Abstract
We addressed the influence of the incubation time (24 h versus 48 h), starting inoculum size (standard inoculum size, ∼103 CFU/ml, versus large inoculum size, ∼104 CFU/ml), and supplementation with 2% glucose of RPMI 1640 medium on the spectrophotometric determination of the MICs of amphotericin B, fluconazole, and itraconazole. We compared the MICs determined spectrophotometrically with those determined by the standard broth macrodilution method (National Committee for Clinical Laboratory Standards approved guideline M27-A). The agreement between the results of the spectrophotometric and standard methods for amphotericin B testing was 100%; this agreement was independent of the inoculum size and incubation time. On the other hand, the agreement for the results for fluconazole testing and itraconazole testing was dependent on the inoculum size and incubation time. With large inoculum size, excellent agreement can be achieved at 24 h. With standard inoculum size, acceptable agreement can be achieved only at 48 h. In contrast to previous observations, the addition of 2% glucose did not have any significant impact on the growth density at 24 h, nor did it improve the agreement with the standard method. Furthermore, supplemental glucose might falsely elevate the MIC at 48 h.
Significant progress has been made in the field of antifungal susceptibility testing over the past decade with the standardization of the testing method, the correlation between in vitro susceptibility results and patients’ outcomes, and the establishment of proposed breakpoint values for antifungal resistance (6, 7, 12). The antifungal susceptibility methods currently recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (6) still have some limitations, however. The major limitation is the determination of endpoint MICs, which is subject to variable interpretations due to trailing phenomenon caused by partial inhibition of fungal growth (13). Several studies have used spectrophotometric determination of endpoints to eliminate such subjective interpretation and revealed good agreement with the NCCLS recommended method (1, 3, 4, 9, 10, 14–16). These studies, however, used various inoculum sizes, glucose concentrations, and incubation times. The variations in these factors might have explained the discrepancies in findings between studies. For example, some studies showed that 48-h incubation was required to yield spectrophotometric results that matched with the NCCLS results (1, 10), whereas others showed that 24-h incubation was sufficient (4, 16). To our knowledge, there has not been any study addressing the combined influence of inoculum sizes, glucose concentrations, and incubation times on the MICs obtained by the spectrophotometric method and their agreement with those determined by the standard broth macrodilution method. Furthermore, most studies tested exclusively Candida albicans isolates (4). Therefore, the ability of the spectrophotometric method to accurately determine the MICs for Candida spp. other than C. albicans is unproven; this information is particularly important since the non-C. albicans spp. often yield ambiguous MICs due to the trailing endpoint.
We studied the impact of the incubation time, starting inoculum size, and glucose concentrations on the growth density of Candida spp. and the influence of the growth density on the spectrophotometric determination of MICs of fluconazole, itraconazole, and amphotericin B. We compared the results of the spectrophotometric method with those obtained by the standard broth macrodilution method (6) for a variety of species of Candida.
MATERIALS AND METHODS
Candida isolates.
Ninety-five Candida isolates recovered from patients with candidemia were tested (8). These included C. albicans (39 isolates), Candida glabrata (21 isolates), Candida tropicalis (19 isolates), Candida parapsilosis (10 isolates), Candida lusitaniae (4 isolates), and Candida krusei (2 isolates). The isolates were maintained at −70°C and passed at least twice onto Sabouraud dextrose agar prior to undergoing susceptibility testing.
Three reference strains (C. parapsilosis ATCC 90018 and C. albicans ATCC 90028 and ATCC 90029) were incorporated into each set of experiments.
Antifungal agents.
Fluconazole (Pfizer Central Research, Groton, Conn.) stock solutions with concentration of 2,000 μg/ml were prepared with distilled water; the concentrations tested ranged from 0.125 to 64 μg/ml. Itraconazole powder (Janssen Research Foundation, Beerse, Belgium) and amphotericin B (Bristol-Myers Squibb, Princeton, N.J.) stock solutions were prepared with dimethyl sulfoxide; the concentrations tested ranged from 0.03 to 16 μg/ml.
Susceptibility testing.
Each Candida isolate was tested simultaneously by macrodilution and microdilution methods. The isolates were subcultured onto Sabouraud dextrose agar plates and grown at 35°C for 24 h prior to testing. Inocula were prepared spectrophotometrically (6).
The broth macrodilution method was performed according to the recommendations of the NCCLS (6). The MICs were determined at 24 and 48 h.
The broth microdilution method was performed according to the recommendations of the NCCLS (6). The medium used was RPMI 1640 with l-glutamine buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer. For the broth microdilution method, two different glucose concentrations were tested: the first contained 0.2% glucose (standard medium), and the second contained an additional 18 g of glucose per liter (medium with 2% glucose). Two different starting inoculum sizes were tested: 103 cells per ml (standard inoculum size) and 104 cells per ml (large inoculum size). The MICs were determined at 24 and 48 h. MICs were determined both visually (visual method) and spectrophotometrically (spectrophotometric method). The MICs by both methods were determined after the wells had been thoroughly mixed by automated pipetting to obtain a homogeneous suspension.
Endpoint determination. (i) Amphotericin B MIC.
By the broth macrodilution and microtiter visual method, the amphotericin B MIC was defined as the lowest concentration of drug that completely inhibited the growth of the isolate. By the spectrophotometric method, the MIC was defined as the lowest concentration at which the optical density (OD) was reduced to ≤90% of the OD of the growth control well (10).
(ii) Fluconazole and itraconazole MICs.
By the broth macrodilution method, the fluconazole and itraconazole MICs were defined as the lowest concentration causing an 80% decrease in turbidity compared to that in the growth control tube. By the microtiter visual method, the MIC was determined according to the 0 to 4 scale, as follows: 0, optically clear; 1, slightly hazy; 2, prominent decrease in turbidity; 3, slight decrease in turbidity; and 4, no reduction in turbidity. The MIC was defined as the lowest concentration of azole drugs with which the score was ≤2. The MIC by the spectrophotometric method was determined by using a microtiter plate spectrophotometer (Titertek Multiskan MCC, Santa Barbara, Calif.) at 492 nm. Previous study has demonstrated that the 50% inhibitory concentration (IC50), the lowest concentration at which the OD was reduced to <50% of that of the growth control well, was superior to the IC80 and IC70 (the lowest concentrations at which the ODs were reduced to <80% and <70%, respectively, of that of the growth control well) as endpoint because it gave a better level of reproducibility and stronger agreement with the NCCLS method (7). We have, therefore, chosen IC50 as the MIC endpoint.
Definition.
Agreement between results determined by different methods was defined as a difference in MICs of less than or equal to fourfold dilution (for two tubes or wells).
RESULTS
Comparison of growth. (i) Incubation for 24 h versus incubation for 48 h.
Growth was evident in the tubes and wells for all isolates after incubation for 24 h, although the growth was heavier after incubation for 48 h than after that for 24 h. This subjective determination of growth was confirmed by the spectrophotometric readings of the microtiter plates (Table 1).
TABLE 1.
Effects of inoculum size, incubation time, and glucose concentration on the growth of Candida spp.
| Medium | Mean OD ± SD (range) for:
|
|||
|---|---|---|---|---|
| Standard inoculum (∼103 CFU/ml) incubated for:
|
Large inoculum (∼104 CFU/ml) incubated for:
|
|||
| 24 h | 48 h | 24 h | 48 h | |
| RPMI 1640 | 0.31 ± 0.11 (0.064–0.48) | 0.48 ± 0.11 (0.31–0.78) | 0.40 ± 0.09 (0.29–0.58) | 0.48 ± 0.07 (0.30–0.68) |
| RPMI 1640 + 2% glucose | 0.36 ± 0.14 (0.06–0.64) | 0.65 ± 0.14 (0.29–0.82) | 0.48 ± 0.12 (0.18–0.68) | 0.68 ± 0.08 (0.49–0.83) |
(ii) Standard inoculum versus large inoculum.
The isolates tested at the large inoculum size yielded a significantly higher OD at 24 h than those tested at the standard inoculum size (analysis of variance [ANOVA], P = 0.001 for inoculum incubated in standard medium and P < 0.0002 for inoculum incubated in medium with 2% glucose) (Table 1).
(iii) Standard medium versus medium with 2% glucose.
For the isolates tested at the inoculum size, the 24-h incubation yielded a higher OD with the addition of glucose (mean OD of 0.40 for the test with standard medium versus 0.48 for the test with medium supplemented with 2% glucose; ANOVA, P = 0.002), although the degree of increase in OD was not as prominent as that for the 48-h incubation (mean OD of 0.48 for the test with standard medium and 0.70 for the test with medium supplemented with 2% glucose; P < 0.0001). For the isolates tested at the standard inoculum size, there was only a marginal increase in OD at 24 h, but there was a significant increase in the OD at 48 h with the addition of glucose (mean OD of 0.48 for test with standard medium versus 0.65 for test with medium containing 2% glucose at 48 h; ANOVA, P < 0.0001) (Table 1).
Selected examples of the improvements in OD with modification of the incubation time, glucose concentration, and inoculum size are presented in Table 2. The OD at 24 h of the control growth of the three selected isolates cultured by the standard method (inoculum size, ∼103 cells/ml; medium, RPMI 1640) were low (range, 0.05 to 0.08). The addition of glucose to standard medium only marginally improved the OD, to 0.06 (one isolate) and 0.15 (two isolates). Extending the incubation time from 24 h to 48 h or using a larger inoculum size substantially increased the OD (Table 2).
TABLE 2.
Effects of incubation time, inoculum size, and glucose concentration on the growth of three Candida isolates that yielded low ODs under standard conditionsa
| Isolate no. | OD at 24 h for:
|
OD at 48 h for:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Standard inoculum (∼103 CFU/ml)
|
Large inoculum (∼104 CFU/ml)
|
Standard inoculum (∼103 CFU/ml)
|
Large inoculum (∼104 CFU/ml)
|
|||||
| RPMI | RPMI-glucose | RPMI | RPMI-glucose | RPMI | RPMI-glucose | RPMI | RPMI-glucose | |
| 32 | 0.05 | 0.06 | 0.30 | 0.41 | 0.31 | 0.71 | 0.33 | 0.69 |
| 12 | 0.07 | 0.15 | 0.39 | 0.49 | 0.37 | 0.75 | 0.46 | 0.76 |
| 28 | 0.08 | 0.15 | 0.44 | 0.52 | 0.44 | 0.76 | 0.44 | 0.82 |
Standard conditions are standard inoculum size (∼103 CFU/ml) and RPMI 1640 medium.
MIC distribution.
When the standard broth macrodilution method was used, the MICs at which 50 and 90% of isolates were inhibited, respectively, 0.5 and 0.5 μg/ml (range, 0.06 to 2 μg/ml) for amphotericin B, 4 and 32 μg/ml (range, 0.125 to >64 μg/ml) for fluconazole, and 0.25 and 4 μg/ml (range, 0.03 to >16 μg/ml) for itraconazole. Based on the proposed breakpoints for fluconazole (12), 85% of the isolates were susceptible, 8% were susceptible dose dependently, and 7% were resistant to fluconazole. Based on the proposed breakpoints for itraconazole (12), 60% of the isolates were susceptible, 23% were susceptible dose dependently, and 17% were resistant to itraconazole.
Fluconazole MIC.
In the tests with the standard inoculum size, the MIC at 48 h determined by the spectrophotometric method displayed a significantly better agreement with the result for the standard broth macrodilution method (94%) than did the MIC at 24 h (72%) (P < 0.001, Fisher’s exact test) (Table 3).
TABLE 3.
Influence of starting inoculum size, supplementation with 2% glucose of RPMI 1640 medium, and incubation time on the MIC determination and its agreement with the macrodilution method
| Drug | % Agreement (no.) with result of standard broth macrodilution method at:
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h
|
48 h
|
||||||||||||||||
| Standard inoculum size
|
Large inoculum size
|
Standard inoculum size
|
Large inoculum size
|
||||||||||||||
| Macrodilution method | Microdilution methoda
|
Spectrophotometric methodb
|
Microdilution method
|
Spectrophotometric method
|
Microdilution method
|
Spectrophotometric method
|
Microdilution method
|
Spectrophotometric method
|
|||||||||
| (−c) | − | +c | − | + | − | + | − | + | − | + | − | + | − | + | − | + | |
| Fluconazole | |||||||||||||||||
| ≤4-foldd | 95 (90) | 93 (88) | 92 (87) | 72 (68) | 76 (72) | 100 (95) | 99 (94) | 96 (91) | 95 (90) | 97 (92) | 92 (87) | 94 (89) | 91 (86) | 99 (94) | 100 (95) | 93 (88) | 95 (90) |
| >4-foldd | 5 (5) | 7 (7) | 8 (8) | 28 (27) | 24 (23) | 0 (0) | 1 (1) | 4 (4) | 5 (5) | 3 (3) | 8 (8) | 6 (6) | 9 (9) | 1 (1) | 0 (0) | 7 (7) | 5 (5) |
| Itraconazole | |||||||||||||||||
| ≤4-fold | 97 (92) | 95 (90) | 91 (86) | 55 (52) | 55 (52) | 97 (92) | 99 (94) | 97 (92) | 96 (91) | 96 (91) | 89 (85) | 78 (74) | 74 (70) | 94 (89) | 95 (90) | 86 (82) | 91 (86) |
| >4-fold | 3 (3) | 5 (5) | 9 (9) | 45 (43) | 45 (43) | 3 (3) | 1 (1) | 3 (3) | 4 (4) | 4 (4) | 11 (10) | 22 (21) | 26 (25) | 6 (6) | 5 (5) | 14 (13) | 9 (9) |
Microtiter, with visual determination of MIC.
Microtiter, with spectrophotometric determination of MIC.
RPMI 1640 with (+) or without (−) supplemental 2% glucose.
Difference between MICs.
In the tests with the large inoculum size, the 24 h MIC determined by the spectrophotometric method displayed a 96% agreement with the result for the standard broth macrodilution method (Table 3). Extending the incubation to 48 h did not significantly improve the agreement with the MIC determined by the standard macrobroth dilution method; indeed, it actually worsened the agreement. For example, five isolates were classified as resistant to fluconazole (MIC, of ≥64 μg/ml) by spectrophotometric reading at 48 h but as susceptible (MIC, 0.25 to 8 μg/ml) at 24 h; they were all classified as susceptible (MIC, 2 to 4 μg/ml) at 48 h by the standard broth macrodilution method. This difference in susceptibility pattern at 48 h was most likely related to the overgrowth of the isolates at 48 h due to the large starting inoculum size.
There was no significant difference in MICs determined in medium with or without 2% glucose. Furthermore, the agreement with the MIC determined by the standard broth macrodilution method did not significantly improve with the addition of 2% glucose (Table 3).
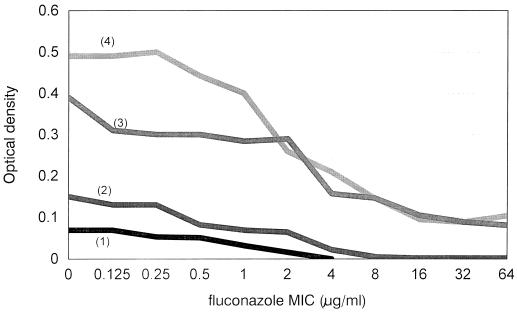
As noted above, the use of a large inoculum significantly improved the growth of the isolates. The OD at 24 h of the growth control 9-30F in RPMI medium was 0.07 for the test with the standard inoculum size and 0.39 for the test with the large inoculum size. The curves of ODs plotted against fluconazole concentrations (Fig. 1) showed a higher starting OD and a steeper decrease in the slope of the curve for the isolate tested with the large inoculum size than for the isolate tested with the standard inoculum size. This, in turn, eliminated the uncertainty of endpoint determination with the test with the standard inoculum size.
FIG. 1.
Influence of inoculum size and glucose concentration on the OD curve and on the fluconazole MIC determination for an isolate of C. albicans. 1, standard inoculum size (∼103 CFU/mL), RPMI 1640 medium; 2, standard inoculum size (∼103 CFU/mL), RPMI 1640 medium supplemented with 2% glucose; 3, large inoculum size (∼104 CFU/mL), RPMI 1640 medium; and 4, large inoculum size (∼104 CFU/mL), RPMI 1640 medium supplemented with 2% glucose. The MICs determined by testing with large inoculum size and medium supplemented with 2% glucose were 2 μg/ml, and the MICs determined by testing under other conditions were 4 μg/ml.
The addition of 2% glucose to RPMI medium only marginally improved the OD of the growth control isolate tested at the the standard inoculum size (OD increased from 0.07 to 0.15); thus, addition of glucose did not eliminate the difficulty of endpoint determination. Although the addition of 2% glucose improved the OD of the growth control isolate tested at the large inoculum size (from 0.39 to 0.49), the slope of the OD curve obtained when medium with 2% glucose was used was essentially similar to that when the standard medium was used.
Itraconazole MIC.
In the test with the standard inoculum size, the agreement between the itraconazole MICs at 24 h determined by the spectrophotometric method and the standardized method was only 55%. Adding 2% glucose to RPMI 1640 medium did not improve the agreement (Table 3). Extending the incubation period to 48 h significantly improved the agreement, to 78% and 74%, respectively, for the tests with standard medium and medium with 2% glucose (P < 0.001, Fisher’s exact test).
In the test with the large inoculum size, the agreement between the MICs at 24 h determined by the spectrophotometric method and the standard method was 97%. Adding 2% glucose did not improve the agreement. Extending the incubation to 48 h significantly worsened the agreement (P = 0.008, Fisher’s exact test) (Table 3).
Amphotericin B MIC.
The agreement between the amphotericin B MICs determined by the standard broth macrodilution with method and the spectrophotometric method in standard and modified media, with either the standard or large inoculum size, was 100%.
DISCUSSION
To our knowledge, this study is the first to address the combined effects of the incubation time, inoculum size, and supplementation with 2% glucose of RPMI 1640 medium on the growth density of Candida spp. and the influence of growth density on the spectrophotometric determination of MICs of fluconazole, itraconazole, and amphotericin B. We demonstrated that the starting inoculum size was the most important factor: large inoculum size led to significantly higher turbidity at 24 h. The supplementation with 2% glucose also had a significant impact on turbidity but only at 48 h.
The significantly higher turbidity seen with a large starting inoculum (∼104 CFU/ml) has translated to an excellent agreement between the MICs determined by the spectrophotometric method and the standard broth macrodilution method at 24 h. The strong agreement (≥97%) was seen for all three drugs tested. On the other hand, the test employing standard inoculum size (∼103 CFU/ml, the inoculum size that is currently recommended by the NCCLS) yielded poor agreement between the 24 h MICs determined spectrophotometrically and by the standard broth macrodilution technique: the agreement was only 75% for fluconazole and 55% for itraconazole (Table 3). Extending the incubation time to 48 h significantly improved the agreement, to 94% for fluconazole and 78% for itraconazole. Our findings confirm the previous observation that, for susceptibility to fluconazole and itraconazole, a 48-h incubation period was required to achieve an acceptable agreement between the results of the spectrophotometric method and the standard broth macrodilution method (10). Our findings also confirm that, for susceptibility to amphotericin B, excellent agreement between the results of the spectrophotometric method and standard broth macrodilution method can be obtained after 24 h (17). In addition, we demonstrated that this agreement was independent of the starting inoculum size and glucose concentrations.
Previous studies have shown that the addition of 2% glucose to RPMI 1640 medium stimulated heavier growth and maximized the distinction between wells with and without significant drug inhibition, thereby facilitating the determination of definitive endpoints (9, 14, 15). We also showed higher turbidity determined by the spectrophotometric readings of isolates incubated in the medium with 2% glucose, but only after 48 h and only with the use of large starting inoculum (Tables 1 and 2). More importantly, isolates that showed suboptimal growth at 24 h under the standard condition showed improved turbidity at 24 h only with the use of large inoculum (Table 2).
In contrast to previous studies, our study did not demonstrate that addition of 2% glucose improved the agreement between the results of the spectrophotometric method and the standard broth macrodilution method at 24 h (Table 3). Indeed, the agreement was no better for the method employing RPMI 1640 alone (agreement of 71% for fluconazole and 55% for itraconazole) and for that employing RPMI supplemented with 2% glucose (76% for fluconazole and 55% for itraconazole). This lack of improved agreement may be attributed to the minimal impact of 2% glucose on the growth of the isolates at 24 h. Furthermore, extending the incubation to 48 h also did not improve the agreement between the results of the spectrophotometric and standard broth macrodilution methods. In fact, we have shown that after addition of the supplemental glucose five isolates were falsely reclassified from susceptible to resistant as determined by the spectrophotometric method; this was most likely due to the overgrowth of the organism in the glucose-supplemented wells after 48 h.
Our study confirms a previous finding (10) that, in itraconazole susceptibility testing, the combination of the spectrophotometric method and the standard inoculum size yielded a poor agreement with the standard broth macrodilution method (agreement of 55% after 24 h and 78% after 48 h). An important finding of our study is that, by using a large starting inoculum, not only can agreement of >95% be achieved but also this agreement is demonstrable after only 24 h. To our knowledge, this study is the first to show good agreement between the spectrophotometrically determined itraconazole MIC and that determined by the standard broth macro- or microdilution method after 24 h.
Given the standardization of the in vitro antifungal susceptibility testing, the establishment of the correlation of in vivo results with the in vitro results, and the emergence of resistance to currently available antifungal agents, the demand for clinical microbiology laboratories to perform antifungal susceptibility testing will increase. To implement such testing, the laboratories must assure the reproducibility of the test. The major source of interlaboratory irreproducibility lies in the endpoint MIC determination, which is reader dependent. The spectrophotometric method offers an advantage over the visual method by providing an objective and quantitative MIC which is based on defined criteria. This objective determination of MIC might provide the best means of enhancing the reproducibility of the test.
REFERENCES
- 1.Barchiesi F, Del Poeta M, Morbiducci V, Ancarani F, Scalise G. Turbidimetric and visual criteria for determining the in vitro activity of six antifungal agents against Candida spp. and Cryptococcus neoformans. Mycopathologia. 1993;124:19–25. doi: 10.1007/BF01103052. [DOI] [PubMed] [Google Scholar]
- 2.Barchiesi F, Colombo A L, McGough D A, Rinaldi M G. Comparative study of broth macrodilution and microdilution techniques for in vitro antifungal susceptibility testing of yeasts by using the National Committee for Clinical Laboratory Standards’ proposed standard. J Clin Microbiol. 1994;32:2494–2500. doi: 10.1128/jcm.32.10.2494-2500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco M T, Pérez-Giraldo C, Blanco J, Morán F J, Hurtado C, Gómez-Garcia A C. In vitro studies of activities of some antifungal agents against Candida albicans ATCC 10231 by the turbidimetric method. Antimicrob Agents Chemother. 1992;36:898–901. doi: 10.1128/aac.36.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espinel-Ingroff A, Rodríguez-Tudela J L, Martínez-Suárez J V. Comparison of two alternative microdilution procedures with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro testing of fluconazole-resistant and -susceptible isolates of Candida albicans. J Clin Microbiol. 1995;33:3154–3158. doi: 10.1128/jcm.33.12.3154-3158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A, Kish C W, Jr, Kerkering T M, Fromtling R A, Bartizal K, Galgiani J N, Villareal K, Pfaller M A, Gerarden T, Rinaldi M G, Fothergill A. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1992;30:3138–3145. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. Development of in vitro susceptibility testing criteria and quality control parameters. Approved guideline M27-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1994. [Google Scholar]
- 7.Nguyen M H, Clancy C J, Yu V L, et al. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J Infect Dis. 1998;177:425–430. doi: 10.1086/514193. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen M H, Peacock J E, Jr, Morris A J, et al. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 9.Odds F C, Vranckx L, Woestenborghs F. Antifungal susceptibility testing of yeasts: evaluation of technical variables for test automation. Antimicrob Agents Chemother. 1995;39:2051–2060. doi: 10.1128/aac.39.9.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller M A, Messer S A, Coffman S. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth microdilution methods to test five antifungal agents, including the new triazole D0870. J Clin Microbiol. 1995;33:1094–1097. doi: 10.1128/jcm.33.5.1094-1097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller M A, Grant C, Morthland V, Rhine-Chalberg J. Comparative evaluation of alternative methods for broth dilution susceptibility testing of fluconazole against Candida albicans. J Clin Microbiol. 1994;32:506–509. doi: 10.1128/jcm.32.2.506-509.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rex J H, Pfaller M A, Galgiani J N, et al. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro and in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 13.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Tudela J L, Martinez-Suarez J V. Improved medium for fluconazole susceptibility testing of Candida albicans. Antimicrob Agents Chemother. 1994;38:45–48. doi: 10.1128/aac.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Tudela J L, Martinez-Suarez J V. Defining conditions for microbroth antifungal susceptibility tests: influence of RPMI and RPMI-2% glucose on the selection of endpoint criteria. J Antimicrob Chemother. 1995;35:739–749. doi: 10.1093/jac/35.6.739. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Tudela J L, Berenguer J, Martínez-Suárez J V, Sanchez R. Comparison of a spectrophotometric microdilution method with RPMI-2% glucose with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro susceptibility testing of amphotericin B, flucytosine, and fluconazole against Candida albicans. Antimicrob Agents Chemother. 1996;40:1998–2003. doi: 10.1128/aac.40.9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tornatore M A, Noskin G A, Hacek D M, Obias A A, Peterson L R. Effects of incubation time and buffer concentration on in vitro activities of antifungal agents against Candida albicans. J Clin Microbiol. 1997;35:1473–1476. doi: 10.1128/jcm.35.6.1473-1476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]