Abstract
Background
The coronavirus disease 2019 (COVID‐19) pandemic has had wide‐ranging health effects and increased isolation. Older with cancer patients might be especially vulnerable to loneliness and poor mental health during the pandemic.
Methods
The authors included active participants enrolled in the longitudinal Thinking and Living With Cancer study of nonmetastatic breast cancer survivors aged 60 to 89 years (n = 262) and matched controls (n = 165) from 5 US regions. Participants completed questionnaires at parent study enrollment and then annually, including a web‐based or telephone COVID‐19 survey, between May 27 and September 11, 2020. Mixed‐effects models were used to examine changes in loneliness (a single item on the Center for Epidemiologic Studies–Depression [CES‐D] scale) from before to during the pandemic in survivors versus controls and to test survivor‐control differences in the associations between changes in loneliness and changes in mental health, including depression (CES‐D, excluding the loneliness item), anxiety (the State‐Trait Anxiety Inventory), and perceived stress (the Perceived Stress Scale). Models were adjusted for age, race, county COVID‐19 death rates, and time between assessments.
Results
Loneliness increased from before to during the pandemic (0.211; P = .001), with no survivor‐control differences. Increased loneliness was associated with worsening depression (3.958; P < .001) and anxiety (3.242; P < .001) symptoms and higher stress (1.172; P < .001) during the pandemic, also with no survivor‐control differences.
Conclusions
Cancer survivors reported changes in loneliness and mental health similar to those reported by women without cancer. However, both groups reported increased loneliness from before to during the pandemic that was related to worsening mental health, suggesting that screening for loneliness during medical care interactions will be important for identifying all older women at risk for adverse mental health effects of the pandemic.
Keywords: anxiety, breast cancer, cancer survivorship, coronavirus disease 2019 (COVID‐19), depression, loneliness, older adults, psychological stress
Short abstract
Older breast cancer survivors and matched noncancer controls experienced similar increases in loneliness from before to during the COVID‐19 pandemic. Women who reported increased loneliness also experienced worsening depression and anxiety symptoms and higher stress during the pandemic.
Introduction
The coronavirus disease 2019 (COVID‐19) pandemic has been a period of unprecedented psychosocial stress for many individuals, including decreased social contact and lower availability of support and services, with the potential for profound impacts on mental health.1, 2, 3, 4, 5 Research suggests that the prevalence of depression and anxiety symptoms in the general population increased during the pandemic compared with estimates from the previous year.1, 2, 4, 6, 7, 8, 9, 10 Loneliness, or feelings of social disconnection resulting from a discrepancy between an individual's actual and desired social relationships, also increased during this time.3, 11, 12, 13 Loneliness has been identified as an independent risk factor for morbidity and mortality that is comparable in its effects on mortality to smoking 15 cigarettes per day in cancer and noncancer populations.14, 15, 16, 17 Research suggests that older adults experience higher levels of loneliness and are more vulnerable to its negative health consequences, including depression, hypertension, and cognitive declines, than younger adults.14
Older adults and individuals with preexisting medical conditions such as cancer were particularly advised to physically isolate from others during the pandemic because of high risk for severe complications of COVID‐19.18 Social isolation thus may contribute to increased feelings of loneliness among older cancer survivors.18 Prepandemic vulnerabilities for loneliness, depression, and anxiety may also be magnified during the pandemic in older breast cancer survivors compared with those without cancer because of concerns about COVID‐19 infection, potential disruptions to medical care, and additional stressors that they might face.5 Reports from other countries suggest that patients with cancer and survivors have experienced increases in loneliness5, 19 and worsening mental health, including depression, anxiety, and stress, during the pandemic compared with their family members and the general population.20 However, there remains little evidence about the impact of the pandemic on loneliness and its association with mental health among older cancer survivors in the United States.
We examined changes in loneliness and mental health during the COVID‐19 pandemic in a sample of older breast cancer survivors and matched noncancer controls enrolled in the longitudinal Thinking and Living With Cancer (TLC) study. We hypothesized that cancer survivors would experience larger increases in loneliness than controls and that increases in loneliness would be associated with worsening depression and anxiety symptoms and higher stress during the pandemic in survivors compared with controls.
Materials and Methods
Participants were enrolled in the ongoing, multisite longitudinal TLC study investigating the effects of cancer treatment on cognitive function in women aged ≥60 years with newly diagnosed nonmetastatic (AJCC stage 0‐III) breast cancer and frequency‐matched noncancer controls. The institutional review boards of all participating study sites (Georgetown University, Memorial Sloan Kettering Cancer Center, Moffitt Cancer Center, City of Hope Comprehensive Cancer Center, Hackensack University Medical Center, and Indiana University School of Medicine) approved the protocol (ClinicalTrials.gov identifier NCT03451383).
Eligibility for the TLC study included no history of stroke, head injury, major psychiatric or neurodegenerative disorder, prior chemotherapy or hormonal therapy, or treatment for another cancer within the past 5 years. Women taking psychoactive medications were eligible if the dose was stable for at least 2 months before study enrollment. Details about the study have been published elsewhere.21, 22 Briefly, breast cancer survivors were enrolled in the study after surgery but before initiation of radiation or systemic therapy. As part of the TLC parent study, all survivors and frequency‐matched noncancer controls completed questionnaires at study enrollment and then annually for up to 60 months.
Of the 1282 women enrolled in the TLC parent study as of March 1, 2020, 533 active participants (ie, those who had not yet completed the study, dropped out, or died) were contacted to complete an additional web‐based or telephone survey during the COVID‐19 pandemic. Compared with nonactive participants, active participants had completed more years of education (mean ± SD: 15.6 ± 2.2 vs 15.2 ± 2.3; P = .006), and a larger proportion was White (83.9% vs 78.1%; P = .011) and was married or living as married (61.0% vs 53.8%; P = .011).
Participants completed the COVID‐19 survey online (80.7%) or by telephone (19.3%) between May 27 and September 11, 2020, to assess the impact of the pandemic on psychosocial functioning. Of the 533 active participants, 279 cancer survivors and 166 noncancer controls responded to the survey. We excluded 13 survivors who were missing cancer treatment information at study enrollment, 3 survivors and 1 control who reported contracting COVID‐19, and 1 survivor who was missing treatment information at enrollment and also reported contracting COVID‐19, for a final sample of 262 survivors (81.6% of active survivors) and 165 controls (85.1% of active controls) (Fig. 1). Compared with responders, nonresponders had higher depression symptoms at study enrollment (mean ± SD: 7.3 ± 6.8 vs 5.3 ± 6.5; P = .011), and a larger proportion was non‐White (27.3% vs 13.8%; P = .002). The proportion of responders differed across study sites for controls (P = .004) but not for survivors (P = .074); therefore, analyses were adjusted for study site using county‐specific COVID‐19 death rates (see description in Measures, below).
Figure 1.
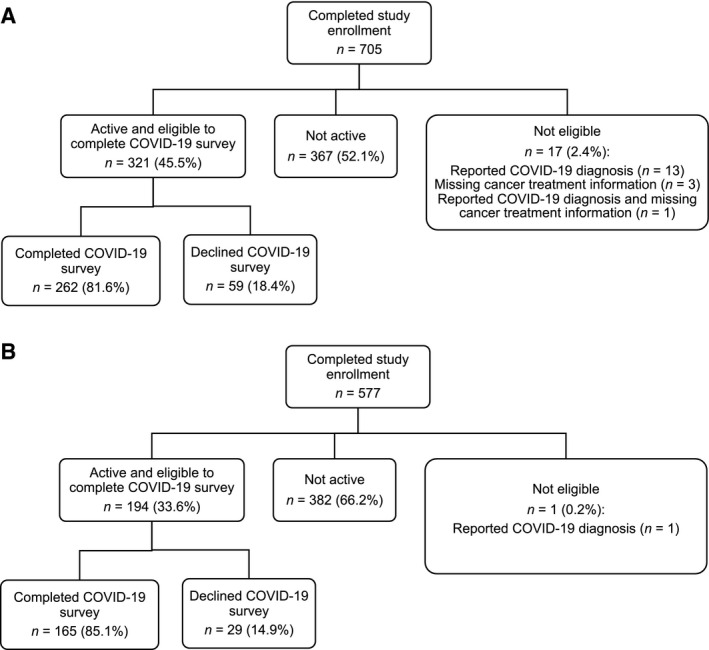
This Consolidated Standards for Reporting Trials (CONSORT) diagram for the Thinking and Living with Cancer Study illustrates the sample of (A) older breast cancer survivors and (B) matched controls without cancer. Participants were excluded if they were not active (ie, had completed the study, dropped out, or died) at the time of the coronavirus disease 2019 (COVID‐19) survey, were missing cancer treatment information at study enrollment, or reported a COVID‐19 diagnosis. The percentages of participants who completed and declined the COVID‐19 survey were calculated based on the number of participants who were active and eligible to complete the survey.
Measures
Loneliness was evaluated using the single item “I felt lonely during the past week” from the Center for Epidemiologic Studies–Depression (CES‐D) Scale,23, 24, 25, 26 which participants completed at all TLC parent study assessments and during the COVID‐19 survey. Response options ranged from 0 (rarely or none of the time) to 3 (most of the time). The COVID‐19 survey also included the 3‐item loneliness scale adapted from the University of California‐Los Angeles loneliness scale.11 Previous research with older adults indicated that the CES‐D loneliness item correlated highly with the 3‐item scale, and the 2 measures were highly correlated in this sample (r = 0.61; P < .001).11 The CES‐D loneliness item was also more highly correlated with items on the 3‐item loneliness scale (r = −0.47 to 0.59; average, r = 0.51) than it was with the other items on the CES‐D scale (r = 0.09‐0.53; average, r = 0.32). All study analyses focused on the CES‐D loneliness item because it was available at TLC study assessments before and during the pandemic.
Participants completed the CES‐D scale23 to assess depressive symptoms at each parent study assessment, and this scale was also included on the COVID‐19 survey, with higher scores indicating greater depression. We removed the loneliness item from the CES‐D for analyses of depression, and internal consistency for the CES‐D remained high both with (Cronbach α = .88) and without (Cronbach α = 0.88) the loneliness item. Participants also completed the State‐Trait Anxiety Inventory (STAI)‐State subscale27 to assess anxiety symptoms at each parent study assessment and on the COVID‐19 survey, with higher scores indicating greater anxiety. As part of the COVID‐19 survey, participants also completed the Perceived Stress Scale,28, 29 which assessed the degree to which women appraised their life as stressful (eg, feeling unable to control important things in life) in the past month.
Several demographic characteristics were considered as covariates, including age, race (non‐White vs White), county rates of COVID‐19 deaths, and time (in months) from participants' last pre–COVID‐19 study assessment to the COVID‐19 survey.30, 31 County COVID‐19 death rates were calculated as the cumulative number of COVID‐19 deaths per 1000 individuals from the start of data reporting in each US county to the date of survey completion for each participant, divided by the 2019 population estimate for that county. Data for cumulative deaths in each county were obtained from The New York Times based on reports from state and local health agencies.32, 33 For the analysis that examined longitudinal changes in loneliness, we included living circumstances (living alone vs with others) and the Medical Outcomes Study Social Support Survey34 emotional (eg, someone to confide in) and tangible (eg, someone to help with daily chores) social support subscales at the time of the COVID‐19 survey as additional covariates.
Statistical Analysis
Descriptive analyses compared survivors and controls at study enrollment, at their last pre–COVID‐19 assessment, and at the COVID‐19 survey. To examine whether there were differences between survivors and controls in longitudinal changes in loneliness from study enrollment to the last pre–COVID‐19 assessment and from the last pre–COVID‐19 assessment to the COVID‐19 survey, we used a piecewise, linear mixed‐effects model with fixed timepoints at study enrollment, the last pre–COVID‐19 study assessment, and the COVID‐19 survey. Because of variation among participants in the number of follow‐up assessments they had completed at the time of the COVID‐19 survey, we aligned outcomes with the last pre–COVID‐19 assessment. The model included interaction terms for timepoint (study enrollment and the COVID‐19 survey vs the last pre–COVID‐19 assessment) and group (survivors vs controls), adjusting for age, race (non‐White vs White), county COVID‐19 death rate, months from the last pre–COVID‐19 assessment to the COVID‐19 survey, living circumstances, and emotional and tangible support.
To test the hypothesis that increases in loneliness would have a greater impact on depression, anxiety, and perceived stress during the pandemic in survivors versus controls, we conducted separate linear mixed‐effects models for each outcome with timepoints at the last pre–COVID‐19 study assessment and at the COVID‐19 survey. For these models, we calculated a loneliness difference score (CES‐D loneliness score at the COVID‐19 survey minus the CES‐D loneliness score at the last pre–COVID‐19 assessment). The models included a 3‐way interaction term for timepoint, loneliness difference score, and group to test for differences between survivors and controls. The models adjusted for age, race, county COVID‐19 death rate, and months from the last pre–COVID‐19 assessment to the COVID‐19 survey. If a 3‐way interaction term was not statistically significant, it was removed from the model and group was added as a covariate. Follow‐up analyses probed significant 2‐way interactions between timepoint and the loneliness difference score on depression and anxiety symptoms to provide point estimates for simple slopes representing change in depression and anxiety symptoms at different levels of the loneliness difference score: a 1‐point increase in loneliness (difference score of +1), no change in loneliness (difference score of 0), and a 1‐point decrease in loneliness (difference score of −1).
All tests of statistical significance were 2‐sided using a P < .05 threshold. Analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
The mean age of survivors and controls was approximately 68 years, and most self‐identified as White, reported being married, and were not currently employed (Table 1). Descriptive statistics for the psychosocial variables appear in Table 2. Consistent with prior reports in noncancer populations, both survivors and controls reported increases in depression and anxiety symptoms during the pandemic.
TABLE 1.
Demographic and Clinical Characteristics Among Older Breast Cancer Survivors and Frequency‐Matched Noncancer Controls
| Demographic and Clinical Variables | No. (%) | P a | |
|---|---|---|---|
| Survivors, n = 262 | Controls, n = 165 | ||
| Age: Mean ± SD, y | 67.9 ± 5.3 | 68.0 ± 6.0 | .834 |
| Race | .274 | ||
| Non‐White | 40 (15.3) | 19 (11.5) | |
| White | 222 (84.7) | 146 (88.5) | |
| Marital status | .184 | ||
| Married or living as married | 156 (64.2) | 91 (57.6) | |
| Other | 87 (35.8) | 67 (42.4) | |
| Education: Mean ± SD, y | 15.6 ± 2.2 | 15.8 ± 2.1 | .361 |
| Employment status | .630 | ||
| Currently employed | 85 (35.0) | 59 (37.3) | |
| Not currently employed | 158 (65.0) | 99 (62.7) | |
| Year of study enrollment | <.001 | ||
| 2014 | 4 (1.5) | 2 (1.2) | |
| 2015 | 31 (11.8) | 1 (0.6) | |
| 2016 | 23 (8.8) | 9 (5.5) | |
| 2017 | 44 (16.8) | 57 (34.5) | |
| 2018 | 90 (34.4) | 47 (28.5) | |
| 2019 | 50 (19.1) | 48 (29.1) | |
| 2020 | 20 (7.6) | 1 (0.6) | |
| AJCC Cancer stage | — | ||
| 0 | 55 (21.8) | — | |
| I | 152 (60.3) | — | |
| II | 40 (15.9) | — | |
| III | 5 (2.0) | — | |
| Treatment group | — | ||
| Chemotherapy | 51 (19.5) | — | |
| Hormonal therapy | 211 (80.5) | — | |
| County COVID‐19 death rate: Mean ± SD | 0.8 ± 0.9 | 0.9 ± 0.8 | .714 |
| Time from the last pre–COVID‐19 assessment to the COVID‐19 survey: Mean ± SD, mo | 10.6 ± 5.6 | 10.4 ± 3.8 | .630 |
Abbreviation: COVID‐19, coronavirus disease 2019.
P values were derived from t tests or χ2 tests.
TABLE 2.
Psychosocial Characteristics Among Older Breast Cancer Survivors and Frequency‐Matched Noncancer Controls
| Psychosocial Variables [Score Range] | Study Enrollment | Last Pre–COVID‐19 Assessment | COVID‐19 Survey | ||||
|---|---|---|---|---|---|---|---|
| Survivors, n = 192 | Controls, n = 122 | Survivors, n = 262 | Controls, n = 165 | Survivors, n = 262 | Controls, n = 165 | P a | |
| Loneliness item [0‐3]: Mean ± SD | 0.3 ± 0.7 | 0.3 ± 0.7 | 0.3 ± 0.6 | 0.4 ± 0.7 | 0.5 ± 0.7 | 0.6 ± 0.8 | <.001 |
| Loneliness difference scoreb [−3 to +3]: Mean ± SD | — | — | — | — | 0.3 ± 0.8 | 0.2 ± 0.8 | .360 |
| Depression symptoms [0‐57]: Mean ± SD | — | — | 6.3 ± 7.0 | 4.5 ± 5.4 | 8.1 ±7.6 | 7.6 ± 8.0 | <.001 |
| Anxiety symptoms [20‐80]: Mean ± SD | — | — | 27.9 ± 6.5 | 27.5 ± 7.1 | 30.1 ± 9.1 | 30.6 ± 9.6 | <.001 |
| Perceived stress [0‐16]: Mean ± SD | — | — | — | — | 4.0 ± 3.0 | 3.9 ± 3.1 | .704 |
| Emotional support [0‐100]: Mean ± SD | — | — | — | — | 75.2 ± 22.0 | 72.2 ± 24.1 | .188 |
| Tangible support [0‐100]: Mean ± SD | — | — | — | — | 78.0 ± 24.2 | 73.5± 26.8 | .076 |
| Living circumstances; No. (%) | .003 | ||||||
| Living alone | — | — | — | — | 68 (26.0) | 65 (39.4) | |
| Living with others | — | — | — | — | 194 (74.0) | 100 (60.6) | |
Abbreviation: COVID‐19, coronavirus disease 2019.
P values were derived from analyses of variance, t tests, or χ2 tests that tested differences between survivors and controls across the available timepoints. Some variables do not have data at study enrollment and the last pre–COVID‐19 assessment because analyses focused on the COVID‐19 survey.
The loneliness difference score was calculated by subtracting the Center for Epidemiologic Studies‐Depression (CES‐D) loneliness item at the last pre–COVID‐19 assessment from the CES‐D loneliness at the COVID‐19 survey.
Longitudinal Changes in Loneliness
Loneliness did not change from study enrollment to the last pre–COVID‐19 assessment (−0.056; P = .421) but significantly increased from the last pre–COVID‐19 assessment to the COVID‐19 survey (0.211; P < .001), even after considering living circumstances (living alone vs with others) and availability of social support at the time of the COVID‐19 survey (Fig. 2) (see Supporting Table 1). However, contrary to our hypothesis, survivors and controls showed similar increases in loneliness from the last pre–COVID‐19 assessment to the COVID‐19 survey (0.064; P = .425), adjusting for covariates.
Figure 2.
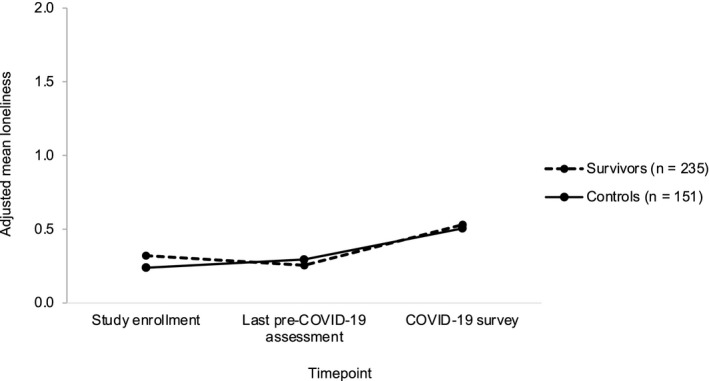
Longitudinal changes in loneliness are illustrated among older breast cancer survivors and noncancer controls. Piecewise mixed‐effects models illustrate adjusted loneliness scores at study enrollment, at the last pre–coronavirus disease 2019 (COVID‐19) assessment, and at the COVID‐19 survey, adjusting for age, race, county COVID‐19 death rate, and months from the last pre–COVID‐19 assessment to the COVID‐19 survey. Loneliness increased from the last pre–COVID‐19 assessment to the COVID‐19 survey (P < .001), with no difference between survivors and controls (P = .425).
Associations Between Loneliness and Mental Health
There were no differences between survivors and controls in associations between changes in loneliness and depression, anxiety, or perceived stress. Changes in loneliness from the last pre–COVID‐19 assessment to the COVID‐19 survey were associated with changes in depression and anxiety symptoms, adjusting for covariates (Table 3). Follow‐up analyses revealed that a 1‐point increase in loneliness (simple slope ± SE, 5.395 ± 0.439; P < .001) and, to a lesser degree, no change in loneliness (simple slope ± SE, 1.437 ± 0.334; P < .001) were associated with worsening depression symptoms, whereas a 1‐point decrease in loneliness was associated with improving depression symptoms (simple slope ± SE, −2.522 ± 0.599; P < .001) (Fig. 3A). Similarly, a 1‐point increase in loneliness (simple slope ± SE, 5.080 ± 0.553; P < .001) and, to a lesser degree, no change in loneliness (simple slope ± SE, 1.838 ± 0.420; P < .001) were associated with worsening anxiety symptoms, whereas a 1‐point decrease in loneliness was marginally associated with improving anxiety symptoms (simple slope ± SE, −1.403 ± 0.755; P = .064) (Fig. 3B). Increases in loneliness from the last pre–COVID‐19 assessment to the COVID‐19 survey were also associated with higher perceived stress (1.172; P < .001) (see Supporting Table 2) during the pandemic, adjusting for covariates.
TABLE 3.
Mixed‐Effects Models With Interactions Between Timepoint and Change in Loneliness (Difference Score) Predicting Change In Depression And Anxiety Symptoms From the Last Pre–Coronavirus Disease 2019 (COVID‐19) Assessment to the COVID‐19 Survey
| Variable | Change in Depression Symptoms | Change in Anxiety Symptoms | ||
|---|---|---|---|---|
| coefficient ± SE | P | coefficient ± SE | P | |
| Timepoint | 1.437 ± 0.334 | <.001 | 1.838 ± 0.420 | <.001 |
| Loneliness difference scorea | −0.720 ± 0.452 | .002 | −0.308 ± 0.519 | .004 |
| Timepoint*loneliness difference score | 3.958 ± 0.405 | <.001 | 3.242 ± 0.511 | <.001 |
| Age | −0.011 ± 0.060 | .856 | 0.029 ± 0.065 | .661 |
| Race: Non‐White vs White | −1.513 ± 0.921 | .101 | −2.098 ± 1.030 | .042 |
| County COVID‐19 death rate | 0.814 ± 0.406 | .046 | 1.638 ± 0.449 | <.001 |
| Time from last pre–COVID‐19 assessment to COVID‐19 survey, mo | 0.026 ± 0.066 | .696 | −0.091 ± 0.074 | .219 |
| Group: Survivors vs controls | 1.022 ± 0.647 | .115 | −0.001 ± 0.722 | .999 |
The loneliness difference score was calculated by subtracting the Center for Epidemiologic Studies‐Depression (CES‐D) loneliness item at the last pre–COVID‐19 assessment from the CES‐D loneliness item at the COVID‐19 survey.
Figure 3.
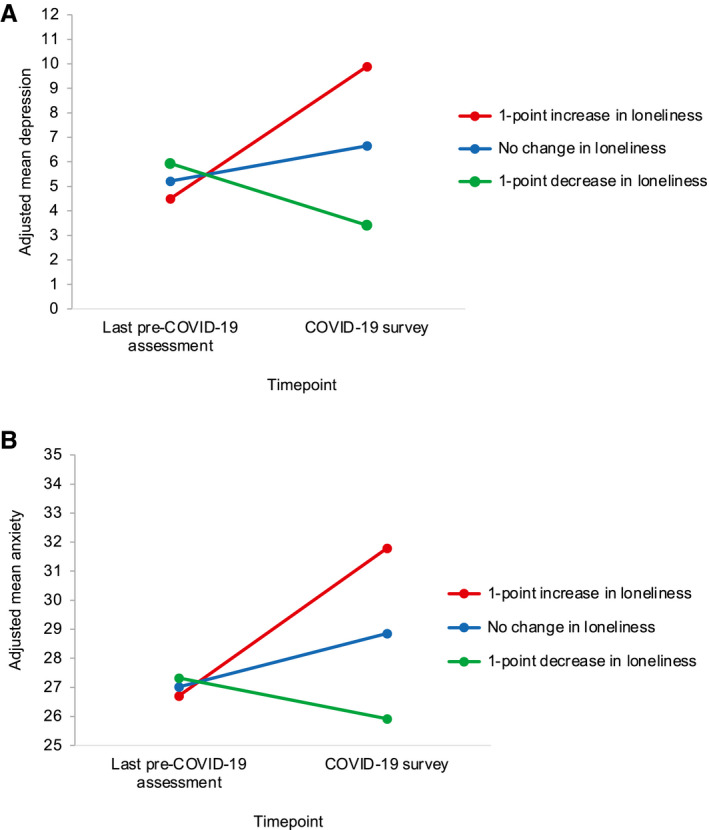
Associations between changes in loneliness and changes in depression and anxiety symptoms during the pandemic are illustrated. Mixed‐effects models show adjusted (A) depression and (B) anxiety symptoms as a function of timepoint and change in loneliness (continuous difference score) from the last pre–coronavirus disease 2019 (COVID‐19) assessment to the COVID‐19 survey, adjusting for age, race, county COVID‐19 death rate, months from the last pre–COVID‐19 assessment to the COVID‐19 survey, and group. Point estimates for simple slopes representing changes in depression and anxiety symptoms from the last pre–COVID‐19 assessment to the COVID‐19 survey suggest that a 1‐point increase (red lines) and no change (blue lines) in loneliness were associated with worsening depression (P < .001) and anxiety (P < .001) symptoms, whereas a 1‐point decrease in loneliness (green lines) was associated with improving depression (P < .001) and anxiety (P = .064) symptoms.
Discussion
This is one of the first US studies to examine changes in loneliness and mental health during the COVID‐19 pandemic in older breast cancer survivors compared with matched controls without cancer. This study is unique because we were able to query women who were part of a well defined longitudinal cohort study of older breast cancer survivors. We found that older women experienced a significant increase in loneliness from before to during the pandemic that was not accounted for by their living circumstances (living alone vs with others) or availability of social support at the time of the COVID‐19 survey. However, contrary to expectation, there was no difference between breast cancer survivors and matched noncancer controls. Also of note, older women in this sample reported significant increases in depression and anxiety symptoms from before to during the pandemic. As hypothesized, women who experienced increases in loneliness also reported worsening depression and anxiety symptoms and higher perceived stress during the pandemic, but, again, there were no differences between survivors and controls.
The proportion of older women in this study who reported feeling lonely during the pandemic (39.0%) is higher than population‐based estimates of loneliness in older adults before the pandemic (19.3%).35 These findings are consistent with the emerging literature on the impact of the COVID‐19 pandemic on loneliness and mental health among cancer survivors. For instance, studies in the Netherlands and China found that patients with cancer and survivors reported increased loneliness and worsening depression and anxiety symptoms during the pandemic.19, 20 Longitudinal research with older adults has also found increases in loneliness, depression, and anxiety symptoms from before to during the pandemic4, 12, 13 as well as over36, 37 the first few months of the pandemic.
In our cohort, older cancer survivors and controls showed similar increases in loneliness and associations with depression, anxiety, and stress. Although one previous study found that patients with cancer and survivors in China had higher psychological symptoms than their family members and the Chinese general population in the early months of the pandemic, another study conducted in Hong Kong found that female breast cancer and colorectal cancer survivors reported fewer depression and anxiety symptoms during the pandemic than noncancer controls.38 One possible explanation for the overall lack of differences between the 2 groups in this study is that cancer survivors may have experienced losses, adapted, and developed adaptive coping responses through their experience of cancer. This could have helped them to cope with future stressors such as the COVID‐19 pandemic, although this remains to be specifically tested. Alternatively, it is also possible that, as older women, COVID‐19–related concerns and isolation affected cancer survivors and noncancer controls equally. Researchers have also proposed that wisdom, which includes compassion, emotion regulation, and self‐reflection, is a resilience factor for older adults during times of stress that is inversely associated with loneliness and may be beneficial for cancer survivors as well.39 The investigation of protective factors that may buffer against increased vulnerability to the adverse effects of the COVID‐19 pandemic on mental health in cancer survivors is an important direction for future research.
Findings from this study should be considered in light of several limitations, which suggest directions for future research. First, our sample was well educated, and nearly all had Medicare, so they may have been buffered from some of the effects of the pandemic. Second, although we identified statistically significant worsening of depression and anxiety symptoms during the pandemic, the average increases in depression and anxiety scores were smaller than minimal clinically important differences published in the literature (ie, >10‐point increase on the CES‐D and STAI)40, 41 and below established cutoffs for clinically meaningful symptoms (ie, CES‐D scores ≥16, STAI scores >44).23, 42 Third, the longitudinal analyses for this study relied on a single‐item measure of loneliness. Although it correlated highly with a longer measure of loneliness on the COVID‐19 survey, the findings should be replicated. Finally, it will be important to assess whether these initial increases in loneliness, depression, and anxiety persist, increase, or decrease over subsequent study visits and whether changes during the COVID‐19 pandemic impact subsequent physical and cognitive health. For instance, loneliness and social isolation have been associated with increased inflammation and decreased antiviral immunity,43, 44, 45, 46 as well as prometastatic molecular profiles in primary tumors (eg, gene expression patterns consistent with epithelial‐mesenchymal transition),47, 48 poorer cognitive function,16 and increased risk for recurrence and mortality17, 49 among cancer survivors. Although older breast cancer survivors and noncancer controls experienced similar increases in loneliness in this study, it remains unclear whether the biologic effects of loneliness may be more detrimental for survivors than controls. Future research might also consider whether individuals undergoing active cancer treatment or those with advanced or metastatic disease experience greater loneliness than noncancer controls given the severity of disease and elevated risk of COVID‐19 complications.
Overall, this is the first study to examine longitudinal changes in loneliness and mental health during the COVID‐19 pandemic in older breast cancer survivors and noncancer controls. We found a high rate of loneliness in both cancer survivors and noncancer controls as well as increases in loneliness, depression, and anxiety symptoms from before to during the pandemic. However, findings from this study demonstrate that older breast cancer survivors do not appear to be at greater risk for loneliness or declines in mental health relative to their peers. Given the associations between loneliness and morbidity and mortality,14, 15 it will be important to screen for loneliness during medical care interactions to identify all women who are at risk for adverse mental health effects of the pandemic.
Funding Support
This research was supported by the National Cancer Institute of the National Institutes of Health through grants R01CA129769, R35CA197289, and R01AG068193 (Jeanne S. Mandelblatt). This study was also supported in part by the National Institutes of Health through grants K01AG065485 (Kelly E. Rentscher), P30AG028716 (Harvey J. Cohen), K01CA212056 (Traci N. Bethea), K08CA241337 (Kathleen M. Van Dyk), K12HD001441 (Zev M. Nakamura), R01CA172119 (Tim A. Ahles), U54CA137788 (Tim A. Ahles), P30CA008748 (Tim A. Ahles), R01CA244673 (Brenna C. McDonald and Andrew J. Saykin), and P30AG010133 (Brenna C. McDonald and Andrew J. Saykin); by the American Cancer Society through grants 17‐023‐01‐CPPB (Sunita K. Patel) and 128660‐RSG‐15‐187‐01‐PCS (Judith E. Carroll); and by the University of California‐Los Angeles Cousins Center for Psychoneuroimmunology at the University of California, Los Angeles (Kelly E. Rentscher and Judith E. Carroll).
Conflict of Interest Disclosures
Brent J. Small reports institutional grant support from the National Institutes of Health outside the submitted work and service as a member of the National Institutes of Health Data Safety Monitoring Board. Asma A. Dilawari attended the Cardinal Health 2020 Advisor Board Oncology Committee. Kathleen M. Van Dyk reports a postdoctoral fellowship award from the American Cancer Society outside the submitted work. Heather S. L. Jim reports grants from the National Institutes of Health, the Department of Defense, the State of Florida, Kite Pharma, and Pfizer Inc; personal fees from Red Hill Biopharma and Janssen Scientific Affairs; personal fees and travel support from Merck all outside the submitted work; has a patent “Methods of Treating Cognitive Impairment” (no. 10806772), issued October 20, 2020; is a member of a data safety monitoring board or advisory board at Northwestern University; and is a member of the Society of Behavioral Medicine. Andrew J. Saykin reports grants from the National Institutes of Health (R01 AG068193, R01 CA129769, and P30 AG010133); service on a Bayer Oncology Advisory Committee; and other support from Avid Radiopharmaceuticals/Eli Lilly all outside the submitted work. Judith E. Carroll reports grants from the STOP Cancer Foundation and the National Institute of Aging outside the submitted work and is a member of the Psychoneuroimmunology Research Society. The other authors made no disclosures.
Author Contributions
Kelly E. Rentscher: Conceptualization, writing–original draft, and writing–review and editing. Xingtao Zhou: Data curation, formal analysis, and writing–review and editing. Brent J. Small: Conceptualization, data curation, formal analysis, and writing–review and editing. Harvey J. Cohen: Funding acquisition, conceptualization, and writing–review and editing. Asma A. Dilawari: Funding acquisition, conceptualization, investigation, and writing–review and editing. Sunita K. Patel: Conceptualization, investigation, and writing–review and editing. Traci N. Bethea: Conceptualization and writing–review and editing. Kathleen M. Van Dyk: Conceptualization and writing–review and editing. Zev M. Nakamura: Conceptualization and writing–review and editing. Jaeil Ahn: Conceptualization, data curation, formal analysis, and writing–review and editing. Wanting Zhai: Data curation and writing–review and editing. Tim A. Ahles: Funding acquisition, investigation, and writing–review and editing. Heather S. L. Jim: Funding acquisition, investigation, and writing–review and editing. Brenna C. McDonald: Funding acquisition, investigation, and writing–review and editing. Andrew J. Saykin: Funding acquisition, investigation, and writing–review and editing. James C. Root: Funding acquisition, investigation, and writing–review and editing. Deena M. A. Graham: Funding acquisition, investigation, and writing–review and editing. Judith E. Carroll: Conceptualization and writing–review and editing. Jeanne S. Mandelblatt: Funding acquisition, conceptualization, investigation, and writing–review and editing.
Supporting information
Tables1‐2
Rentscher KE, Zhou X, Small BJ, Cohen HJ, Dilawari AA, Patel SK, Bethea TN, Van Dyk KM, Nakamura ZM, Ahn J, Zhai W, Ahles TA, Jim HSL, McDonald BC, Saykin AJ, Root JC, Graham DMA, Carroll JE, Mandelblatt JS. Loneliness and mental health during the COVID‐19 pandemic in older breast cancer survivors and noncancer controls. Cancer. 2021. 10.1002/cncr.33687
The last 2 authors contributed equally to this article as co‐senior authors.
[Correction added on 9 August 2021, after first online publication: The spelling of name of Xingtao Zhou's name has been updated in the author byline.]
References
- 1.Czeisler ME, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID‐19 pandemic—United States, June 24‐30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1049‐1057. doi: 10.15585/mmwr.mm6932a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke JE, Eirich R, Racine N, Madigan S. Prevalence of posttraumatic and general psychological stress during COVID‐19: a rapid review and meta‐analysis. Psychiatry Res. 2020;292:113347. doi: 10.1016/j.psychres.2020.113347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Killgore WDS, Cloonan SA, Taylor EC, Miller MA, Dailey NS. Three months of loneliness during the COVID‐19 lockdown. Psychiatry Res. 2020;293:113392. doi: 10.1016/j.psychres.2020.113392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong SYS, Zhang D, Sit RWS, et al. Impact of COVID‐19 on loneliness, mental health, and health service utilisation: a prospective cohort study of older adults with multimorbidity in primary care. Br J Gen Pract. 2020;70:e817‐e824. doi: 10.3399/bjgp20X713021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swainston J, Chapman B, Grunfeld EA, Derakshan N. COVID‐19 lockdown and its adverse impact on psychological health in breast cancer. Front Psychol. 2020;11:2033. doi: 10.3389/fpsyg.2020.02033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salari N, Hosseinian‐Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID‐19 pandemic: a systematic review and meta‐analysis. Global Health. 2020;16:57. doi: 10.1186/s12992-020-00589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groarke JM, Berry E, Graham‐Wisener L, McKenna‐Plumley PE, McGlinchey E, Armour C. Loneliness in the UK during the COVID‐19 pandemic: cross‐sectional results from the COVID‐19 Psychological Wellbeing Study. PLoS One. 2020;15:e0239698. doi: 10.1371/journal.pone.0239698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killgore WDS, Cloonan SA, Taylor EC, Dailey NS. Loneliness: a signature mental health concern in the era of COVID‐19. Psychiatry Res. 2020;290:113117. doi: 10.1016/j.psychres.2020.113117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li LZ, Wang S. Prevalence and predictors of general psychiatric disorders and loneliness during COVID‐19 in the United Kingdom. Psychiatry Res. 2020;291:113267. doi: 10.1016/j.psychres.2020.113267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith BJ, Lim MH. How the COVID‐19 pandemic is focusing attention on loneliness and social isolation. Public Heal Res Pract. 2020;30:3022008. doi: 10.17061/phrp3022008 [DOI] [PubMed] [Google Scholar]
- 11.Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys results from two population‐based studies. Res Aging. 2004;26:655‐672. doi: 10.1177/0164027504268574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krendl AC, Perry BL. The impact of sheltering in place during the COVID‐19 pandemic on older adults' social and mental well‐being. J Gerontol B Psychol Sci Soc Sci. 2021;76:e53‐e58. doi: 10.1093/geronb/gbaa110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Tilburg TG, Steinmetz S, Stolte E, van der Roest H, de Vries DH. Loneliness and mental health during the COVID‐19 pandemic: a study among Dutch older adults. J Gerontol B Psychol Sci Soc Sci. Published online August 5, 2020. doi: 10.1093/geronb/gbaa111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong AD, Uchino BN, Wethington E. Loneliness and health in older adults: a mini‐review and synthesis. Gerontology. 2016;62:443‐449. doi: 10.1159/000441651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holt‐Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta‐analytic review. Perspect Psychol Sci. 2015;10:227‐237. doi: 10.1177/1745691614568352 [DOI] [PubMed] [Google Scholar]
- 16.Jaremka LM, Peng J, Bornstein R, et al. Cognitive problems among breast cancer survivors: loneliness enhances risk. Psychooncology. 2014;23:1356‐1364. doi: 10.1002/pon.3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutgendorf SK, De Geest K, Bender D, et al. Social influences on clinical outcomes of patients with ovarian cancer. J Clin Oncol. 2012;30:2885‐2890. doi: 10.1200/JCO.2011.39.4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81:e16‐e25. doi: 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schellekens MPJ, van der Lee ML. Loneliness and belonging: exploring experiences with the COVID‐19 pandemic in psycho‐oncology. Psychooncology. 2020;29:1399‐1401. doi: 10.1002/pon.5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Zhou F, Zhang L, Su Y, Mao L. Psychological symptoms of cancer survivors during the COVID‐19 outbreak: a longitudinal study. Psychooncology. 2021;30:378‐384. doi: 10.1002/pon.5588 [DOI] [PubMed] [Google Scholar]
- 21.Mandelblatt JS, Small BJ, Luta G, et al. Cancer‐related cognitive outcomes among older breast cancer survivors in the Thinking and Living With Cancer study. J Clin Oncol. 2018;36:3211‐3222. doi: 10.1200/JCO.18.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandelblatt JS, Zhou X, Small BJ, et al. Deficit accumulation frailty trajectories of older breast cancer survivors and non‐cancer controls: the Thinking and Living with Cancer study. J Natl Cancer Inst. Published online January 23, 2021. doi: 10.1093/jnci/djab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385‐401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 24.Donovan NJ, Wu Q, Rentz DM, Sperling RA, Marshall GA, Glymour MM. Loneliness, depression and cognitive function in older U.S. adults. Int J Geriatr Psychiatry. 2017;32:564‐573. doi: 10.1002/gps.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiovitz‐Ezra S, Ayalon L. Use of direct versus indirect approaches to measure loneliness in later life. Res Aging. 2012;34:572‐591. doi: 10.1177/0164027511423258 [DOI] [Google Scholar]
- 26.Thurston RC, Kubzansky LD. Women, loneliness, and incident coronary heart disease. Psychosom Med. 2009;71:836‐842. doi: 10.1097/PSY.0b013e3181b40efc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spielberger C. Manual for the State‐Trait Anxiety Inventory (STAI: Form Y). Consulting Psychologists Press; 1983. [Google Scholar]
- 28.Puterman E, Lin J, Blackburn E, O'Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010;5:e0010837. doi: 10.1371/journal.pone.0010837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warttig SL, Forshaw MJ, South J, White AK. New, normative, English‐sample data for the Short Form Perceived Stress Scale (PSS‐4). J Health Psychol. 2013;18:1617‐1628. doi: 10.1177/1359105313508346 [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi LC, Cohen HJ, Zhai W, et al. Cognitive function prior to systemic therapy and subsequent well‐being in older breast cancer survivors: longitudinal findings from the Thinking and Living with Cancer Study. Psychooncology. 2020;29:1051‐1059. doi: 10.1002/pon.5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll JE, Small BJ, Tometich DB, et al. Sleep disturbance and neurocognitive outcomes in older patients with breast cancer: interaction with genotype. Cancer. 2019;125:4516‐4524. doi: 10.1002/cncr.32489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The New York Times . Coronavirus (COVID‐19) Data in the United States. Accessed December 2, 2020. https://github.com/rearc‐data/covid‐19‐nyt‐data‐in‐usa
- 33.US Department of Agriculture Economic Research Services (USDA ERS) . Population Estimates for the U.S., States, and Counties, 2010‐19. USDA; 2020. Accessed September 28, 2020. https://www.ers.usda.gov [Google Scholar]
- 34.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705‐714. doi: 10.1016/0277-9536(91)90150-b [DOI] [PubMed] [Google Scholar]
- 35.Theeke LA. Predictors of loneliness in U.S. adults over age sixty‐five. Arch Psychiatr Nurs. 2009;23:387‐396. doi: 10.1016/j.apnu.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 36.Luchetti M, Lee JH, Aschwanden D, et al. The trajectory of loneliness in response to COVID‐19. Am Psychol. 2020;75:897‐908. doi: 10.1037/amp0000690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotwal AA, Holt‐Lunstad J, Newmark RL, et al. Social isolation and loneliness among San Francisco Bay Area older adults during the COVID‐19 shelter‐in‐place orders. J Am Geriatr Soc. 2021;69:20‐29. doi: 10.1111/jgs.16865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng DWL, Chan FHF, Barry TJ, et al. Psychological distress during the 2019 coronavirus disease (COVID‐19) pandemic among cancer survivors and healthy controls. Psychooncology. 2020;29:1380‐1383. doi: 10.1002/pon.5437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vahia IV, Jeste DV, Reynolds CF 3rd. Older adults and the mental health effects of COVID‐19. JAMA. 2020;324:2253‐2254. doi: 10.1001/jama.2020.21753 [DOI] [PubMed] [Google Scholar]
- 40.Busch AM, Wagener TL, Gregor KL, Ring KT, Borrelli B. Utilizing reliable and clinically significant change criteria to assess for the development of depression during smoking cessation treatment: the importance of tracking idiographic change. Addict Behav. 2011;36:1228‐1232. doi: 10.1016/j.addbeh.2011.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taghizadeh N, Tremblay A, Cressman S, et al. Health‐related quality of life and anxiety in the PAN‐CAN lung cancer screening cohort. BMJ Open. 2019;9:e024719. doi: 10.1136/bmjopen-2018-024719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korfage IJ, Essink‐Bot ML, Janssens A, De Koning HJ. Anxiety and depression after prostate cancer diagnosis and treatment: 5‐year follow‐up. Br J Cancer. 2006;94:1093‐1098. doi: 10.1038/sj.bjc.6603057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:1‐13. doi: 10.1186/gb-2007-8-9-r189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole SW, Levine ME, Arevalo JMG, Ma J, Weir DR, Crimmins EM. Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology. 2015;62:11‐17. doi: 10.1016/j.psyneuen.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole SW, Capitanio JP, Chun K, Arevalo JMG, Ma J, Cacioppo JT. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc Natl Acad Sci U S A. 2015;112:15142‐15147. doi: 10.1073/pnas.1514249112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaremka LM, Fagundes CP, Peng J, et al. Loneliness promotes inflammation during acute stress. Psychol Sci. 2013;24:1089‐1097. doi: 10.1177/0956797612464059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bower JE, Shiao SL, Sullivan P, et al. Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectr. 2018;2:pky029. doi: 10.1093/jncics/pky029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lutgendorf SK, Penedo F, Goodheart MJ, et al. Epithelial‐mesenchymal transition polarization in ovarian carcinomas from patients with high social isolation. Cancer. 2020;126:4407‐4413. doi: 10.1002/cncr.33060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kroenke CH, Michael YL, Poole EM, et al. Postdiagnosis social networks and breast cancer mortality in the After Breast Cancer Pooling Project. Cancer. 2017;123:1228‐1237. doi: 10.1002/cncr.30440 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables1‐2


