Abstract
Background
P-wave signal-averaged electrocardiography (P-SAECG) quantifies atrial electrical activity. P-SAECG measures and their clinical correlates and heritability have had limited characterization in community-based cohorts.
Objective
The purpose of this study was to (1) establish reference values; (2) identify clinical risk factors associated with P-SAECG; and (3) estimate genetic heritability for P-SAECG traits.
Methods
We performed P-SAECG in 2 generations of Framingham Heart Study participants. We performed backward elimination regression models to assess associations of clinical factors with each SAECG trait (P-wave [PW] duration, root mean square voltage in terminal 40 ms [RMS40], terminal 30 ms RMS30, terminal 20 ms RMS20, RMS PW, and PW integral). We estimated the adjusted genetic heritability of P-SAECG measures using the Sequential Oligogenic Linkage Analysis Routines (SOLAR) program.
Results
We included 4307 participants (age 55 ± 14 years; 56% female). The reference values were derived from 1752 participants without cardiovascular risk factors. Median (2.5th percentile; 97.5th percentile) total PW duration was 118 ms (93; 146) in women and 128 ms (104; 158) in men in the reference sample, and 121 ms (94; 151) in women and 129 ms (103; 159) in the entire study cohort (broad sample). In the broad sample, after adjusting for age and sex, total PW duration was positively associated with height, weight, prevalent heart failure, history of atrial fibrillation (AF), and atrioventricular node blockers, and negatively associated with smoking, waist circumference, heart rate, and diabetes. The estimated heritability of P-SAECG traits was moderate, ranging from 11.9% for RMS30 to 24.9% for PW integral.
Conclusion
P-SAECG traits are associated with multiple AF-related risk factors and are moderately heritable.
Keywords: Electrocardiography, Epidemiology, Heritable traits, P wave, Range reference, Signal-averaged electrocardiography
Introduction
P-wave signal-averaged electrocardiography (P-SAECG) is a noninvasive, high-fidelity measurement of atrial electric function. It has shown potential to predict atrial fibrillation (AF) risk after myocardial infarction,1 cardiothoracic surgery,2 cardioversion,3 and catheter ablation.4 P-wave (PW) indices and PR interval are commonly used electrocardiographic (ECG) measures reflecting intermediate phenotypes for AF.5 Longer PW duration and lower PW amplitude are associated with incident AF,6,7 as well as with increased rates of cardiovascular and all-cause mortality.8 A meta-analysis of PW indices showed limited improvement in risk prediction for AF compared to a robust risk score for the condition, initially derived from multiple community-based cohorts.7 However, the results are inconsistent,9, 10, 11 and most P-SAECG studies are limited by small-to-modest sample sizes with incomplete anthropometric and clinical characterization12,13 or technical issues.14,15 Also, which clinical risk factors are associated with P-SAECG measurements is unclear, and the heritability of P-SAECG measurements remains known.
The objective of our investigation was to ascertain P-SAECG assessments in a large, multigenerational cohort that has had extensive characterization of clinical risk factors. We studied P-SAECG traits to (1) establish reference values; (2) identify associated clinical risk factors; and (3) estimate the genetic heritability, as well as genetic and environmental correlations between traits.
Methods
Study population
The Framingham Heart Study (FHS) is an observational epidemiologic study located in Framingham, Massachusetts. It was initiated by the US Public Health Service in 1948 to identify risk factors for cardiovascular disease. The study enrolled Framingham residents in the Original cohort (n = 5209); Offspring cohort—children of the Original cohort and their spouses (n = 5124); and Third Generation cohort—adult children from the Offspring cohort (n = 4095). To reflect the more diverse nature of contemporary Framingham, 2 multiethnic cohorts were recruited with examination 4 of the Offspring (Omni 1) and examination 1 of the Third Generation (Omni 2). Offspring and Third Generation, and Omni 1 and 2 multiethnic cohorts were studied every 4–8 years with standardized FHS examinations. At each examination, data on medical history, physical examination, and laboratory tests were collected. Regular health status updates for cardiovascular disease included requests for hospital admission or outpatient records and ECGs.
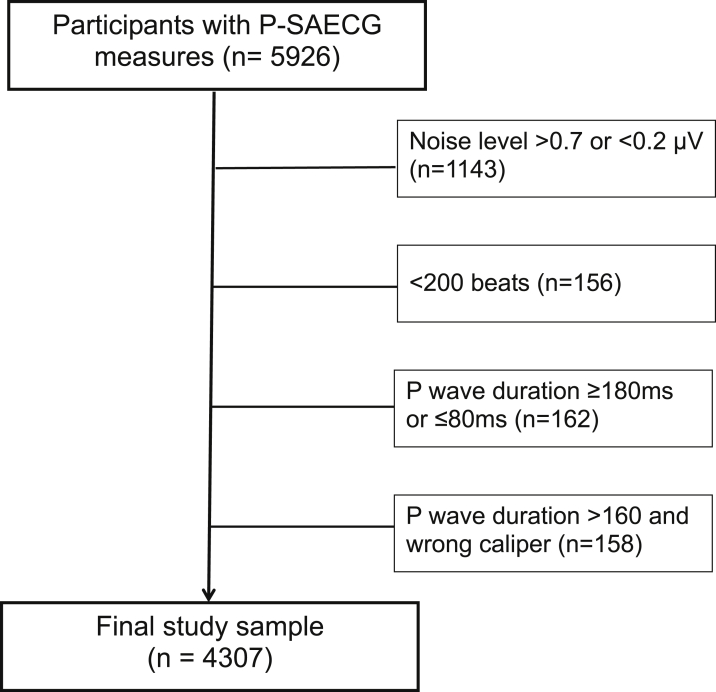
SAECG was assessed in participants from the Offspring (examination 9, 2011–2014), Omni 1 (examination 4, 2011–2014), Offspring Spouse (examination 2, 2008–2011), Generation 3 (examination 2, 2008–2011), and Omni 2 (examination 2, 2009–2011) cohorts (Figures 1 and 2). Exclusion of individuals with SAECG was based on criteria presented in Supplemental Tables 1 to 3. Reference values were analyzed in a subgroup of individuals without cardiovascular disease or risk factors (eg, without obesity, smoking, hypertension, AF, diabetes, and cardiac medication use).
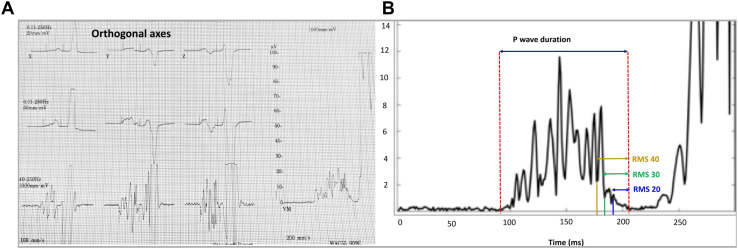
Figure 1.
Representative P-wave signal-averaged electrocardiography (P-SAECG) (A) and main P-SAECG traits (B) in the Framingham Heart Study. RMS20 = root mean square voltage in terminal 20 ms; RMS30 = root mean square voltage in terminal 30 ms; RMS40 = root mean square voltage in terminal 40 ms.
Figure 2.
Flowchart of the study cohort. P-SAECG = P-wave signal-averaged electrocardiography.
The FHS protocol was approved by the Boston University Medical Campus Institutional Review Board, and participants signed informed consent.
P-SAECG protocol and heritability estimation
Detailed P-SAECG protocol and heritability estimation are given in the Supplemental Methods.
Covariate measurement
Research staff recorded age, sex, height, weight, body mass index, diabetes, systolic and diastolic blood pressures (BPs), self-reported smoking status, moderate/severe alcohol consumption, and antihypertensive medication use during each examination. Triglycerides and total/high-density lipoprotein (HDL) cholesterol ratio were measured at the same examination when SAECG analysis was performed. Moderate/severe alcohol use was defined by self-report as weekly consumption of >14 drinks for men or >7 drinks for women. Heart rate was measured at rest from the 12-channel ECG performed on the day of SAECG analysis. If needed, further information was obtained from records supplied by the hospital, attending physician, pathologist, medical examiner, or family. At least 2 clinical researchers reviewed FHS research examination results and outside clinician and hospital records to adjudicate a history of heart failure and myocardial infarction, as previously described.16
Statistical analysis
Reference values were defined as the median (2.5th and 97.5th percentiles). Linear mixed models were used to study the association of P-SAECG traits with multiple clinical factors, including age, sex, cohort, height, weight, smoking, systolic and diastolic BPs, triglycerides, total/HDL cholesterol, waist circumference, heart rate, alcohol consumption, diabetes, hypertension treatment, history of myocardial infarction, heart failure, and AF, atrioventricular (AV) node blockers (beta-blockers and calcium antagonists of nondihydropyridine type).
We examined the association of multiple clinical factors with P-SAECG traits by backward elimination. The model started with all clinical factors, and the association of each clinical factor with P-SAECG traits was assessed. The one with the least significant association was eliminated, and the associations of the remaining clinical factors with P-SAECG traits were reassessed. The procedure continued until all the remaining clinical factors were at least nominally 2-sided significant (P <.05). All models were adjusted for age, sex, and cohort. All statistical analyses were performed using R software Version 3.6.0 (https://www.r-project.org/).
Results
Study population
In total, 4307 individuals were included in the analysis. The clinical characteristics of the study sample are listed in Table 1. Mean age of the study cohort was 55 ± 14 years, 56% were women, 31% received antihypertensive treatment, 4% had previous myocardial infarction, and 3% had prevalent AF. P-SAECG traits are listed in Table 2.
Table 1.
Baseline characteristics of the whole study sample (n = 4307)
| Age (y) | 55 ± 14 |
| Female | 2417 (56.1) |
| Systolic BP (mm Hg) | 120 ± 16 |
| Diastolic BP (mm Hg) | 74 ± 9 |
| Height (inch) | 66 ± 4 |
| Weight (lb) | 174 ± 40 |
| Current smoking | 338 (7.8) |
| Triglycerides (mg/dL) | 113 ± 73 |
| Total/HDL cholesterol | 3.3. ± 1.1 |
| Waist circumference (inch/cm) | 39 ± 6/98 ± 14 |
| Heart rate (bpm) | 64 ± 9 |
| Moderate/severe alcohol consumption | 657 (15.3) |
| Diabetes | 370 (8.6) |
| Hypertension treatment | 1324 (30.7) |
| Prevalent myocardial infarction | 182 (4.2) |
| Prevalent heart failure | 28 (0.7) |
| Prevalent AF | 109 (2.5) |
| AV node blockers | 769 (17.9) |
Values are given as n (%) for dichotomous variables and mean ± SD or median (25th, 75th percentiles) for continuous variables
AF = atrial fibrillation; AV = atrioventricular; BP = blood pressure; HDL = high-density lipoprotein.
∗P value was calculated by the Fisher exact test for categorical variables or Mann-Whitney U test for continuous variables.
Table 2.
Values of P-SAECG traits in the reference sample without cardiovascular disease or risk factors∗
| 2.5% | 25% | 50% | 75% | 97.5% | ||
|---|---|---|---|---|---|---|
| Total P-wave duration (ms) | Women | 93 | 111 | 118 | 127 | 146 |
| Men | 104 | 120 | 128 | 140 | 158 | |
| Standardized P-wave duration (ms) | Women | 90 | 105 | 114 | 123 | 146 |
| Men | 101 | 115 | 124 | 138 | 157 | |
| Integral of P wave (μVms) | Women | 252 | 504 | 637 | 798 | 1116 |
| Men | 286 | 512 | 666 | 810 | 1170 | |
| Root mean square voltage in terminal 20 ms (mV) | Women | 1 | 2 | 3 | 4 | 8 |
| Men | 1 | 2 | 3 | 4 | 8 | |
| Root mean square voltage in terminal 30 ms (mV) | Women | 1 | 3 | 4 | 6 | 9 |
| Men | 1 | 2 | 4 | 5 | 10 | |
| Root mean square voltage in terminal 40 ms (mV) | Women | 2 | 4 | 5 | 7 | 10 |
| Men | 2 | 3 | 4 | 6 | 11 | |
| Root mean square voltage in the entire P wave (mV) | Women | 3 | 6 | 7 | 9 | 12 |
| Men | 3 | 5 | 7 | 8 | 12 |
P-SAECG = P-wave signal-averaged electrocardiography.
Reference sample, n = 1752 (1079 women and 673 men).
Reference values of P-SAECG traits
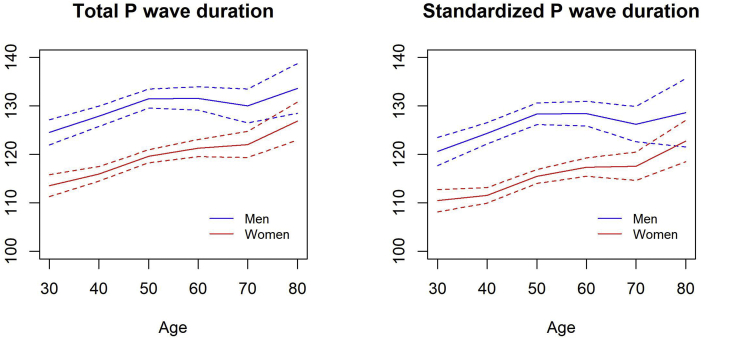
We derived the reference values of P-SAECG traits from 1752 participants without cardiovascular risk factors. As shown in Table 2, for total PW duration, median (2.5th percentile; 97.5th percentile) was 118 ms (93; 146) in women and 128 ms (104; 158) in men. For standardized PW duration, median (2.5th percentile; 97.5th percentile) was 114 ms (90; 146) in women and 124 ms (101; 157) in men. Whereas PW durations were significantly higher in men, all 3 RMS voltage traits in the terminal portion of the signal-averaged P wave (20, 30, and 40 ms) were higher in women (all P <.001). Men and women had similar PW integrals. Distribution of total and standardized PW durations across different age groups are given in Supplemental Table 4 and Figure 3. Means of both traits were higher with advancing age (P <.001). P-SAECG trait values in the entire broad study sample are given in Supplemental Table 5.
Figure 3.
Distribution of total (A) and standardized (B) P-wave durations across different ages of healthy participants.
Association of P-SAECG traits with clinical factors and resting ECG traits
Table 3 lists clinical factors significantly associated with each P-SAECG trait. Total and standardized PW durations both were positively associated with height, weight, history of heart failure, history of AF, and AV node blockers, and negatively associated with smoking, waist circumference, heart rate, and diabetes. RMS PW was positively associated with smoking and heart rate, and negatively associated with weight, total cholesterol, and history of AF. PW integral was positively associated with smoking and diastolic BP, and negatively associated with weight and total cholesterol. All 3 RMS traits in the terminal portion of the signal-averaged P wave (20, 30, and 40 ms) were positively associated with waist circumference and heart rate, and negatively associated with weight. RMS20 was additionally associated with smoking. There was no evidence of association between systolic BP, triglycerides, alcohol consumption, hypertension treatment, and history of myocardial infarction with any SAECG traits in multivariable models. The association of each P-SAECG trait with each individual clinical factor is given in Supplemental Table 6.
Table 3.
Multiple regression coefficients for SAECG traits
| Total P-wave duration | Standardized P-wave duration | Integral of P wave | RMS20 | RMS30 | RMS40 | RMS PW | |
|---|---|---|---|---|---|---|---|
| Height (inch) | 0.30 | 0.24 | |||||
| Weight (lb) | 0.09 | 0.10 | –0.72 | –0.01 | –0.01 | –0.01 | –0.01 |
| Current smoking | –1.58 | –1.70 | 36.63 | 0.28 | 0.41 | ||
| Diastolic BP (mm Hg) | 0.92 | ||||||
| Total/HDL cholesterol | –11.68 | –0.10 | |||||
| Waist circumference (inch) | –0.32 | –0.33 | 0.04 | 0.04 | 0.04 | ||
| Heart rate (bpm) | –0.30 | –0.46 | 0.02 | 0.03 | 0.03 | 0.02 | |
| Diabetes | –1.96 | –2.40 | |||||
| Prevalent heart failure | 6.82 | 6.45 | |||||
| Prevalent AF | 4.55 | 5.08 | –0.52 | ||||
| AV node blockers | 2.03 | 1.93 |
Blank cells represent clinical factors that were not significant and thus eliminated into the final backward models. Regression coefficients per unit (continuous variables) or presence of condition (binary variables). Age, sex, and cohort were forced in all the models.
The following factors were not retained in any of the models: Systolic blood pressure, triglycerides, moderate/severe alcohol consumption, hypertension treatment, and prevalence myocardial infarction.
RMS20 = root mean square voltage in terminal 20 ms; RMS30 = root mean square voltage in terminal 30 ms; RMS40 = root mean square voltage in terminal 40 ms; RMS PW = root mean square voltage of the entire P wave; SAECG = signal-averaged electrocardiogram; other abbreviations as in Table 1.
The associations adjusted for age, sex, and cohort between P-SAECG and 12-lead resting ECG traits are given in Supplemental Table 7. Total and standardized PW durations both were positively associated with PR interval in 12-leads ECG, whereas RMS20, RMS30, RMS40, and RMS PW were negatively associated with PR interval.
Heritability
Table 4 lists the heritability estimation for each P-SAECG trait. In the simple model that was adjusted for age, sex, and cohort, total PW duration, standardized PW duration, PW integral, and RMS PW all showed modest heritability, whereas PW integral had the highest heritability of 26.1%. All 3 RMS traits in the terminal portion of the signal-averaged P wave (20, 30, and 40 ms) had limited but still significant heritability (all P <.0001). The heritabilities were slightly attenuated in the multivariable model. PW integral remained the most heritable trait, with 24.9% heritability.
Table 4.
Heritability and correlation between traits
| Heritability |
Total P-wave duration | Standardized P-wave duration | Integral of P wave | RMS20 | RMS30 | RMS40 | RMS PW | ||
|---|---|---|---|---|---|---|---|---|---|
| Simple model∗ (%) | Multivariable model† (%) | ||||||||
| Total P-wave duration | 24.6 | 21.6 | — | 0.99 ± 0.01 | 0.20 ± 0.11 | –0.41 ± 0.13 | –0.42 ± 0.13 | –0.29 ± 0.15 | –0.05 ± 0.12 |
| Standardized P-wave duration | 20.8 | 18.0 | 0.93 ± 0.00 | — | 0.14 ± 0.12 | –0.51 ± 0.13 | –0.57 ± 0.12 | –0.42 ± 0.14 | –0.11 ± 0.12 |
| Integral of P-wave | 26.1 | 24.9 | 0.12 ± 0.04 | 0.03 ± 0.04 | — | 0.22 ± 0.03 | 0.38 ± 0.03 | 0.50 ± 0.03 | 0.50 ± 0.03 |
| RMS20 | 15.0 | 14.3 | –0.54 ± 0.03 | –0.52 ± 0.03 | 0.34 ± 0.15 | — | 0.84 ± 0.01 | 0.72 ± 0.02 | 0.34 ± 0.03 |
| RMS30 | 12.7 | 11.9 | –0.49 ± 0.03 | –0.48 ± 0.03 | 0.44 ± 0.14 | 0.98 ± 0.04 | — | 0.89 ± 0.01 | 0.49 ± 0.03 |
| RMS40 | 12.2 | 12.3 | –0.45 ± 0.03 | –0.45 ± 0.03 | 0.72 ± 0.10 | 0.76 ± 0.10 | 0.88 ± 0.05 | — | 0.60 ± 0.02 |
| RMS PW | 24.7 | 23.0 | –0.13 ± 0.04 | –0.19 ± 0.04 | 0.72 ± 0.10 | 0.44 ± 0.14 | 0.56 ± 0.12 | 0.80 ± 0.09 | — |
Values are given as correlation coefficient ± standard error. Upper diagonal shows genetic correlations, and lower shaded diagonal shows environmental correlations.
Simple model: Adjusted for age, sex, and cohort.
Multivariable model: Additionally adjusted for height, weight, smoking, systolic and diastolic blood pressure, triglycerides, total/HDL cholesterol, waist circumference, heart rate, moderate/severe alcohol consumption, diabetes, hypertension treatment, prevalent myocardial infarction, prevalent AF, prevalent heart failure, and AV node blockers.
Table 4 also lists the genetic and environmental correlations between P-SAECG traits pairs. Strong genetic correlation (0.99) and environmental correlation (0.93) were observed between total and standardized PW durations. Both measures were negatively associated with RMS20, RMS30, RMS30, and RMS PW, and positively associated with PW integral. For most trait pairs, similar patterns were observed between genetic and environmental correlations, and genetic correlations tended to be slightly stronger than environmental correlations.
Discussion
In the current study, we established reference values for P-SAECG traits in a large community-based cohort. This assessment of P-SAECG in a highly phenotyped and genotyped, multigenerational community-based cohort represents the most extensive assessment of these measures to date. In the whole study sample, P-SAECG traits were associated with multiple AF-related risk factors, such as diabetes, obesity, smoking, and history of heart failure. The traits were also moderately heritable, with the highest heritability observed for RMS PW.
Comparison with previous studies
Previous studies analyzed PW mostly using standardized ECG techniques. However, accuracy of PW analysis was limited as very low electric potentials in atrial myocardium were suppressed by 12-lead ECG filters.15 Therefore, high-resolution SAECG was introduced to record very low (microvolt) amplitude signals to increase the accuracy of PW measurement. The main issue behind P-SAECG was identification of individuals at high risk for AF and with AF-related outcomes (especially thromboembolic complications). Also, it was important to determine predisposing endophenotypes that can be dissected for mechanistic insights, including genetic risk, with the hope of identifying risk in the community.
A summary of clinical P-SAECG studies is given in Supplemental Table 8. First attempts to use P-SAECG in AF were performed in late 1980s and were mainly negative, probably due to small sample sizes, inaccurate PW sampling, and SAECG data processing.14 Later technical improvements allowed the transferring from QRS-triggered to PW-triggered testing, and “P-wave layout” was introduced.17,18 Besides a filtered PW duration analysis, SAECG allows more advanced characteristics of PW traits, such as amplitude of the terminal part of atrial depolarization (ie, terminal 40, 30, or 20 ms of the PW).19 In a first prospective study analyzing AF prediction, P-SAECG was significantly correlated with incident AF in patients undergoing cardiac surgery.2 Although PW duration was not prolonged in the 12-lead resting ECG, filtered PW duration using SAECG was significantly prolonged in individuals developing AF. Furthermore, AF incidence was ∼4-fold higher in patients with P-SAECG >140 ms. Later SAECG studies demonstrated an association between PW indices with recurrent AF after cardioversion3,20 or progression to chronic AF.21,22
We observed that all P-SAECG traits were associated with at least 1 clinical risk factor related with AF. PW duration and PR interval in 12-lead ECG were significantly higher in men than in women, which could be partly explained by the larger heart and body size of men. In contrast, PW terminal depolarization traits were significantly higher in women, possibly due to the impact of neurohormonal processes.
Heritability
Heritability of ECG traits is essential for understanding population variance in disease presentation. Several common variants modulate heart rate, PR interval, and QRS complex durations.23, 24, 25 Heritability of ECG traits was analyzed using 12-lead resting ECGs, which can be considered as a 10-second “snapshot” of cardiac electrical activity. Others have analyzed ECG heritability using 24-hour Holter monitoring.26 Heritability of different ECG traits varies from high (eg, RR interval 40%–98%) to moderate (eg, QT/QTc 25%–67%; PR interval 34%–46%; QRS 33%–43%).23,27,28
To date, our analysis is the largest study investigating heritability of PW traits using P-SAECG. In contrast to previous studies analyzing heritability among different 12-lead ECG traits,27 we found only mild heritability among P-SAECG traits. The heritability of PW duration was only 22%. Nevertheless, the knowledge of P-SAECG heritability underscores genetic contributions to PW endophenotypes. Understanding the genetic predisposition to endophenotypes for complex diseases such as AF may lead to targeted medical therapy for AF prevention or treatment. Furthermore, the genetic and environmental correlations contribute understanding, whether some traits are co-regulated by genetic or environmental factors.
Clinical implication
Atrial cardiomyopathy describes an atrial dysfunction due to structural (anatomic) and electrical remodeling.29 Although atrial cardiomyopathy can be a cause of AF, it may exist without AF as well.30 Thus, atrial premature beats or atrial tachycardia are markers of underlying atrial substrate and are associated with increased risk of stroke beyond AF.31 Therefore, invasive or noninvasive atrial ECGs may help to identify individuals with underlying atrial cardiomyopathy and consequently with increased risk for cerebrovascular thromboembolic complications.
Currently, the CHA2DS2-VASc score is widely used to stratify an increased risk for thromboembolic complications in individuals with AF. However, those individuals at increased risk for stroke but without a history of AF usually are not considered for oral anticoagulation. In such cases, identification or detection of an atrial cardiomyopathy may enhance risk assessment for thromboembolic stroke or identify individuals meriting increased routine screening for unrecognized AF. In contrast, individuals without features of an atrial cardiomyopathy may not require oral coagulation independent on their CHA2DS2-VASc score. In contrast, identification of atrial cardiomyopathy after apparently successful catheter ablation (eg, no arrhythmia recurrences but with features of atrial cardiomyopathy) would indicate necessity of continuation of oral anticoagulation. Therefore, P-SAECG as a high-fidelity analysis of the P wave might be useful to predict and prevent adverse outcomes in individuals at risk.
Study strengths and limitations
The current study represents the largest epidemiologic cohort with extensive measures of P-SAECG. With P-SAECG measures in >4000 participants, we characterized distributions of different P-SAECG traits and identified multiple clinical factors associated with P-SAECG traits. The pedigree structure in FHS also allows the heritability estimation of P-SAECG traits, which may motivate the identification of genetic variants associated with P-SAECG traits and enable future targeted medical therapy.
We also acknowledge several limitations. First, the vast majority of the study participants are of European ancestry; thus, the generalizability to other races/ethnicities is unknown. Also, the study population at the time of SAECG analysis had a mean age of 55 years. Therefore, the reference ranges and heritability results might differ in samples of older patients. The study was cross-sectional and observational in nature; we cannot exclude residual confounding in our analyses of factors associated with the SAECG traits (eg, sleep apnea, not routinely ascertained during examinations). Advantages of SAECG include the incorporation of information from hundreds of datapoints and essential improvement of the reliability and accuracy. However, as for disadvantages, SAECG requires highly specialized equipment that is not in wide clinical usage. Also, to reach at least 250 signals for the qualitative registration and accurate analysis, acquisition times of up to 10 minutes are necessary for each individual. Furthermore, the nominal significance cutoff in our study was used to define significant associations, such that the association of some of clinical risk factors with P-SAECG traits may be by chance. Also, the difference in P-SAECG values was significant but modest. Finally, more informed knowledge about the SAECG and facility of its application may increase its clinical usage, as the ECG is a widely available, noninvasive, and low-cost assessment. Clinical significance needs to be established in future large-scale cohorts.
Conclusion
In our large community-based cohort, we observed that P-SAECG traits are associated with multiple AF-related risk factors such as height, weight, smoking, blood pressure, and prevalent heart failure. The P-SAECG traits were moderately heritable, and the highest heritability was observed for RMS PW. Further studies analyzing the impact of P-SAECG predicting incident AF and AF-related complications are needed. Also, the role of P-SAECG in evaluating association with atrial cardiomyopathy in individuals at risk remains an unmet clinical need.
Acknowledgments
We acknowledge the dedication of the FHS study participants without whom this research would not be possible. We thank GE representatives Robert M. Farrell and Michael J. Mysliwy for their constructive advice and help during manuscript revision addressing technical aspects of P-SAECG analysis.
Footnotes
Funding sources: The Framingham Heart Study acknowledges the support of contracts NO1-HC-25195, HHSN268201500001I, and 75N92019D00031 from the National Heart, Lung, and Blood Institute. Dr Kornej received funding from the Marie Sklodowska-Curie Actions under the European Union’s Horizon 2020 research and innovation programme (Agreement No. 838259). Dr Magnani is supported by National Institutes of Health (NIH) Grants R01HL143010 and R33HL144669. Dr Preis is supported by NIH Grant 5R01HL128914-04. Dr Trinquart is supported by the American Heart Association (18SFRN34150007). Dr Ko is supported by American College of Cardiology Foundation/Merck Research Fellowship in Cardiovascular Diseases and Cardiometabolic Disorders. Dr Benjamin was supported by NIH Grants 2R01 HL092577, 1R01 HL141434 01A1, 2U54HL12016, and 1R01AG066010; and American Heart Association AHA_18SFRN34110082. Dr Lin is supported by the European Commission Grant (Agreement No 847770).
Disclosures: The authors have no conflicts of interest to disclose.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrthm.2021.05.009.
Appendix. Supplementary data
References
- 1.Rosiak M., Ruta J., Bolinska H. Usefulness of prolonged P-wave duration on signal averaged ECG in predicting atrial fibrillation in acute myocardial infarction patients. Med Sci Monit. 2003;9 MT85–88. [PubMed] [Google Scholar]
- 2.Steinberg J.S., Zelenkofske S., Wong S.C., Gelernt M., Sciacca R., Menchavez E. Value of the P-wave signal-averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation. 1993;88:2618–2622. doi: 10.1161/01.cir.88.6.2618. [DOI] [PubMed] [Google Scholar]
- 3.Budeus M., Hennersdorf M., Perings C., Wieneke H., Erbel R., Sack S. Prediction of the recurrence of atrial fibrillation after successful cardioversion with P wave signal-averaged ECG. Ann Noninvasive Electrocardiol. 2005;10:414–419. doi: 10.1111/j.1542-474X.2005.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa M., Kumagai K., Vakulenko M., et al. Reduction of P-wave duration and successful pulmonary vein isolation in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:931–938. doi: 10.1111/j.1540-8167.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher K., Dagres N., Hindricks G., Husser D., Bollmann A., Kornej J. Characteristics of PR interval as predictor for atrial fibrillation: association with biomarkers and outcomes. Clin Res Cardiol. 2017;106:767–775. doi: 10.1007/s00392-017-1109-y. [DOI] [PubMed] [Google Scholar]
- 6.Magnani J.W., Johnson V.M., Sullivan L.M., et al. P wave duration and risk of longitudinal atrial fibrillation in persons >/= 60 years old (from the Framingham Heart Study) Am J Cardiol. 2011;107:917–921 e1. doi: 10.1016/j.amjcard.2010.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnani J.W., Zhu L., Lopez F., et al. P-wave indices and atrial fibrillation: cross-cohort assessments from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2015;169:53–61 e1. doi: 10.1016/j.ahj.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnani J.W., Gorodeski E.Z., Johnson V.M., et al. P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National Health and Nutrition Examination Survey. Heart Rhythm. 2011;8:93–100. doi: 10.1016/j.hrthm.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arroja J.D., Burri H., Park C.I., Giraudet P., Zimmermann M. Electrophysiological abnormalities in patients with paroxysmal atrial fibrillation in the absence of overt structural heart disease. Indian Pacing Electrophysiol J. 2016;16:152–156. doi: 10.1016/j.ipej.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babaev A.A., Vloka M.E., Sadurski R., Steinberg J.S. Influence of age on atrial activation as measured by the P-wave signal-averaged electrocardiogram. Am J Cardiol. 2000;86:692–695. doi: 10.1016/s0002-9149(00)01056-0. A9. [DOI] [PubMed] [Google Scholar]
- 11.Gang Y., Hnatkova K., Mandal K., Ghuran A., Malik M. Preoperative electrocardiographic risk assessment of atrial fibrillation after coronary artery bypass grafting. J Cardiovasc Electrophysiol. 2004;15:1379–1386. doi: 10.1046/j.1540-8167.2004.04084.x. [DOI] [PubMed] [Google Scholar]
- 12.Barbosa E.C., Barbosa P.R., Ginefra P., et al. The frequency analysis of signal-averaged ECG of P wave as predictor of efficacy of class III antiarrhythmic drugs to maintain sinus rhythm in recurrent idiopathic atrial fibrillation. Ann Noninvasive Electrocardiol. 2001;6:43–49. doi: 10.1111/j.1542-474X.2001.tb00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbosa E.C., Benchimol-Barbosa P.R., Bomfim Ade S., Rocha P.J., Boghossian S.H., Albuquerque D.C. Reversal atrial electrical remodeling following cardioversion of long-standing lone atrial fibrillation. Arq Bras Cardiol. 2009;93:213–220. doi: 10.1590/s0066-782x2009000900004. [DOI] [PubMed] [Google Scholar]
- 14.Engel T.R., Vallone N., Windle J. Signal-averaged electrocardiograms in patients with atrial fibrillation or flutter. Am Heart J. 1988;115:592–597. doi: 10.1016/0002-8703(88)90809-5. [DOI] [PubMed] [Google Scholar]
- 15.Palano F., Adduci C., Cosentino P., Silvetti G., Boldini F., Francia P. Assessing atrial fibrillation substrates by P wave analysis: a comprehensive review. High Blood Press Cardiovasc Prev. 2020;27:341–347. doi: 10.1007/s40292-020-00390-1. [DOI] [PubMed] [Google Scholar]
- 16.Kenchaiah S., Evans J.C., Levy D., et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 17.Michelucci A., Padeletti L., Chelucci A., et al. Influence of age, lead axis, frequency of arrhythmic episodes, and atrial dimensions on P wave triggered SAECG in patients with lone paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1996;19:758–767. doi: 10.1111/j.1540-8159.1996.tb03357.x. [DOI] [PubMed] [Google Scholar]
- 18.Stafford P.J., Turner I., Vincent R. Quantitative analysis of signal-averaged P waves in idiopathic paroxysmal atrial fibrillation. Am J Cardiol. 1991;68:751–755. doi: 10.1016/0002-9149(91)90648-5. [DOI] [PubMed] [Google Scholar]
- 19.Fukunami M., Yamada T., Ohmori M., et al. Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave-triggered signal-averaged electrocardiogram. Circulation. 1991;83:162–169. doi: 10.1161/01.cir.83.1.162. [DOI] [PubMed] [Google Scholar]
- 20.Dixen U., Joens C., Parner J., Rasmussen V., Pehrson S.M., Jensen G.B. Prolonged signal-averaged P wave duration after elective cardioversion increases the risk of recurrent atrial fibrillation. Scand Cardiovasc J. 2004;38:147–151. doi: 10.1080/14017430410028645. [DOI] [PubMed] [Google Scholar]
- 21.Budeus M., Felix O., Hennersdorf M., Wieneke H., Erbel R., Sack S. Prediction of conversion from paroxysmal to permanent atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:243–252. doi: 10.1111/j.1540-8159.2007.00656.x. [DOI] [PubMed] [Google Scholar]
- 22.Dixen U., Larsen M.V., Ravn L., Parner J., Jensen G.B. Signal-averaged P wave duration and the long-term risk of permanent atrial fibrillation. Scand Cardiovasc J. 2008;42:31–37. doi: 10.1080/14017430701652282. [DOI] [PubMed] [Google Scholar]
- 23.Holm H., Gudbjartsson D.F., Arnar D.O., et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet. 2010;42:117–122. doi: 10.1038/ng.511. [DOI] [PubMed] [Google Scholar]
- 24.Pfeufer A., van Noord C., Marciante K.D., et al. Genome-wide association study of PR interval. Nat Genet. 2010;42:153–159. doi: 10.1038/ng.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darbar D., Hardy A., Haines J.L., Roden D.M. Prolonged signal-averaged P-wave duration as an intermediate phenotype for familial atrial fibrillation. J Am Coll Cardiol. 2008;51:1083–1089. doi: 10.1016/j.jacc.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodkinson E.C., Neijts M., Sadrieh A., et al. Heritability of ECG biomarkers in the Netherlands Twin Registry measured from Holter ECGs. Front Physiol. 2016;7:154. doi: 10.3389/fphys.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva C.T., Kors J.A., Amin N., et al. Heritabilities, proportions of heritabilities explained by GWAS findings, and implications of cross-phenotype effects on PR interval. Hum Genet. 2015;134:1211–1219. doi: 10.1007/s00439-015-1595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith J.G., Lowe J.K., Kovvali S., et al. Genome-wide association study of electrocardiographic conduction measures in an isolated founder population: Kosrae. Heart Rhythm. 2009;6:634–641. doi: 10.1016/j.hrthm.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zipes D.P. Atrial fibrillation. A tachycardia-induced atrial cardiomyopathy. Circulation. 1997;95:562–564. doi: 10.1161/01.cir.95.3.562. [DOI] [PubMed] [Google Scholar]
- 30.Kottkamp H. Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J Cardiovasc Electrophysiol. 2012;23:797–799. doi: 10.1111/j.1540-8167.2012.02341.x. [DOI] [PubMed] [Google Scholar]
- 31.Larsen B.S., Kumarathurai P., Falkenberg J., Nielsen O.W., Sajadieh A. Excessive atrial ectopy and short atrial runs increase the risk of stroke beyond incident atrial fibrillation. J Am Coll Cardiol. 2015;66:232–241. doi: 10.1016/j.jacc.2015.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.