Abstract
Objectives:
Genetics, environment, and their interactions (GxE) impact Autism Spectrum Disorder (ASD) etiology. Smoking is a supsected ASD risk factor due to biological plausibility and high prevalence.
Methods:
Using two large epidemiological samples, we examined whether ASD was associated with prenatal paternal smoking in a Discovery Sample (DS: N=10,245) and an independent Replication Sample (RS: N=29,773). Paternal smoking was retrospectively assessed with questionnaires. Likelihood of having ASD was estimated with the Autism Spectrum Screening Questionnaire (ASSQ) at three levels: low (ASSQ<10), intermediate (ASSQ=10-14), and high (ASSQ≥15). Ordinal regression was used to examine the relationship between prenatal paternal smoking and likelihood of having ASD, adjusting for confounders.
Results:
36.5% of DS fathers and 63.3% of RS fathers smoked during the pregnancy period (PP). 7% of the RS smoker fathers smoked during the pre-conception period (PCP) but quit during PP. DS prenatal paternal smoking significantly increased likelihood of having ASD in their offspring (adjusted odds ratio [aOR]=1.27). This was confirmed in the RS with aOR 1.15 among smoking PCP+PP fathers. 14.4% and 11.1% increased high likelihood of ASD was attributable to prenatal paternal smoking in DS and RS, respectively.
Conclusion:
Smoking prevention, especially in pregnancy planning, may decrease ASD risk in offspring.
INTRODUCTION
Autism Spectrum Disorder (ASD), an early-onset neurodevelopmental disorder (NDD) with prevalence 1.6-3.0% worldwide, is characterized by pervasive impairment in social communication and the presence of restricted and repetitive behaviors/interests [Fombonne, 2009, Kim&Leventhal et al., 2011, Maenner&Rice et al., 2014, Zablotsky&Black et al., 2015, Christensen, 2016]. Epidemiological and genomic analyses demonstrate substantial etiologic contributions from additive genetic factors [Gaugler&Klei et al., 2014, Tick&Bolton et al., 2016], with the remainder likely explained by a combination of non-additive genetic factors, environmental factors, and interactions, including gene-environment interactions (GxE) [Gaugler&Klei et al., 2014].
The pre- and perinatal period appears to be a critical nexus of risk for the genesis of ASD [Willsey&Sanders et al., 2013, Lyall&Schmidt et al., 2014]. Studies have reported associations between ASD and maternal prenatal exposure to some medications, toxins, and intrapartum rubella infection, suggesting that exposure to exogenous agents during critical developmental periods contribute to ASD susceptibility [Chess, 1971, Stromland&Nordin et al., 1994, Rodier&Ingram et al., 1996, Rodier&Ingram et al., 1997, Ingram&Peckham et al., 2000, Moore&Turnpenny et al., 2000, Bescoby-Chambers&Forster et al., 2001, Williams&King et al., 2001, Lee&Newschaffer et al., 2008, Williams&Helmer et al., 2008].
Prior studies examining relationships between perinatal risks and ASD have reported inconsistent findings between increased ASD risks and prenatal maternal factors, including complicated birth histories [Hultman&Sparen et al., 2002, Glasson&Bower et al., 2004, Larsson&Eaton et al., 2005, Lee&Newschaffer et al., 2008, Schendel and Bhasin, 2008, Williams&Helmer et al., 2008, Bilder&Pinborough-Zimmerman et al., 2009, Burstyn&Sithole et al., 2010, Hultman&Sandin et al., 2010, Mann&McDermott et al., 2010, Cheslack-Postava&Liu et al., 2011, Dodds&Fell et al., 2011] as well as maternal smoking and alcohol exposure [Eliasen&Tolstrup et al., 2010, Lee&Gardner et al., 2012, Tran&Lehti et al., 2013, Singer&Aylsworth et al., 2017]. Inconsistent findings are likely the result of methodological shortcomings, including small, clinical samples with phenotype heterogeneity, and confounding by comorbidity, including intellectual disability [ID]. A recent meta-analysis, examining over 60 perinatal and neonatal risk factors, suggested that the following increased ASD risk: abnormal presentation, umbilical-cord complications, fetal distress, birth injury or trauma, multiple birth, maternal hemorrhage, summer birth, low birth weight, small for gestational age, congenital malformation, low 5-minute Apgar score, feeding difficulties, meconium aspiration, neonatal anemia, ABO or Rh incompatibility, and hyperbilirubinemia [Gardener&Spiegelman et al., 2011]; another meta-analysis with 15 studies suggested no association between maternal smoking exposure and ASD [Rosen&Lee et al., 2015].
In prior studies, the majority of perinatal risks were limited to maternal exposures. With increased paternal age as an identified risk for ASD [Hultman&Sandin et al., 2011], paternal perinatal exposures may increase ASD risk. ASD risk attributable to prenatal paternal smoking is of particular interest due to: 1) Biological plausibility through cumulative toxicity to the male germline [Linschooten&Verhofstad et al., 2013] and secondary exposure to toxins [Clifford&Lang et al., 2012]; 2) High smoking prevalence (43% in Korea and 15.1% in US) [KOSIS, 2010, Jamal&King et al., 2016]; 3) Highest prevalence male smoking, 20–39 years, overlaps with peak age for reproduction [Li&Lin et al., 2011]; 4) Availablity of animal models [Nixon&Stanger et al., 2015, Esakky and Moley, 2016]; 5) Relatively accurate retrospective recall of exposure [Krall&Valadian et al., 1989]; and, 6) Availability of interventions for smoking cessation yielding other health benefits [Hopkins&Briss et al., 2001, Phs Guideline Update Panel and Staff, 2008].
The adverse impact of perinatal paternal smoking on fetal and newborn health has been reported (e.g., low birth weight, prematurity, heart defects, and childhood cancer) but little is known about its impact on ASD risk [Ahluwalia&Grummer-Strawn et al., 1997, Windham&Hopkins et al., 2000, Larsson&Weiss et al., 2009, Lee&Ward et al., 2009, Newman&Momirova et al., 2010, Zhang&Lv et al., 2010, Duan&Yao et al., 2014, Kaur, 2014, Thacher&Gruzieva et al., 2014, Ion&Wills et al., 2015, Luh Putu Rihayani Budi, 2015, Forest and Priest, 2016]. Four studies have examined paternal smoking effects including second hand smoking on offspring ASD risk (Table 1). The small number of subjects and lack of control for known confounders (e.g., maternal smoking and parental age) make it difficult to arrive at conclusions.
Table 1.
Summary of prior studies that examined the relationship between prenatal paternal smoking and offspring autism risks
| Reference | Country | Time period | Definition of smoking | Diagnostic assessment | Odds of smoking in ASD (%) |
Odds of smoking in control (%) |
Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
Study design |
|---|---|---|---|---|---|---|---|---|---|
| Larsson, 2009 | Sweden | 2000/2005a | Father’s smoking during pregnancy | Diagnosis history | 1/49 (2.0) | 50/3846 (1.28) | 1.73 (0.93-3.20) | NA | case/control |
| Zhang, 2010 | China | 2007 | Secondhand smoking during pregnancy | CARS (childhood autism rating scale) | 18/77 (18.9) | 6/89 (6.32) | No Information | 3.53 (1.30- 9.56)b | case/control |
| Duan, 2014 | China | 2011~2013 | Passive smoking on perinatal period | CARS (childhood autism rating scale) | 60/226 (21.0) | 17/269 (5.94) | 3.07 (0.51-3.62) | 1.58 (1.02-2.63)c | case/control |
| Budi, 2015 | Indonesia | 2013 | Father smoking during pregnancy | DSM-IV-TR | 28/22 (56.0) | 33/67 (33.0) | 2.6 (1.3-5.2) | 3.2 (1.5-6.9)d | case/control |
Initial assessment in 2000 and follow up in 2005
Adjusted for paternal age at delivery, gender, birth year
Adjusted for father and mother’s education level, father and mother’s age, father and mother’s character, family history of psychiatric disorder, mental stress, anxiety and nervousness, pregnancy complications, edema, threatened abortion, infection with fever during pregnancy, premature rupture of fetal membranes, premature delivery, cesarean, umbilical cord around the neck, birth asphyxia, severe jaundice
Adjusted for paternal age at pregnancy, history of asphyxia
We attempted to overcome these shortcomings by using an internal replication design, with two large, community cohorts to: 1) Test the hypothesis that paternal prenatal smoking increases likelihood for offspring with having ASD, in a “Discovery sample (DS);” and, 2) Use a “Replication Sample (RS)” to confirm the initial findings while determining if the timing of paternal smoking timing modifies the risk.
METHODS
Participants
DS and RS were two independent, epidemiologically-ascertained cohorts of school-aged children. DS participants are from a Simons Foundation Autism Research Initiative (SFARI) project; The RS subjects are from the Korean Environmental Risk and Children’s Health Project.
The 15,981target subjects in the DS were drawn from the 7-13-year-old children participating in the SFARI project in 13 cities representative of South Korea in 2009-2011 (Figure 1). Of 12,447 questionnaires distributed to children with up-to-date contact information, 10,503 parents agreed to participate in the survey (84.4% response). 10,245 questionnaires were used for the final analyses, after deleting those with missing data (Autism Spectrum Screening Questionnaire [ASSQ] > 5 missing items [N=141], gender [N=108], age [N=9]). RS subjects were ascertained between 2007 and 2008 from Cheonan City, a mixed urban and rural area in the center of South Korea (population = ~629,000) [Statistics Korea, 2015]. The target population was 49,570 children attending in 65 elementary schools. Of 42,746 questionnaires distributed, 30,552 were retrieved from the parents via the school system (71.5% response). After excluding subjects with missing data (ASSQ [N=510], gender and/or age [N=269]), 29,773 questionnaires were used in final analyses. Missing values for parental smoking and maternal drinking were treated as “no response” and included in the final analyses. Questionnaires were completed by principal care-givers, (usually mothers) in both the DS and RS samples.
Figure 1.
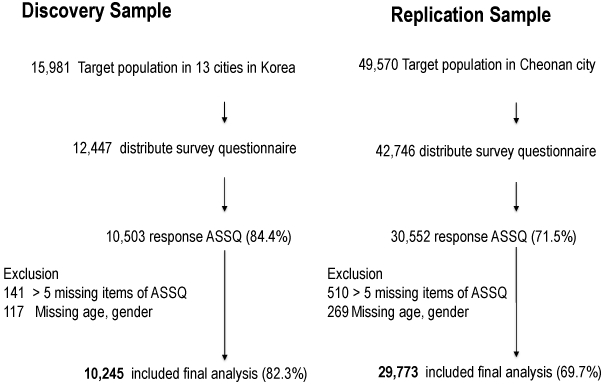
Sample Ascertainment in Discovery and Replication Samples
The Yale and Dankook University Institutional Review Boards approved the study; informed consent was obtained from parents.
Measurement
Predictor: Parental Smoking
Parental smoking data were retrospectively collected, using a questionnaire: “Did the father smoke during pregnancy with an index child (response: Yes/No)?” in the DS, and “Has father ever smoked (responses: never, ex-smoker, current smoker)?” and “Did father smoke during pregnancy?” in the RS. The same questions were asked about the mother. Using a method of an indicator variable for missingness of categorical predictor [Gelman A, 2006], missing DS and RS data were coded as “no response” and were included in the analyses.
Use of two items in the RS allowed refinement of timing for parental smoking into four groups: “never smoker,” “smoking before (pre-conceptual period [PCP]) and throughout the entire pregnancy period [PP],” “Smoked but quit during pregnancy (PCP only),” and “smoking of unknown exposure timing” (supplementary Figure 1).
In the RS, three additional questions were asked about the amount and duration of smoking: “How many cigarettes did father/mother smoke?” “When did father/mother start to smoke?” “When did father/mother quit smoking?”
Outcome: Autism Spectrum Phenotyping
The ASSQ, a 27-item questionnaire for ASD, measures social interaction, communication problems, RRB’s, and associated features. Each item is rated from 0 to 2, (total score = 0-54). The ability of the ASSQ to distinguish ASD from other diagnoses is well-established for European and Korean children [Ehlers&Gillberg et al., 1999] [Mattila&Kielinen et al., 2007, Yim, 2012] and assessing environmental effects on ASD [Lyall&Schmidt et al., 2014]. ASSQ scores in the upper 5th percentile (≥15) defined children as “screen positive;” this definition demonstrated optimal agreement with best estimate diagnoses of ASD in Korean children [Kim&Leventhal et al., 2011]. We categorized ASSQ scores into three groups for an ASD diagnosis: 1) “High likelihood” score ≥15 (≥5th percentile); 2) “Intermediate likelihood” score 10-14 (10th-6th percentile); and 3) “Low likelihood” score <10 (<10th percentile).
Potential Confounders
Based on a review of the literature, potential confounders were selected to include parental age at pregnancy, maternal smoking and drinking during pregnancy, and family history of psychiatric disorders [Durkin&Maenner et al., 2008, Ornoy&Weinstein-Fudim et al., 2015, Modabbernia&Velthorst et al., 2017, Singer&Aylsworth et al., 2017]. These potential confounders were included in our final analyses.
Demographic Covariates
Childrens’ age, gender, and parental demographic characteristics (education and marital status) were included in our final analyses.
Statistical analysis
The Pearson chi square test was used for comparing categorical demographic variables between ASD likelihood groups. While the ASSQ’s skewed distribution does not meet the assumption for linear regression, it met proportional odds assumptions for ordinal logistic regression, which was used to examine the relationship between paternal smoking and the incremental increase in likelihood of having ASD in their offspring (“low,” “intermediate,” and “high” likelihood). Potential confounders and demographic covariates were controlled in a multivariable model. In order to avoid use of inaccurate assumptions for missing data imputations, we used “no response” as data points for missing responses in predictor variables in multivariable regression.
In subsample from the RS (N=4,660: PCP only=439, PCP+PP=3,675, Unknown timing=546) who completed additional items about the duration and number of cigarettes smoked, one-way ANOVA was performed to determine whether the pack-years of smoking by the time of pregnancy (a proxy for smoking exposure dose) was associated with smoking cessation during pregnancy.
Additionally, the Attributional Risk Fraction (ARF) was computed to examine the proportion of offspring at high likelihood of having ASD attributable to paternal smoking in the study population.
All analyses were conducted using STATA (version 13.0).
Community Invovlement: There is no community involvement in this study.
RESULTS
Study Subjects
In the DS and RS males accounted for 51.5% and 49.2% of participants, respectively while the mean ages were 9.61 (±1.69), and 9.19 (±1.74) years, respectively. In the DS, 6.9% were at intermediate and 4.3% at high likelihood of having ASD. In the RS, 7.2% were at intermediate and 5.3% at high likelihood of having ASD (Supplement Table 2).
Parental Smoking
Of the 35.0% of DS fathers who smoked during pregnancy, 88% had children at low likelihood for ASD, 7% had children at intermediate likelihood, and 5% had children at high likelihood for ASD (Supplement Table 2). Among RS fathers, 56.3% smoked before and during pregnancy (PCP+PP). Of these, 87% of their children were low likelihood for ASD, 8% of their children were at intermediate likelihood, and 5% had children at high ASD likelihood. It was also noted that 7.0 % of RS fathers smoked only during the pre-conceptual period (PCP only); for this group, 90% of offspring were at low likelihood of having ASD while 6% of the children were at intermediate likelihood and 4% were at high ASD likelihood.
53.1 % of RS fathers were current smokers, and 75.7% had a smoking history. In comparison, prevalence of current smoking and smoking history in the general population of Korean males are 43% and 69%, respectively. In the RS, age-specific prevalence of current smokers was 62% and 80.4% had past smoking histories <29 years-old. In 30-39-year-old males, the current smoking rate was 50.5% and 74.5% smoked in the past. For those over 40-years-old, 47.2 % were current smokers and 73.0% smoked in the past. Similar smoking patterns have been reported for males in the general Korean population: For 20-29-year-olds, 53.5% currently smoke, 63% previously smoked; for 30-39-year-olds 54% currently smoke and 73% previously smoke; and, for those over 40. 48% currently smoke and 75% smoke previously [Corporation, 2010]. In the RS, when compared to fathers who smoked but stopped smoking during pregnancy (PCP only), those who continued smoking during the pregnancy period (PCP+PP) had significantly higher levels of exposure to smoking, as measured in pack-years in the sub-group analysis (Supplementary Table 1). By the time of conception, the average pack-years were 7.71±6.04 in the PCP+PP group, 6.65±6.02 in the PCP group, and 5.03±5.17 in the unknown timing group (p=1.04e-22).
In DS, compared to 4.49% of non-smoking fathers, 7.08% of smoking fathers had family psychiatric histories (p<.001). Similarily, more smoking fathers (0.59%) had paternal psychiatric histories than non-smoking fathers (0.30%, p<.001) in DS (Table 2). In the RS, 5.24% of PCP+PP smokers, 4.77% of PCP-only smoker fathers, and 4.80% of non-smoking fathers had family psychiatric histories (p=0.127). Additionally, 1.84% of PCP+PP smoker fathers, 1.53% of PCP-only smoker fathers, and, 1.19% of non-smoking fathers had previous psychiatric diagnoses histories in RS (p=0.011).
Table 2.
Demographic and Risk Characteristics of Subjects by Paternal Smoking Status
| Discovery sample | Replication Sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Total (N=10245) |
No smoking (n=4944) |
Smoking (n=3587) |
NRd (n=1714) |
Total (N=29773) |
Never Smoker (n=6896) |
PCP onlya (n=2096) |
PCP+PPb (n=16768) |
UKc (n=3103) |
NRd (n=910) |
|
| Children Characteristics | |||||||||||
| Age, mean(SD), y | 9.61(1.69) | 9.83(1.66) | 9.30(1.64) | 9.63(1.76) | 9.19(1.74) | 9.16(1.74) | 9.11(1.79) | 9.18(1.73) | 9.38(1.78) | 9.23(1.76) | |
| Male sex, N(%) | 5277(51.46) | 2493(50.42) | 1876(52.30) | 903(52.68) | 14871(49.95) | 3385(49.09) | 1076(51.34) | 8395(50.07) | 1541(49.66) | 474(52.09) | |
| Prematurity | |||||||||||
| Yes | 463(4.52) | 205(4.15) | 183(5.10) | 75(4.38)*** | 1489(5.00) | 315(4.57) | 89(4.25) | 872(5.20) | 167(5.38) | 46(5.05)*** | |
| No | 8659(84.52) | 4353(88.05) | 3177(88.57) | 1129(65.87) | 27502(92.37) | 6402(92.84) | 1972(94.08) | 15550(92.74) | 2842(91.59) | 736(80.88) | |
| Unknown | 1123(10.96) | 386(7.81) | 227(6.33) | 510(29.75) | 782(2.63) | 179(2.60) | 35(1.67) | 346(2.06) | 94(3.03) | 128(14.07) | |
| Birth order | |||||||||||
| First | 4633(45.22) | 2303(46.58) | 1627(45.36) | 703(41.02)*** | 14781(49.65) | 3387(48.12) | 993(47.38) | 8454(50.42) | 1463(50.37) | 384(42.20)*** | |
| Second | 4113(40.15) | 2039(41.24) | 1505(41.96) | 569(33.20) | 12414(41.70) | 2852(41.36) | 920(43.89) | 7004(41.77) | 1287(41.48) | 351(38.57) | |
| Third | 1006(9.82) | 513(10.38) | 401(11.18) | 92(5.37) | 2253(7.57) | 586(8.50) | 157(7.49) | 1191(7.10) | 230(7.41) | 89(9.78) | |
| >_fourth | 131(1.28) | 64(1.29) | 47(1.31) | 20(1.17) | 214(0.72) | 60(0.87) | 26(1.24) | 96(0.57) | 16(0.52) | 16(1.76) | |
| unknown | 362(3.53) | 25(0.51) | 7(0.20) | 330(19.25) | 111(0.37) | 11(0.16) | 0 | 23(0.14) | 7(0.23) | 70(7.69) | |
| Parents Characteristics | |||||||||||
| Parental marriage status | |||||||||||
| Unmarried | 366(3.57) | 178(3.60) | 133(3.71) | 55(3.21)*** | 2164(7.27) | 539(7.82) | 183(8.73) | 1178(7.03) | 221(7.12) | 43(4.73)*** | |
| Married/cohabitation | 8667(84.60) | 4288(86.73) | 3104(86.53) | 1275(74.39) | 24560(82.49) | 5796(84.05) | 1792(85.50) | 14186(84.60) | 2425(78.15) | 361(39.67) | |
| Separation/divorce/widowed | 639(6.24) | 296(5.99) | 207(5.77) | 136(7.93) | 1443(4.85) | 249(3.61) | 35(1.67) | 724(4.32) | 201(6.48) | 234(25.71) | |
| unknown | 573(5.59) | 182(3.68) | 143(3.99) | 248(14.47) | 1606(5.39) | 312(4.52) | 86(4.10) | 680(4.06) | 256(8.25) | 272(29.89) | |
| Fa e age at pregnancy, yr | |||||||||||
| Mean, y(SD) | 30.91(4.46) | 30.62(4.52) | 31.13(4.54) | 31.31(3.88)*** | 32.11(3.97) | 32.61(3.89) | 32.81(3.94) | 31.87(3.92) | 31.75(4.14) | 32.47(4.73)*** | |
| <20 | 40(0.39) | 21(0.42) | 17(0.47) | 2(0.12)*** | 17(0.06) | 1(0.01) | 0 | 11(0.07) | 4(0.13) | 1(0.11)*** | |
| 20-29 | 3706(36.17) | 1983(40.11) | 1337(37.27) | 386(22.52) | 7231(24.29) | 1375(19.94) | 401(19.13) | 4490(26.78) | 855(27.55) | 110(12.09) | |
| 30-34 | 4054(39.57) | 1905(38.53) | 1536(42.82) | 613(35.76) | 14639(49.17) | 3530(51.19) | 1080(51.53) | 8341(49.74) | 1477(47.60) | 211(23.19) | |
| 35-39 | 1286(12.55) | 616(12.46) | 506(14.11) | 164(9.57) | 5625(18.89) | 1487(21.56) | 462(22.04) | 3063(19.27) | 529(17.05) | 84(9.23) | |
| >40 | 295(2.88) | 135(2.73) | 126(3.51) | 34(1.98) | 1183(3.97) | 302(4.38) | 116(5.53) | 604(3.60) | 122(3.93) | 39(4.29) | |
| unknown | 864(8.43) | 284(5.74) | 65(1.81) | 515(30.05) | 1078(3.62) | 201(2.91) | 37(1.77) | 259(1.54) | 116(3.74) | 465(51.10) | |
| Mo f age at pregnancy, yr | |||||||||||
| Mean, y(SD) | 27.96(4.21) | 27.58(4.31) | 28.22(4.21) | 28.68(3.59)*** | 29.27(3.71) | 29.72(3.68) | 29.77(3.56) | 29.06(3.66) | 29.00(3.82) | 29.53(4.46)*** | |
| <20 | 179(1.75) | 107(2.16) | 68(1.90) | 4(0.23)*** | 67(0.23) | 8(0.12) | 2(0.10) | 44(0.26) | 10(0.32) | 3(0.33)*** | |
| 20-29 | 6250(1.01) | 3193(64.58) | 2283(63.65) | 774(45.16) | 16194(54.39) | 3454(50.09) | 1045(49.86) | 9637(57.47) | 1734(55.88) | 324(35.60) | |
| 30-34 | 2411(23.53) | 1098(22.21) | 939(26.18) | 374(21.82) | 10063(33.80) | 2595(37.63) | 810(38.65) | 5497(32.78) | 956(30.81) | 205(22.53) | |
| 35-39 | 453(4.42) | 207(4.19) | 192(5.35) | 54(3.15) | 2017(6.77) | 539(7.82) | 171(8.16) | 1043(6.22) | 192(6.19) | 72(7.91) | |
| >40 | 60(0.59) | 26(0.53) | 24(0.67) | 10(0.58) | 341(1.15) | 96(1.39) | 22(1.05) | 172(1.03) | 38(1.22) | 13(1.43) | |
| unknown | 892(8.71) | 313(6.33) | 81(2.26) | 498(29.05) | 1091(3.66) | 204(2.96) | 46(2.19) | 375(2.24) | 173(5.58) | 293(32.20) | |
| Fa education level, yr | |||||||||||
| <12 | 233(2.27) | 115(2.33) | 94(2.62) | 24(1.40)*** | 601(2.02) | 99(1.44) | 33(1.57) | 370(2.21) | 76(2.45) | 23(2.53)*** | |
| 12 | 3421(33.39) | 1632(33.01) | 1401(39.06) | 388(22.64) | 11074(37.19) | 2074(30.08) | 649(30.96) | 6871(40.98) | 1244(40.09) | 236(25.93) | |
| >12 | 5747(56.10) | 2947(59.61) | 2003(55.84) | 797(46.50) | 17126(57.52) | 4573(66.31) | 1371(65.41) | 9260(55.22) | 1668(53.75) | 254(27.91) | |
| unknown | 844(8.24) | 250(5.06) | 89(2.48) | 505(29.46) | 972(3.26) | 150(2.18) | 43(2.05) | 267(1.59) | 115(3.71) | 397(43.63) | |
| Mo education level, yr | |||||||||||
| <12 | 225(2.20) | 130(2.63) | 73(2.04) | 22(1.28)*** | 609(2.05) | 105(1.52) | 31(1.48) | 359(2.14) | 68(2.19) | 46(5.05)*** | |
| 12 | 4314(42.11) | 2083(42.13) | 1706(47.56) | 525(30.63) | 15599(52.39) | 3198(46.37) | 945(45.09) | 9366(55.86) | 1655(53.34) | 435(47.80) | |
| >12 | 4815(47.00) | 2441(49.37) | 1701(47.42) | 673(39.26) | 12526(42.07) | 3432(49.77) | 1069(51.00) | 6637(39.58) | 1190(38.35) | 198(21.76) | |
| unknown | 891(8.70) | 290(5.87) | 107(2.98) | 494(28.82) | 1039(3.49) | 16192.33) | 51(2.43) | 406(2.42) | 190(6.12) | 231(25.38) | |
| Mo drinking Pregnancy | |||||||||||
| Yes | 289(2.82) | 170(3.44) | 106(2.96) | 13(0.76)*** | 2942(9.88) | 492(7.13) | 161(7.68) | 1789(10.67) | 382(12.31) | 118(12.97)*** | |
| No | 3238(31.61) | 2252(45.55) | 885(24.67) | 101(5.89) | 22047(74.05) | 5100(73.96) | 1524(72.71) | 12720(75.86) | 2208(71.16) | 495(54.40) | |
| No reponse | 6718(65.57) | 2522(51.01) | 2596(72.37) | 1600(93.39) | 4784(16.07) | 1304(18.91) | 411(19.61) | 2259(13.47) | 513(16.53) | 297(32.64) | |
| Fhx of Psychiatric dis g | |||||||||||
| Yes | 598(5.84) | 222(4.49) | 254(7.08) | 122(7.12)*** | 1513(5.08) | 331(4.80) | 100(4.77) | 878(5.24) | 145(4.67) | 59(6.48) | |
| Fa Psychiatric hx | |||||||||||
| Yes | 56(0.55) | 15(0.30) | 21(0.59) | 20(1.17)*** | 485(1.63) | 82(1.19) | 32(1.53) | 308(1.84) | 49(1.58) | 14(1.54)* | |
| Mo smoking Pregnacy | |||||||||||
| Yes | 26(0.25) | 10(0.20) | 12(0.33) | 4(0.23)*** | PCP+PP | 47(0.16) | 1(0.01) | 1(0.05) | 38(0.23) | 1(0.03) | 6(0.66)*** |
| No | 3627(35.40) | 2530(51.17) | 1004(27.99) | 93(5.43) | PCP only | 78(0.26) | 3(0.04) | 11(0.52) | 50(0.30) | 11(0.35) | 3(0.33) |
| No response | 6592(64.34) | 2404(48.62) | 2571(71.68) | 1617(94.34) | UK | 114(0.38) | 11(0.16) | 3(0.14) | 65(0.39) | 23(0.74) | 12(1.32) |
| NR | 2003(6.73) | 148(2.15) | 80(3.82) | 1072(6.39) | 305(9.83) | 398(43.73) | |||||
| Never | 27531(92.47) | 6733(97.64) | 2001(95.47) | 15543(92.69) | 2763(89.04) | 491(53.96) | |||||
p<0.05
p<0.01
p<0.001, chi-square between no smoking, smoking, and smoking unknown timing groups
PCP only, Pre-Conception Period only
PCP+PP, PCP + Pregnancy Period
UK, Smoking unknown timing
NR, no response
Fa, Father
Mo, Mother
Family history of Psychiatric disorder, included father, mother and siblings with NDD, schizophrenia, depressive disorder, bipolar disorder, anxiety disorder, substance use and addictive disorder, neurocognitive disorders, trauma related disorder, and other neuropsychiatric disorder by DSM-V
Frequencies of maternal smoking during pregnancy were low in both the DS and RS groups: In the DS, 0.25% overall and in the RS 0.3% for PCP only and 0.2% for PP+P.
Association between Paternal Smoking and Offspring likelihood of having ASD
In the DS, paternal smoking during pregnancy was associated with a higher likelihood to have offspring with ASD: crude Odds Ratio [OR]= 1.21 (95%CI: 1.06-1.39). The significant association held in the subsequent model adjusting other confounders and demographic covariates, with adjusted OR [aOR]=1.27 (95% CI, 1.10-1.47, p=0.001) (Table 3). This finding was replicated in the RS: crude OR of offspring having higher likelihood for ASD =1.26 (95% CI 1.16-1.38, p<0.001) and the aOR = 1.15 (95% CI 1.05-1.25, p=0.003) among fathers who smoked during the PCP+PP. ARFs of paternal smoking during pregnancy for likelihoods of having offspring with ASD in DS and RS were 14.4 and 11.1%, respectively (supplement method 1).
Table 3.
Ordinal logistic regression analysisa to examine the relationship between prenatal paternal smoking exposure and offspring likelihood of having ASD.
| Discovery Sample (N=10245) | Replication Sample (N=29773) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjustedb | Unadjusted | Adjustedb | ||||||
| OR(95% CI) | p-value | aOR a( 95% CI ) | p-value | OR(95% CI) | p-value | aORa ( 95% CI ) | p-value | ||
| Main predictors | |||||||||
| Fa c smoking Pregnancy | |||||||||
| No | 1 [reference] | NA | 1 [reference] | NA | Never | 1 [reference] | NA | 1 [reference] | NA |
| Yes | 1.21(1.06-1.39) | 0.005 | 1.27(1.10-1.47) | 0.001 | PCP+PP | 1.26(1.16-1.38) | <0.001 | 1.15(1.05-1.25) | 0.003 |
| No response | 1.31(1.11-1.56) | 0.001 | 1.07(0.87-1.30) | 0.520 | PCP only | 0.96(0.82-1.13) | 0.635 | 0.95(0.81-1.11) | 0.507 |
| UK timing | 1.22(1.07-1.39) | 0.002 | 1.03(0.90-1.18) | 0.657 | |||||
| No response | 1.61(1.33-1.96) | <0.001 | 0.86(0.68-1.08) | 0.199 | |||||
| Covariates | |||||||||
| Characteristics of Children | |||||||||
| Age | 1.04(1.00-1.08) | 0.033 | 0.98(0.93-1.02) | 0.284 | 1.05(1.03-1.08) | <0.001 | 1.05(1.03-1.07) | <0.001 | |
| Sex | |||||||||
| Female | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | ||
| Male | 1.59(1.40-1.80) | <0.001 | 1.63(1.43-1.85) | <0.001 | 1.42(1.32-1.52) | <0.001 | 1.43(1.33-1.53) | <0.001 | |
| Characteristics of Parents | |||||||||
| Fa age at pregnancy, yr | |||||||||
| 20-29 | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | |
| <20 | 1.86(0.82-4.22) | 0.135 | 0.75(0.30-1.86) | 0.534 | 2.88(1.02-8.13) | 0.045 | 1.52(0.50-4.64) | 0.459 | |
| 30-34 | 1.00(0.86-1.16) | 0.979 | 1.11(0.93-1.30) | 0.252 | 0.83(0.77-0.91) | <0.001 | 0.93(0.84-1.02) | 0.107 | |
| 35-39 | 1.05(0.85-1.30) | 0.629 | 1.04(0.80-1.34) | 0.769 | 0.90(0.81-1.00) | 0.044 | 0.91(0.80-1.03) | 0.136 | |
| >40 | 2.03(1.48-2.77) | <0.001 | 1.88(1.26-2.79) | 0.002 | 1.51(1.29-1.78) | <0.001 | 1.16(0.93-1.43) | 0.180 | |
| Unknown | 2.33(1.92-2.84) | <0.001 | 1.79(1.10-2.91) | 0.019 | 1.67(1.42-1.97) | <0.001 | 0.99(0.75-1.30) | 0.929 | |
| Mo d age at pregnancy, yr | |||||||||
| 20-29 | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | |
| <20 | 2.20(1.51-3.20) | <0.001 | 1.80(1.19-2.74) | 0.006 | 2.35(1.32-4.19) | 0.004 | 1.61(0.88-2.97) | 0.125 | |
| 30-34 | 1.02(0.87-1.19) | 0.839 | 0.97(0.81-1.16) | 0.719 | 0.94(0.87-1.01) | 0.095 | 1.04(0.95-1.14) | 0.409 | |
| 35-39 | 1.69(1.29-2.21) | <0.001 | 1.23(0.88-1.73) | 0.222 | 1.29(1.13-1.47) | <0.001 | 1.20(1.02-1.42) | 0.029 | |
| >40 | 0.77(0.31-1.92) | 0.571 | 0.37(0.14-0.99) | 0.049 | 1.80(1.38-2.36) | <0.001 | 1.19(0.87-1.67) | 0.254 | |
| Unknown | 2.41(2.01-2.89) | <0.001 | 1.07(0.67-1.73) | 0.767 | 2.33(2.01-2.70) | <0.001 | 1.74(1.35-2.24) | <0.001 | |
| Characteristics of Parents | |||||||||
| Parental marriage status | |||||||||
| Married/cohabitation | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | |
| Unmarried | 1.26(0.92-1.74) | 0.153 | 1.19(0.86-1.65) | 0.295 | 1.18(1.04-1.34) | 0.012 | 1.10(0.97-1.26) | 0.151 | |
| Separation/divorce/widowed | 2.59(2.13-3.16) | <0.001 | 1.90(1.53-2.34) | <0.001 | 2.36(2.08-2.68) | <0.001 | 1.59(1.38-1.83) | <0.001 | |
| Unknown | 1.72(1.37-2.18) | <0.001 | 1.12(0.85-1.47) | 0.421 | 1.28(1.11-1.48) | 0.001 | 1.04(0.88-1.23) | 0.632 | |
| Fa education level, yr | |||||||||
| 12 | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | |
| <12 | 2.37(1.72-3.25) | <0.001 | 1.39(0.96-2.04) | 0.089 | 2.79(2.34-3.34) | <0.001 | 1.77(1.44-2.19) | <0.001 | |
| >12 | 0.76(0.66-0.87) | <0.001 | 0.85(0.72-1.00) | 0.062 | 0.65(0.61-0.70) | <0.001 | 0.78(0.71-0.85) | <0.001 | |
| Unknown | 1.78(1.46-2.17) | <0.001 | 0.82(0.49-1.37) | 0.450 | 1.22(1.02-1.45) | 0.028 | 1.05(0.79-1.38) | 0.759 | |
| Mo education level, yr | |||||||||
| 12 | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | |
| <12 | 2.50(1.81-3.46) | <0.001 | 1.67(1.13-2.46) | 0.010 | 2.61(2.18-3.12) | <0.001 | 1.40(1.13-1.73) | 0.002 | |
| >12 | 0.82(0.72-0.95) | 0.006 | 0.98(0.83-1.15) | 0.784 | 0.70(0.65-0.76) | <0.001 | 0.89(0.82-0.98) | 0.016 | |
| Unknown | 1.98(1.64-2.39) | <0.001 | 1.55(0.94-2.55) | 0.084 | 1.58(1.35-1.86) | <0.001 | 1.03(0.79-1.33) | 0.844 | |
| Family Psychiatric History e | |||||||||
| No | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | |
| Yes | 1.44(1.14-1.81) | 0.002 | 1.53(1.20-1.94) | 0.001 | 1.95(1.72-2.22) | <0.001 | 1.69(1.48-1.93) | <0.001 | |
| Mo drinking at pregnancy | |||||||||
| No | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | 1 [reference] | NA | |
| Yes | 1.17(0.82-1.67) | 0.387 | 1.02(0.70-1.48) | 0.913 | 1.75(1.58-1.94) | <0.001 | 1.55(1.40-1.72) | <0.001 | |
| No response | 0.93(0.81-1.06) | 0.264 | 0.87(0.65-1.17) | 0.370 | Exposure time | 0.90(0.82-1.00) | 0.049 | 0.83(0.75-0.93) | 0.001 |
| Mo smoking Pregnancy | |||||||||
| No | 1 [reference] | NA | 1 [reference] | NA | Never | 1 [reference] | NA | 1 [reference] | NA |
| Yes | 2.46(0.98-6.15) | 0.055 | 1.61(0.62-4.14) | 0.327 | PCP+PPf | 3.80(2.06-6.99) | <0.001 | 2.00(1.07-3.76) | 0.031 |
| No response | 0.93(0.82-1.06) | 0.283 | 0.94(0.70-1.27) | 0.694 | PCP onlyg | 3.54(2.23-5.60) | <0.001 | 2.74(1.71-4.40) | <0.001 |
| UK timing | 3.04(2.04-4.54) | <0.001 | 1.92(1.27-2.92) | 0.002 | |||||
| No response | 1.77(1.57-1.99) | <0.001 | 1.30(1.14-1.49) | <0.001 | |||||
Proportional odds assumption upheld
Adjusted for children’s age, sex, parents’ age, education level and marital status, family history of psychiatric disorder, maternal smoking and drinking during pregnancy
Father
Mother
Family history of Psychiatric disorder, included father, mother and siblings with NDD, schizophrenia, depressive disorder, bipolar disorder, anxiety disorder, substance use and addictive disorder, neurocognitive disorders, trauma related disorder, and other neuropsychiatric disorder by DSM-V
PCP only, Pre-Conception Period only
PCP+PP, PCP + Pregnancy Period
Analyses were repeated with two additional missing data methods (complete-case analyses and chained multiple imputation analyses), and the results remained identical (supplement method 2).
DISCUSSION
Male smoking is associated with many adverse health consequences, including adverse reproductive outcomes. However, the impact of paternal smoking on the risk for having offspring with ASD has not been systematically studied. Compared to earlier research examining small numbers of children in case-control study designs [Zhang&Lv et al., 2010, Duan&Yao et al., 2014, Luh Putu Rihayani Budi, 2015], our study included 40,000 community-ascertained children, using a two-step, internal replication design. Our results demonstrate that prenatal paternal smoking is associated with a modestly increased risk (OR=1.15, CI 1.05-1.26, p=0.003) for having offspring at high likelihood for ASD. When combined with the high prevalence of paternal smoking, modest increases in risk may contribute meaningfully to increased ASD prevalence. Based on our findings, we estimate that, in our Discovery Sample, 14.4% of children at high likelihood of having ASD are attributable to prenatal paternal smoking; similarly, in the Replication Sample, 11.1 % of high likelihood of having ASD is due to prenatal paternal smoking.
Initial associations between prenatal paternal smoking and offspring likelihood of having ASD in the DS were confirmed in the RS. These findings have public health implications because smoking is a common and modifiable risk factor.
The observed association between prenatal paternal smoking and offspring likelihood to have ASD may shed light on potential biological mechanisms underlying the genesis of ASD, if, indeed, the observed association reflects a causal effect, even though it has not been established in the current study. While there are many possibilities, our findings suggest possible three mechanisms: (1) Paternal smoking is a marker for unmeasured inherited genetic risk for ASD; (2) de novo mutations generated by prenatal paternal smoking lead to germline disruptions which contribute to development of ASD; and, (3) Direct toxic effects via maternal exposure to secondhand smoke during pregnancy may underly ASD etiology.
To examine the first mechanism, family and paternal psychiatric histories (as a marker for genetic risks for ASD) [Robinson&Samocha et al., 2014] were compared, based on paternal smoking status in both the DS and RS (supplementary Table 1). Family and paternal histories of psychiatric disorders were more common for DS smoker fathers: 7.08% of smoking fathers vs. 4.49% of non-smoking fathers had family psychiatric histories (p<.001), and 0.59% of smoking fathers vs. 0.30% of non-smoking fathers had paternal psychiatric histories (p<.001). In the RS, there were no differences in family psychiatric histories (5.24% of fathers who smoked in PCP+PP; 4.77% for PCP only smokers; and 4.80% for non-smoking fathers, p=0.127). However, there were differences noted in the the fathers’ personal histories of psychiatric disorder: 1.84% of PCP+PP smoker fathers; 1.53% of PCP-only smoker fathers; and, 1.19% of non-smoking fathers (p=0.011). To adjust for unmeasured inherited genetic risk for ASD in smoking fathers, family psychiatric history that included both maternal and paternal psychiatric histories was included in our final model. Family history was a significant factor for having a child at high likelihood of having ASD. (For DS: aORs=1.52 [95% CI 1.20-1.93, p=0.001]; For RS aOR=1.68 [95% CI 1.47-1.92, p<.001]). Prenatal paternal smoking remained as a risk factor, independent of parental psychiatric histories.
Paternal exposure to chemical substances is known to affect spermatogenesis [Fabia and Thuy, 1974] in humans and increase mutations in sperm in the mouse epididymis [Nixon&Stanger et al., 2015], due to the genesis of de novo mutations in the sperm. Tobacco contains more than 7,000 chemicals, many of which have been identified as systemic mutagens in human [DeMarini, 2004]. Cigarette smoking affects the genomic components of sperm and contributes to developmental defects in offspring [Esakky and Moley, 2016]. In rodent studies, cigarette smoking increases the variability in copy number at a hypermutable genetic locus, potentially through inducing mutations in sperm DNA which are passed on to offspring; these permanent, irreversible changes in the genetic composition of the offspring persist in subsequent generations [Yauk&Berndt et al., 2007]. From human studies, male smokers frequently demonstrate several anomalies in spermatogenesis, including increased levels of oxidative DNA damage [Fraga&Motchnik et al., 1996, Shen&Chia et al., 1997], sperm DNA strand breaks [Potts&Newbury et al., 1999], DNA adducts [Horak&Polanska et al., 2003], chromosomal abnormalities [Robbins&Vine et al., 1997, Rubes&Lowe et al., 1998] and decreased viability and fertility [Kunzle&Mueller et al., 2003].
For smoking-induced de novo mutations to occur in the sperm and increase risk for offspring with ASD, it appears most likely that paternal smoking exposure occurs prior to conception; from the present study this includes the PCP only and/or the PCP+PP groups. In the RS, this prediction was partially supported by evidence for increased risks of having offspring at high likelihood of having ASD in the PCP+PP group. The PCP only group did not have significant associations (p=0.537); indeed, there seemed to be a mild protective effect (aOR=0.95 with 95% CI 0.81-1.12), albeit statistically not significant; this unexpected finding in the PCP only group may be an artifact due to relatively small sample size (7% of the RS sample). Dose response in smoking exposure is also a possible explanation for increasing ASD likelihood. At the time of conception, smoking fathers who continue to smoke during pregnancy (PCP+PP) had higher exposure to smoking (7.71 pack-years), compared to 6.65 pack-years for the smoking fathers who stopped smoking during pregnancy (PCP only) (Supplementary Table 2, p<.001). This suggests that PCP+PP exposure is a marker not only for exposure timing, but also for the exposure dose. Ultimately, sequencing DNA from nuclear families will be necessary to demonstrate the presence of de novo mechanisms in ASD risk.
Alternatively, significant findings in the PCP+PP, but not in PCP only, may indicate that the direct toxic effect via maternal exposure to second-hand smoke during pregnancy might play a role in increasing offspring ASD risk. While the research findings are inconclusive [Rosen&Lee et al., 2015], there is evidence to suggest a role for maternal smoking during pregnancy and the development of other NDDs such as Attention Deficit Hyperactivity Disorder, conduct/antisocial disorders, alcohol abuse, depressive disorder, anxiety, aggression, and cognitive impairment in their offspring [Wakschlag&Lahey et al., 1997, Perera&Tang et al., 2007, Carter&Paterson et al., 2008, Cornelius and Day, 2009, Hsieh&Jeng et al., 2010]. Future studies designed to examine the independent impact of timing (pre-conception, pregnancy and postnatal periods) and dose of maternal and paternal, direct and second-hand smoke exposures can help further understand the role of smoking, and possibly other toxins, in the underlying mechanisms for offspring ASD risk.
Our study has several strengths. First, our two-step, internal replication provides greater confidence in the observed associations between prenatal paternal smoking and having offspring at high likelihood of having ASD. Second, study subjects were drawn from epidemiologically-ascertained, representative samples with greater than 70% response rates. Third, study subjects were assessed using a dimensional instrument, ASSQ, with three incremental likelihood categories. Such methods are likely to reduce phenotype heterogeneity [Abrahams and Geschwind, 2008, Losh&Sullivan et al., 2008] and sampling bias including missed ASD cases [Berkson, 2014]. The observed association between prenatal paternal smoking and having offspring at high likelihood for ASD persisted even after the adjusting for potential confounders, such as maternal smoking and drinking, and a family history of psychiatric disorders (a proxy for genetic risk for ASD).
Limitations of our study include the retrospective collection of prenatal paternal smoking data, ASD outcome measurement by questionnaire only, missing data from nonresponders and the potential impact of unmeasured genetic risks. Esepcially, missing rate of maternal smoking are high since mothers, main primary caregiver, reluctant to reply to the smoking status of themselves. In this sense, maternal smoking was higher odds ratio for having a children with a likelihood of having ASD than paternal smoking. Prenatal exposure data for parental smoking were collected by questionnaire, retrospectively, and the majority of questionnaires were completed by mothers. While the reliability and validity of the short-term and long-term recall of perinatal events, as well as recall of spouse smoking status are well-accepted in epidemiologic research [Yawn&Suman et al., 1998, Buka&Goldstein et al., 2004, Sou&Chen et al., 2006, Mejia&Braun et al., 2017], there is still potential for misclassification of paternal smoking. Such misclassification is likely to be random in a cohort study design, which might have attenuated observed associations [Hennekens CH, 1987]. ASD phenotypes were measured with a 27-item screening questionnaire, not by direct clinical examination. While our prior Korean prevalence study demonstrated that the ASSQ is an excellent screening instrument with good positive predictive values for the best estimate diagnoses of ASD [Kim & Fombonne et al., 2014], in our samples, the diagnoses of the children at high and intermediate likelihood of having ASD were not clinically validated. Therefore, children without ASD could have been included in the high and/or intermediate ASD likelihood groups; this might diminish the magnitude of the observed associations. While participation rates in both the DS and the RS are >70% at every stage, we do not have data on non-participants. It is possible that unknown characteristics in the non-respondents could have affected the observed relationships between prenatal paternal smoking and having increased offspring ASD likelihood. Finally, we attempted to account for genetic risk by controlling for family psychiatric histories. While psychiatric history is correlated with polygenetic risks of NDDs, including ASD [Robinson&St Pourcain et al., 2016], we cannot rule out the potential impact of unmeasured genetic risks on the observed associations.
CONCLUSIONS
Using two independent, large community samples of children and their families, our study demonstrates that prenatal paternal smoking increases risk for having a child at high likelihood for ASD. While independent replication is warranted, our findings add further support for the importance of education and intervention to reduce smoking. This is especially crucial for individuals planning to have children as the elimination of paternal smoking can reduce the risk of having a child at high likelihood for ASD by as much as 11-14%.
Supplementary Material
Figure 2. Prenatal Paternal Smoking in Discovery and Replication Samples (%).
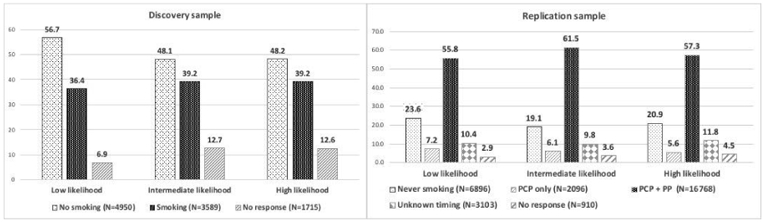
Abbreviations: PCP only, Pre-Conception Period only; PCP+PP, PCP + Pregnancy Period
Acknowledgements and Financial Disclosure
This project has been funded by NIEHS R01 Award (R01 ES021462-01), SFARI Pilot Award (M134793), NIMH K01 Award (MH079317), NCRR CTSA Grant (UL1 RR024139) in US, and Ministry of Environment Grant, in Korea. Authors are indebted to Ms. Jihyun Kim at Korea Institute for Children’s Social Development for survey field work and data management and to Dr. Hyelee Kim at Harvard T.H. Chan School of Public Health for data analyses; the Board of Education that helped implementation of the school survey; and children and their families who participated in the study.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report in relation to the research presented in this manuscript.
References
- Abrahams BS and Geschwind DH (2008). Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 9(5): 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia IB, Grummer-Strawn L and Scanlon KS (1997). Exposure to environmental tobacco smoke and birth outcome: increased effects on pregnant women aged 30 years or older. Am J Epidemiol 146(1): 42–47. [DOI] [PubMed] [Google Scholar]
- Berkson J (2014). Limitations of the application of fourfold table analysis to hospital data. Int J Epidemiol 43(2): 511–515. [DOI] [PubMed] [Google Scholar]
- Bescoby-Chambers N, Forster P and Bates G (2001). ‘Foetal valproate syndrome and autism: additional evidence of an association’. Dev Med Child Neurol 43(12): 847. [DOI] [PubMed] [Google Scholar]
- Bilder D, Pinborough-Zimmerman J, Miller J and McMahon W (2009). Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics 123(5): 1293–1300. [DOI] [PubMed] [Google Scholar]
- Buka SL, Goldstein JM, Spartos E and Tsuang MT (2004). The retrospective measurement of prenatal and perinatal events: accuracy of maternal recall. Schizophr Res 71(2-3): 417–426. [DOI] [PubMed] [Google Scholar]
- Burstyn I, Sithole F and Zwaigenbaum L (2010). Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can 30(4): 125–134. [PubMed] [Google Scholar]
- Carter S, Paterson J, Gao W and Iusitini L (2008). Maternal smoking during pregnancy and behaviour problems in a birth cohort of 2-year-old Pacific children in New Zealand. Early Hum Dev 84(1): 59–66. [DOI] [PubMed] [Google Scholar]
- Cheslack-Postava K, Liu K and Bearman PS (2011). Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics 127(2): 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess S (1971). Autism in children with congenital rubella. J Autism Child Schizophr 1(1): 33–47. [DOI] [PubMed] [Google Scholar]
- Christensen DL (2016). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR. Surveillance Summaries 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford A, Lang L and Chen R (2012). Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicol Teratol 34(6): 560–570. [DOI] [PubMed] [Google Scholar]
- Cornelius MD and Day NL (2009). Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol 22(2): 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarini DM (2004). Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res 567(2-3): 447–474. [DOI] [PubMed] [Google Scholar]
- Dodds L, Fell DB, Shea S, Armson BA, Allen AC and Bryson S (2011). The Role of Prenatal, Obstetric and Neonatal Factors in the Development of Autism. J Autism Dev Disord. [DOI] [PubMed] [Google Scholar]
- Duan G, Yao M, Ma Y and Zhang W (2014). Perinatal and background risk factors for childhood autism in central China. Psychiatry Res 220(1-2): 410–417. [DOI] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Newschaffer CJ, Lee LC, Cunniff CM, Daniels JL, Schieve LA (2008). Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol 168(11): 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S, Gillberg C and Wing L (1999). A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord 29(2): 129–141. [DOI] [PubMed] [Google Scholar]
- Eliasen M, Tolstrup JS, Nybo Andersen AM, Gronbaek M, Olsen J and Strandberg-Larsen K (2010). Prenatal alcohol exposure and autistic spectrum disorders--a population-based prospective study of 80,552 children and their mothers. Int J Epidemiol 39(4): 1074–1081. [DOI] [PubMed] [Google Scholar]
- Esakky P and Moley KH (2016). Paternal smoking and germ cell death: A mechanistic link to the effects of cigarette smoke on spermatogenesis and possible long-term sequelae in offspring. Mol Cell Endocrinol 435: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabia J and Thuy TD (1974). Occupation of father at time of birth of children dying of malignant diseases. Br J Prev Soc Med 28(2): 98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E (2009). Epidemiology of pervasive developmental disorders. Pediatr Res 65(6): 591–598. [DOI] [PubMed] [Google Scholar]
- Forest S and Priest S (2016). Intrauterine Tobacco Smoke Exposure and Congenital Heart Defects. J Perinat Neonatal Nurs 30(1): 54–63; quiz E52. [DOI] [PubMed] [Google Scholar]
- Fraga CG, Motchnik PA, Wyrobek AJ, Rempel DM and Ames BN (1996). Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat Res 351(2): 199–203. [DOI] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D and Buka SL (2011). Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 128(2): 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, Buxbaum JD (2014). Most genetic risk for autism resides with common variation. Nat Genet 46(8): 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hill J (2006). Missing-data imputation. In Data Analysis Using Regression and Multilevel/Hierarchical Models (Analytical Methods for Social Research, pp. 529–544). Cambridge University Press; Cambridge. doi: 10.1017/CBO9780511790942.031 [DOI] [Google Scholar]
- Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G and Hallmayer JF (2004). Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry 61(6): 618–627. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, B. J., Mayre SL. (1987). Epidemiology in Medicine. Lippincott Williams & Wilkins; Philadelphia, PA. [Google Scholar]
- Hopkins DP, Briss PA, Ricard CJ, Husten CG, Carande-Kulis VG, Fielding JE, … Task Force on Community Preventive, S. (2001). Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. Am J Prev Med 20(2 Suppl): 16–66. [DOI] [PubMed] [Google Scholar]
- Horak S, Polanska J and Widlak P (2003). Bulky DNA adducts in human sperm: relationship with fertility, semen quality, smoking, and environmental factors. Mutat Res 537(1): 53–65. [DOI] [PubMed] [Google Scholar]
- Hsieh CJ, Jeng SF, Su YN, Liao HF, Hsieh WS, Wu KY and Chen PC (2010). CYP1A1 modifies the effect of maternal exposure to environmental tobacco smoke on child behavior. Nicotine Tob Res 12(11): 1108–1117. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sandin S, Levine SZ, Lichtenstein P and Reichenberg A (2010). Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sandin S, Levine SZ, Lichtenstein P and Reichenberg A (2011). Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol Psychiatry 16(12): 1203–1212. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sparen P and Cnattingius S (2002). Perinatal risk factors for infantile autism. Epidemiology 13(4): 417–423. [DOI] [PubMed] [Google Scholar]
- Ingram JL, Peckham SM, Tisdale B and Rodier PM (2000). Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol 22(3): 319–324. [DOI] [PubMed] [Google Scholar]
- Ion RC, Wills AK and Bernal AL (2015). Environmental Tobacco Smoke Exposure in Pregnancy is Associated With Earlier Delivery and Reduced Birth Weight. Reprod Sci 22(12): 1603–1611. [DOI] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD and Graffunder CM (2016). Current Cigarette Smoking Among Adults - United States, 2005-2015. MMWR Morb Mortal Wkly Rep 65(44): 1205–1211. [DOI] [PubMed] [Google Scholar]
- Kaur B (2014). The Association between Autism Spectrum Disorders and Secondhand Tobacco Exposure. Master of Public Health Program, Wright State University, Dayton, Ohio. Master of Public Health. [Google Scholar]
- Kim YS, Fombonne E, Koh YJ, Kim SJ, Cheon KA and Leventhal BL (2014). A comparison of DSM-IV pervasive developmental disorder and DSM-5 autism spectrum disorder prevalence in an epidemiologic sample. J Am Acad Child Adolesc Psychiatry 53(5): 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, Grinker RR (2011). Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry 168(9): 904–912. [DOI] [PubMed] [Google Scholar]
- Korean Statistical Information Service [KOSIS] (2010). National Health Screening Statistics, 2010. From http://kosis.kr/eng/statisticsList/statisticsList_01List.jsp?vwcd=MT_ETITLE&parentId=D-SubCont. [Google Scholar]
- Krall EA, Valadian I, Dwyer JT and Gardner J (1989). Accuracy of recalled smoking data. Am J Public Health 79(2): 200–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzle R, Mueller MD, Hanggi W, Birkhauser MH, Drescher H and Bersinger NA (2003). Semen quality of male smokers and nonsmokers in infertile couples. Fertil Steril 79(2): 287–291. [DOI] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, Mortensen PB (2005). Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol 161(10): 916–925; discussion 926-918. [DOI] [PubMed] [Google Scholar]
- Larsson M, Weiss B, Janson S, Sundell J and Bornehag CG (2009). Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6-8 years of age. Neurotoxicology 30(5): 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Gardner RM, Dal H, Svensson A, Galanti MR, Rai D, Magnusson C (2012). Brief report: maternal smoking during pregnancy and autism spectrum disorders. J Autism Dev Disord 42(9): 2000–2005. [DOI] [PubMed] [Google Scholar]
- Lee KM, Ward MH, Han S, Ahn HS, Kang HJ, Choi HS, Kang D (2009). Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res 33(2): 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LC, Newschaffer CJ, Lessler JT, Lee BK, Shah R and Zimmerman AW (2008). Variation in season of birth in singleton and multiple births concordant for autism spectrum disorders. Paediatr Perinat Epidemiol 22(2): 172–179. [DOI] [PubMed] [Google Scholar]
- Li Y, Lin H, Li Y and Cao J (2011). Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril 95(1): 116–123. [DOI] [PubMed] [Google Scholar]
- Linschooten JO, Verhofstad N, Gutzkow K, Olsen AK, Yauk C, Oligschlager Y, Godschalk RW (2013). Paternal lifestyle as a potential source of germline mutations transmitted to offspring. FASEB J 27(7): 2873–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Sullivan PF, Trembath D and Piven J (2008). Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol 67(9): 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luh Putu Rihayani Budi MNS, Gusti Ayu Trisna Windiani I (2015). Paternal and maternal age at pregnancy and autism spectrum disorders in offspring. Paediatrica Indonesiana 55(6): 345–351. [Google Scholar]
- Lyall K, Schmidt RJ and Hertz-Picciotto I (2014). Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol 43(2): 443–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner MJ, Rice CE, Arneson CL and et al. (2014). Potential impact of dsm-5 criteria on autism spectrum disorder prevalence estimates. JAMA Psychiatry 71(3): 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JR, McDermott S, Bao H, Hardin J and Gregg A (2010). Pre-eclampsia, birth weight, and autism spectrum disorders. J Autism Dev Disord 40(5): 548–554. [DOI] [PubMed] [Google Scholar]
- Mattila ML, Kielinen M, Jussila K, Linna SL, Bloigu R, Ebeling H and Moilanen I (2007). An epidemiological and diagnostic study of Asperger syndrome according to four sets of diagnostic criteria. J Am Acad Child Adolesc Psychiatry 46(5): 636–646. [DOI] [PubMed] [Google Scholar]
- Mejia RM, Braun S, Pena L, Gregorich SE and Perez-Stable EJ (2017). Validation of Non-Smoking Status by Spouse Following a Cessation Intervention. J Smok Cessat 12(1): 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modabbernia A, Velthorst E and Reichenberg A (2017). Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T and Dean JC (2000). A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet 37(7): 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RB, Momirova V, Dombrowski MP, Schatz M, Wise R, Landon M, Human Development Maternal-Fetal Medicine Units, N. (2010). The effect of active and passive household cigarette smoke exposure on pregnant women with asthma. Chest 137(3): 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B, Stanger SJ, Mihalas BP, Reilly JN, Anderson AL, Tyagi S, McLaughlin EA (2015). The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol Reprod 93(4): 91. [DOI] [PubMed] [Google Scholar]
- Ornoy A, Weinstein-Fudim L and Ergaz Z (2015). Prenatal factors associated with autism spectrum disorder (ASD). Reprod Toxicol 56: 155–169. [DOI] [PubMed] [Google Scholar]
- Perera FP, Tang D, Rauh V, Tu YH, Tsai WY, Becker M, Lederman SA (2007). Relationship between polycyclic aromatic hydrocarbon-DNA adducts, environmental tobacco smoke, and child development in the World Trade Center cohort. Environ Health Perspect 115(10): 1497–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phs Guideline Update Panel, L. and Staff (2008). Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care 53(9): 1217–1222. [PubMed] [Google Scholar]
- Potts RJ, Newbury CJ, Smith G, Notarianni LJ and Jefferies TM (1999). Sperm chromatin damage associated with male smoking. Mutat Res 423(1-2): 103–111. [DOI] [PubMed] [Google Scholar]
- Robbins WA, Vine MF, Truong KY and Everson RB (1997). Use of fluorescence in situ hybridization (FISH) to assess effects of smoking, caffeine, and alcohol on aneuploidy load in sperm of healthy men. Environ Mol Mutagen 30(2): 175–183. [DOI] [PubMed] [Google Scholar]
- Robinson EB, Samocha KE, Kosmicki JA, McGrath L, Neale BM, Perlis RH and Daly MJ (2014). Autism spectrum disorder severity reflects the average contribution of de novo and familial influences. Proc Natl Acad Sci U S A 111(42): 15161–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, St Pourcain B, Anttila V, Kosmicki JA, Bulik-Sullivan B, Grove J, Daly MJ (2016). Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet 48(5): 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B and Croog VJ (1997). Linking etiologies in humans and animal models: studies of autism. Reprod Toxicol 11(2-3): 417–422. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S and Romano J (1996). Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol 370(2): 247–261. [DOI] [PubMed] [Google Scholar]
- Rosen BN, Lee BK, Lee NL, Yang Y and Burstyn I (2015). Maternal Smoking and Autism Spectrum Disorder: A Meta-analysis. J Autism Dev Disord 45(6): 1689–1698. [DOI] [PubMed] [Google Scholar]
- Rubes J, Lowe X, Moore D 2nd, Perreault S, Slott V, Evenson D, Wyrobek AJ (1998). Smoking cigarettes is associated with increased sperm disomy in teenage men. Fertil Steril 70(4): 715–723. [DOI] [PubMed] [Google Scholar]
- Schendel D and Bhasin TK (2008). Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics 121(6): 1155–1164. [DOI] [PubMed] [Google Scholar]
- Shen HM, Chia SE, Ni ZY, New AL, Lee BL and Ong CN (1997). Detection of oxidative DNA damage in human sperm and the association with cigarette smoking. Reprod Toxicol 11(5): 675–680. [DOI] [PubMed] [Google Scholar]
- Singer AB, Aylsworth AS, Cordero C, Croen LA, DiGuiseppi C, Fallin MD, Daniels JL (2017). Prenatal Alcohol Exposure in Relation to Autism Spectrum Disorder: Findings from the Study to Explore Early Development (SEED). Paediatr Perinat Epidemiol 31(6): 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sou SC, Chen WJ, Hsieh WS and Jeng SF (2006). Severe obstetric complications and birth characteristics in preterm or term delivery were accurately recalled by mothers. J Clin Epidemiol 59(4): 429–435. [DOI] [PubMed] [Google Scholar]
- Statistics Korea (2015). Population and Housing Census.
- Stromland K, Nordin V, Miller M, Akerstrom B and Gillberg C (1994). Autism in thalidomide embryopathy: a population study. Dev Med Child Neurol 36(4): 351–356. [DOI] [PubMed] [Google Scholar]
- Thacher JD, Gruzieva O, Pershagen G, Neuman A, Wickman M, Kull I, Bergstrom A (2014). Pre- and postnatal exposure to parental smoking and allergic disease through adolescence. Pediatrics 134(3): 428–434. [DOI] [PubMed] [Google Scholar]
- Tick B, Bolton P, Happe F, Rutter M and Rijsdijk F (2016). Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry 57(5): 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PL, Lehti V, Lampi KM, Helenius H, Suominen A, Gissler M, Sourander A (2013). Smoking during pregnancy and risk of autism spectrum disorder in a Finnish National Birth Cohort. Paediatr Perinat Epidemiol 27(3): 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA and Leventhal BL (1997). Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry 54(7): 670–676. [DOI] [PubMed] [Google Scholar]
- Williams G, King J, Cunningham M, Stephan M, Kerr B and Hersh JH (2001). Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol 43(3): 202–206. [PubMed] [Google Scholar]
- Williams K, Helmer M, Duncan GW, Peat JK and Mellis CM (2008). Perinatal and maternal risk factors for autism spectrum disorders in New South Wales, Australia. Child Care Health Dev 34(2): 249–256. [DOI] [PubMed] [Google Scholar]
- Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, State MW (2013). Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155(5): 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Hopkins B, Fenster L and Swan SH (2000). Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology 11(4): 427–433. [DOI] [PubMed] [Google Scholar]
- Yauk CL, Berndt ML, Williams A, Rowan-Carroll A, Douglas GR and Stampfli MR (2007). Mainstream tobacco smoke causes paternal germ-line DNA mutation. Cancer Res 67(11): 5103–5106. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Suman VJ and Jacobsen SJ (1998). Maternal recall of distant pregnancy events. J Clin Epidemiol 51(5): 399–405. [DOI] [PubMed] [Google Scholar]
- Yim G (2012). Validation of the Autism Spectrum Screening Questionnaire (ASSQ) in a School-Aged Population in Korea. School of Public Health, Yale University MPH. [Google Scholar]
- Zablotsky B, Black LI, Maenner MJ, Schieve LA and Blumberg SJ (2015). Estimated Prevalence of Autism and Other Developmental Disabilities Following Questionnaire Changes in the 2014 National Health Interview Survey. National health statistics reports(87): 1–20. [PubMed] [Google Scholar]
- Zhang X, Lv CC, Tian J, Miao RJ, Xi W, Hertz-Picciotto I and Qi L (2010). Prenatal and perinatal risk factors for autism in China. J Autism Dev Disord 40(11): 1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


