Abstract
Adverse life events (ALEs) are a risk factor for chronic pain; however, mechanisms underlying this association are not understood. This study examined whether cumulative ALE exposure impairs endogenous inhibition of pain (assessed from pain report) and spinal nociception (assessed from nociceptive flexion reflex; NFR) in healthy, pain-free Native Americans (n=124) and non-Hispanic Whites (n=129) during a conditioned pain modulation (CPM) task. Cumulative ALE exposure was assessed prior to testing by summing the number of potentially traumatic events experienced by each participant across their lifespan. Multilevel modeling found that ALEs were associated with NFR modulation during the CPM task even after controlling for general health, body mass index, sex, age, blood pressure, sleep quality, stimulation intensity, stimulus number, perceived stress, and psychological distress. Low exposure to ALEs was associated with NFR inhibition, whereas high exposure to ALEs was associated with NFR facilitation. By contrast, pain perception was inhibited during the CPM task regardless of the level of ALE exposure. Race/ethnicity did not moderate these results. Thus, ALEs may be pronociceptive for both Native Americans and non-Hispanic Whites by impairing descending inhibition of spinal nociception. This could contribute to a chronic pain risk phenotype involving latent spinal sensitization.
Perspective:
This study found that adverse life events were associated with impaired descending inhibition of spinal nociception in a sample of Native Americans and non-Hispanic Whites. These findings expand on previous research linking adversity to chronic pain risk by identifying a proximate physiological mechanism for this association.
Keywords: Adverse life events, nociceptive flexion reflex, conditioned pain modulation, trauma, pain
Introduction
Accumulating evidence suggests a dose-dependent relationship between ALEs and myriad negative sequalae, including sleep disturbances1, immune dysregulation31, somatic problems1, lower quality of life1, and psychopathology17. ALEs are also considered to be a risk factor for chronic pain52,70, yet few proximate mechanisms have been identified to explain the link. Nonetheless, the cumulative number of ALEs is associated with pain risk above and beyond the severity and magnitude of each individual event67,70.
Yarnitsky et al. (2014) have argued that greater pain facilitation (assessed by temporal summation [TS]) and impaired endogenous pain inhibition (assessed by conditioned pain modulation [CPM]) promote risk for chronic pain. TS measures the extent to which repeated noxious stimuli amplify pain64, an effect that is believed due to spinal neuron hyperexcitability (i.e., wind-up in animals)37. Some have found that TS of pain (TS-pain) is enhanced in people reporting more ALEs68. Furthermore, greater ALE exposure is associated with enhanced TS of the nociceptive flexion reflex (TS-NFR), a withdrawal reflex used to assess spinal nociception46,55. This supports the notion that ALEs increase spinal neuron hyperexcitability in a dose-dependent manner55. Consistent with this, other studies have found that ALEs were associated with greater capsaicin-induced secondary hyperalgesia/allodynia (markers of spinal sensitization)68,69. These studies suggest ALEs may increase risk for chronic pain by amplifying spinal nociception39, but the effect on endogenous inhibition is less clear.
Endogenous mechanisms exist that allow CNS inhibition of spinal nociception2,29,41,58. When impaired, incoming nociceptive signals are more likely to be experienced as painful and thus could promote chronic pain2,58. The CPM task compares the degree to which a painful test stimulus is inhibited by a painful conditioning stimulus at a distal location. In humans, CPM-related pain inhibition (CPM-pain) is typically assessed using self-report pain ratings, and thus may not exclusively reflect descending inhibition of spinal nociception41,51. Nonetheless, CPM can inhibit the spinally-mediated NFR12,29, so the effect of the CPM task on NFR (CPM-NFR) can be used to assess descending inhibition of spinal nociception. To our knowledge, only one study has examined the link between adversity and CPM-NFR and found that a specific type of adversity (i.e., sexual assault) was associated with disrupted CPM-NFR inhibition22. Thus, it is plausible that ALEs may confer a dose-dependent pain risk by increasing spinal hyperexcitability (TS-NFR)55 and impairing inhibition of spinal nociception (CPM-NFR).
Moreover, establishing the relationship between ALEs and chronic pain risk may improve our understanding of pain disparities among racial/ethnic minorities. For example, African-Americans experience considerable ALEs36,53, and also suffer from a significant pain disparity38,45. Their pain disparity may at least partially result from impaired CPM-pain inhibition6, perhaps due to exposure to adversity. Similarly, Native Americans (NAs) are more likely to experience ALEs than non-Hispanic whites (NHWs)33 and are more likely to experience chronic pain25. Thus, ALEs could promote pronociceptive mechanisms (e.g., enhanced TS, impaired CPM) contributing to higher rates of chronic pain in NAs. However, it is unclear whether ALEs have a dose-dependent effect on CPM-related inhibition, and whether this effect is stronger in NAs. To address these issues, data from healthy, pain-free NAs and NHWs who participated in the Oklahoma Study of Native American Pain Risk (OK-SNAP) were analyzed. It was hypothesized that ALEs would be associated with impaired CPM-NFR and CPM-pain. Given that NAs have a higher risk of chronic pain, we also hypothesized that the effect of ALEs on CPM would be stronger in NAs. However, it is also possible that the effect of ALEs on CPM is similar in NAs and NHWs given that race/ethnicity did not moderate the effect of ALEs on TS55.
Materials and Methods
Participants
OK-SNAP was a two-day study designed to assess risk factors (e.g., pain sensitivity, measures of central sensitization, and measures of endogenous inhibition) for chronic pain in NAs. Pain-free NA and NHW participants were recruited so that risk factors for chronic pain could be identified prior to the onset of disease when racial/ethnic differences could be confounded by differences in disease severity, access to health care, or other factors. Data were collected from March 2014 through October 2018.
Of the 329 found eligible in OK-SNAP, 2 participants’ data were lost due to a computer malfunction, 22 participants were non-NA minorities and thus were excluded from analyses, and 3 were later excluded for having Type 1 or Type 2 diabetes. This resulted in 302 participants in the final sample. Of these, 253 (120 men) completed some or all of CPM and are used in the current study (differences between completers and non-completers are reported in the Results section and Table 1). Papers reporting on the racial/ethnic group differences in pain sensitivity, central sensitization, and endogenous inhibition (without considering ALEs) can be found elsewhere49,50. A subset of these data were used to explore the relationship between sexual assault and CPM (prior to completion of data collection in OK-SNAP)22, but the current hypotheses and analyses were novel in that they: 1) tested a dose-dependent relationship between cumulative ALE exposure and CPM (regardless of ALE type) and 2) examined whether race/ethnicity moderates the ALEs-CPM relationships.
Table 1.
Comparison of Participants with and without CPM Data on Continuous Background Variables
| Completed CPM (n=251) |
Did not Complete CPM (n=51) |
||||||
|---|---|---|---|---|---|---|---|
| Continuous Variable | M | SD | M | SD | t | p | d |
| Age (years) | 28.928 | 12.796 | 32.882 | 14.271 | 1.834 | 0.071 | 0.303 |
| Adverse Life Events (ALEs) | 2.084 | 1.612 | 2.000 | 1.575 | −0.345 | 0.731 | −0.052 |
| Body Mass Index (kg/m2) | 24.920 | 4.232 | 25.492 | 4.158 | 0.891 | 0.376 | 0.136 |
| Mean Arterial Pressure (mmHg) | 85.274 | 9.149 | 86.901 | 7.968 | 1.251 | 0.215 | 0.181 |
| Dispositional Pain Catastrophizing (PCS; 0–52) | 9.624 | 7.573 | 9.143 | 7.853 | −0.394 | 0.695 | −0.063 |
| Negative Affect (PANAS; 0–40) | 2.900 | 2.604 | 2.939 | 2.520 | 0.098 | 0.922 | 0.015 |
| Positive Affect (PANAS; 0–40) | 18.536 | 7.275 | 19.235 | 8.326 | 0.338 | 0.740 | 0.095 |
| State Anxiety (STAI; 20–80) | 32.736 | 7.098 | 31.694 | 7.335 | −0.914 | 0.364 | −0.146 |
| SCL-90 - Global Severity Index (0–4) | 0.125 | 0.087 | 0.096 | 0.082 | −2.223 | 0.041 | −0.331 |
| Perceived Stress (PSS; 0–40) | 13.852 | 6.005 | 13.326 | 6.031 | −0.529 | 0.599 | −0.088 |
| SF-36 Body Pain Scale (0–100) | 90.200 | 10.802 | 93.023 | 8.139 | 1.993 | 0.050 | 0.270 |
| SF-36 General Health Scale (0–100) | 79.480 | 13.765 | 79.070 | 14.811 | −0.169 | 0.866 | −0.029 |
| Subjective Sleep Quality (0–3) | 1.106 | 0.764 | 1.167 | 0.753 | 0.194 | 0.853 | 0.079 |
Note. PCS=Pain Catastrophizing Scale. PANAS=Positive and Negative Affect Schedule. STAI=State Trait Anxiety Inventory. SCL-90= Symptom Checklist 90. PSS=Perceived Stress Scale. SF-36= Medical Outcomes Short Study From, 36-item.
Prior to performing any study procedures, all participants provided verbal and written informed consent. As compensation, each participant received $100 per testing day completed. If a subject withdrew during a testing day, they received $10 for each hour completed that day. All study procedures were approved by the Institutional Review Boards at the University of Tulsa, the Cherokee Nation, and the Indian Health Service Oklahoma City Area Office. Participants were recruited via fliers, newspaper ads, emails, Craigslist ads, Facebook ads, and in person meetings with NA groups.
The study excluded people who were younger than 18 years old, people with a history of cardiovascular, neuroendocrine, musculoskeletal, or neurological disorders, people with acute or chronic pain, people who were unable to read or write fluently in English, and people with a body mass index (BMI) greater than or equal to 35 (due to difficulties recording NFR). People who used antidepressant, anxiolytic, stimulant, or antihypertensive medications within four half-lives of the respective drug prior to testing were also excluded. Use of over-the-counter analgesics was exclusionary if used within 24 hours of testing, and use of prescription analgesics was exclusionary if used within two weeks prior to testing. Additionally, people who endorsed having current psychotic symptoms or substance abuse were excluded. NA participants were required to provide verification of their tribal affiliation for inclusion in the NA group (e.g., Certificate of Degree of Indian Blood Card, tribal affiliation card). NA participants in the current study represent tribal nations predominately from the southern plains and eastern Oklahoma tribes. To respect tribal confidentiality, tribal affiliations are not reported.
Testing Day Procedures
OK-SNAP consisted of two testing days that each lasted approximately 4–6 hours. For a detailed overview of all OK-SNAP procedures, see50, and for a detailed description of the CPM day procedures, see57. To briefly summarize, participants provided informed consent and then sensors were applied for recording NFR. Following sensor application, participants completed questionnaires (i.e., dispositional catastrophizing, PANAS, STAI) before undergoing procedures to determine the electric test stimulus intensity used during the CPM task (NFR threshold, Pain30 [Pain30 was only administered if NFR threshold stimuli did not evoke pain that was at least a 30 out of 100 on a VAS], and 3-stimulation threshold).
Afterwards, participants underwent a battery of pain tasks (responses to heat pulses, single electric stimulations, and TS-NFR) and then completed the SF-36 questionnaire. Finally, participants underwent CPM and emotional controls of nociception (ECON) tasks, the order of which was randomized. The SCL-90 and PSS were completed between these two tasks. Mandatory breaks were taken after tasks to reduce the likelihood of carryover effects.
Apparatus, Electrode Application, and Signal Acquisition
All procedures were controlled on a dual monitor computer using an analog-to-digital board (USB-6212 BNC; National Instruments, Austin, TX, USA) with LabVIEW software (National Instruments). Study procedures took place in a sound-attenuated and electrically shielded experiment room, and participants used a monitor and computer mouse to complete electronic questionnaires, except that pain ratings were made verbally during CPM testing. Additionally, participants wore sound-attenuating headphones to communicate with the researcher and to receive pre-recorded instructions. While testing occurred, researchers in an adjacent control room monitored the participant physiology that was displayed on a second monitor. To ensure protocol compliance, researchers monitored participants during tasks using a video camera.
At the beginning of the CPM testing day, a medical grade device (Dinamap; Tampa, FL) was used to measure mean arterial pressure (MAP) 3 times at rest with 3-minute intervals between each test. The average MAP of these three readings was used as a control variable in the current analyses since NAs experienced slightly higher blood pressure than NHWs.
Conditioned Pain Modulation (CPM)
Similar to prior research, the CPM paradigm used cold water as the conditioning stimulus (CS) and an electric stimulation as the test stimulus26,32. The paradigm involved 3 phases (baseline, conditioning, posttest) lasting 2-mins each. Each phase began with a 20-second waiting period. During the baseline phase, participants were exposed to 5 electrical stimulations delivered at an interstimulus interval of 8–12 seconds. After each stimulation, participants were instructed to verbally report the pain felt due to the stimulation between 0–100 on a numerical rating scale (NRS) with anchors every 20 points (0=no pain, 20=mild pain, 40=moderate pain, 60=severe pain, 80=very severe pain, 100=worst possible pain)50. At the end of the baseline phase, participants rested for 2-min before beginning the conditioning phase, at which point they were instructed to submerge their right hand (palms down, fingers spread) into painfully cold 10 ± 0.1°C C water CS up to their forearm32. During the 2-min of hand submersion, 5 electrical stimulations (test stimuli) were delivered at an interstimulus interval of 8–12 seconds. Participants gave verbal pain ratings in response to each stimulation using the same 0–100 NRS used during the baseline phase. After a 5-min rest period, participants began the posttest phase (data not presented), which was identical to the baseline phase. Following the posttest phase, participants were instructed to use the NRS to rate the pain they experienced due to the cold water. All test stimuli were set at the highest of 1.2× NFR threshold, 1.2× 3-stimulation threshold, or 1× Pain30 (described below). Experimenters recorded the participants’ verbal ratings in response each stimulation, which were used for CPM-pain analyses. NFR magnitude was measured in response to each stimulation and was used for CPM-NFR analyses.
Conditioning stimulus (CS).
A regulated 10 ± 0.1°C cold water bath was used as the CS during CPM (Thermo Fisher Scientific, Pittsburgh, PA). Participants were asked to submerge their right hand up to their forearm in the cold water for two minutes. They were instructed to place their palm face-down and spread their fingers during CPM conditioning. On average, the CS evoked moderate to severe pain (mean NRS rating = 54) and ratings did not differ by ethnic group (Table 2).
Table 2.
Comparison of Non-Hispanic white (NHW) and Native American (NA) Participants on Background Variables
| NHW (n=129) | NA (n=124) | ||||||
|---|---|---|---|---|---|---|---|
| Continuous Variable | M | SD | M | SD | t | p | d |
| Age (years) | 28.519 | 13.569 | 29.339 | 11.925 | −0.509 | 0.611 | 0.064 |
| Adverse Life Events (ALEs) | 1.754 | 1.473 | 2.139 | 1.602 | −1.970 | 0.050 | 0.250 |
| Body Mass Index (kg/m2) | 24.210 | 3.757 | 25.717 | 4.570 | −2.845 | 0.005 | 0.361 |
| Mean Arterial Pressure (mmHg) | 82.459 | 7.268 | 88.300 | 9.819 | −5.333 | <0.001 | 0.676 |
| Dispositional Pain Catastrophizing (PCS; 0–52) | 9.806 | 7.562 | 9.545 | 7.604 | 0.274 | 0.785 | 0.034 |
| Negative Affect (PANAS; 0–40) | 2.806 | 2.613 | 3.057 | 2.625 | −0.760 | 0.448 | 0.096 |
| Positive Affect (PANAS; 0–40) | 17.938 | 6.875 | 19.114 | 7.669 | −1.283 | 0.201 | 0.162 |
| State Anxiety (STAI; 20–80) | 32.411 | 6.903 | 33.146 | 7.288 | −0.823 | 0.411 | 0.104 |
| SCL-90 - Global Severity Index (0–4) | 0.336 | 0.332 | 0.445 | 0.416 | −3.059 | 0.002 | 0.291 |
| Perceived Stress (PSS; 0–40) | 13.039 | 5.631 | 14.697 | 6.265 | −2.207 | 0.028 | 0.279 |
| SF-36 Body Pain Scale (0–100) | 91.221 | 9.708 | 89.184 | 11.247 | 1.543 | 0.124 | 0.142 |
| SF-36 General Health Scale (0–100) | 81.047 | 13.385 | 78.033 | 13.504 | 1.881 | 0.061 | 0.294 |
| Subjective Sleep Quality (0–3) | 0.948 | 0.630 | 1.268 | 0.849 | −3.219 | 0.001 | −0.429 |
| Suprathreshold Stimulus Intensity (0–50mA) | 25.034 | 12.558 | 27.425 | 12.156 | −1.538 | 0.125 | 0.193 |
| Cold Water Pain (0–100) | 51.820 | 24.266 | 55.910 | 24.281 | −1.334 | 0.184 | 0.168 |
| Categorical Variable | N | % | N | % | χ 2 | p | |
| Sex (male) | 65 | 50.4% | 55 | 44.4% | 0.923 | 0.337 | |
| Education | 2.890 | 0.409 | |||||
| High School Graduate | or Less | 15 | 11.7% | 22 | 17.9% | ||
| Some College | 68 | 53.1% | 54 | 43.9% | |||
| College Graduate | 34 | 26.6% | 36 | 29.3% | |||
| Graduate/Professional School | 11 | 8.6% | 11 | 8.9% | |||
| Employment | 3.863 | 0.145 | |||||
| ≥40 Hours per Week | 28 | 22.0% | 39 | 32.0% | |||
| <40 Hours per Week | 60 | 47.2% | 45 | 36.9% | |||
| Retired | 39 | 30.7% | 38 | 31.1% | |||
| Income | 9.491 | 0.091 | |||||
| <$9,999 | 49 | 38.6% | 30 | 25.0% | |||
| $10,000–$14,999 | 15 | 11.8% | 15 | 12.5% | |||
| $15,000–$24,999 | 16 | 12.6% | 15 | 12.5% | |||
| $25,000–$34,999 | 10 | 7.9% | 15 | 12.5% | |||
| $35,000–$49,999 | 10 | 7.9% | 21 | 17.5% | |||
| ≥$50,000 | 27 | 21.3% | 24 | 20.0% | |||
| Marital Status | 5.553 | 0.062 | |||||
| Single | 97 | 75.2% | 79 | 64.8% | |||
| Married | 22 | 17.1% | 22 | 18.0% | |||
| Other | 10 | 8.8% | 21 | 17.2% | |||
Note. NHW=non-Hispanic white. NA= Native American. PCS=Pain Catastrophizing Scale. PANAS=Positive and Negative Affect Schedule. STAI=State Trait Anxiety Inventory. SCL-90= Symptom Checklist 90. PSS=Perceived Stress Scale. SF-36= Medical Outcomes Short Study From, 36-item.
Test stimuli.
A constant current nerve stimulator (Digimeter DS7A; Hertfordshire, England) and a bipolar electrode (Nicolet, Model #019-40400, Madison, WI, USA) that was placed on the left ankle over the retromalleolar pathway of the sural nerve, was used to deliver electric stimuli. The timing of stimulations was controlled by computer. All stimulations were delivered as trains of five 1-ms rectangular wave pulses at 250-Hz; these were perceived as a single stimulus. To ensure participant safety, the stimulus intensity of electric stimulations was capped at 50-mA.
NFR Recording
NFR activity was measured using electromyography (EMG) of the left biceps femoris, which is located approximately 10-cm superior to the popliteal fossa. To record this activity, two Ag-AgCl electrodes were placed over the left biceps femoris, where signals were recorded, filtered (10-Hz to 300-Hz), and amplified (×10,000) using a Grass Technologies (West Warwick, RI, USA) Model 15LT amplifier (with AC Module 15A54). A ground electrode was also placed on the lateral epicondyle of the femur. Prior to sensor and stimulating electrode application, a researcher cleaned the participant’s skin with alcohol and exfoliated (NuPrep gel; Weaver and Company, Aurora, CO, USA) before a conductive gel (EC60, Grass Technologies) was placed onto all electrodes and sensors to achieve impedances ≤ 5kΩ. NFR magnitude was calculated using a d-score (NFR d= [mean rectified EMG of 90–150 ms poststimulation interval minus mean of rectified EMG from −60 to 0 ms prestimulation interval] divided by the average standard deviation of the rectified EMG from the 2 intervals).
NFR threshold.
To determine the intensity of the CPM test stimulus, NFR threshold was determined according to three ascending-descending staircases method46. Beginning at 0 mA, participants underwent a single electric stimulation that increased in 2 mA increments until an NFR was detected, which occurred when the rectified EMG activity in the 90–150 ms poststimulus interval was 1.4 standard deviations greater than the rectified EMG activity in the −60–0 ms prestimulus interval. After obtaining the first NFR, the stimulations decreased in 1 mA intervals until an NFR was no longer detected. Then, the second and third ascending-descending staircases obtained NFRs using 1 mA increments. The current study defined NFR threshold as the average stimulus intensity (mA) of the peaks and troughs obtained during the second and third ascending-descending staircases.
Pain30.
During the staircases of NFR threshold, participants also rated the pain caused by the electric stimulations using the visual analog scale (VAS), which ranged from 0 (no pain) to 100 (maximum tolerable pain). If the average VAS rating of NFR threshold was not greater than or equal to 30, stimulations continued and increased at 2 mA increments until this rating was obtained (Pain30).
3-stimulation threshold.
Following NFR threshold and Pain30 testing, a 3-stimulation NFR threshold was obtained. Beginning at 0-mA and increasing by 2-mA increments, a series of 3 electric stimulations was delivered with an interstimulus interval of 0.5 seconds until an NFR was elicited on the last stimulus in the series.
Questionnaires
Demographics and health exclusion.
As part of the screening process and to provide descriptive information of the sample, participants provided background information (e.g., sex, age, SES, and health status). In addition, height and weight were measured using a medical scale to calculate BMI.
Psychological problems.
Psychological problems were measured using the Global Severity Index (GSI) of the Symptom Checklist-90-Revised (SCL-90-R). The SCL-90-R consists of 90 questions that broadly address different areas of psychopathology (e.g., somatic complaints, obsessive-compulsive symptoms, depression, phobic anxiety, paranoia, and psychoticism), and it has been widely utilized across treatment and research settings11,43.
Perceived stress.
The Perceived Stress Scale (PSS), a 10 item measure assessing perceived stress in the past month, was given to participants. Scores range from 0–40; higher scores are indicative of more perceived stress8.
Perceptions of physical health.
The General Health (11 items) and Body Pain (2 items) subscales of the Medical Outcomes Study 36-item Short Form Health Survey (SF-36) were used to measure participants’ physical health60. Scores on each subscale range from 0–100, and lower scores broadly indicate worse health60. Specifically, lower scores on the General Health subscale suggest that an individual believes that their health is generally poor and that it is unlikely to improve60, and low scores on the Body Pain subscale suggest than an individual experiences severe and/or disabling pain60.
Perceived sleep quality.
Subjective sleep quality was assessed using the sleep quality item of the Pittsburgh Sleep Quality Index (PSQI)5. Participants rated their sleep quality during the past month on a scale of 0 (very good) to 3 (very bad).
Adverse life events (ALEs).
To assess ALEs, the Life Events Checklist (LEC) for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision, was administered4. The LEC is a self-report measure containing 17 items asking participants whether they have directly experienced, witnessed, learned about, or have not heard about various ALEs in their lifetime; each item assesses a single stressful or traumatic event. The LEC has been found to be a reliable and valid measure of exposure to potentially traumatic events19. In the current study, ALEs were operationally defined as having direct exposure (answering “happened to me”) to any items on the LEC, with a possible range of 0–17. The distribution of scores for ALEs was highly skewed, primarily driven by outliers in the positive direction. To address this, outliers were winsorized to the nearest non-outlier value (i.e., 5), so the winsorized ALE variable ranged from 0 to 5 (see description of outlier detection in the Data Analysis section).
Data Analysis
All analyses were performed using SPSS v25. Before being analyzed, all variables were first examined for non-normality, and skewed distributions were corrected using transformations. Outliers were identified using Wilcox’s61 MAD-median procedure using a cutoff of 2.24 and were winsorized to the nearest non-outlier value. An alpha level of p < .05 (two-tailed) was used in all analyses. To determine between-group differences between NAs and NHWs, independent samples t-tests were conducted on continuous variables; variables demonstrating group differences were grand mean centered and used as control variables in the model. Categorical variables were analyzed using chisquare analyses.
For the primary analyses, the current study compared data from the baseline and conditioning phases of CPM (the only conditions needed to assess descending inhibition) using regression-based multilevel models. Multilevel models are advantageous because they can simultaneously model the intra- and inter-individual variance in subjective reactions (i.e., pain ratings) and physiological responses (i.e., NFR magnitudes)47. Thus, each repeated observation of the primary dependent variables (i.e., verbal pain ratings for CPM-pain, NFR magnitude for CPM-NFR) served as a level 1 unit and was given its own row, such that each participant (level 2 unit) who completed the baseline and conditioning phases of CPM had 10 rows of data (1 row for each stimulation). A first-order autoregressive matrix (AR1) was used to model the within-subject variance-covariance structure in order to account for autocorrelations across repeated measures. Since a regression-based approach was taken, all predictors were treated as if they were continuous-like. The primary independent variable in this study, CPM phase, was dummy coded 0 (baseline) and 1 (conditioning) and used as a continuous-like predictor in order to code for the slope of CPM-related modulation, which varied across level 2 units (participants). The ALEs variable was entered as a continuous-like variable and was centered at 3 so that the interaction term could be created between ALEs and CPM phase without causing multicollinearity. Stimulus number (Stim1 to Stim5) which coded for the 5 stimulations in each CPM phase was entered as a continuous-like control variable to account for habituation/sensitization in response to electric stimuli delivered during CPM. Interactions between CPM phase and ALEs were tested to determine whether inhibition during CPM was affected by the number of ALEs endorsed by each participant. Additionally, race/ethnicity was coded as a continuous-like predictor (−1=NHW, 1=NA), and a three-way interaction was tested to determine whether race/ethnicity moderated the relationship between ALEs and CPM phase. In the event of a significant ALEs × CPM phase interaction, simple effects for CPM phase were calculated for each of the low, medium, and high values of the ALE variable (i.e., 0, 3, ≥5)42 to evaluate the effect of ALE exposure on the CPM effect.
Results
Sample characteristics
Of the enrolled participants, 29 did not attend any of the CPM testing day, and 23 of the enrolled participants attended the CPM testing day but withdrew prior to CPM. Only one participant dropped out during the CPM task because of the pain from the cold water. See Table 1 for differences between CPM completers and non-completers. The final sample of participants with some CPM data totaled 253 (129 NHW, 124 NA).
Of the participants with CPM data, 52 (20 NAs) reported 0 ALEs, 61 (33 NAs) reported 1 ALE, 58 (27 NAs) reported 2 ALEs, 38 (17 NAs) reported 3 ALEs, 19 (11 NAs) reported 4 ALEs, and 24 (16NAs) reported ≥5 ALEs. One NHW did not complete the LEC.
Characteristics of the 129 NHW and 124 NA participants are presented in Table 2. Although the differences between NAs and NHWs on exposure to ALEs did not reach statistical significance (p = .05), it was in the predicted direction and represents a 22% greater exposure to ALEs within the NA group, which at the population level is clearly significant. Analysis of group differences found that NA participants had higher BMI, higher MAP, higher levels of psychological distress (SCL-90 GSI), and higher perceived stress (PSS).
Variable Conditioning
For the current analyses, psychological problems (GSI) were log transformed due to positive skew. Outliers were identified and subsequently winsorized for ALEs (as noted previously), perceived stress (PSS), sleep problems (PSQI), the General Health subscale of the SF-36, NFR magnitudes, and pain ratings. To minimize potential bias in the results, four participants were excluded from CPM-pain analyses due to having CPM baseline pain ratings at ceiling (NRS rating ≥ 95) or floor (NRS rating ≤ 5). In addition, individual stimulation trials with an averaged pre-stimulus baseline EMG ≥ 3μV (i.e., excess muscle tension) were excluded from NFR analysis (4.12% of 2377 trials).
Control Variables
Given that NAs and NHWs differed on BMI, mean arterial pressure (MAP), subjective sleep quality (PSQI), self-reported psychological distress (SCL-90-R GSI), and perceived stress levels (PSS), these variables were controlled for in the analyses. Further, sex, age, and general health (general health scale of the SF-36) were also controlled for given their potential influence on CPM28,34. And finally, suprathreshold stimulus intensity differed for each participant so it was controlled for in all analyses.
CPM-NFR
Table 3 reports the results of the multilevel model of NFR and provides summary statistics for our data. Although there was no significant main effect of CPM phase, a significant interaction between ALEs and CPM phase was found (p = .003). Consistent with a regression-based approach, Figure 1 depicts CPM-NFR at high (i.e., ≥5), medium (i.e., 3), and low (i.e., 0) ALEs. CPM phase was associated with significant NFR inhibition for people with 0 ALEs (p =.001). For people with 3 ALEs, CPM phase was not found to significantly modulate NFR (ps > .05) (note: because the interaction was significant, the main effect of CPM phase reflects the simple effect of CPM phase when ALEs =3, due to ALEs being centered at 3). But, for people with ≥5 ALEs, CPM phase was associated with a significant facilitation of NFR (p = .031). There were no main effects or interactions with race, indicating the relationship between ALEs and CPM-NFR was statistically equivalent in NAs and NHWs.
Table 3.
Results of multilevel growth curve analysis of CPM-NFR
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Fixed Effects | Estimate | SE | lower | upper |
| Intercept | 1.028* | 0.071 | 0.888 | 1.168 |
| ALEs | −0.042 | 0.038 | −0.116 | 0.032 |
| CPM Phase | 0.040 | 0.048 | −0.053 | 0.134 |
| Stimulus Number | −0.045* | 0.006 | −0.057 | −0.034 |
| Suprathreshold Stimulus Intensity | 0.015* | 0.003 | 0.010 | 0.021 |
| Age | < −0.001 | 0.003 | −0.006 | 0.006 |
| Sex | −0.029 | 0.035 | −0.097 | 0.040 |
| Body Mass Index (kg/m2) | −0.003 | 0.009 | −0.021 | 0.014 |
| Mean Arterial Pressure (mmHg) | −0.005 | 0.004 | −0.014 | 0.004 |
| Sleep Quality (PSQI) | 0.097 | 0.052 | −0.004 | 0.199 |
| Perceived Stress (PSS) | −0.001 | 0.009 | −0.018 | 0.016 |
| Psychological Distress (log GSI) | −0.176 | 0.635 | −1.424 | 1.073 |
| General Health (SF-36-GH) | < −0.001 | 0.003 | −0.007 | 0.007 |
| Race (NA) | 0.027 | 0.097 | −0.163 | 0.218 |
| ALEs × CPM Phase | 0.075* | 0.025 | 0.026 | 0.124 |
| ALEs × Race (NA) | 0.052 | 0.049 | −0.045 | 0.149 |
| CPM Phase × Race (NA) | −0.014 | 0.064 | −0.140 | 0.112 |
| ALEs × CPM Phase × Race (NA) | −0.064 | 0.034 | −0.131 | 0.003 |
| 95% Confidence Interval | ||||
| Random Effects | Estimate | SE | lower | upper |
| AR1 diagonal | 0.157* | 0.006 | 0.146 | 0.168 |
| AR1 rho | 0.083* | 0.030 | 0.025 | 0.142 |
| Intercept Variance | 0.299* | 0.031 | 0.245 | 0.365 |
| Intercept and CPM Phase Covariance | −0.064* | 0.017 | −0.097 | −0.031 |
| CPM Phase Variance | 0.094* | 0.016 | 0.068 | 0.130 |
Note. Estimates show the unstandardized relationship between each predictor and the criterion. Bolded text indicates significance at *p < .05. SE=Standard error of estimate/coefficient. PSQI=Pittsburgh Sleep Quality Index. PSS=Perceived Stress Scale. GSI=Global Severity Index of the Symptom Checklist 90. SF-36-GH=General Health Scale of the Short Form Health Survey. NA=Native American. ALEs= Adverse Life Events. CPM=Conditioned Pain Modulation. Sex was coded −1=male and 1=female. Race was coded 0=non-Hispanic white and 1=Native American. AR1=first-order autoregressive structure.
Figure 1.
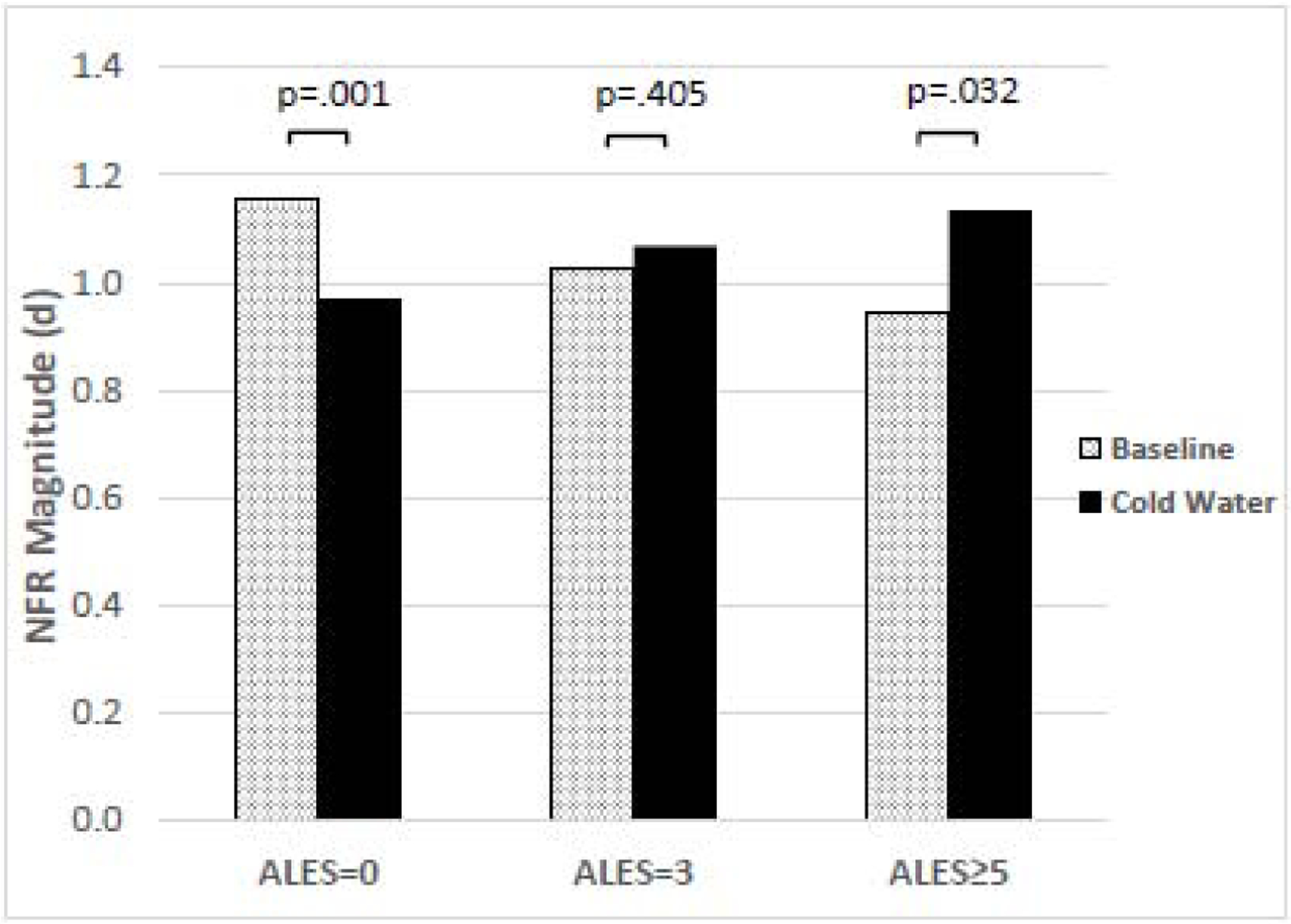
Effect of adverse life events (ALEs) on nociceptive flexion reflex (NFR) magnitudes during conditioned pain modulation (CPM). Results suggest a significant interaction between ALEs and pain modulation evoked by the cold water conditioning stimulus (CS) in the CPM paradigm (p=0.003). People with 0 ALEs showed statistically significant inhibition of NFR (p=0.001) when exposed to the CS phase of CPM, whereas people with 5 or more ALEs showed statistically significant facilitation of NFR (p=0.031) when exposed to the CS phase of CPM. People with 3 ALEs showed no statistically significant changes in NFR magnitude in response to the CS phase of CPM.
The significant main effect of stimulus number (p < .001), indicated habituation of NFR within each CPM phase. The significant main effect of stimulus intensity (p < .001) indicated that stronger electric stimulations were associated with larger NFRs. The model intercept was significant (p < .001) indicating that NFR magnitudes were significantly different from zero when all predictors were controlled.
As for the random effects, the significant diagonal and rho effects indicate that there was significant repeated measures (within-subject) variance and significant across-time covariance in NFR, respectively. The significant intercept variance indicates that there was significant unexplained between-subject variance in NFR during CPM baseline to be explained. The significant CPM phase slope variance indicates that there was significant unexplained between-subject variability in the CPM-related modulation of NFR. And finally, the significant negative covariance between the intercept and CPM phase slope indicates that those with higher NFRs during the CPM baseline were more likely to show greater inhibition of NFR during CPM, and vice versa.
CPM-pain
Table 4 reports the results of the multilevel model of pain and provides summary statistics for our data. A significant main effect of CPM phase was found (p < .001), indicating participants found electrical stimulations to be less painful during the cold water phase of CPM. But unlike CPM-NFR, ALEs did not moderate the relationship between CPM phase and pain ratings (Figure 2). There were also no significant effects containing race, indicating NAs and NHWs did not differ statistically in their pain inhibition.
Table 4.
Results of multilevel growth curve analysis of CPM of electric pain ratings
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Fixed Effects | Estimate | SE | lower | upper |
| Intercept | 37.778* | 2.029 | 33.782 | 41.775 |
| ALEs | 1.274 | 1.068 | −0.830 | 3.377 |
| CPM Phase | −6.420* | 1.143 | −8.682 | −4.158 |
| Stimulus Number | 0.753* | 0.103 | 0.550 | 0.955 |
| Suprathreshold Stimulus Intensity | 0.187* | 0.075 | 0.038 | 0.335 |
| Age | 0.144 | 0.087 | −0.026 | 0.315 |
| Sex | 0.948 | 0.964 | −0.949 | 2.846 |
| Body Mass Index (kg/m2) | 0.058 | 0.249 | −0.433 | 0.549 |
| Mean Arterial Pressure (mmHg) | −0.083 | 0.124 | −0.321 | 0.161 |
| Sleep Quality (PSQI) | −1.801 | 1.423 | −4.604 | 1.003 |
| Perceived Stress (PSS) | −0.103 | 0.236 | −0.568 | 0.361 |
| Psychological Distress (log GSI) | 35.598* | 17.402 | 1.326 | 69.871 |
| General Health (SF-36-GH) | −0.139 | 0.097 | −0.329 | 0.052 |
| Race (NA) | 0.227 | 2.747 | −5.183 | 5.637 |
| ALEs × CPM Phase | −0.568 | 0.584 | −1.722 | 0.586 |
| ALEs × Race (NA) | −1.358 | 1.408 | −4.131 | 1.415 |
| CPM Phase × Race (NA) | −1.087 | 1.502 | −4.056 | 1.882 |
| ALEs × CPM Phase × Race (NA) | 0.780 | 0.799 | −0.800 | 2.359 |
| 95% Confidence Interval | ||||
| Random Effects | Estimate | SE | lower | upper |
| AR1 diagonal | 71.303* | 9.754 | 54.534 | 93.228 |
| AR1 rho | 0.660* | 0.052 | 0.545 | 0.751 |
| Intercept Variance | 238.585* | 25.217 | 193.944 | 293.502 |
| Intercept and CPM Phase Covariance | −57.001* | 12.077 | −80.672 | −33.330 |
| CPM Phase Variance | 53.967* | 10.021 | 37.503 | 77.658 |
Note. Estimates show the unstandardized relationship between each predictor and the criterion. Bolded text indicates significance at *p < .05. SE=Standard error of estimate/coefficient. PSQI=Pittsburgh Sleep Quality Index. PSS=Perceived Stress Scale. GSI=Global Severity Index of the Symptom Checklist 90. SF-36-GH= General Health Scale of the Short Form Health Survey. NA=Native American. ALEs=Adverse Life Events. CPM=Conditioned Pain Modulation. Sex was coded −1=male and 1=female. Race was coded 0=non-Hispanic White and 1=Native American. AR1=first-order autoregressive structure.
Figure 2.
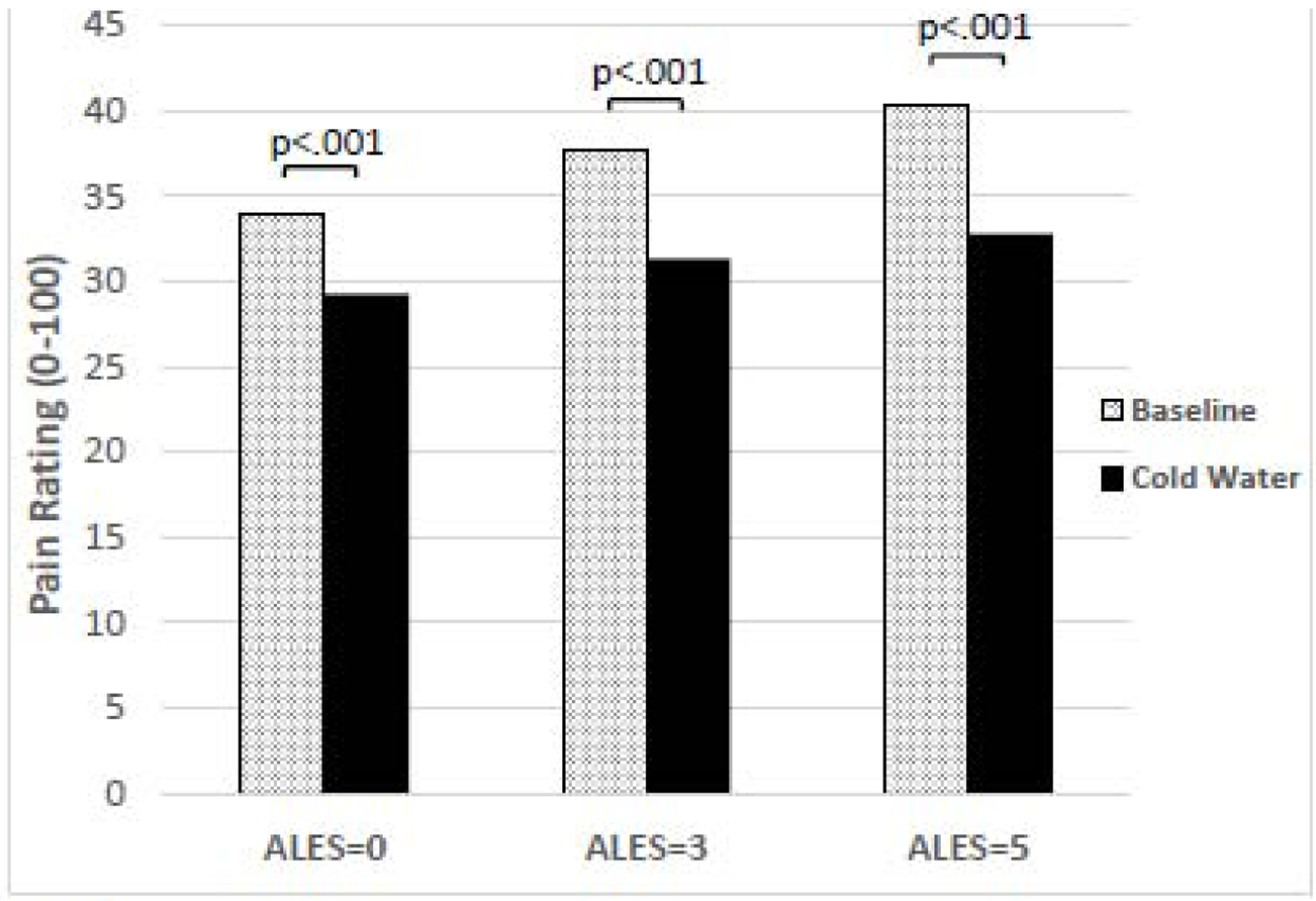
Effect of adverse life events (ALEs) on pain ratings during conditioned pain modulation (CPM). ALEs did not moderate the relationship between CPM phase and subjective pain ratings (p=.332). Regardless of ALEs, the cold water conditioning stimulus led to significant inhibition of pain ratings.
The significant main effect of stimulus number (p < .001) suggested sensitization of pain ratings during each CPM phase. The significant main effect of stimulus intensity (p = .016) indicated that higher electrical stimulation intensity was associated with higher pain ratings. Additionally, the significant main effect of psychological distress (p = .027) suggested that greater psychological distress was associated with higher pain ratings. The model intercept was significant (p < .001) indicating that pain ratings were significantly different from zero when all predictors were controlled.
As for the random effects, the significant diagonal and rho effects indicate that there was significant repeated measures (within-subject) variance and significant across-time covariance in pain ratings, respectively. The significant intercept variance indicates that there was significant unexplained between-subject variance in pain during CPM baseline to be explained. The significant CPM phase slope variance indicates that there was significant unexplained between-subject variability in the CPM-related modulation of pain. And finally, the significant negative covariance between the intercept and CPM phase slope indicates that those with higher pain ratings during the CPM baseline were more likely to show greater inhibition of pain during CPM, and vice versa.
Discussion
This study was the first to assess whether cumulative ALEs had a dose-dependent relationship with endogenous inhibition of spinal nociception (NFR) and pain in NAs. It was hypothesized that modulation of pain perception and NFR would be associated with ALEs, but ALEs were only associated with NFR modulation. Higher exposure to ALEs was associated with less CPM-NFR inhibition, and even NFR facilitation at high ALEs (Figure 1). Together, these findings suggest ALEs may confer chronic pain risk by disrupting descending (cerebrospinal) inhibition of spinal nociception without altering pain experience.
Adverse Life Events May Promote Pronociceptive Mechanisms
Combined with other OK-SNAP findings22,55, ALEs appear to promote a pronociceptive phenotype by enhancing TS-NFR and disrupting CPM-NFR. Some have argued that TS and CPM are experimental predictors of chronic pain onset66, and 3 studies have prospectively demonstrated CPM’s ability to predict future chronic pain. Yarnitsky et al. showed that disrupted CPM-pain (test stimulus=hot thermode on volar forearm, CS=46.5°C water) prospectively predicted the onset of chronic pain in a sample of 62 thoracotomy patients65. Landau et al replicated these findings showing that a pre-surgical CPM assessment in a sample of 75 pregnant women could predict post-Cesarian pain27. Finally, less inhibition during CPM with electric and pressure test stimuli and painful cold water CS was found to predict future chronic pain in a study of 20 participants undergoing elective abdominal surgery62.
Indeed, CPM-pain appears to be a predictor of chronic pain, and while it does not appear that many studies have used CPM-NFR to predict pain onset, preliminary follow-up data collected from OK-SNAP indicate that CPM-NFR may also predict chronic pain onset, even above and beyond CPM-pain23. Given the clinical utility of identifying individuals at risk for developing chronic pain, additional prospective research is warranted. Furthermore, these at-risk individuals may benefit from interventions that specifically increase descending inhibition of spinal nociception, such as relaxation or biofeedback14,15,48.
ALEs Impact CPM-NFR, but not CPM-Pain: Possible Latent Spinal Sensitization?
Although NFR and pain are correlated7,20,63, they assess different processes. NFR serves as a proxy measure for spinal nociception, whereas pain ratings reflect a combination of incoming spinal nociceptive signaling and supraspinal processing29,63. To support this notion, NFR and pain ratings have been shown to differ under pharmacological40 and psychological10 conditions. Furthermore, a study by Piché et al41 identified distinct neural circuits for CPM-NFR (cerebrospinal circuit) and CPM-pain (fully supraspinal circuit). Thus, a dysfunction in cerebrospinal descending inhibitory circuits that are uniquely associated with NFR inhibition may mediate the dose-dependent association between ALEs and risk for chronic pain.
Given that separate mechanisms may mediate CPM-NFR vs. CPM-pain, then it is possible that CPM-NFR impairment may occur earlier than CPM-pain impairment in a cascade of events that promote chronic pain onset. Indeed, the present study contributes to accumulating evidence that, in healthy, pain-free participants, adversity may promote spinal sensitization without concomitant pain amplification21,22,55,68,69. This apparent disconnect between spinal nociception and pain perception could be explained by intact supraspinal pain inhibitory processes that keep amplified spinal nociception from being experienced as more painful (Figure 3).
Figure 3.
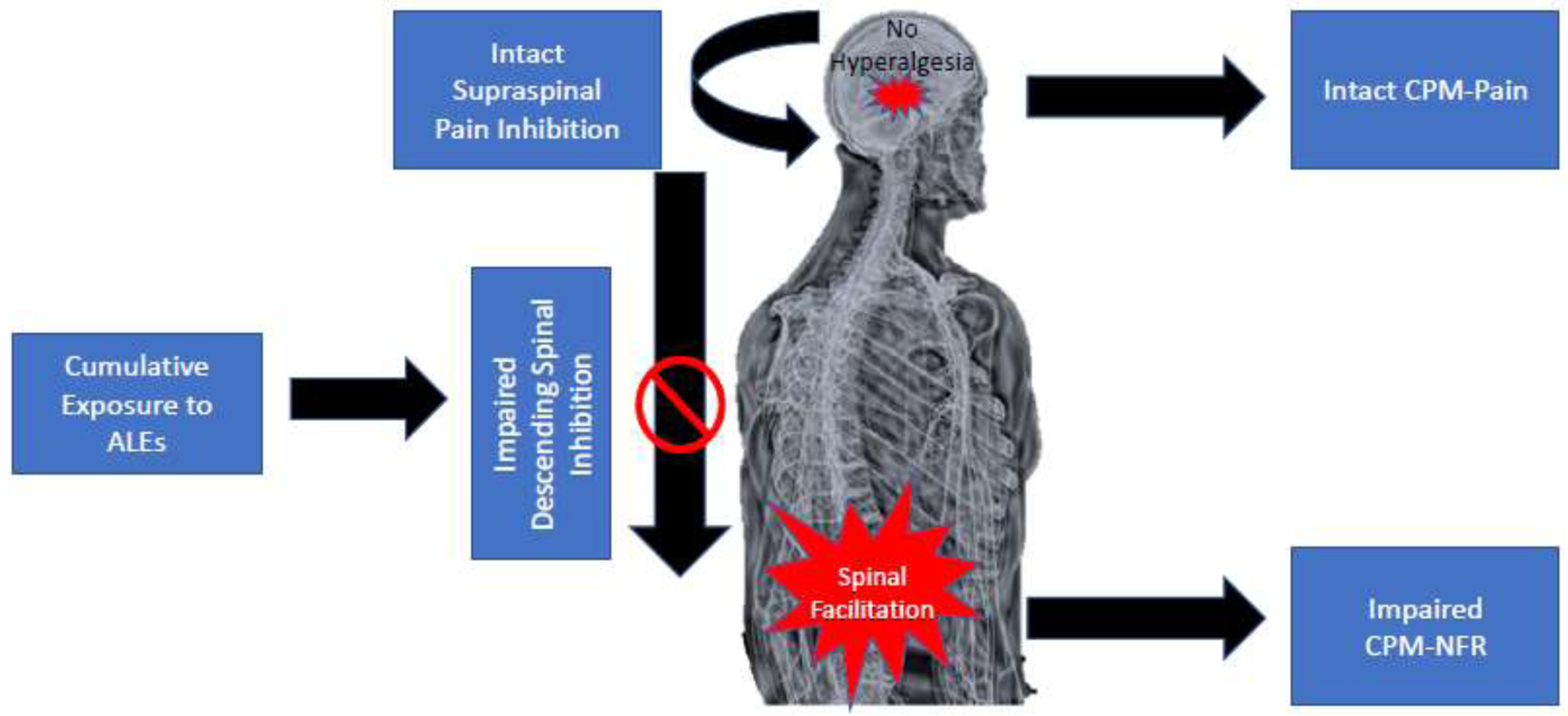
Proposed heuristic model for how adverse life events (ALEs) may confer chronic pain risk in currently healthy, pain-free individuals. Experiencing ALEs may promote spinal facilitation, reflected by impaired inhibition during conditioned pain modulation of the nociceptive flexion reflex (CPM-NFR). Given that CPM-Pain remains intact, supraspinal inhibitory circuits could mitigate the enhanced spinal nociception so that hyperalgesia does not occur.
This notion is akin to the rodent model of chronic pain vulnerability called latent sensitization35,44,56. In rodents, exposure to a major stressor or inflammatory insult leads to a sensitization of spinal nociception and hyperalgesia9,56. But after a few days, the hyperalgesia remits suggesting the insult has been resolved. However, if an opioid antagonist (e.g., naloxone, naltrexone) is administered during the apparent state of remission, the animal returns to a hyperalgesic state, yet animals not exposed to stress or an inflammatory insult do not show this same response to opioid blockade56. According to this model, the spinal sensitization that promotes hyperalgesia does not subside, but is instead kept suppressed by endogenous inhibitory mechanisms (e.g., endogenous opioids)56. Nonetheless, this “latent” spinal sensitization places the animal at risk for future chronic pain. Subsequent exposure to environmental stress is one triggering event that can unveil the latent spinal sensitization to cause chronic pain in rodents30.
Thus, the current study contributes to this emerging story and shows that ALE exposure, in otherwise healthy and pain-free individuals, may promote latent spinal sensitization in humans by promoting descending facilitation of spinal nociception, without concomitant hyperalgesia. Further, the lack of association between ALEs and pain inhibition could indicate that pain inhibitory mechanisms (e.g., purely supraspinal circuitry) are intact to suppress the spinal sensitization in healthy, pain-free persons exposed to high levels of ALEs.
Psychological Distress does not Fully Explain the Relationship between ALEs and Pronociceptive Mechanisms
ALEs were associated with CPM-NFR even after controlling for several physiological (i.e., BMI, MAP, sleep quality, general health) and psychosocial variables (i.e., psychological distress, perceived stress). Consistent with a growing body of research55,68, psychological distress did not sufficiently account for the effects of ALEs during experimental pain tasks. Indeed, other studies have observed that exposure to stressful life events (i.e., trauma) may lead to a cascade of long-lasting adverse physiological consequences, including epigenetic changes in immune dysregulation54 and hyperresponsivity in the hypothalamic-pituitary-adrenal axis59. That is, there is growing support for the notion that exposure to traumatic or potentially traumatic events may lead to physiological changes that are not wholly explained by psychological impairment. The present study is consistent with this literature and suggests that ALEs may lead to a disruption of descending, cerebrospinal, inhibitory circuitry, an effect that is at least partially independent of psychological distress/stress.
ALEs Appears to Confer Pain Risk in Native Americans and non-Hispanic Whites
The absence of a significant interaction with race suggests that ALEs may confer a similar level of chronic pain risk for NAs and NHWs. In other words, racial/ethnic group differences between NAs and NHWs appear to neither promote nor protect from the pronociceptive phenotype associated with ALEs. Nonetheless, NAs do experience more ALEs than NHWs on average33; thus, the greater frequency of ALEs for NAs may still contribute to observed disparities in chronic pain prevalence between these groups.
Strengths and Limitations
Several strengths in the current study are noted. First, this study benefited from using statistically powerful analyses (multilevel modeling) on a large, diverse sample. Next, subjective and physiological CPM outcomes were recorded (pain ratings and NFR), which allowed the study to assess perceptual versus spinal processes. In addition, physiological and background data were collected for each subject, so that analyses were able to control for variables known to affect CPM, and variables that differed between racial/ethnic groups. However, this study faced limitations as well.
Future studies may benefit from using alternative measures of ALEs, as the LEC does not measure all aspects of ALE exposure (e.g., symptom presence, severity, chronicity, or duration) or the age at which an ALE was experienced. The age at which an ALE occurs may have an effect on its impact, as changes in epigenetic expression— especially during periods of significant neurodevelopmental plasticity— may worsen the lasting pathophysiological consequences of ALEs3,18; this process is known as biological embedding. Also, the LEC does not directly ask about other specific stressful life events that may yield similar pathophysiological consequences (e.g., parental incarceration, divorce, poverty). Thus, the range of ALEs may have been restricted due to our use of the LEC. Nonetheless, the LEC is similar to the format of the questionnaire that has been used to assess the impact of adverse childhood experiences1,13,16.
Furthermore, CPM was only completed by 253 of the 302 study participants in OK-SNAP, indicating a potential selection effect; however, very few group differences were observed between CPM completers and CPM non-completers thus tempering this concern (Table 1). It is also worthy to note that our NA participants were recruited mostly from northeastern Oklahoma. It is not clear if our results will generalize to NAs from other geographical regions. Finally, the sample was healthy and pain-free, limiting the generalizability of these findings. For instance, people with more ALEs are susceptible to health conditions that were excluded for in OK-SNAP1,16,31, such that participants included in the study may have been less prone to negative sequelae from ALEs than the general population. Thus, caution is warranted in generalizing these findings to chronic pain populations. However, longitudinal data are being collected for this sample to determine whether these variables predict chronic pain onset.
Conclusions
This study suggests ALEs impair descending inhibition of spinal nociception, and high ALE exposure may even promote descending facilitation. By contrast, inhibition of pain perception was not associated with ALE exposure. Consistent with other findings from OK-SNAP24,55,57, racial/ethnic group did not moderate these effects. These results have at least 3 important contributions: 1) they contribute to accumulating evidence that adversity promotes a pain risk phenotype that involves sensitization of spinal nociception, 2) they provide preliminary first evidence that latent spinal sensitization, an animal model of pain vulnerability, can be observed in humans, and 3) they extend our understanding of pronociceptive mechanisms in NAs.
Highlights.
Native Americans (NAs) are at higher risk for chronic pain than other ethnic groups
NAs report more adverse life events (ALEs), which are associated with pain risk
The mechanism(s) by which ALEs confer pain risk are not fully understood
ALEs were associated with impaired descending inhibition of spinal nociception
The effect if ALEs was not moderated by ethnicity
Acknowledgements
The authors would like to thank and vette G ereca, Heather B. Coleman, Kathryn A. Thompson, Jessica M. Fisher, Samuel P. Herbig, Ky’Lee B. Barnoski, Garrett Newsom, and Lucinda Chee for their help with data collection, as well as Dr. John M. Chaney for his consultation on the project.
Disclosures:
This research was supported by the National Institute on Minority Health and Health Disparities of the National Institute of Health under Award Number R01MD007807. Edward Lannon was supported by a National Science Foundation Graduate Research Fellowship Program and is currently funded by a T32 from the National Institute On Drug Abuse (T32DA035165). The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health, National Science Foundation, Indian Health Service, or the Cherokee Nation. Aspects of this research have been presented at the International Association for the 2020 American Psychosomatic Society conference. The authors report no conflicts of interest. The authors report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH: The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Psychiatry Clin Neurosci Springer; 256:174–86, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K: Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–84, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Berens AE, Jensen SKG, Nelson CA 3rd: Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med [Internet] BioMed Central; 15:135–35, 2017. Available from: https://pubmed.ncbi.nlm.nih.gov/28724431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM: The development of a clinician-administered PTSD scale. J Trauma Stress [Internet] John Wiley & Sons, Ltd; 8:75–90, 1995. [cited 2020 Apr 30]. Available from: 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- 5.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ: The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Res 28:193–213, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB: Ethnic differences in diffuse noxious inhibitory controls. J Pain 9:759–66, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan CW, Dallaire M: Subjective pain sensation is linearly correlated with the flexion reflex in man. Brain Res 479:145–50, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Kamarck T, Mermelstein R: Perceived stress scale. Meas Stress Guide Health Soc Sci , 1994. [PubMed] [Google Scholar]
- 9.Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf J, Mogil J, Storm D: Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science American Association for the Advancement of Science; 341:1394–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danziger N, Fournier E, Bouhassira D, Michaud D, De Broucker T, Santarcangelo E, Carli G, Chertock L, Willer JC: Different strategies of modulation can be operative during hypnotic analgesia: a neurophysiological study. Pain 75:85–92, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Derogatis LR: SCL-90-R : symptom checklist-90-R : administration, scoring & procedures manual. [Minneapolis, Minn.]: [National Computer Systems, Inc.]; 1994. [Google Scholar]
- 12.Dhondt E, Van Oosterwijck S, Coppieters I, Danneels L, Van Oosterwijck J: Effects of Conditioned Pain Modulation on the Nociceptive Flexion Reflex in Healthy People. Clin J Pain Wolters Kluwer; 35:794–807, 2019. [DOI] [PubMed] [Google Scholar]
- 13.Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH: Childhood Abuse, Household Dysfunction, and the Risk of Attempted Suicide Throughout the Life SpanFindings From the Adverse Childhood Experiences Study. JAMA [Internet] 286:3089–96, 2001. [cited 2020 Oct 11]. Available from: 10.1001/jama.286.24.3089 [DOI] [PubMed] [Google Scholar]
- 14.Emery CF, France CR, Harris J, Norman G, Vanarsdalen C: Effects of progressive muscle relaxation training on nociceptive flexion reflex threshold in healthy young adults: a randomized trial. Pain [Internet] 138:375–9, 2008. Available from: http://0-search.ebscohost.com.library.utulsa.edu/login.aspx?direct=true&db=cmedm&AN=18291584&site=ehost-live [DOI] [PubMed] [Google Scholar]
- 15.Emery CF, Keefe FJ, France CR, Affleck G, Waters S, Fondow MD, McKee DC, France JL, Hackshaw KV, Caldwell DS, Stainbrook D: Effects of a brief coping skills training intervention on nociceptive flexion reflex threshold in patients having osteoarthritic knee pain: a preliminary laboratory study of sex differences. J Pain Symptom Manage 31:262–9, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS: Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14:245–58, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Flouri E, Kallis C: Adverse life events and psychopathology and prosocial behavior in late adolescence: testing the timing, specificity, accumulation, gradient, and moderation of contextual risk. J Am Acad Child Adolesc Psychiatry Elsevier; 46:1651–9, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Fox SE, Levitt P, Nelson CA III: How the timing and quality of early experiences influence the development of brain architecture. Child Dev Wiley Online Library; 81:28–40, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray MJ, Litz BT, Hsu JL, Lombardo TW: Psychometric Properties of the Life Events Checklist. Assessment 11:330–41, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Guieu R, Blin P, Pouget J, Serratrice G: High level sportsmen and nociceptive flexion reflex of the lower limb. Can J Neurosci 19:69–71, 1992. [Google Scholar]
- 21.Hellman N, Kuhn BL, Lannon EW, Payne MF, Sturycz CA, Palit S, Shadlow JO, Rhudy JL: Emotional Modulation of Pain and Spinal Nociception in Sexual Assault Survivors. Psychosom Med , 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellman N, Sturycz CA, Lannon EW, Kuhn BL, Güereca YM, Toledo TA, Payne MF, Huber FA, Demuth M, Palit S, Shadlow JO, Rhudy JL: Conditioned Pain Modulation in Sexual Assault Survivors. J Pain [Internet] Elsevier; 20:1027–39, 2019. [cited 2020 Mar 23]. Available from: 10.1016/j.jpain.2019.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber F, Kuhn B, Lannon E, Sturycz C, Payne M, Hellman N, Toledo T, Guereca Y, DeMuth M, Palit S: Less efficient endogenous inhibition of spinal nociception predicts chronic pain onset: A prospective analysis from the Oklahoma Study of Native American Pain Risk (OK-SNAP). Am Pain Soc , 2019. [Google Scholar]
- 24.Huber FA, Kell PA, Kuhn BL, Lannon EW, Palit S, Payne MF, Hellman N, Sturycz CA, Güereca YM, Toledo TA, Demuth MJ, Hahn BJ, Shadlow JO, Rhudy JL: The Association Between Adverse Life Events, Psychological Stress, and Pain-Promoting Affect and Cognitions in Native Americans: Results from the Oklahoma Study of Native American Pain Risk. J Racial Ethn Health Disparities [Internet] , 2021. Available from: 10.1007/s40615-020-00945-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez N, Garroutte E, Kundu A, Morales L, Buchwald D: A Review of the Experience, Epidemiology, and Management of Pain among American Indian, Alaska Native, and Aboriginal Canadian Peoples. J Pain 12:511–22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice AS: Reliability of conditioned pain modulation: a systematic review. Pain Wolters Kluwer Health; 157:2410, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landau R, Kraft JC, Flint LY, Carvalho B, Richebé P, Cardoso M, Lavand’homme P, Granot M, Yarnitsky D, Cahana A: An experimental paradigm for the prediction of Post-Operative Pain (PPOP). J Vis Exp Jove [Internet] , 2010. Available from: http://0-search.ebscohost.com.library.utulsa.edu/login.aspx?direct=true&db=cmedm&AN=20107427&site=ehost-live [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larivière M, Goffaux P, Marchand S, Julien N: Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain [Internet] 23:506–10, 2007. Available from: http://0-search.ebscohost.com.library.utulsa.edu/login.aspx?direct=true&db=psyh&AN=2007-09998-006&site=ehost-liveSerge.Marchand@USherbrooke.ca [DOI] [PubMed] [Google Scholar]
- 29.Le Bars D, Willer J-C: Diffuse Noxious Inhibitory Controls (DNIC). Senses Compr Ref 5:762–73, 2010. [Google Scholar]
- 30.Le Roy C, Laboureyras E, Gavello-Baudy S, Chateauraynaud J, Laulin J-P, Simonnet G: Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization Via a NMDA-dependent process. J Pain Elsevier; 12:1069–79, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Lin JE, Neylan TC, Epel E, O’Donovan A: Associations of childhood adversity and adulthood trauma with C-reactive protein: A cross-sectional population-based study. Brain Behav Immun Elsevier; 53:105–12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manresa JAB, Fritsche R, Vuilleumier PH, Oehler C, Mørch CD, Arendt-Nielsen L, Andersen OK, Curatolo M: Is the conditioned pain modulation paradigm reliable? A test-retest assessment using the nociceptive withdrawal reflex. PloS One Public Library of Science; 9:e100241, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manson SM, Beals J, Klein SA, Croy CD, Ai-superpfp Team: Social epidemiology of trauma among 2 American Indian reservation populations. Am J Public Health 95:851–9, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martel MO, Wasan AD, Edwards RR: Sex differences in the stability of conditioned pain modulation (CPM) among patients with chronic pain. Pain Med 14:1757–68, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marvizon JC, Walwyn W, Minasyan A, Chen W, Taylor BK: Latent sensitization: a model for stress-sensitive chronic pain. Curr Protoc Neurosci [Internet] 71:9.50.1–9.50.14, 2015. Available from: https://pubmed.ncbi.nlm.nih.gov/25829356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, Kessler RC: Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry Elsevier; 52:815–30, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendell LM, Wall PD: Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature [Internet] 206:97, 1965. Available from: http://search.epnet.com/login.aspx?direct=true&db=cmedm&an=14334366 [DOI] [PubMed] [Google Scholar]
- 38.Mossey JM: Defining racial and ethnic disparities in pain management. Clin Orthop Relat Res Springer; 469:1859–70, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ossipov MH, Morimura K, Porreca F: Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 8:143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen-Felix S, Arendt-Nielsen L, Bak P, Roth D, Fischer M, Bjerring P, Zbinden AM: Analgesic effect in humans of subanaesthetic isoflurane concentrations evaluated by experimentally induced pain. Br J Anaesthesiol 75:55–60, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Piché M, Arsenault M, Rainville P: Cerebral and cerebrospinal processes underlying counterirritation analgesia. J Neurosci 29:14236–46, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preacher KJ, Curran PJ, Bauer DJ: Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat Sage Publications; Sage CA: Los Angeles, CA; 31:437–48, 2006. [Google Scholar]
- 43.Prinz U, Nutzinger DO, Schulz H, Petermann F, Braukhaus C, Andreas S: Comparative psychometric analyses of the SCL-90-R and its short versions in patients with affective disorders. BMC Psychiatry Springer; 13:104, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichling DB, Levine JD: Critical role of nociceptor plasticity in chronic pain. Trends Neurosci [Internet] 2009/09/24 ed. 32:611–8, 2009. Available from: https://pubmed.ncbi.nlm.nih.gov/19781793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO: Pain in aging community-dwelling adults in the United States: non-Hispanic whites, non-Hispanic blacks, and Hispanics. J Pain Elsevier; 8:75–84, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhudy JL, France CR: Defining the nociceptive flexion reflex (NFR) threshold in human participants: A comparison of different scoring criteria. Pain 128:244–53, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhudy JL, France CR, Bartley EJ, McCabe KM, Williams AE: Psychophysiological responses to pain: Further validation of the nociceptive flexion reflex (NFR) as a measure of nociception using multilevel modeling. Psychophysiology 46:939–48, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Rhudy JL, Hellman N, Sturycz CA, Toledo TA, Palit S: Modified Biofeedback (Conditioned Biofeedback) Promotes Antinociception by Increasing the Nociceptive Flexion Reflex Threshold and Reducing Temporal Summation of Pain: A Controlled Trial. J Pain Elsevier; , 2019. [DOI] [PubMed] [Google Scholar]
- 49.Rhudy JL, Lannon EW, Kuhn BL, Palit S, Payne MF, Sturycz CA, Hellman N, Güereca YM, Toledo TA, Coleman HB: Sensory, affective, and catastrophizing reactions to multiple stimulus modalities: results from the Oklahoma study of native American pain risk. J Pain Elsevier; 20:965–79, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhudy JL, Lannon EW, Kuhn BL, Palit S, Payne MF, Sturycz CA, Hellman N, Güereca YM, Toledo TA, Huber FA, Demuth MJ, Hahn BJ, Chaney JM, Shadlow JO: Assessing peripheral fibers, pain sensitivity, central sensitization, and descending inhibition in Native Americans: Main findings from the Oklahoma Study of Native American Pain Risk. Pain 161:388–404, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson ME, Myers CD, Sadler IJ, Riley JLI, Kvaal SA, Geisser ME: Bias Effects in Three Common Self-Report Pain Assessment Measures. Clin J Pain [Internet] 13:, 1997. Available from: https://journals.lww.com/clinicalpain/Fulltext/1997/03000/Bias__Effects_in_Three_Common_Self_Report_Pain.10.aspx [DOI] [PubMed] [Google Scholar]
- 52.Sachs-Ericsson N, Kendall-Tackett K, Hernandez A: Childhood abuse, chronic pain, and depression in the National Comorbidity Survey. Child Abuse Negl Elsevier; 31:531–47, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Slopen N, Shonkoff JP, Albert MA, Yoshikawa H, Jacobs A, Stoltz R, Williams DR: Racial disparities in child adversity in the US: Interactions with family immigration history and income. Am J Prev Med Elsevier; 50:47–56, 2016. [DOI] [PubMed] [Google Scholar]
- 54.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ: Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet [Internet] John Wiley & Sons, Ltd; 156:700–8, 2011. [cited 2020 Mar 23]. Available from: 10.1002/ajmg.b.31212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sturycz C, Hellman N, Payne M, Kuhn B, Hahn B, Lannon E, Palit S, Güereca Y, Toledo T, Shadlow J, Rhudy J: Race/ethnicity does not moderate the relationship between adverse life experiences and temporal summation of the nociceptive flexion reflex and pain: Results from the Oklahoma Study of Native American Pain Risk (OK-SNAP). J Pain 20:, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor B, Corder G: Endogenous Analgesia, Dependence, and Latent Pain Sensitization. Curr Top Behav Neurosci 20:, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toledo TA, Kuhn BL, Payne MF, Lannon EW, Palit S, Sturycz CA, Hellman N, Güereca YM, Demuth MJ, Huber F, Shadlow JO, Rhudy JL: The Effect of Pain Catastrophizing on Endogenous Inhibition of Pain and Spinal Nociception in Native Americans: Results From the Oklahoma Study of Native American Pain Risk. Ann Behav Med [Internet] , 2020. [cited 2020 Apr 4]. Available from: 10.1093/abm/kaaa004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tracey I, Mantyh PW: The cerebral signature for pain perception and its modulation. Neuron [Internet] 55:377–91, 2007. Available from: http://0-search.ebscohost.com.library.utulsa.edu/login.aspx?direct=true&db=cmedm&AN=17678852&site=ehost-live [DOI] [PubMed] [Google Scholar]
- 59.Walsh K, Galea S, Koenen KC: Mechanisms Underlying Sexual Violence Exposure and Psychosocial Sequelae: A Theoretical and Empirical Review. Clin Psychol Sci Pract [Internet] John Wiley & Sons, Ltd; 19:260–75, 2012. [cited 2020 Mar 23]. Available from: 10.1111/cpsp.12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ware JE, Snow KK, Kosinski M, Gandek B: SF-36 Health Survey manual and interpretation guide. Boston: The Health Institute New England Medical Center; 1993. [Google Scholar]
- 61.Wilcox RR: Understanding and applying basic statistical methods using R. John Wiley & Sons; 2016. [Google Scholar]
- 62.Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L: Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Pharmacother 24:119–28, 2010. [DOI] [PubMed] [Google Scholar]
- 63.Willer JC: Comparative study of perceived pain and nociceptive flexion reflex in man. Pain 3:69–80, 1977. [DOI] [PubMed] [Google Scholar]
- 64.Woolf CJ, Thompson SW: The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain Elsevier; 44:293–9, 1991. [DOI] [PubMed] [Google Scholar]
- 65.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y: Prediction of chronic post-operative pain: preoperative DNIC testing identifies patients at risk. Pain [Internet] , 2008. Available from: http://www.sciencedirect.com/science/article/pii/S0304395907006501 [DOI] [PubMed] [Google Scholar]
- 66.Yarnitsky D, Granot M, Granovsky Y: Pain modulation profile and pain therapy: between pro-and antinociception. Pain 155:663–5, 2014. [DOI] [PubMed] [Google Scholar]
- 67.You DS, Albu S, Lisenbardt H, Meagher MW: Cumulative Childhood Adversity as a Risk Factor for Common Chronic Pain Conditions in Young Adults. Pain Med Malden Mass [Internet] Oxford University Press; 20:486–94, 2019. Available from: https://pubmed.ncbi.nlm.nih.gov/30011037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.You DS, Creech SK, Meagher MW: Enhanced Area of Secondary Hyperalgesia in Women with Multiple Stressful Life Events: A Pilot Study. Pain Med [Internet] 17:1859–64, 2016. [cited 2020 Apr 24]. Available from: 10.1093/pm/pnw049 [DOI] [PubMed] [Google Scholar]
- 69.You DS, Meagher MW: Childhood adversity and pain facilitation. Psychosom Med LWW; 80:869–79, 2018. [DOI] [PubMed] [Google Scholar]
- 70.You DS, Meagher MW: Childhood Adversity and Pain Sensitization. Psychosom Med [Internet] 78:, 2016. Available from: https://journals.lww.com/psychosomaticmedicine/Fulltext/2016/11000/Childhood_Adversity_and_Pain_Sensitization.13.aspx [DOI] [PubMed] [Google Scholar]


