serum phosphate levels negatively influence bone turnover. This effect may be mediated via the parathyroid hormone effect on RANKL-directed osteoclastogenesis or even via stimulating inflammation. Dietary phosphate loading increased serum TNF-α in uremic rats.41 After parathyroidectomy, bone expression of TNF-α significantly decreases in patients on HD.42
Sclerostin, a potent inhibitor of the Wnt/β-catenin pathway,43 showed an early reduction in plasma concentrations with convective clearance.24 Children on HDF in this study had sclerostin levels comparable to those with moderate to severe CKD in the series published by Doyon et al.,14 although levels in both groups were lower than in age-matched healthy children.44 Serum sclerostin levels in healthy children were inversely associated with cortical volumetric bone mineral density and cortical thickness,44 and lower sclerostin levels were predictive of high bone turnover even in adults with CKD.45
FGF23, a phosphaturic hormone produced by osteocytes, is a 32-kDa protein that is known to be cleared by HDF. Comparable to previous studies, we showed ~25% lower FGF23 levels in HDF children,29 whereas levels increased by >100% in HD children. As seen with the dialytic clearance of other large middle-sized molecules compounds such as FGF23, IL-6, and TNF-α, although an early reduction in plasma concentrations is seen with convective clearance,24 this is not sufficient to further reduce plasma concentrations in the long-term, perhaps because production of these compounds overwhelms the clearance capacity of thrice-weekly dialysis.28 Although protective in early CKD, FGF23 is known to have several off-target effects, independent of changes in mineral metabolism, on cardiac myocytes,46 with an increased prevalence of left ventricular hypertrophy47 and premature death in adults with all stages of CKD,48 stressing the importance of reducing FGF23 levels by HDF. Importantly, these changes in FGF23 levels were independent of serum phosphate, which did not change in the HD or HDF cohorts. Nevertheless, serum phosphate levels are a poor proxy of phosphate exposure, and other groups have shown that phosphate control is better in patients on HDF compared with HD.49 Similarly, FGF23 levels are significantly lower in patients on short daily HD compared with those on conventional thrice-weekly HD, suggesting that FGF23 levels may be a more sensitive biomarker of cumulative phosphate burden.50
FGF23 expression is markedly increased in acute inflammation, but osteocytes are able to maintain normal serum levels of biologically active FGF23, despite the rise in FGF23 transcription, by commensurately increasing FGF23 cleavage.20 In contrast, chronic inflammation also increases biologically active intact FGF23 levels, perhaps because sustained periods of FGF23 overproduction overwhelm the capacity of the FGF23 cleavage apparatus in osteocytes. Measurement of FGF23 fragments may have revealed a stronger association between FGF23 concentrations and inflammation. Cytokine profiling in a cohort of adults with predialysis CKD has shown that several cytokines may play a role in increasing the production of FGF23.51,52 Furthermore, inflammation may also indirectly stimulate the production of FGF23 through the activation of hepcidin and induction of hypoferremia.20 Thus, in patients on dialysis, FGF23 can aggravate the bone disease of CKD and lose any protective role it may have in countering hyperphosphatemia in the early stages of CKD.53, 54, 55 Importantly, the relationship between inflammation and CKD-MBD may be bidirectional and self-perpetuating: FGF23 stimulates hepatocytes to increase secretion of IL-6 and CRP, and FGF23 may itself be affected by inflammatory cytokines.55,56
Conventional HD evokes an inflammatory response by releasing inflammatory cytokines.6,57,58 In our study, the inflammatory cytokines IL-6, TNF-α, and hs-CRP were higher in HD than in HDF patients at baseline and continued to increase significantly in HD even after adjusting for baseline levels, but no change was seen in the HDF cohort over the 12-month follow-up. As with all dialysis studies, the “incident” patients in our cohort were on dialysis for a median of 1 month to stabilize and achieve the optimal dialysis program before the first study measures were recorded. The inflammatory markers showed a strong correlation with β2M, a prototype middle molecule, indicating that clearance on HDF largely accounted for lower levels in HDF.
Similar results were shown in the CONvective TRAnsport Study (CONTRAST) study: although CRP and IL-6 concentrations increased in patients treated with HD during the 3-year study period, they remained stable in the HDF cohort, suggesting that HDF maintains a sustained reduction in inflammatory activity.57,59,60 Notably, we found lower levels of hsCRP, a large middle-sized molecule, in HDF compared with HD patients. The molecular weight of hsCRP is higher than that of albumin, so its clearance is very unlikely, but removal of smaller molecules that trigger hsCRP generation may have led to an improved antioxidant–oxidant balance and reduced levels in patients on HDF.
Children on extracorporeal dialysis are a “rare disease” cohort, with only ~450 children across Europe (according to the European Renal Registry 2018 data), and usually have a short dialysis vintage before transplantation, making longitudinal studies difficult. The 3H study recruited 40% of this pediatric dialysis cohort,23 and to adjust for possible differences in country-specific practices, all regression models were adjusted for country as well as baseline values.
However, some limitations of the current study must be acknowledged. As with all pediatric studies, the scarcity of hard end points, such as fractures, necessitates the use of surrogate biomarkers of bone health. Biomarkers are easy to measure, relatively cheap, and can be repeatedly assessed in the same patient, but may be affected by age, pubertal stage, circadian rhythm, renal or liver function, and recent fractures. The impact of inflammatory markers on the pathway from dialysis modality to bone growth was investigated by simple adjustment in the subgroup of participants with measurements available for all markers. Future work considering approaches such as causal mediation analysis to explore these pathways in detail could provide further insight into these potential mechanisms. In the 3H study, there was no difference in residual renal function in HD and HDF cohorts,23 and this did not influence markers of mineral metabolism.
Although it would be ideal to compare children on similar high-flux dialyzers and provide ultrapure water for HD patients too, in the 3H study 27% of HD patients were dialyzed with mid- or low-flux dialyzers, and only 51% had ultrapure water for HD,23 reflecting the real-life situation of pediatric dialysis across Europe, but rendering very small numbers for subgroup analysis. Unfortunately, it has not been possible to analyze the impact of high-flux versus low-flux HD or of the effect of immunosuppressant therapy, given small patient numbers in each category.
Conclusion
We have shown that children on HDF have increased bone formation and lower FGF23/klotho ratios compared with those on HD, which may be at least partly explained by the improved clearance of large and middle molecular weight inflammatory cytokines by HDF. Further long-term studies are required to determine whether the improved bone biomarker profile improves key patient-level outcomes such as statural growth, bone pain, and fractures.
Disclosures
RS has an investigator-initiated study funded by Fresenius Medical Care and has received speaker honoraria from Fresenius Medical Care and Amgen. CPS and FS receive funding for investigator-initiated research from Fresenius Medical Care, and CPD also from Amgen.
Acknowledgments
The 3H study was sponsored by Kidney Research UK. Part sponsorship was obtained from Fresenius Medical Care, who approved the study protocol, but had no role in data collection, data analysis, or drafting the manuscript. RS is funded by a National Institute for Health Research, Career Development Fellowship for this research project. This publication presents independent research funded by the National Institute for Health Research. The views expressed are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health and Social Care. A part of the work took place in the Biomedical Research Centre at Great Ormond Street Hospital for Children National Health Service Foundation Trust and University College London.
Author Contributions
SB, AAg, AAn, BA, VA, AB, IKB, NC, AD, SH, SK, CL, ML, LO, FP, BR, CSa, MS, MDS, BS, CJS, EV, AY, MF, FS, CS, and RS contributed patients to the study. RS is the principal investigator and obtained funding. RS, CPS, and FS designed the study. DCF and AR performed all biomarker analyses. CS performed the statistical analyses. RS, DCF, and CS drafted the paper. All authors read and approved the final manuscript.
Footnotes
Figure S1. Flow chart of study populations.
Table S1. Demographics of the study population at baseline.
Table S2. Routine CKD mineral bone disease related measures at baseline and 12 months in the HD and HDF cohorts.
Table S3. Bone-specific biomarkers at baseline and 12-months in the HD and HDF cohorts.
Table S4. Factors associated with height SD score at 12-month follow-up.
STROBE Checklist
Supplementary Material
Figure S1. Flow chart of study populations.
Table S1. Demographics of the study population at baseline.
Table S2. Routine CKD mineral bone disease related measures at baseline and 12 months in the HD and HDF cohorts.
Table S3. Bone-specific biomarkers at baseline and 12-months in the HD and HDF cohorts.
Table S4. Factors associated with height SD score at 12-month follow-up.
STROBE Checklist
References
- 1.Bacchetta J., Harambat J., Cochat P., Salusky I.B., Wesseling-Perry K. The consequences of chronic kidney disease on bone metabolism and growth in children. Nephrol Dial Transplant. 2012;27:3063–3071. doi: 10.1093/ndt/gfs29927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wesseling-Perry K. Defective skeletal mineralization in pediatric CKD. Curr Osteoporos Rep. 2015;13:98–105. doi: 10.1007/s11914-015-0253-4. [DOI] [PubMed] [Google Scholar]
- 3.Groothoff J.W., Offringa M., Van Eck-Smit B.L. Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int. 2003;63:266–275. doi: 10.1046/j.1523-1755.2003.00727.x.63. [DOI] [PubMed] [Google Scholar]
- 4.Haffner D., Schaefer F., Nissel R. Effect of growth hormone treatment on the adult height of children with chronic renal failure. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. N Engl J Med. 2000;343:923–930. doi: 10.1056/NEJM200009283431304. [DOI] [PubMed] [Google Scholar]
- 5.Denburg M.R., Kumar J., Jemielita T. Fracture burden and risk factors in childhood CKD: results from the CKiD Cohort Study. J Am Soc Nephrol. 2016;27:543–550. doi: 10.1681/ASN.2015020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shroff R., Long D.A., Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 2013;24:179–189. doi: 10.1681/ASN.2011121191. [DOI] [PubMed] [Google Scholar]
- 7.Bakkaloglu S.A., Bacchetta J., Lalayiannis A.D. Bone evaluation in paediatric chronic kidney disease: clinical practice points from the European Society for Paediatric Nephrology CKD-MBD and Dialysis working groups and CKD-MBD working group of the ERA-EDTA. Nephrol Dial Transplant. 2021;36:413–425. doi: 10.1093/ndt/gfaa210. [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(suppl 3):S1–S202. [PubMed] [Google Scholar]
- 9.Lalayiannis A.D., Crabtree N.J., Fewtrell M. Assessing bone mineralisation in children with chronic kidney disease: what clinical and research tools are available? Pediatr Nephrol. 2020;35:937–957. doi: 10.1007/s00467-019-04271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalayiannis AD, Crabtree NJ, Ferro CJ, et al. Routine serum biomarkers, but not dual-energy X-ray absorptiometry, correlate with cortical bone mineral density in children and young adults with chronic kidney disease. Nephrol Dial Transplant. Published online October 23, 2020. https://doi.org/10.1093/ndt/gfaa199 [DOI] [PubMed]
- 11.Kleerekoper M. Biochemical markers of bone turnover: why theory, research, and clinical practice are still in conflict. Clin Chem. 2001;47:1347–1349. [PubMed] [Google Scholar]
- 12.Singer F.R., Eyre D.R. Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med. 2008;75:739–750. doi: 10.3949/ccjm.75.10.73975. [DOI] [PubMed] [Google Scholar]
- 13.Fischer D.C., Mischek A., Wolf S. Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Ann Clin Biochem. 2012;49:546–553. doi: 10.1258/acb.2012.011274. [DOI] [PubMed] [Google Scholar]
- 14.Doyon A., Fischer D.C., Bayazit A.K. Markers of bone metabolism are affected by renal function and growth hormone therapy in children with chronic kidney disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0113482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 16.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann C. Transcriptional networks controlling skeletal development. Curr Opin Genet Dev. 2009;19:437–443. doi: 10.1016/j.gde.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Kubota T., Michigami T., Ozono K. Wnt signaling in bone metabolism. J Bone Miner Metab. 2009;27:265–271. doi: 10.1007/s00774-009-0064-8. [DOI] [PubMed] [Google Scholar]
- 19.Brandenburg V.M., D'Haese P., Deck A. From skeletal to cardiovascular disease in 12 steps-the evolution of sclerostin as a major player in CKD-MBD. Pediatr Nephrol. 2016;31:195–206. doi: 10.1007/s00467-015-3069-7. [DOI] [PubMed] [Google Scholar]
- 20.David V., Martin A., Isakova T. In inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89:135–146. doi: 10.1038/ki.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukasaki M., Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol. 2019;19:626–642. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 22.Shroff R., Bayazit A., Stefanidis C.J. Effect of haemodiafiltration vs conventional haemodialysis on growth and cardiovascular outcomes in children–the HDF, Heart and Height (3H) study. BMC Nephrol. 2018;19:199. doi: 10.1186/s12882-018-0998-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shroff R., Smith C., Ranchin B. Effects of hemodiafiltration versus conventional hemodialysis in children with ESKD: the HDF, Heart and Height Study. J Am Soc Nephrol. 2019;30:678–691. doi: 10.1681/ASN.2018100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ağbaş A., Canpolat N., Çalışkan S. Hemodiafiltration is associated with reduced inflammation, oxidative stress and improved endothelial risk profile compared to high-flux hemodialysis in children. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Shroff R., Fewtrell M., Heuser A. Naturally occurring stable calcium isotope ratios in body compartments provide a novel biomarker of bone mineral balance in children and young adults. J Bone Miner Res. 2021;36:133–142. doi: 10.1002/jbmr.4158. [DOI] [PubMed] [Google Scholar]
- 27.Schaible J., Wigger M., Staude H. Serum fetuin-A and vitamin D in children with mild-to-severe chronic kidney disease: a cross-sectional study. Nephrol Dial Transplant. 2012;27:1107–1113. doi: 10.1093/ndt/gfr382. [DOI] [PubMed] [Google Scholar]
- 28.Carlson N., Mortensen O.H., Axelsen M., Pedersen R.S., Heaf J.G. Clearance of sclerostin, osteocalcin, fibroblast growth factor 23, and osteoprotegerin by dialysis. Blood Purif. 2017;44:122–128. doi: 10.1159/000465513. [DOI] [PubMed] [Google Scholar]
- 29.Pérouse de Montclos T., Ranchin B., Leclerc A.L. Online hemodiafiltration in children and hypoparathyroidism: a single-centre series of cases. Article in French. Nephrol Ther. 2014;10:35–38. doi: 10.1016/j.nephro.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Maduell F., Moreso F., Pons M. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–497. doi: 10.1681/ASN.2012080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang C. The use of bone turnover markers in chronic kidney disease-mineral and bone disorders. Nephrology (Carlton) 2017;22(suppl 2):11–13. doi: 10.1111/nep.13014. [DOI] [PubMed] [Google Scholar]
- 32.Meza K., Biswas S., Zhu Y.S. Tumor necrosis factor-alpha is associated with mineral bone disorder and growth impairment in children with chronic kidney disease. Pediatr Nephrol. 2021;36:1579–1587. doi: 10.1007/s00467-020-04846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding J., Ghali O., Lencel P. TNF-alpha and IL-1beta inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009;84:499–504. doi: 10.1016/j.lfs.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Lam J., Takeshita S., Barker J.E., Kanagawa O., Ross F.P., Teitelbaum S.L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osta B., Benedetti G., Miossec P. Classical and paradoxical effects of TNF-alpha on bone homeostasis. Front Immunol. 2014;5:48. doi: 10.3389/fimmu.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krum S.A., Chang J., Miranda-Carboni G., Wang C.Y. Novel functions for NFkappaB: inhibition of bone formation. Nat Rev Rheumatol. 2010;6:607–611. doi: 10.1038/nrrheum.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin L.M., Thayu M., Baldassano R.N. Improvements in bone density and structure during anti-TNF-alpha therapy in pediatric Crohn’s disease. J Clin Endocrinol Metab. 2015;100:2630–2639. doi: 10.1210/jc.2014-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simonini G., Giani T., Stagi S., de Martino M., Falcini F. Bone status over 1 yr of etanercept treatment in juvenile idiopathic arthritis. Rheumatology (Oxford) 2005;44:777–780. doi: 10.1093/rheumatology/keh592. [DOI] [PubMed] [Google Scholar]
- 39.Tynjala P., Lahdenne P., Vahasalo P., Kautiainen H., Honkanen V. Impact of anti-TNF treatment on growth in severe juvenile idiopathic arthritis. Ann Rheum Dis. 2006;65:1044–1049. doi: 10.1136/ard.2005.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malik S., Ahmed S.F., Wilson M.L. The effects of anti-TNF-alpha treatment with adalimumab on growth in children with Crohn’s disease (CD) J Crohns Colitis. 2012;6:337–344. doi: 10.1016/j.crohns.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Yamada S., Tokumoto M., Tatsumoto N. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am J Physiol Renal Physiol. 2014;306:F1418–F1428. doi: 10.1152/ajprenal.00633.2013. [DOI] [PubMed] [Google Scholar]
- 42.Santos F.R., Moyses R.M., Montenegro F.L., Jorgetti V., Noronha I.L. IL-1beta, TNF-alpha, TGF-beta, and bFGF expression in bone biopsies before and after parathyroidectomy. Kidney Int. 2003;63:899–907. doi: 10.1046/j.1523-1755.2003.00835.x. [DOI] [PubMed] [Google Scholar]
- 43.Drueke T.B., Lafage-Proust M.H. Sclerostin: just one more player in renal bone disease? Clin J Am Soc Nephrol. 2011;6:700–703. doi: 10.2215/CJN.01370211. [DOI] [PubMed] [Google Scholar]
- 44.Kirmani S., Amin S., McCready L.K. Sclerostin levels during growth in children. Osteoporos Int. 2012;23:1123–1130. doi: 10.1007/s00198-011-1669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cejka D., Herberth J., Branscum A.J. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol. 2011;6:877–882. doi: 10.2215/CJN.06550810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faul C., Amaral A.P., Oskouei B. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutiérrez O.M., Januzzi J.L., Isakova T. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutiérrez O.M., Mannstadt M., Isakova T. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lornoy W., De Meester J., Becaus I. Impact of convective flow on phosphorus removal in maintenance hemodialysis patients. J Ren Nutr. 2006;16:47–53. doi: 10.1053/j.jrn.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Zaritsky J., Rastogi A., Fischmann G. Short daily hemodialysis is associated with lower plasma FGF23 levels when compared with conventional hemodialysis. Nephrol Dial Transplant. 2014;29:437–441. doi: 10.1093/ndt/gft382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egli-Spichtig D., Imenez Silva P.H., Glaudemans B. Tumor necrosis factor stimulates fibroblast growth factor 23 levels in chronic kidney disease and non-renal inflammation. Kidney Int. 2019;6:890–905. doi: 10.1016/j.kint.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Wallquist C., Mansouri L., Norrbäck M. Associations of fibroblast growth factor 23 with markers of inflammation and leukocyte transmigration in chronic kidney disease. Nephron. 2018;138:287–295. doi: 10.1159/000485472. [DOI] [PubMed] [Google Scholar]
- 53.Leifheit-Nestler M., Richter B., Basaran M. Impact of altered mineral metabolism on pathological cardiac remodeling in elevated fibroblast growth factor 23. Front Endocrinol (Lausanne) 2018;9:333. doi: 10.3389/fendo.2018.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leifheit-Nestler M., Große Siemer R., Flasbart K. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant. 2016;31:1088–1099. doi: 10.1093/ndt/gfv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharaf El Din U.A., Salem M.M., Abdulazim D.O. Is fibroblast growth factor 23 the leading cause of increased mortality among chronic kidney disease patients? A narrative review. J Adv Res. 2017;8:271–278. doi: 10.1016/j.jare.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh S., Grabner A., Yanucil C. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016;90:985–996. doi: 10.1016/j.kint.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leurs P., Lindholm B., Stenvinkel P. Effects of hemodiafiltration on uremic inflammation. Blood Purif. 2013;35(suppl 1):11–17. doi: 10.1159/000346359. [DOI] [PubMed] [Google Scholar]
- 58.Dai L., Golembiewska E., Lindholm B., Stenvinkel P. End-stage renal disease, inflammation and cardiovascular outcomes. Contrib Nephrol. 2017;191:32–43. doi: 10.1159/000479254. [DOI] [PubMed] [Google Scholar]
- 59.Santoro A., Mancini E. Is hemodiafiltration the technical solution to chronic inflammation affecting hemodialysis patients? Kidney Int. 2014;86:235–237. doi: 10.1038/ki.2014.81. [DOI] [PubMed] [Google Scholar]
- 60.den Hoedt C.H., Bots M.L., Grooteman M.P. Online hemodiafiltration reduces systemic inflammation compared to low-flux hemodialysis. Kidney Int. 2014;86:423–432. doi: 10.1038/ki.2014.9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.