Abstract
Radioiodine (131I) has been used to ablate thyroid tissue not removed by surgery or to treat differentiated thyroid cancer that has metastasized to other parts of the body for the past 80 years. However, the Na+/I− symporter (NIS), which mediates active iodide uptake into thyroid follicular cells, is also expressed in several non-thyroidal tissues. This NIS expression permits 131I accumulation and radiation damage in these non-target tissues, which accounts for the adverse effects of radioiodine therapy. We will review the data regarding the expression, function, and regulation of NIS in non-thyroidal tissues. We will explain the seemingly paradoxical adverse effects induced by 131I: the self-limited gastrointestinal adverse effects in contrast to the permanent salivary dysfunction that is seen after 131I therapy. We propose that prospective studies are needed to uncover the time-course of pathological processes underlying development and progression or ultimate resolution of 131I-induced salivary ductal obstruction and nasolacrimal duct obstruction. Finally, preventive measures and early therapeutic interventions that can be applied potentially to eliminate or alleviate long-term radioiodine adverse effects will be discussed.
Keywords: Na+/I− symporter, non-thyroid tissues, radioiodine adverse effect, salivary ductal obstruction, nasolacrimal duct obstruction
Introduction
The thyroid gland is the only tissue in the body that utilizes iodine (Berman et al. 1968); radioiodine (131I) is therefore, a logical and highly specific targeted therapy for remnant ablation after total thyroidectomy and/or treatment of metastatic lesions derived from well-differentiated, follicular cell-derived thyroid cancer (Mazzaferri & Jhiang 1994). After administration, radioiodine taken into the thyroid is retained via iodine organification (Berman et al. 1968). The majority of the remaining radioiodine not taken up by thyroid tissues is eliminated in the urine within two days (Berman et al. 1968). For the first two days after radioiodine administration, notable radioiodine accumulation is also found in the salivary glands (Myant 1960) and stomach (Berman et al. 1968), which leads to the early complications of sialadenitis (Goolden et al. 1957, Van Nostrand et al. 1986) and gastrointestinal symptoms (Van Nostrand et al. 1986). Gastrointestinal symptoms typically resolve quickly, yet salivary gland dysfunction may recur and can persist in some patients (Van Nostrand et al. 1986; Clement et al. 2015). Additional adverse effects of radioiodine therapy include xerostomia, bone marrow suppression, gonadal damage, dry eye(s), nasolacrimal duct obstruction, and secondary cancer (Fard-Esfahani et al. 2014, Clement et al. 2015). While the incidence and severity of the aforementioned adverse effects are generally correlated with the cumulative radioactivity administered (Clement et al. 2015, Hollingsworth et al. 2016), factors contributing to variations in incidence and severity of radioiodine adverse effects among individual patients are yet to be delineated. Molecular cloning of the Na+/I− symporter (NIS) that mediates active iodide uptake into thyroid follicular cells (Dai et al. 1996; Smanik et al. 1996), as well as the subsequent development of NIS antibodies (Levy et al. 1997; Jhiang et al. 1998), have made it possible to investigate NIS expression, function, and regulation in non-thyroidal tissues. A better understanding of NIS expression and modulation in non-thyroid tissues may uncover the underlying pathological process of 131I-induced adverse effects and allow us to devise better strategies to prevent 131I-induced adverse effects.
NIS expression in non-thyroidal tissues
NIS expression in thyroidal and non-thyroidal tissues may be detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) or Northern blot analysis at the mRNA level, and by immunohistochemical (IHC) staining or Western blot analysis at the protein level. RT-qPCR is the most sensitive method that can detect minute quantities of NIS mRNA. However, the physiological or functional significance of such an infinitesimal detection is unclear. IHC is superior in terms of detecting NIS expression restricted to a specific cell type and in revealing plasma membrane localization of NIS, where NIS mediates radioiodine uptake (Jhiang et al. 1998, Wapnir et al. 2003). However, IHC results can be compromised by cross-reactivity to non-NIS proteins due to variations in NIS antibody specificity and stringency of antibody incubation and washing conditions. Both Northern and Western blot analyses suffer from limited detection sensitivity, but may reveal alternative spliced transcripts and post-translational modifications, respectively. For example, with those tissues composed of multiple cell types, NIS expression may not be detected by Northern or Western blot analyses, particularly if its expression is restricted to a specific cell type that comprises only a minor component of the overall tissue composition (Spitzweg et al. 1998). Taken together, RT-qPCR accompanied by IHC staining using a NIS antibody of high specificity can be informative for investigating NIS expression in non-thyroidal tissues at different developmental stages and in various pathological conditions.
According to the website https://www.proteinatlas.org/ENSG00000105641-SLC5A5/tissue, thyroid, salivary gland, and stomach are tissues with significant NIS detection at both mRNA and protein levels. The https://www.genecards.org/cgi-bin/carddisp.pl?gene=SLC5A5&keywords=SLC5A5 website indicates that RNAseq analysis has identified numerous tissues expressing NIS mRNA including blood cells, lymph nodes, brain, cortex, cerebellum, spinal cord, tibial nerve, heart, artery, skeletal muscle, small intestine, colon, adipocyte, kidney, liver, lung, spleen, stomach, esophagus, bladder, pancreas, salivary gland, adrenal gland, pituitary, breast, skin, ovary, uterus, prostate and testis. Among these tissues, NIS mRNA level was the highest in stomach, followed by thyroid, salivary glands, and cerebellum.
NIS expression in salivary gland tissues:
It was recognized that NIS is expressed in the salivary glands before NIS molecule cloning. In patients with autosomal recessive, congenital dyshormongenetic goiters, the abnormal saliva/serum iodide ratio as well as the lack of 99mTcO4− uptake in both thyroid and salivary glands were used to reveal the underlying iodide transport defect (Wolff 1983). However, the localization of NIS expression in salivary ductal cells rather than acinar cells was not discovered until the development of NIS antibodies. IHC staining demonstrates that NIS is highly expressed in the basolateral membranes of the striated ducts (Jhiang et al. 1998, Fig 1a), and is weakly expressed in intercalated and excretory ductal cells (La Perle et al. 2013). Accordingly, 131I is concentrated from blood circulation into saliva in the ductal lumen mainly from salivary striated ductal cells. In sialoadenitis, NIS expression in striated ducts has been demonstrated to be heterogeneously decreased or absent (La Perle et al. 2013, Fig 1b), suggesting that inflammation decreases NIS expression in striated ductal cells. This pathologic process translates well with clinical observations of decreased uptake and impaired clearance of 99mTcO4− that is seen after RAI therapy (Mandel & Mandel, 2003, Fig 1c). The 131I-induced inflammatory infiltrate causes increased periductal pressure with duct constriction and sialadenitis, which usually manifests as bilateral parotid swelling and pain with decreased salivary flow (Goolden et al. 1957, Mandel & Mandel 2003). For some patients, resolution of the inflammatory process and improvement in symptoms occurs within days, while other patients experience subclinical inflammation with delayed symptoms (Mandel & Mandel 2003). The latter group is more likely to suffer from recurrent/persistent sialadenitis that eventually leads to permanent xerostomia with progressive susceptibility to dental caries and periodontal disease (Mandel & Mandel 2003). It is important to note that both acute periductal inflammation and chronic sclerosis in the salivary glands may be seen in thyroid cancer patients who have been treated with131I therapy.
Figure 1:
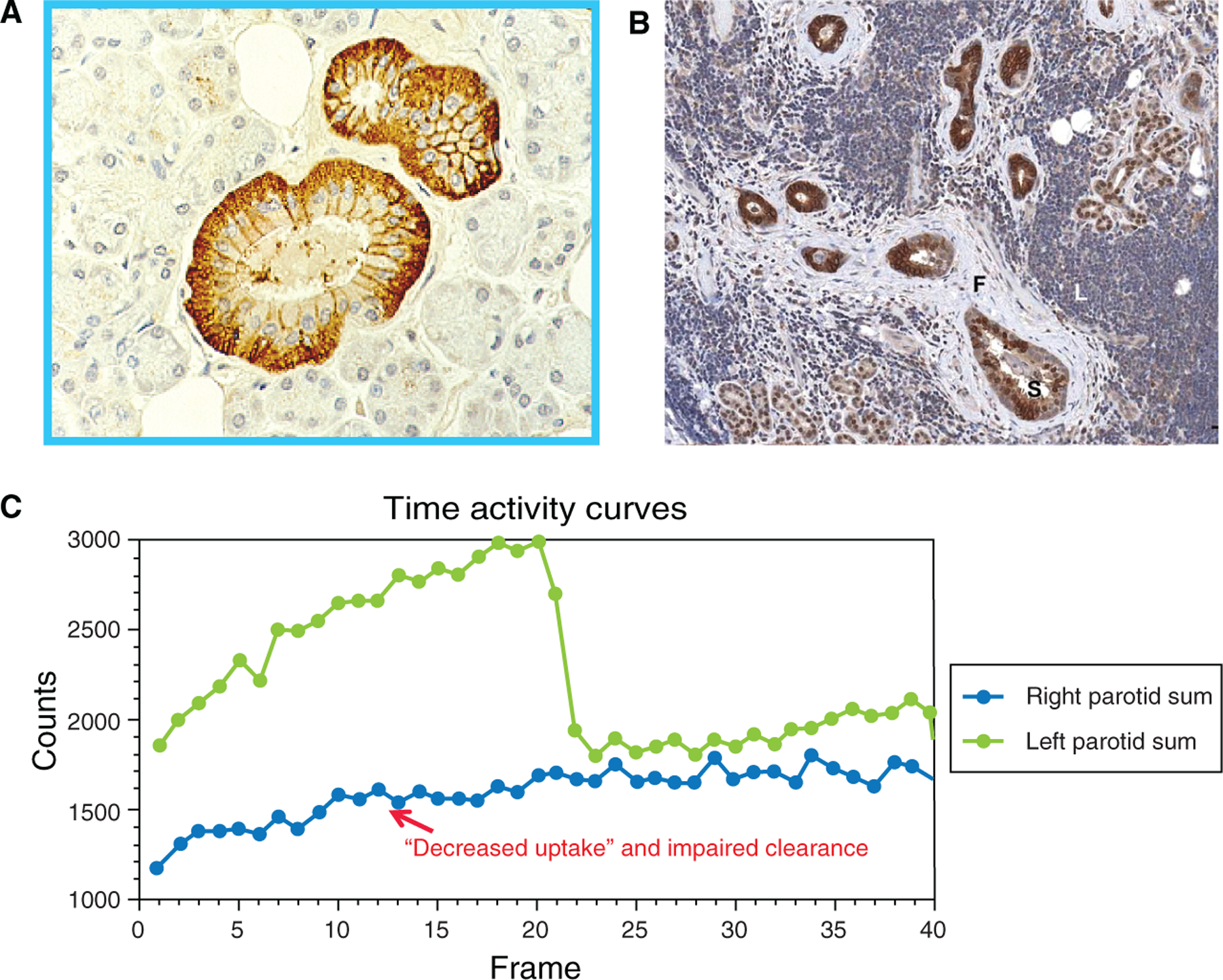
NIS expression and function in normal and inflamed salivary glands. (a) NIS immunostaining (brown color) is detected in the basolateral membranes of the normal striated ducts; (b) NIS immunostaining (brown color) is heterogeneously decreased or absent in inflamed striated ductal cells during sialoadenitis ; and (c) Technetium-99m pertechnetate (TPT) time activity graph shows normal uptake and secretory clearance of the TPT radioisotope in the functioning left parotid (curve of green color). The inflamed right parotid (curve of blue color) had minimal uptake and no secretory clearance, indicating reduced salivary NIS expression and salivary ductal obstruction, respectively. Lemon candy was given at frame 20 to stimulate secretory clearance.
Our study of 123I single-photon-emission computed tomography/anatomic imaging of computed tomography (SPECT/CT) images from 50 thyroid cancer patients prior to radioiodine therapy showed that the ratio of 123I cumulative radioactivity at 24 hr after administration between parotid and submandibular glands is 2.38±0.19 (La Perle et al. 2013). However, the corresponding ratio of 123I accumulation normalized by volume of interest is 1.19±0.06. The percentage of NIS-positive striated ductal cells in submandibular salivary glands was statistically greater than in parotid salivary glands, suggesting a higher clearance rate of 131I in submandibular than parotid salivary glands. Indeed, approximately 65–70% of saliva in the oral cavity is produced by the submandibular glands, even though they are much smaller than the parotid glands. These findings are consistent with the recent study showing that damage to the parotid glands is more common than damage to submandibular salivary tissue among radioiodine treated patients when examined by neck ultrasound (Horvath et al. 2020).
We also demonstrated that most ductal salivary gland tumors do not express NIS. The exception is Warthin’s tumors of striated duct origin which exhibit consistent and intense NIS staining and avid radioactive iodine uptake (La Perle et al. 2013). Taken together, NIS expression in salivary ductal cells appears to be mainly modulated by ductal cell differentiation and NIS expression is retained in salivary tumors derived from striated ducts.
NIS expression in gastrointestinal tissues:
In the gastrointestinal tract, NIS is expressed in the basolateral membrane of gastric mucin-producing cells (Wapnir et al. 2003). However, in the small intestine, NIS is functionally expressed on the apical surface of enterocytes (Nicola et al. 2009), suggesting that iodide from food sources as well as iodide concentrated in gastric juice is likely to be absorbed and reabsorbed, respectively, via NIS at apical membrane of the small intestine into the blood circulation. The gastrointestinal epithelium is a constitutively developing tissue, constantly differentiating from stem cells in a progenitor pool throughout the life of the organism (de Santa Barbara et al. 2003). The high turnover rate of enterocytes and the gastric mucosa may explain why gastrointestinal symptoms generally resolve quickly after radioiodine therapy, as injured gastric mucosa and enterocytes are quickly replenished with newly differentiated gastric mucosa and enterocytes.
The pathogenesis of gastric cancer has been associated with abnormal patterns of gastric differentiation and with chronic tissue injury (Mills & Shivdasani 2010). Altorjay et al. (2007) found that NIS staining was observed in gastric mucin-producing cells in gastritis, both in the presence and absence of Helicobacter pylori. However, NIS expression was absent in gastric cancer, independent of its histological type. They also observed that focal NIS staining in the direct vicinity of gastric tumors increased gradually and became linear with escalating distance from the tumor, suggesting that loss of NIS expression precedes microscopically identifiable morphological changes. Recently, Shiozaki et al. (2019) examined NIS expression by IHC staining in 145 primary gastric cancer samples, and showed that 37% of the samples were NIS-positive yet NIS immunostaining was detected in the cytoplasm and non-polarized cell membrane. They further demonstrated that strong NIS expression was associated with a poor prognosis in gastric cancer. A separate study reported a similar prognostic role of NIS expression in gastric cancers (Mishra & Shrivastava, 2020).
In the normal gastrointestinal tract, NIS expression is restricted to terminal differentiated cells with a rapid turnover rate. For NIS-negative gastric cancer, one could interpret that malignant transformation suppresses NIS expression or malignant transformation occurs in NIS-negative gastric precursor cells at a less-differentiated stage. For NIS-positive gastric cancer, it is possible that signaling nodes and/or transcription factors that initiate NIS expression in gastric mucosa are aberrantly activated during malignant transformation or malignant transformation occurs after precursor cells are committed to differentiated gastric mucosa.
NIS expression in breast tissues:
NIS expression and radioiodide uptake in breast tissues are at the highest levels during active lactation (Cho et al. 2000, Tazebay et al. 2000). NIS proteins are detected at the basolateral membrane of breast ductal epithelium; iodide is transported from the mother’s blood circulation to the ductal lumen where milk is accumulated. This process is critical to ensure sufficient iodide is provided to the newborn for thyroid hormone synthesis. The NIS protein level in breast tissue declines significantly during a post-lactational involution process until it is at an undetectable level in non-lactating normal breast tissues (Cho et al. 2000).
Incidental findings of radioiodine uptake in non-breastfeeding women have been reported (Hammami et al. 1996), yet the underlying reason(s) remains to be elucidated. Among pathological breast tissues, our unpublished data and others (Kilbane et al. 2000, Berger et al. 2006, Ryan et al. 2011) detected high level of NIS expression at the basolateral membrane of fibroadenoma, a benign breast tumor which may change in size during pregnancy, breastfeeding, or while taking hormone replacement therapy. Fibroadenoma is one of the most common benign tumors of the breast in women under 30 years of age. NIS-positive fibroadenomas may account for some of the incidental findings of radioiodine uptake in non-breastfeeding women (Hammami & Bakheet 1996, Kim et al. 2017)
Based on communication with our previous collaborator, Dr. Juha Kononen at National Human Genome Research Institute, our analysis of NIS IHC staining in tissue microarray composed of 650 breast tumors showed that 17% of 650 breast tumors had strong plasma membrane NIS staining that did not correlate with the presence or absence of estrogen receptor, progesterone receptor, nor human epidermal growth factor receptor 2 (HER2) (unpublished data). In contrast, Wapnir et al. (2003) reported a significant number of breast cancers with cytoplasmic NIS immunostaining. It is possible that detectability of plasma membrane NIS and cytoplasmic NIS is epitope-dependent and can vary among NIS antibodies. However, the identity of cytoplasmic NIS immunostaining in both thyroid and breast cancers had been questioned (Peyrottes et al. 2009). If possible, an additional method of NIS detection, such as RT-qPCR with an acceptable Ct value (cycle threshold), needs to be applied to confirm NIS identity in breast cancers with exclusively cytoplasmic NIS staining. Of interest, Smith et al. (2009 & 2013) reported that inhibition of PTTG binding factor (PBF) increases NIS plasma membrane localization with improved radioiodine uptake in thyroid and other tumors. Recently, Fletcher et al. (2020) reported that an inhibitor for valosin-containing protein (VCP) significantly increases NIS cell surface localization and results in a marked increase in radioiodine uptake activity in both mouse and human thyroid models.
NIS expression in lacrimal sac and nasolacrimal duct:
Nasolacrimal duct obstruction has been associated with high dose radioiodine therapy (Kloos et al. 2002, Ali 2016, da Fonseca et al. 2016). Morgenstern et al. (2005) showed that NIS mRNA was detected in normal lacrimal sacs via RT-PCR and NIS immunostaining was detected at the basolateral membrane of the stratified columnar epithelial cells in the lacrimal sac (Fig 2a) and nasolacrimal duct (Fig 2b). In contrast, NIS immunostaining was not detected in the lacrimal glands (Fig 2c), Wolfring or Krause glands, conjunctiva, canaliculus, nor nasal mucosa. In lacrimal sacs removed from radioiodine-treated patients, NIS-expressing cells were absent and marked fibrosis was noted at sites of the stratified columnar epithelial cells (Morgenstern et al. 2005). Morgenstern et al. (2005) concluded that 131I accumulation at the lacrimal sac and nasolacrimal duct is responsible for the development of nasolacrimal drainage system obstruction, as patients diagnosed with nasolacrimal obstruction typically do not demonstrate signs of proximal canalicular stenosis. Instead, the association of dry eye with radioiodine therapy could be attributed to increasing risk of eye infection or inflammation from improper tear drainage (Morgenstern et al. 2005). As illustrated in Fig 2d, tears produced by the lacrimal glands is secreted into excretory ducts which empty into the superior conjunctival fornix and then spread over to the cornea by the process of blinking. However, NIS expression in the excretory ducts has not been examined. If NIS is expressed in the excretory ducts, it would explain the detection of radioactive tears after 131I administration and 131I-induced excretory duct obstruction may lead to dry eyes. Since dry eyes can be triggered by multiple factors, such as aging, medications, allergy, menopause, etc., the role of 131I in the development of dry eyes in 131I-treated patients is uncertain.
Figure 2:
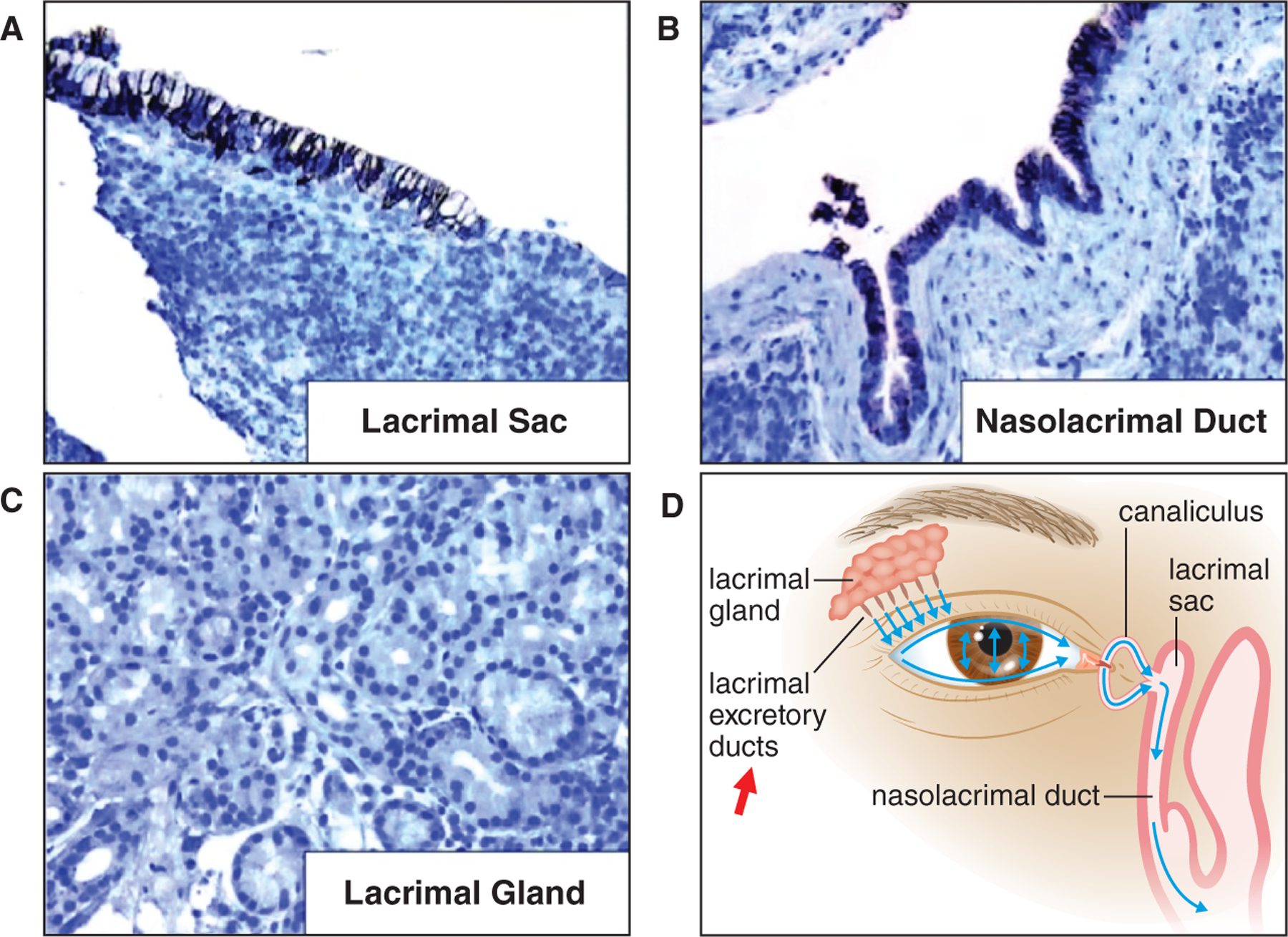
NIS expression in normal lacrimal drainage tissues. NIS immunostaining (brown color) is detected at the basolateral membrane of the stratified columnar epithelial cells in (a) the lacrimal sac, and (b) the nasolacrimal duct. NIS immunostaining is absent in (c) lacrimal glands. NIS expression was not investigated in (d) lacrimal excretory ducts (red arrow).
NIS expression in other non-thyroid tissues:
Using a very sensitive detection method, RT-PCR followed by Southern hybridization, Spitzweg et al. (1998) reported that NIS expression was detected in pituitary gland, pancreas, testis, mammary gland, gastric mucosa, prostate and ovary, adrenal gland, heart, thymus, and lung. Wapnir et al. (2003) reported apical membrane NIS staining in placenta cytotrophoblasts and renal distal and collecting tubules, as well as NIS intracellular immunostaining in many normal tissues including bladder mucosa, colonic mucosal cells, bile duct, etc. However, the implications of cytoplasmic NIS immunostaining without concomitant detection of equivalent NIS mRNA levels remain speculative. Likewise, the physiological and clinical significance of extremely low tissue expression of NIS mRNA without unequivocal plasma membrane NIS immunostaining within a specific cell type remains uncertain.
NIS function in non-thyroidal tissues
NIS function in the thyroid gland is well defined; NIS mediates active iodide uptake against its concentration gradient from the blood circulation into thyroid follicular cells for thyroid hormone synthesis. Since iodine is a rare but essential element for thyroid hormone synthesis, the reabsorption of iodide is a critical step for maintaining a circulating pool. The iodine that has not yet been retained by the thyroid gland can be re-absorbed into the blood circulation and may be eventually taken up via NIS into thyroid follicular cells. Indeed, iodide is taken from blood circulation by NIS at salivary ducts and gastric mucosa into saliva and gastric juice, respectively. Iodide containing saliva and gastric fluids that move into the small intestine are then resorbed into bloodstream via NIS at the apical surface of enterocytes to complete entero-thyroid circulation of iodide (Josefsson et al. 2002). Taken together, NIS function in the salivary gland and gastrointestinal tract plays an important role in iodide conservation for thyroid hormone synthesis.
In the placenta, NIS in the apical membrane of cytotrophoblasts mediates iodide transport from maternal circulation to fetal circulation for thyroid hormone synthesis by the fetus. In lactating breast tissue, NIS in the basolateral membrane transports iodide from maternal blood circulation to maternal milk so that the newborn has sufficient iodine for his/her own thyroid hormone synthesis. Maternal iodine deficiency has been reported to have negative impact on infant’s thyroid function (Dei-Tutu et al. 2020).
Additionally, it has been proposed that a function of NIS in non-thyroidal tissues is to provide antimicrobial protection and healing (Venturi & Venturi 2009). However, the clinical impact of this possible role is unclear. The clinical phenotype for ITD patients with low or absent radioiodide uptake by the thyroid and other NIS-expressing organs has been limited to consequence of thyroid hormone deficiency only. To date, there is no evidence to support increased susceptibility to oral or gastrointestinal infections nor pathological dysfunction in NIS-expressing non-thyroid organs for ITD patients. Similarly, the phenotype of NIS-knock out mice is characterized by hypothyroidism, with reduced thyroid hormone and increased thyroid stimulating hormone levels (Ferrandino et al. 2017). Taken together, NIS function appears not to be critical in NIS-expressing non-thyroidal tissues beyond iodide conservation for thyroid hormone synthesis.
NIS regulation in non-thyroidal tissues
Among NIS-expressing tissues, NIS expression is generally elevated and restricted to highly functioning differentiated cells. In the thyroid, NIS is most prominent in thyrocytes of patients with Graves’ disease (Jhiang et al. 1998), when thyrocytes are actively synthesizing and releasing thyroid hormones. In breast tissue, NIS is most abundant during lactation (Cho et al. 2000, Wapnir et al. 2003) when providing iodine to the infant. In the stomach, NIS is restricted to the gastric mucosa (Wapnir et al. 2003) where gastric juice is secreted to prepare for food digestion. In salivary glands, NIS is most prominent in striated ducts (Jhiang et al. 1998, La Perle et al. 2013), which plays a major role in the modification of the ion composition of the primary saliva produced by acini. Taken together, it is reasonable to postulate that NIS modulation is tissue-specific and is likely modulated by factor(s) driving lineage commitment to the highly functioning differentiated cells.
Among NIS-expressing non-thyroid tissues, breast is the most extensively studied tissue for NIS modulation. Tazebay et al. (2000) showed that subcutaneous injection of 1 IU oxytocin for 3 consecutive days significantly increased NIS expression and radioiodide uptake in the mammary gland of nubile mice. For ovariectomized nubile mice, the combination of oxytocin, prolactin and 17-β-estradiol stimulated maximal NIS induction. Our previous study demonstrates that radioiodide uptake in the lactating mammary glands of rats is partially decreased by a selective oxytocin antagonist or bromocriptine that inhibits prolactin release (Cho et al. 2000). In MCF-7 cells, an estrogen receptor positive human breast cancer cell line, all-trans retinoic acid (ATRA) induces NIS expression and radioiodide uptake (Kogai et al. 2000). ATRA-induced NIS expression can be further enhanced by co-treatment with dexamethasone (Kogai et al. 2005, Unterholzner et al. 2006). Additional factors that modulate NIS expression in ATRA+Dexamethasone treated MCF-7 cells include miR-339–5p (Lakshmanan et al. 2015), an MEK inhibitor (Zhang et al. 2013), a staurosporine-related protein kinase inhibitor, KT5823 (Beyer et al. 2011), and a potent activator of pregnane-X-receptor, carbamazepine (Willhauck et al. 2011). A histone deacetylase inhibitor was reported to increase NIS mRNA in a variety of established cell lines (Liu & Xing 2012, Kelkar et al. 2016). Examining NIS expression in mammary gland tumors from various genetically engineered mouse models, cAMP or PI3K activation appears to associate with NIS up-regulation (Knostman et al. 2004).
In contrast to breast, selective modulation of NIS expression in other non-thyroid tissues has not been investigated except that NIS expression is driven by lineage commitment to the highly functioning differentiated cells. La Perle et al. (2013) reported that inflammation decreases NIS expression in salivary striated ductal cells. It will be of interest to investigate if NIS expression in other non-thyroid tissues is also reduced by acute or chronic inflammation.
Similar to the thyroid, NIS expression is likely modulated by iodide concentration in non-thyroid tissues that facilitate dietary iodide absorption or iodide conservation for thyroid hormone synthesis. Indeed, iodide excess downregulates NIS protein levels and NIS-mediated iodide absorption in rat’s small intestine as well as in small intestinal cell line (Nicola et al. 2009 & 2012). This finding emphasizes the important role that dietary iodine plays in the enterocyte physiology, as it controls its own NIS-mediated absorption and, by extension, manipulating the supply of iodine to the body (Nicola et al. 2015). NIS in gastric mucosa and salivary ductal cells mediates iodide uptake from blood circulation into epithelial cells such that iodide not taken by thyroid can be released into gastric juice and saliva, respectively. Iodide return to the gastrointestinal lumen can then be reabsorbed via NIS in small intestines. It would be of interest to investigate if NIS expression is modulated by iodide concentration in other non-thyroid tissues.
Impact of NIS expression in non-thyroidal tissues on thyroid cancer radioiodine therapy
NIS expression in non-thyroid tissues makes these tissues susceptible to radiation damage during radioiodine therapy for thyroid cancer patients. Thus, transiently silencing/reducing NIS expression or inhibiting NIS function in non-thyroid tissues for 24–48 hours after 131I administration would prevent or alleviate 131I-induced adverse effects. However, the proper mechanism to achieve transient, targeted, and restricted delivery of small interfering RNA (siRNA) or an NIS inhibitor to target cells without compromising the 131I therapeutic efficacy on thyroid cancer remains to be elucidated. Nevertheless, identifying the specific cell type in which NIS is abundantly expressed in non-thyroidal tissues has provided a better understanding of the development and progression of 131I-induced adverse effects. For example, dry mouth is likely a secondary effect of salivary duct obstruction, as NIS expression is restricted to ductal cells. Indeed, loss of acinar cells and salivary gland atrophy occurs quickly after ligation of salivary excretory ducts, however, acinar cells can be regenerated after ductal ligation is removed in an experimental animal model (Cotroneo et al. 2010, Weng et al. 2018). Accordingly, if salivary duct obstruction could be recognized and relieved in a timely manner, not only could the discomfort of full-blown salivary gland swelling and pain be alleviated, 131I-induced dry mouth could also potentially be prevented. Uncovering the pathological process and intervention at an early, potentially reversible stage is an alternate therapeutic intervention to reduce the severity of 131I-induced adverse effects. Taken together, we are now in a better position to devise more effective strategies to alleviate the adverse effects of radioiodine therapy and thereby improve the quality of life for thyroid cancer survivors.
Among radioiodine adverse effects, salivary and lacrimal complications are relatively common and have a major negative impact on the quality of life for thyroid cancer survivors. As stated in a recent joint multidisciplinary clinical consensus statement of otolaryngology, ophthalmology, nuclear medicine and endocrinology (Singer et al. 2020), there is a need to better understand the true incidence, optimal treatments and importantly prevention of salivary gland and lacrimal dysfunction elicited by radioiodine therapy. Since salivary duct and nasolacrimal drainage ductal system are relatively accessible for local delivery of reagents, the incidence and severity of these adverse effects can be significantly reduced with collective effort from our community in pursuing personalized preventive measure and therapeutic intervention
Radioiodine-induced salivary duct obstruction:
Many thyroid cancer survivors suffer from life-long morbidity of radioiodine-induced salivary gland dysfunction, including recurrent sialadenitis, persistent xerostomia, and progressive susceptibility to dental caries and periodontal disease. The prevalence of chronic symptomatic salivary dysfunction (16–54%) and abnormal salivary gland scintigraphic findings (37%–72%) vary among studies (Clement et al. 2015). Various prevention strategies for 131I-induced salivary gland (SG) dysfunction have been implemented, including salivary stimulation, radioprotection, and anti-inflammatory medications. Currently, the most common practice includes adequate hydration and initiation of salivary stimulation, such as sucking sour candy or lemon juice, for the first 48 hrs after 131I treatment. However, the efficacy and optimal timing of salivary stimulation has not been systematically evaluated and currently remains controversial (Nakada et al. 2005, Jentzen et al. 2010, Van Nostrand et al. 2010). The fact that chronic SG dysfunction is a common adverse effect among patients who have received high doses of 131I indicates that novel approaches with better efficacy need to be developed.
Since salivary accumulation of 131I is absent in NIS knock-out mice and in patients with a congenital iodide transport defect who carry NIS loss-of-function mutation, one could hypothesize that 131I-induced salivary gland damage can be avoided if salivary NIS expression/activity is temporarily inhibited during the time period when blood circulating 131I is high. The salivary ductal system, the primary injury site of 131I, is accessible for local delivery of NIS silencing or NIS inhibition reagent. However, the challenge is to ensure that NIS silencing or NIS inhibition will be retained solely within the salivary glands without entering blood circulation to compromise radioiodine therapeutic efficacy for the thyroid cancer. For 131I-treated patients who suffer from radioiodine-induced sialadenitis with ductal stenosis and mucus plugs, sialendoscopy with dilatation, saline irrigation, and steroid injection have resulted in clinical improvement of symptoms for 75–100% of patients (Bhayani et al. 2015, Cung et al. 2017). Sialendoscopy interventions at an early stage of sialadenitis have a better outcome than patients who already have developed chronic salivary gland dysfunction. Of interest, sialendoscopy also shown to be effective in salivary gland protection by reducing the inflammatory effects of PSMA targeting alpha therapy in prostate cancer patients (Rathke et al. 2019). Since not all patients are at risk to develop 131I-induced chronic salivary dysfunction, it is important to identify which patients will benefit from preventive measures prior to radioiodine therapy and which patients will require sialendoscopy intervention at an early stage of sialadenitis.
The incidence and severity of 131I-induced salivary dysfunction in thyroid cancer survivors increases with cumulative 131I radioactivity received (Clement et al. 2015). In addition to cumulative 131I radioactivity, we found that female gender, previous incidence of sialadenitis, and personal history of autoimmune disease associated with Sjogren’s syndrome are risk factors for the development of sialadenitis after 131I therapy (Hollingsworth et al. 2016). Excluding patients with pre-excising salivary gland diseases, Horvath et al. (2020) reported ~25% patients had anatomical damage in salivary gland(s) revealed by neck ultrasonography conducted prior to and after 131I therapy. They found that the risk of sialadenitis was significantly correlated with 131I radioactivity administered and female gender. Salivary gland atrophy was found in 21%, 47%, 78% of patients receiving 100 mCI, 150 mci, and >= 200 mCi radioactivity, respectively. Therefore, the challenge is to identify individual patients who are susceptible to 131I-induced chronic salivary dysfunction for prevention or early intervention.
The underlying pathobiological process associated with transient, recurrent, and chronic sialadenitis induced by 131I have not been studied. This can be accomplished by conducting prospective studies investigating changes in answers to subjective questions related to the health status of the salivary gland (Le Roux et al. 2020), along with changes in objective evaluation of saliva composition (Klein Hesselink et al. 2016), such as saliva electrolyte and molecule profiles, scintigraphic functional imaging (Jeong et al. 2013), and neck ultrasound imaging (Horvath et al. 2020), prior to radioiodine therapy and at follow-up and then correlate these findings with the development of sialadenitis and/or dry mouth. By doing so, each patient can serve as their own control to identify the magnitude of changes in saliva composition and imaging characteristics by scintigraphy and/or ultrasonic images that lead to transient, recurrent, or chronic sialadenitis induced by 131I. If we could use quantifiable saliva biomarkers or changes in salivary gland anatomic/functional images to monitor the onset and progression of salivary gland dysfunction induced by 131I, intervention strategies can be applied to patients in a timely manner. We can investigate whether salivary biomarkers detected in the early stages of salivary dysfunction induced by 131I are predictive of future progression to chronic disease. We may also be able to distinguish which patients are more susceptible to developing life-long 131I-induced salivary gland dysfunction. The acquired insight on mechanisms underlying the development and progression of salivary gland dysfunction induced by 131I can lead to alternative preventive measures and/or therapeutic interventions.
Radioiodine-induced nasolacrimal duct obstruction:
The incidence of 131I-induced nasolacrimal duct obstruction is 2–18% and typically occurs months after radioiodine therapy (Ali 2016). Preventive measures to reduce 131I-induced nasolacrimal duct obstruction have not been proposed. A recent study demonstrated that a patient with pre-existing asymptomatic and incomplete nasolacrimal duct obstruction was able to be identified on the pre-therapy 123I whole-body scan and SPECT/CT and further confirmed in post-therapy 131I whole-body scan and SPECT/CT of head and neck (Kay et al. 2020). Thus it is reasonable to assume that retrospective studies examining the pre-therapy and post-therapy whole body scans along with SPECT/CT of head and neck from patients with multiple radioiodine treatments may help determine the prevalence of pre-existing nasolacrimal duct obstruction and 131I-induced asymptomatic nasolacrimal duct obstruction among thyroid cancer patients. It would be possible to investigate whether additional 131I therapy further exacerbates the severity of asymptomatic nasolacrimal duct obstruction, leading to symptomatic disease. With a better understanding of the development and progression of 131I-induced nasolacrimal duct obstruction, we will be poised to devise appropriate prevention measures and intervention strategies.
Conclusion
NIS expression in non-thyroidal tissues allows radioiodine accumulation in these tissues after radioiodine administration. Consequently, these tissues are also vulnerable to radiation damage in 131I-treated thyroid cancer patients. NIS expression in non-thyroidal tissues is generally restricted to or enriched in functioning differentiated cells and tissue damage elicited by radioiodine are caused by direct or indirect radiation-induced inflammation. The severity and duration of adverse effects in these non-thyroidal tissues depends on the extent and duration of radioiodine accumulation, the tissue radiosensitivity, the turnover rate of radioiodine injured cells, and the individual tissue repair capacity. While NIS silencing in non-thyroidal tissues prior to 131I administration can eliminate 131I-induced adverse effects, additional technology is needed to ensure restricted delivery of NIS siRNA within targeted cells. Fully uncovering the pathological processes underlying 131I-induced damage is critical to devising preventive measures and/or early therapeutic interventions that will facilitate complete resolution of radiation-induced inflammation. Finally, the incidence and severity of 131I-induced adverse effects vary among individual patients. Risk factors in developing long-term salivary dysfunction or nasolacrimal duct obstruction other than cumulative radioactivity administered need to be identified to aid in the clinical management and decision-making.
Acknowledgments
Funding
This work was, in part, supported by National Institute of Health (NIH) Grant P50CA168505 (Project 3 leader: SM Jhiang, PI: MD Ringel) and the Ohio State University Comprehensive Cancer Center Bridge Fund.
Footnotes
Declaration of interest
SJ and JS declare no competing interests.
References
- Ali MJ 2016Iodine-131 Therapy and Nasolacrimal Duct Obstructions: What We Know and What We Need to Know. Ophthalmic Plast Reconstr Surg 32243–8. [DOI] [PubMed] [Google Scholar]
- Altorjay A, Dohán O, Szilágyi A, Paroder M, Wapnir IL & Carrasco N 2007. Expression of the Na+/I− symporter (NIS) is markedly decreased or absent in gastric cancer and intestinal metaplastic mucosa of Barrett esophagus. BMC Cancer 7 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Unterholzner S, Diebold J, Knesewitsch P, Hahn K & Spitzweg C. 2006. Mammary radioiodine accumulation due to functional sodium iodide symporter expression in a benign fibroadenoma. Biochem Biophys Res Commun 349 1258–63. [DOI] [PubMed] [Google Scholar]
- Berman M, Hoff E, Barandes M, Becker DB, Sonenberg M, Benua R & Koutras DA. 1968. Iodine kinetics in man--a model. J Clin Endocrinol Metab 28 1–14. [DOI] [PubMed] [Google Scholar]
- Beyer S, Lakshmanan A, Liu YY, Zhang X, Wapnir I, Smolenski A, & Jhiang S. 2011. KT5823 differentially modulates sodium iodide symporter expression, activity, and glycosylation between thyroid and breast cancer cells. Endocrinology 152 782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhayani MK, Acharya V, Kongkiatkamon S, Farah S, Roberts DB, Sterba J, Chambers MS & Lai SY. 2015. Sialendoscopy for Patients with Radioiodine-Induced Sialadenitis and Xerostomia. Thyroid 25 834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Léveillé R, Kao R, Rousset B, Parlow AF, Burak WE Jr, Mazzaferri EL & Jhiang SM. 2000. Hormonal regulation of radioiodide uptake activity and Na+/I− symporter expression in mammary glands. J Clin Endocrinol Metab 85 2936–43. [DOI] [PubMed] [Google Scholar]
- Clement SC, Peeters RP, Ronckers CM, Links TP, van den Heuvel-Eibrink MM, Nieveen van Dijkum EJ, van Rijn RR, van der Pal HJ, Neggers SJ, Kremer LC et al. 2015Intermediate and long-term adverse effects of radioiodine therapy for differentiated thyroid carcinoma--a systematic review. Cancer Treat Rev 41925–34. [DOI] [PubMed] [Google Scholar]
- Cotroneo E, Proctor GB & Carpenter GH. 2010. Regeneration of acinar cells following ligation of rat submandibular gland retraces the embryonic-perinatal pathway of cytodifferentiation. Differentiation 79 120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cung TD, Lai W, Svider PF, Hanba C, Samantray J, Folbe AJ, Shkoukani M & Raza SN. 2017. Sialendoscopy in the Management of Radioiodine Induced Sialadenitis: A Systematic Review. Ann Otol Rhinol Laryngol 126 768–773. [DOI] [PubMed] [Google Scholar]
- da Fonseca FL, Yamanaka PK, Kato JM & Matayoshi S. 2016. Lacrimal System Obstruction After Radioiodine Therapy in Differentiated Thyroid Carcinomas: A Prospective Comparative Study. Thyroid 26 1761–1767. [DOI] [PubMed] [Google Scholar]
- Dai G, Levy O & Carrasco N. 1996. Cloning and characterization of the thyroid iodide transporter. Nature 379 458–60. [DOI] [PubMed] [Google Scholar]
- de Santa Barbara P, van den Brink GR & Roberts DJ. 2003. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci 60 1322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dei-Tutu SA, Manful A, Heimburger DC, Malechi H, Moore DJ, Oppong SA, Russell WE & Aliyu MH. 2020. Correlating maternal iodine status with neonatal thyroid function in two hospital populations in Ghana: a multicenter cross-sectional pilot study. BMC Pediatr 20 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fard-Esfahani A, Emami-Ardekani A, Fallahi B, Fard-Esfahani P, Beiki D, Hassanzadeh-Rad A & Eftekhari M. 2014. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl Med Commun 35 808–17. [DOI] [PubMed] [Google Scholar]
- Ferrandino G, Kaspari RR, Reyna-Neyra A, Boutagy NE, Sinusas AJ & Carrasco N. 2017. An extremely high dietary iodide supply forestalls severe hypothyroidism in Na+/I− symporter (NIS) knockout mice. Sci Rep 7 5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A, Read ML, Thornton CEM, Larner DP, Poole VL, Brookes K, Nieto HR, Alshahrani M, Thompson RJ, Lavery GG et al. 2020Targeting Novel Sodium Iodide Symporter Interactors ADP-Ribosylation Factor 4 and Valosin-Containing Protein Enhances Radioiodine Uptake. Cancer Res 80102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goolden AW, Mallard JR & Farran HE. 1957. Radiation sialitis following radioiodine therapy. Br J Radiol 30 210–2. [DOI] [PubMed] [Google Scholar]
- Hammami MM, Bakheet S. Radioiodine breast uptake in nonbreastfeeding women: clinical and scintigraphic characteristics.1996J Nucl Med 3726–31. [PubMed] [Google Scholar]
- Hollingsworth B, Senter L, Zhang X, Brock GN, Jarjour W, Nagy R, Brock P, Coombes KR, Kloos RT, Ringel MD et al. 2016Risk Factors of 131I-Induced Salivary Gland Damage in Thyroid Cancer Patients. J Clin Endocrinol Metab 1014085–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath E, Skoknic V, Majlis S, Tala H, Silva C, Castillo E, Whittle C, Niedmann JP & González P. 2020. Radioiodine-Induced Salivary Gland Damage Detected by Ultrasonography in Patients Treated for Papillary Thyroid Cancer: Radioactive Iodine Activity and Risk. Thyroid 30 1646–1655. [DOI] [PubMed] [Google Scholar]
- Jentzen W, Balschuweit D, Schmitz J, Freudenberg L, Eising E, Hilbel T, Bockisch A & Stahl A. 2010. The influence of saliva flow stimulation on the absorbed radiation dose to the salivary glands during radioiodine therapy of thyroid cancer using 124I PET(/CT) imaging. Eur J Nucl Med Mol Imaging 37 2298–306. [DOI] [PubMed] [Google Scholar]
- Jeong SY, Kim HW, Lee SW, Ahn BC & Lee J. 2013. Salivary gland function 5 years after radioactive iodine ablation in patients with differentiated thyroid cancer: direct comparison of pre- and postablation scintigraphies and their relation to xerostomia symptoms. Thyroid 23 609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhiang SM, Cho JY, Ryu KY, DeYoung BR, Smanik PA, McGaughy VR, Fischer AH & Mazzaferri EL. 1998. An immunohistochemical study of Na+/I− symporter in human thyroid tissues and salivary gland tissues. Endocrinology 139 4416–9 [DOI] [PubMed] [Google Scholar]
- Josefsson M, Grunditz T, Ohlsson T & Ekblad E. 2002. Sodium/iodide-symporter: distribution in different mammals and role in entero-thyroid circulation of iodide. Acta Physiol Scand 175 129–37. [DOI] [PubMed] [Google Scholar]
- Kay MD, Morris-Wiseman LF, Beazer A, Winegar BA & Kuo PH. 2020. Primary Nasolacrimal Duct Obstruction Visualized on 123I Preablation Scan for Papillary Thyroid Carcinoma. J Nucl Med Technol 48 77–78. [DOI] [PubMed] [Google Scholar]
- Kelkar MG, Senthilkumar K, Jadhav S, Gupta S, Ahn BC & De A. 2016. Enhancement of human sodium iodide symporter gene therapy for breast cancer by HDAC inhibitor mediated transcriptional modulation. Sci Rep 6 19341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbane MT, Ajjan RA, Weetman AP, Dwyer R, McDermott EW, O’Higgins NJ & Smyth PP. 2000. Tissue iodine content and serum-mediated 125I uptake-blocking activity in breast cancer. J Clin Endocrinol Metab 85 1245–50. [DOI] [PubMed] [Google Scholar]
- Kim MH, Kim HS & Park SA. 2017. Extrathyroidal Radioiodine Accumulation in a Fibroadenoma of the Breast. Clin Nucl Med 42 e123–e125. [DOI] [PubMed] [Google Scholar]
- Klein Hesselink EN, Brouwers AH, de Jong JR, van der Horst-Schrivers AN, Coppes RP, Lefrandt JD, Jager PL, Vissink A & Links TP. 2016. Effects of Radioiodine Treatment on Salivary Gland Function in Patients with Differentiated Thyroid Carcinoma: A Prospective Study. J Nucl Med 57 1685–1691. [DOI] [PubMed] [Google Scholar]
- Kloos RT, Duvuuri V, Jhiang SM, Cahill KV, Foster JA & Burns JA. 2002. Nasolacrimal drainage system obstruction from radioactive iodine therapy for thyroid carcinoma. J Clin Endocrinol Metab 87 5817–20. [DOI] [PubMed] [Google Scholar]
- Knostman KA, Cho JY, Ryu KY, Lin X, McCubrey JA, Hla T, Liu CH, Di Carlo E, Keri R, Zhang M et al. 2004Signaling through 3’,5’-cyclic adenosine monophosphate and phosphoinositide-3 kinase induces sodium/iodide symporter expression in breast cancer. J Clin Endocrinol Metab 895196–203. [DOI] [PubMed] [Google Scholar]
- Kogai T, Schultz JJ, Johnson LS, Huang M & Brent GA. 2000. Retinoic acid induces sodium/iodide symporter gene expression and radioiodide uptake in the MCF-7 breast cancer cell line. Proc Natl Acad Sci U S A 97 8519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogai T, Kanamoto Y, Li AI, Che LH, Ohashi E, Taki K, Chandraratna RA, Saito T & Brent GA. 2005. Differential regulation of sodium/iodide symporter gene expression by nuclear receptor ligands in MCF-7 breast cancer cells. Endocrinology 146 3059–69. [DOI] [PubMed] [Google Scholar]
- La Perle KM, Kim DC, Hall NC, Bobbey A, Shen DH, Nagy RS, Wakely PE Jr, Lehman A, Jarjoura D & Jhiang SM. 2013. Modulation of sodium/iodide symporter expression in the salivary gland. Thyroid 23 1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A, Wojcicka A, Kotlarek M, Zhang X, Jazdzewski K & Jhiang SM. 2015. microRNA-339–5p modulates Na+/I− symporter-mediated radioiodide uptake. Endocr Relat Cancer 22 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux MK, Graillon N, Guyot L, Taieb D, Galli P, Godio-Raboutet Y, Chossegros C & Foletti JM. 2020. Salivary side effects after radioiodine treatment for differentiated papillary thyroid carcinoma: Long-term study. Head Neck 42 3133–3140. [DOI] [PubMed] [Google Scholar]
- Levy O, Dai G, Riedel C, Ginter CS, Paul EM, Lebowitz AN & Carrasco N. 1997. Characterization of the thyroid Na+/I− symporter with an anti-COOH terminus antibody. Proc Natl Acad Sci U S A 94 5568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z & Xing M. 2012. Induction of sodium/iodide symporter (NIS) expression and radioiodine uptake in non-thyroid cancer cells. PLoS One 7 e31729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel SJ & Mandel L. 2003. Radioactive iodine and the salivary glands. Thyroid 13 265–71. [DOI] [PubMed] [Google Scholar]
- Mazzaferri EL & Jhiang SM. 1994. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97 418–28. [DOI] [PubMed] [Google Scholar]
- Mills JC & Shivdasani RA. 2011. Gastric epithelial stem cells. Gastroenterology 140 412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A & Shrivastava A. 2020. Prognostic Significance of Sodium Iodide Symporter and Deiodinase Enzymes mRNA Expression in Gastric Cancer. Int J Appl Basic Med Res 10 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern KE, Vadysirisack DD, Zhang Z, Cahill KV, Foster JA, Burns JA, Kloos RT & Jhiang SM. 2005. Expression of sodium iodide symporter in the lacrimal drainage system: implication for the mechanism underlying nasolacrimal duct obstruction in I(131)-treated patients. Ophthalmic Plast Reconstr Surg 21 337–44 [DOI] [PubMed] [Google Scholar]
- Myant NB. 1960Iodine metabolism of salivary glands. Ann N Y Acad Sci 85208–14. [DOI] [PubMed] [Google Scholar]
- Nakada K, Ishibashi T, Takei T, Hirata K, Shinohara K, Katoh S, Zhao S, Tamaki N, Noguchi Y & Noguchi S. 2005. Does lemon candy decrease salivary gland damage after radioiodine therapy for thyroid cancer? J Nucl Med 46 261–6. [PubMed] [Google Scholar]
- Nicola JP, Basquin C, Portulano C, Reyna-Neyra A, Paroder M & Carrasco N. 2009. The Na+/I− symporter mediates active iodide uptake in the intestine. Am J Physiol Cell Physiol 296 C654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola JP, Reyna-Neyra A, Carrasco N, Masini-Repiso AM. 2012Dietary iodide controls its own absorption through post-transcriptional regulation of the intestinal Na+/I− symporter. J Physiol 590:6013–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola JP, Carrasco N, Masini-Repiso AM. 2015Dietary I(−) absorption: expression and regulation of the Na(+)/I(−) symporter in the intestine. Vitam Horm 98:1–31. [DOI] [PubMed] [Google Scholar]
- Peyrottes I, Navarro V, Ondo-Mendez A, Marcellin D, Bellanger L, Marsault R, Lindenthal S, Ettore F, Darcourt J & Pourcher T. 2009. Immunoanalysis indicates that the sodium iodide symporter is not overexpressed in intracellular compartments in thyroid and breast cancers. Eur J Endocrinol 160 215–25. [DOI] [PubMed] [Google Scholar]
- Rathke H, Kratochwil C, Hohenberger R, Giesel FL, Bruchertseifer F, Flechsig P, Morgenstern A, Hein M, Plinkert P, Haberkorn U et al. 2019Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur J Nucl Med Mol Imaging 46139–147. [DOI] [PubMed] [Google Scholar]
- Ryan J, Curran CE, Hennessy E, Newell J, Morris JC, Kerin MJ & Dwyer RM. 2011. The sodium iodide symporter (NIS) and potential regulators in normal, benign and malignant human breast tissue. PLoS One 6 e16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki A, Ariyoshi Y, Iitaka D, Kosuga T, Shimizu H, Kudou M, Konishi T, Shoda K, Arita T, Konishi H et al. 2019Functional analysis and clinical significance of sodium iodide symporter expression in gastric cancer. Gastric Cancer 22473–485. [DOI] [PubMed] [Google Scholar]
- Singer MC, Marchal F, Angelos P, Bernet V, Boucai L, Buchholzer S, Burkey B, Eisele D, Erkul E, Faure F et al. 2020Salivary and lacrimal dysfunction after radioactive iodine for differentiated thyroid cancer: American Head and Neck Society Endocrine Surgery Section and Salivary Gland Section joint multidisciplinary clinical consensus statement of otolaryngology, ophthalmology, nuclear medicine and endocrinology. Head Neck 423446–3459. [DOI] [PubMed] [Google Scholar]
- Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL & Jhiang SM. 1996. Cloning of the human sodium lodide symporter. Biochem Biophys Res Commun 226 339–45. [DOI] [PubMed] [Google Scholar]
- Smith VE, Read ML, Turnell AS, Watkins RJ, Watkinson JC, Lewy GD, Fong JC, James SR, Eggo MC, Boelaert K et al. 2009A novel mechanism of sodium iodide symporter repression in differentiated thyroid cancer. J Cell Sci 1223393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VE, Sharma N, Watkins RJ, Read ML, Ryan GA, Kwan PP, Martin A, Watkinson JC, Boelaert K, Franklyn JA et al. 2013Manipulation of PBF/PTTG1IP phosphorylation status; a potential new therapeutic strategy for improving radioiodine uptake in thyroid and other tumors. J Clin Endocrinol Metab 982876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzweg C, Joba W, Eisenmenger W & Heufelder AE. 1998. Analysis of human sodium iodide symporter gene expression in extrathyroidal tissues and cloning of its complementary deoxyribonucleic acids from salivary gland, mammary gland, and gastric mucosa. J Clin Endocrinol Metab 83 1746–51. [DOI] [PubMed] [Google Scholar]
- Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, Zhao QH, Deng HF, Amenta PS, Fineberg S, Pestell RG et al. 2000The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med 6871–8. [DOI] [PubMed] [Google Scholar]
- Unterholzner S, Willhauck MJ, Cengic N, Schütz M, Göke B, Morris JC & Spitzweg C. 2006. Dexamethasone stimulation of retinoic Acid-induced sodium iodide symporter expression and cytotoxicity of 131-I in breast cancer cells. J Clin Endocrinol Metab 91 69–78. [DOI] [PubMed] [Google Scholar]
- Van Nostrand D, Neutze J & Atkins F. 1986. Side effects of “rational dose” iodine-131 therapy for metastatic well-differentiated thyroid carcinoma. J Nucl Med 27 1519–27. [PubMed] [Google Scholar]
- Van Nostrand D, Bandaru V, Chennupati S, Wexler J, Kulkarni K, Atkins F, Mete M & Gadwale G. 2010. Radiopharmacokinetics of radioiodine in the parotid glands after the administration of lemon juice. Thyroid 20 1113–9. [DOI] [PubMed] [Google Scholar]
- Venturi S & Venturi M. 2009. Iodine in evolution of salivary glands and in oral health. Nutr Health 20 119–34. [DOI] [PubMed] [Google Scholar]
- Wapnir IL, van de Rijn M, Nowels K, Amenta PS, Walton K, Montgomery K, Greco RS, Dohán O & Carrasco N. 2003. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J Clin Endocrinol Metab 88 1880–8. [DOI] [PubMed] [Google Scholar]
- Weng PL, Aure MH, Maruyama T & Ovitt CE. 2018. Limited Regeneration of Adult Salivary Glands after Severe Injury Involves Cellular Plasticity. Cell Rep 24 1464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willhauck MJ, O Kane DJ, Wunderlich N, Göke B, Spitzweg C. 2011Stimulation of retinoic acid-induced functional sodium iodide symporter (NIS) expression and cytotoxicity of ¹³¹I by carbamazepine in breast cancer cells. Breast Cancer Res Treat 125:377–86. [DOI] [PubMed] [Google Scholar]
- Wolff J 1983Congenital goiter with defective iodide transport. Endocr Rev 4240–54. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Beyer S & Jhiang SM. 2013. MEK inhibition leads to lysosome-mediated Na+/I− symporter protein degradation in human breast cancer cells. Endocr Relat Cancer 20 241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


