Abstract
Rumination, a form of passive, repetitive negative thinking, predicts the development of depressive disorders in non-autistic individuals, and recent work suggests higher levels of rumination may contribute to elevated rates of depression in the autistic population. Utilizing psychological network analysis, this study sought to investigate the structure of rumination in autistic individuals and the relationships between rumination and individual depressive symptoms. Non-regularized partial correlation networks were estimated using cross-sectional data from 608 autistic adults who completed the Ruminative Responses Scale and Beck Depression Inventory–II. Node centrality indices were calculated to determine which specific symptoms may have a disproportionate influence on the network of repetitive negative thoughts. Nodes were also grouped into communities, and specific “bridge” nodes were identified that most strongly connected these different communities. Results demonstrated strong positive relationships between all facets of ruminative thinking, similar to a prior study in non-autistic adults. Self-directed negative cognitions appeared to be particularly central in this network. The depression symptoms most strongly related to rumination in autistic adults were sadness and guilt. Although these findings are preliminary, they highlight specific facets of rumination that warrant future study as depression risk factors and potential intervention targets in the autistic population.
Lay Abstract
Autistic adults are substantially more likely to develop depression than individuals in the general population, and recent research has indicated that certain differences in thinking styles associated with autism may play a role in this association. Rumination, the act of thinking about the same thing over and over without a functional outcome, is a significant risk factor for depression in both autistic and non-autistic adults. However, little is known about how different kinds of rumination relate to each other and to depressive symptoms in the autistic population specifically. To fill this gap in knowledge, we recruited a large online sample of autistic adults, who completed questionnaire measures of both the tendency to ruminate and symptoms of depression. By examining the interacting network of rumination and depression symptoms this study was able to identify particular aspects of rumination—such as thinking repetitively about one’s guilty feelings or criticizing oneself—that may be particularly important in maintaining these harmful thought patterns in autistic adults. Although further study is needed, it is possible that the symptoms identified as most “influential” in the network may be particularly good targets for future interventions for mood and anxiety disorders in the autistic population.
Depression is among the most clinically significant outcomes for autistic1 adults, with a lifetime prevalence in this population nearly four times that of the general population (Hollocks et al., 2019; Hudson et al., 2019). In autistic individuals, depression has been linked to lower quality of life (Lawson et al., 2020; Park et al., 2019), greater service use (Joshi et al., 2013), lost work days (Park et al., 2019), increased caregiver burden (Cadman et al., 2012), self-injury, and suicidality (Cassidy et al., 2018). Stakeholders in the autism community, particularly autistic adults, have consistently called for better understanding of and interventions for this condition (Benevides et al., 2020; Crane et al., 2019; Pellicano et al., 2014; Van Hees et al., 2015). One road to improving prevention and treatment efforts is to better understand the cognitive factors that may influence depression onset and maintenance in autistic adults.
Rumination is a type of repetitive thinking that focuses on one’s distress without making active efforts to solve the problem (Nolen-Hoeksema et al., 2008); this type of passive repetitive cognition has been shown to prospectively predict depression (Nolen-Hoeksema, 1991, 2000), anxiety (Michl et al., 2013; Nolen-Hoeksema, 1991), and other negative health outcomes (Brosschot et al., 2006; Nolen-Hoeksema, 2012) in the general population. While rumination is studied most frequently in non-autistic populations, there is evidence to suggest both that autistic adults engage in more negative repetitive thinking than non-autistic peers (Crane et al., 2013; Gotham et al., 2014) and that rumination scores are associated with depression symptom endorsement in these adults (Gotham et al., 2018; Keenan et al., 2018). In a general population sample, Keenan et al. (2018) found that perseverative thinking predicted depression regardless of autistic trait level (i.e., autistic traits did not moderate the relationship between rumination and depression); however those with more autistic traits tended to report higher levels of rumination and perseveration, and rumination was found to partially mediate the relationship between elevated autistic traits and more depressive symptoms. This suggests that negative repetitive thinking is associated with depression across neurotypes, but that autistic individuals may be at increased likelihood of demonstrating this cognitive pattern (Keenan et al., 2018). Similarly, in a recent review, Smith and White (2020) found that autistic adolescents and adults ruminated more than non-autistic individuals, additionally noting that rumination was similarly predictive of depressive symptomatology in autistic individuals as it was in the general population.
However, not all repetitive cognition or rumination results in negative outcomes (Burns et al., 2019; Watkins, 2008), and prior research in non-autistic populations has supported a more nuanced understanding of this construct. In particular, Bernstein et al. (2019) proposed the reconceptualization of trait rumination as a network of interacting thoughts, which cohere together due to causal relations among the thoughts themselves rather than being indicators of a latent “common cause” (McNally, 2021; Robinaugh et al., 2020). By emphasizing analyses of symptoms rather than the underlying rumination subconstructs, network analyses have the potential to generate novel insights about psychopathological constructs, such as the identification of “central” symptoms with high network-wide influence.
Based on a network analysis of the Ruminative Responses Scale (RRS; Nolen-Hoeksema & Morrow, 1991), Bernstein and colleagues (2019) identified the most central rumination symptoms as (a) thinking repetitively about one’s lack of motivation, (b) concerns about not being able to concentrate in the future, (c) analyzing one’s personality to understand why one is depressed, and (d) wondering why one is not better able to cope with stressors. In addition, both unconstructive and constructive forms of repetitive thinking (as described by Watkins, 2008) were highly interrelated, at least cross-sectionally (Bernstein et al., 2019). Based on these findings, these authors suggested that ostensibly adaptive forms of “reflective pondering” could be benign at low levels, but due to the strong connections between these thoughts and maladaptive brooding, persistent reflection in the absence of problem-solving may still lead to suboptimal outcomes by activating these more traditionally problematic ruminative thoughts. Another study in non-autistic adults examined the network structure of depression in relation to single nodes representing the frequency of repetitive negative thinking and positive reappraisal (Everaert & Joormann, 2019). In this study, repetitive negative thinking was most strongly associated with the symptoms of guilty feelings, changes in appetite, agitation, self-criticalness, and sadness. Notably, these analyses were conducted in a community sample, and it remains unclear whether the findings of this prior study are generalizable to clinical populations such as autistic adults.
In the current study, we sought to assess the network structure of rumination as defined by the RRS within the autistic population, identifying the specific nodes that appear to most strongly maintain the network of maladaptive repetitive thoughts in these individuals. Two prior network studies in the autism literature have examined the relationships between autistic traits and depression symptoms, producing mixed results regarding whether symptom networks differ meaningfully between autistic and non-autistic samples (Montazeri et al., 2020; van Heijst et al., 2020). However, neither of these studies examined relations between depression and rumination at the symptom level, and no study to date has examined the structure of rumination symptoms within the autistic population. By conceptualizing rumination in autistic adults as a network of interconnected symptoms, the present study seeks to generate new perspectives on the centrality of particular symptoms and the associations between subcomponents of rumination. Moreover, we extend previous work on the network structure of rumination by examining the combined network of rumination and depressive symptoms, seeking to identify the specific nodes that most strongly connect the two constructs. Although predominantly exploratory, the results of these network analyses will generate new hypotheses about the relationships between cognitive patterns and various aspects of internalizing psychopathology in the autistic population, laying groundwork for additional research on causal relationships between symptoms and moving closer to the development of novel targeted interventions for depression and other psychopathology in the autistic population.
Methods
Participants
Autistic adults between the ages of 18 and 45 years were recruited from the Simons Foundation Powering Autism Research for Knowledge (SPARK) cohort (Feliciano et al., 2018) via the SPARK Research Match service. The upper age limit of 45 was chosen to increase the validity of autism diagnoses in our sample (due to the difficulty inherent in diagnosing older individuals without caregiver reports) and avoid many of the complex emotional and physical issues specific to older age, such as menopause (Moseley et al., 2020). All individuals self-reported a prior professional diagnosis of autism spectrum disorder or equivalent condition (e.g., Asperger syndrome, PDD-NOS). Notably, although these diagnoses are not independently validated by SPARK, the majority of participants are recruited from university autism clinics and thus have a very high likelihood of valid autism diagnosis (Feliciano et al., 2018). Furthermore, validation of diagnoses in the Interactive Autism Network, a similar participant pool now incorporated into SPARK, found that 98% of registry participants were able to produce valid clinical documentation of self-reported diagnoses when requested (Daniels et al., 2012). Autistic participants in our study completed a series of online surveys, which included the RRS (Nolen-Hoeksema & Morrow, 1991), Beck Depression Inventory–II (BDI-II; Beck et al., 1996), Social Responsiveness Scale–Second Edition (SRS-2; Constantino & Gruber, 2012), and a large number of additional self-report questionnaires. Participants were compensated with $50 in Amazon gift cards for completion of these surveys. All participants gave informed consent, and all study procedures were approved by the institutional review board at Vanderbilt University Medical Center.
These data were collected during winter and spring of 2019 as part of a larger study on repetitive thinking in autistic adults (project number RM0030Gotham) and the SPARK participants in the current study overlap partially with those described in previous investigations (Williams et al., 2020; Williams & Gotham, 2020a, 2020b, 2021). A total of 1,012 individuals enrolled in the study, although only 895 completed the three measures analyzed in the current study (RRS, BDI-II, and SRS-2). We further excluded participants who (a) had been told by a professional they do not meet criteria for autism after their initial diagnosis (n=43), (b) did not self-report a professional diagnosis of autism on our study-specific demographics form (n=20), (c) answered “Yes” or “Suspected” to a question regarding being diagnosed with Alzheimer’s disease (which given the age of participants in our study almost certainly indicated random or careless responding; n=9), (d) provided demographic information on the study-specific form that differed substantially from that provided initially to SPARK (n=5), (e) both self-referred to SPARK (i.e., was not referred by a family member, provider, etc.) and did not report an autism diagnosis by a qualified professional (i.e., physician, psychologist, interdisciplinary clinic/school team; n=43), (f) reported a total T-score on the SRS below 60 (i.e., <1 SD above the general population mean; n=104), and (g) indicated careless responding as determined by incorrect answers to two instructed-response items (e.g., Please respond ‘Strongly Agree’ to this question; n=64). The final sample used in the current study thus included a total of 608 adults with a high likelihood of valid autism diagnoses (Table 1). Notably, the mean age, sex ratio, and age of autism diagnosis were similar between the 608 included participants and the additional 404 who were excluded for the above reasons.
Table 1.
Sociodemographic and Clinical Characteristics of the Sample and Relevant Subgroups
| Males (n=211) | Females (n=397) | Childhood Diagnosis (n=250) | Adult Diagnosis (n=357) | Total (N=608) | |
|---|---|---|---|---|---|
|
| |||||
| Age (Years) | 31.42 (7.15) | 31.16 (6.75) | 27.34 (5.88) | 34.02 (6.16) | 31.25 (6.88) |
| Gender Identity | 50.6% Women | 59.1% Women | 55.4% Women | ||
| Cisgender | 191 (91.0%) | 335 (84.4%) | 213 (85.2%) | 313 (87.7%) | 650 (87.1%) |
| Transgender | 2 (1.0%) | 12 (3.0%) | 8 (3.2%) | 6 (1.6%) | 17 (2.3%) |
| Non-binary | 17 (8.1%) | 50 (12.6%) | 29 (11.6%) | 38 (10.6%) | 65 (8.7%) |
| Race/Ethnicity | |||||
| American Indian/Alaska Native | 13 (6.2%) | 26 (6.5%) | 11 (4.4%) | 28 (7.8%) | 39 (6.4%) |
| Asian | 10 (4.7%) | 11 (2.8%) | 8 (3.2%) | 13 (3.6%) | 21 (3.5%) |
| Black/African American | 11 (5.2%) | 17 (4.3%) | 13 (5.2%) | 15 (4.2%) | 28 (4.6%) |
| Native Hawaiian/Pacific Islander | 3 (1.4%) | 0 (0.0%) | 0 (0.0%) | 3 (0.8%) | 3 (0.5%) |
| White | 197 (93.4%) | 373 (94.0%) | 234 (93.6%) | 335 (93.8%) | 570 (93.8%) |
| Other Race | 5 (2.4%) | 14 (3.5%) | 6 (2.4%) | 13 (3.6%) | 19 (3.1%) |
| Hispanic/Latino | 15 (7.1%) | 34 (8.6%) | 20 (8.0%) | 29 (8.1%) | 49 (8.1%) |
| Education | |||||
| No High School Diploma | 9 (4.3%) | 10 (2.5%) | 13 (5.2%) | 6 (2.6%) | 19 (3.1%) |
| High School Diploma/GED | 51 (24.2%) | 60 (15.1%) | 69 (27.6%) | 42 (12.9%) | 111 (18.3%) |
| Trade/Vocational Certificate | 8 (3.8%) | 28 (7.1%) | 18 (7.2%) | 18 (4.9%) | 36 (5.9%) |
| Some College | 41 (19.4%) | 120 (30.2%) | 65 (26.0%) | 95 (28.3%) | 161 (26.5%) |
| Associate Degree | 24 (11.4%) | 36 (9.1%) | 33 (13.2%) | 38 (12.0%) | 60 (9.9%) |
| Bachelor’s Degree | 47 (22.3%) | 92 (23.2%) | 50 (20.0%) | 89 (27.7%) | 139 (22.9%) |
| Graduate/Professional Degree | 31 (14.7%) | 51 (12.8%) | 13 (5.2%) | 69 (22.0%) | 82 (13.5%) |
| Age of Autism Diagnosis (Years) | 20.15 (12.17) | 20.94 (10.29) | 9.57 (4.74) | 28.44 (6.49) | 20.67 (10.97) |
| Recruited from SPARK Clinical Sitea | 112 (53.1%) | 153 (38.5%) | 113 (45.2%) | 151 (42.3%) | 265 (43.6%) |
| Received Special Education Services | 79 (37.6%) | 106 (26.7%) | 161 (64.4%) | 24 (6.7%) | 185 (30.5%) |
| Received Any Autism-specific Services | 125 (59.5%) | 190 (47.9%) | 173 (69.2%) | 142 (39.8%) | 315 (51.9%) |
| SRS-2 Total T-score | 72.34 (7.93) | 74.54 (8.22) | 72.09 (8.14) | 74.95 (8.03) | 73.77 (8.18) |
| BDI-II Autism-specific T-score | 50.89 (8.58) | 52.98 (8.93) | 51.68 (9.57) | 52.68 (8.31) | 52.25 (8.86) |
| RRS Total Score | 54.17 (14.09) | 57.72 (13.44) | 55.67 (14.71) | 57.06 (13.07) | 56.49 (13.76) |
| RRS Brooding Score | 12.85 (4.06) | 13.71 (3.85) | 13.35 (4.08) | 13.46 (3.84) | 13.41 (3.94) |
| RRS Reflective Pondering Score | 11.21 (3.71) | 11.94 (3.63) | 11.43 (3.66) | 11.87 (3.67) | 11.69 (3.67) |
| Current Psychiatric Conditionsb | |||||
| Anxiety | 119 (56.4%) | 297 (74.8%) | 154 (61.6%) | 262 (73.4%) | 416 (68.4%) |
| ADHD | 73 (34.6%) | 152 (38.3%) | 101 (40.4%) | 124 (34.7%) | 225 (37.0%) |
| Bipolar Disorder | 20 (9.5%) | 55 (13.9%) | 32 (12.8%) | 43 (12.0%) | 75 (12.3%) |
| Depressive Disorder | 111 (52.6%) | 247 (62.2%) | 130 (52.0%) | 228 (63.9%) | 358 (58.9%) |
| Obsessive-compulsive Disorder | 24 (11.4%) | 84 (21.2%) | 50 (20.0%) | 58 (16.2%) | 108 (17.8%) |
| Posttraumatic Stress Disorder | 32 (15.2%) | 138 (34.8%) | 53 (21.2%) | 117 (32.8%) | 170 (28.0%) |
Note. All information gathered by self-report. “Males” and “Females” refer to sex assigned at birth rather than gender.
“Childhood” and “Adult” diagnosis refer to autism diagnosis before or after age 18 years. One individual with missing data for age of diagnosis question is excluded from those columns. Values are presented as M (SD) for continuous variables and N (%) for categorical variables. SRS-2 = Social Responsiveness Scale–Second Edition; BDI-II = Beck Depression Inventory–II; RRS = Ruminative Responses Scale; ADHD = attention deficit/hyperactivity disorder.
Indicates a high likelihood that autism diagnosis was verified using rigorous clinical methods (e.g., multidisciplinary evaluation)
Defined as receiving a professional diagnosis in the past and either (a) having symptoms in the past three months or (b) currently receiving treatment for this condition. Values may over-estimate disorders currently in partial or full remission with active treatment.
Measures
Ruminative Responses Scale (RRS)
The RRS (Nolen-Hoeksema & Morrow, 1991; Treynor et al., 2003) is a 22-item self-report questionnaire that captures the tendency to ruminate when feeling “down, sad, or depressed.” Items are rated on a four-point Likert scale ranging from 1 (almost never) to 4 (almost always). Treynor et al. (2003) grouped the items from the RRS into three subscales, measuring brooding, reflective pondering, and broader depressive symptoms, respectively. Although this form has not undergone psychometric validation in a sample of autistic adults, it is the most frequently used measure of repetitive thinking employed in this population (Crane et al., 2013; Gotham et al., 2014, 2018; Ho et al., 2021; Unruh et al., 2020). A previous network analysis of the RRS in a sample of non-autistic adults found that the items could be described by three communities that somewhat approximate the three-subscale structure of the form (Bernstein et al., 2019). Scores on each of the 22 individual RRS items were used as input to the initial network analysis.
Beck Depression Inventory–II (BDI-II)
The BDI-II (Beck et al., 1996) is a widely used 21-item self-report measure of depressive symptoms experienced over the past two weeks. The severity of each symptom is rated on a four-point Likert scale with unique anchors for each question. This measure is typically modeled as a bifactor structure, with two specific factors representing “cognitive-affective” and “somatic-vegetative” symptoms of depression (Huang & Chen, 2015), although almost all reliable variance is attributable to the general depression factor (Brouwer et al., 2013; Williams et al., 2020). The BDI-II is a reliable and valid measure of depression symptom severity in the autistic adult population, and normed latent trait scores on the general depression factor have been derived specifically to quantify general depression symptomatology in autistic adults (Williams et al., 2020). It has also been utilized in previous network analyses in the general population that examined the relationships between repetitive thinking and depressive symptoms (Everaert & Joormann, 2019). Scores on each of the 21 individual BDI-II items were used as input to the combined rumination-depression network analysis.
Social Responsiveness Scale-Second Edition (SRS-2)
The SRS-2 Adult Self Report (Bruni, 2014; Constantino & Gruber, 2012) is a widely used 65-item measure of quantitative autistic traits designed for use both in samples of autistic and general population adults. Items are scored on a 4-point Likert scale from Not true to Almost always true, with higher scores indicating higher levels of autistic symptomatology. T-scores (M = 50, SD = 10) are also available for individuals based on sex and the specific form used. In the current study, total T-scores on the SRS-2 of 60 or higher were required for inclusion as a T-score of 65 (a recommended screening cutoff; Constantino & Gruber, 2012) demonstrates poor sensitivity for autism diagnosis in self-reporting adults (Bezemer et al., 2020). Though this questionnaire was not examined using network analysis, it was used to determine whether autistic trait levels moderated the parameters derived from our symptom networks.
Network Analyses
In the current study, we analyzed items from the RRS and BDI-II using psychological network techniques (Contreras et al., 2019; Epskamp et al., 2018; Epskamp & Fried, 2018). In order to examine the structure of rumination in the autistic adult population, we first estimated a partial correlation network based on the 22 RRS items, similar to that reported by Bernstein et al. (2019). In addition to the rumination network, we also jointly estimated a partial correlation network containing both RRS and BDI-II items in order to better understand the links between rumination and depression in the autistic population and identify key symptoms that bridge these constructs. As the proportion of missing data in these items was very small (<0.2%), we handled missing values using pairwise deletion when calculating polychoric correlations.
Identification of Redundant Nodes
Recent discussion in the psychological network literature has highlighted the issue of node redundancy (i.e., two nodes that represent the same underlying symptom or construct) as a particular problem in some psychological networks (Christensen et al., 2020; Hallquist et al., 2019). In particular, if two nodes truly do represent one underlying construct, the partial correlations of either “redundant” node with all other nodes will have the common variance of the other “redundant” node residualized out, leaving very little reliable variance and resulting in noisy and unrepresentative correlations. In addition, when two redundant nodes are present in a partial correlation network, the semantic variance shared between the two of them may cause their shared edge to be exceptionally large. Some authors have warned that these artifactually strong connections distort network measures such as node centrality, upwardly biasing metrics such as expected influence (Hallquist et al., 2019).
Using the a combination of zero-order correlations and proportions of significantly different correlations for each node pair, the previous RRS network analysis by Bernstein et al. (2019) identified multiple pairs of redundant nodes that they collapsed together within their network model. However, in the current study, we utilized a somewhat different approach to redundancy detection by focusing specifically on the partial correlations between each node. As redundant items appear to bias network parameters primarily by inflating inter-item correlations, we defined redundant items as those exhibiting high intercorrelations (rp>0.4) after controlling for all other items in the network. We also calculated a network-based metric of weighted topological overlap (wTO; Gysi et al., 2018; Zhang & Horvath, 2005) using the full partial correlation matrix in order to determine the similarity of observed correlations between so-called “redundant” nodes. In cases where the wTO of a node pair was 0.3 or greater (indicating a high degree of overlap; Gysi et al., 2018), we combined the two items into a single “testlet” node representing the sum of both item scores. In cases where items were intercorrelated at rp>0.4 but wTO was below 0.3, we removed one of the items from the network entirely, retaining the node with the higher centrality (based on the sum of all pairwise partial correlations).
Four pairs of nodes in the RRS network met the criteria for redundancy (items 11/21: rp=0.613, wTO=0.501; 7/20: rp=0.514, wTO=0.512; 15/16: rp=0.469, and 5/15: rp=0.445, wTO=0.361; Table 2), and the first three of these pairs were summed together to form seven-point composite nodes (items 5 and 15 were not merged due to item 15 being merged into item 16). After collapsing these nodes, all partial correlations remained below 0.4. The joint RRS-BDI network (already including the three collapsed RRS nodes) included four additional redundant item pairs (BDI-II items 15/20: rp=0.524, wTO=0.351; BDI-II items 7/8: rp=0.418, wTO=0.239; RRS item 4/BDI-II item 19: rp=0.492, wTO=0.267; and RRS item 5/BDI-II item 6: rp= 0.444, wTO=0.224). Based on the observed wTO values, we thus collapsed BDI-II items 15/20, and removed BDI-II item 8 and RRS items 4 and 5. The resulting network displayed two additional redundant node pairs (RRS items 2/14: rp=0.420, wTO=0.337; BDI-II items 11/17: rp=0.420, wTO=0.265). After combining RRS items 2/14 and removing BDI-II item 11, the RRS-BDI network displayed no additional partial correlations with values greater than 0.4. The final RRS and RRS-BDI networks were thus composed of 19 and 36 nodes, respectively.
Table 2.
Network Nodes and Associated Item Content
| Node Label | Item Content | RRS Community |
|---|---|---|
|
| ||
| RRS | ||
| angry | Think about how angry you are with yourself. | 1 |
| deserve | Think ‘What am I doing to deserve this?’ | 1 |
| fault | Think about all your shortcomings, failings, faults, mistakes. | 1 |
| lonely | Think about how alone you feel. | 1 |
| react | Think ‘Why do I always react this way?’ | 1 |
| thnksad | Think about how sad you feel. | 1 |
| whyme (combined) | Think ‘Why do I have problems other people don’t have?’ | 1 |
| Think ‘Why can’t I handle things better?’ | ||
| wish | Think about a recent situation, wishing it had gone better. | 1 |
| alone (combined) | Go away by yourself and think about why you feel this way. | 2 |
| Go someplace alone to think about your feelings. | ||
| analyze (combined) | Analyze recent events to try to understand why you are depressed. | 2 |
| Analyze your personality to try to understand why you are depressed. | ||
| write | Write down what you are thinking about and analyze it. | 2 |
| cantdo | Think ‘I won’t be able to do my job if I don’t snap out of this.’ | 3 |
| concen | Think about how hard it is to concentrate. | 3 |
| feel | Think about how you don’t feel up to doing anything. | 3 |
| fut | Think ‘I won’t be able to concentrate if I keep feeling this way.’ | 3 |
| going | Think ‘Why can’t I get going?’ | 3 |
| numb | Think about how you don’t seem to feel anything anymore. | 3 |
| passive | Think about how passive and unmotivated you feel. | 3 |
| phys | Think about your feelings of fatigue and achiness. | 3 |
| beable (combined)a | Think ‘I won’t be able to do my job if I don’t snap out of this.’ | — |
| Think ‘I won’t be able to concentrate if I keep feeling this way.’ | ||
| BDI-II b | ||
| app | Changes in Appetite | — |
| conc | Concentration Difficulty | — |
| cry | Crying | — |
| decide | Indecisiveness | — |
| dislk | Self-Dislike | — |
| enrgy (combined) | Loss of Energy | — |
| Tiredness or Fatigue | ||
| fail | Past Failure | — |
| guilt | Guilty Feelings | — |
| intrst | Loss of Interest | — |
| irtbl | Irritability | — |
| pess | Pessimism | — |
| pleas | Loss of Pleasure | — |
| punish | Punishment Feelings | — |
| sad | Sadness | — |
| sex | Loss of Interest in Sex | — |
| sleep | Changes in Sleeping Pattern | — |
| suic | Suicidal Thoughts or Wishes | — |
| wrthls | Worthlessness | — |
Note. “alone,” “analyze,” “beable,” “whyme,” “enrgy,” and “irtbl” are combinations of the two items listed. Node values are the sum of the two item scores. RRS = Ruminative Responses Scale; BDI-II = Beck Depression Inventory–II
Node present only in combined RRS-BDI network (replaced by “cantdo” and “fut” in the RRS network)
Excludes items 8 (Self-Criticalness) and 11 (Agitation) due to overlap with items 7 (Self-Dislike) and 17 (Irritability), respectively
Network Estimation and Inference
To explore the relationships among nodes in our network we computed Gaussian Graphical Models (GGMs) using the qgraph package (Epskamp et al., 2012) in the R statistical computing environment (R Core Team, 2020). In these partial correlation networks, each node (symptom) is connected to a number of other nodes with edges (lines), the size of which signify the partial polychoric correlation between the two symptoms after controlling for all other variables in the network (Epskamp & Fried, 2018). A non-regularized network was fit using the ggmModSelect function in the qgraph package (Isvoranu & Epskamp, 2021), performing a stepwise specification search that added and subtracted edges from the network until the Bayesian Information Criterion (BIC; Schwarz, 1978) was minimized. This method overcomes one of the primary limitations of the more popular graphical LASSO method (Epskamp et al., 2018), which has a low specificity for detecting true edges when samples are large (Isvoranu & Epskamp, 2021). Network plot layouts were created using multidimensional scaling (MDS; Jones et al., 2018), which allows the distance between two nodes to be interpreted as the similarity between those nodes. A small repulsion force was added to each network plot to avoid node overlap.
In order to quantify node importance (centrality) within the network, we used the networktools R package (Jones, 2020) to compute one- and two-step expected influence statistics (EI1 and EI2, respectively; Robinaugh et al., 2016). Higher EI1 and EI2 values indicate greater influence in the network, as quantified by the sum of signed edge weights for a given node, and in the case of EI2, its neighbors (Robinaugh et al., 2016). The bootnet R package (Epskamp, 2020) was additionally used to generate 95% confidence intervals for EI1 values based on a percentile bootstrap (1000 resamples). Nodes with EI1 or EI2 values greater than one standard deviation above the mean were considered particularly influential in the network. Nodes with high expected influence are hypothesized to contribute an outsized amount to the symptom network, and in cases where causal relationships between these nodes and others can be established, such nodes may be preferred targets for psychological interventions. However, in cross-sectional symptom networks such as ours, there are many limitations to the interpretation of central nodes as causally important (Dablander & Hinne, 2019; Fried et al., 2018; Hallquist et al., 2019; Rodebaugh et al., 2018). Nevertheless, we believe the identification of central nodes in our network is important, both to compare our results to previous studies of non-autistic adults (Bernstein et al., 2019; Everaert & Joormann, 2019) and to highlight symptoms whose causal relationships may be worth elucidating further.
We also conducted several sensitivity analyses to ensure the validity of our centrality estimates. Using a case-dropping bootstrap procedure implemented in the bootnet R package (Epskamp et al., 2018), we additionally investigated the stability of all centrality estimates by calculating the correlation stability coefficient (CS), the proportion of cases that must be dropped before fewer than 95% of 2000 bootstrapped correlations between true and resampled2 centrality indices are less than 0.7. CS values of 0.25 are minimally required for interpretation of these indices, with CS > 0.5 preferred for most applications (Epskamp et al., 2018).
In addition, centrality estimates can be biased if certain nodes are restricted in range and/or nodes differ substantially in variance (Terluin et al., 2016). Following the suggestions of Iverson et al. (2020), we investigated this possibility by calculating the (Pearson) correlations between node strength centrality and node variance, with significant positive correlations (i.e., higher-variance nodes are more central) indicative of this problem.
Community Detection and Bridge Centrality
Within each of our networks, we examined the community structure using the spin glass algorithm, γ=0.5, start temperature=1, stop temperature=.01, cooling factor=0.99, spins=20 (Reichardt & Bornholdt, 2006), as implemented in the igraph R package (Csárdi & Nepusz, 2006). Given the stochastic nature of this algorithm, the procedure was conducted 1000 times, and the modal number of communities was selected as the final community structure. We then identified important “bridge” nodes that have the strongest connections between the various communities by calculating one- and two-step bridge expected influence (BEI1 and BEI2, respectively; Jones et al., 2019) using the bridge function in the networktools R package (Jones, 2020). These bridge centrality indices are calculated in the same manner as EI1 and EI2, except that they utilize only edges that connect nodes in separate communities, thereby excluding influence that a node may have within its own community. Nodes with BEI1 or BEI2 values greater than one standard deviation above the mean were considered “bridge” nodes in the current study. We were particularly interested in the bridge centrality of nodes in the combined RRS-BDI network, as the ruminative symptoms most closely related to depression are potentially those whose modification could have the largest impact on depressive symptoms in the autistic population, provided that the direction of causality points from rumination to depression as it does in the general population (Barlow et al., 2014; Nolen-Hoeksema et al., 2008b; Sarin et al., 2005).
Structural Network Differences Across Subgroups
We also investigated differences in network structure between subpopulations of our autistic sample, focusing specifically on (a) sex differences (male vs. female) and (b) age of autism diagnosis (diagnosed in adulthood [≥18 years old] vs. diagnosed in childhood [<18 years old]), and (c) level of autistic traits (SRS-2 T-score ≤70 vs >70). These comparisons were tested using the permutation testing procedure proposed by van Borkulo et al. (2015, 2017) and implemented in the NetworkComparisonTest (NCT) R package (van Borkulo et al., 2019). Using a modification of the NCT for polychoric correlation matrices (based on 500 permutations; Williams, 2021), we tested for group differences in (a) global network structure, (b) global network strength (the sum of all absolute edge weights in the network), and (c) global EI1 and EI2 (as operationalized by the sum of EI1 or EI2 values for all nodes in a given network). In cases where fewer than 10 permuted values of a parameter exceeded the observed parameter value, we utilized the method of Knijnenburg et al., (2009) to estimate the permuted p-value using the generalized Pareto distribution. Statistically significant (p<0.05) NCT results for global centrality were followed up with post-hoc tests of node centrality differences, with the Benjamini-Hochberg (1995) p-value correction implemented to control the false discovery rate (FDR) at 5%. Edges from nodes with significant group differences in centrality (pFDR<0.05) were further examined for group differences, again using the FDR correction for each node separately. Lastly, we examined group differences in the previously identified “bridge” nodes (i.e., BEI1/BEI2 Z-score≥1.0) to determine whether these nodes were equally influential across all subsets of the autistic population.
Autistic Community Involvement
The current study was conducted as a collaboration between autistic and non-autistic researchers. Author ZJW, who is autistic, conceptualized and designed the study, cleaned and processed the data, performed all statistical analyses, and drafted the initial manuscript. He has also been heavily involved in the larger project on repetitive thinking from which the current sample is drawn, assisting author KOG in designing the survey battery and validating the psychometric tools employed in this project.
Results
Participants and Demographics
RRS data were available for 608 autistic adults (65.3% female sex), with a mean (SD) age of 31.25 (6.88) years. Demographics and clinical characteristics of the participants, overall and divided according to biological sex and age of diagnosis, are presented in Table 1. The mean (SD) total T-score on the SRS-2 was 73.77 (8.18), with slightly higher scores in females compared to males (d=0.271, CI95% [0.103,0.438]). The mean (SD) autism-specific T-score on the BDI-II (Williams et al., 2020) was 52.25 (8.86), with females reporting significantly higher levels of depressive symptoms than males (d=0.237, CI95% [0.069,0.404]). Additionally, females scored higher on the RRS total score than males (d=0.260, CI95% [0.092,0.427]), with similar patterns for both subscale scores (Brooding: d=0.219, CI95% [0.052,0.387]; Reflective Pondering: d=0.199, CI95% [0.031,0.366]).
Network Structure and Centrality
RRS/BDI-II items and the abbreviated names used in figures are presented in Table 2, and the non-regularized partial correlation networks for the RRS and combined RRS-BDI networks are presented in Figures 1A and 2A, respectively (see Supplemental Material for full edgelists). Within the RRS network, most connections between symptoms were positive, indicating that higher levels of one type of repetitive thinking predict unique variance in many other types of repetitive thinking as well. Although the nodes representing items from the “reflective pondering” subscale are thought to generally reflect more adaptive repetitive thinking patterns (Treynor et al., 2003), only the write node in this subscale demonstrated negative connections with other RRS nodes (passive: rp=−0.184, fault: rp=−0.098). Additionally, in the combined RRS-BDI network, the three “reflective pondering” nodes (write, analyze, alone) did display some negative connections with various BDI-II items, though all were relatively small in magnitude (all rp>−0.1).
Figure 1. Network of Ruminative Responses Scale (RRS) Items and Expected Influence Metrics.
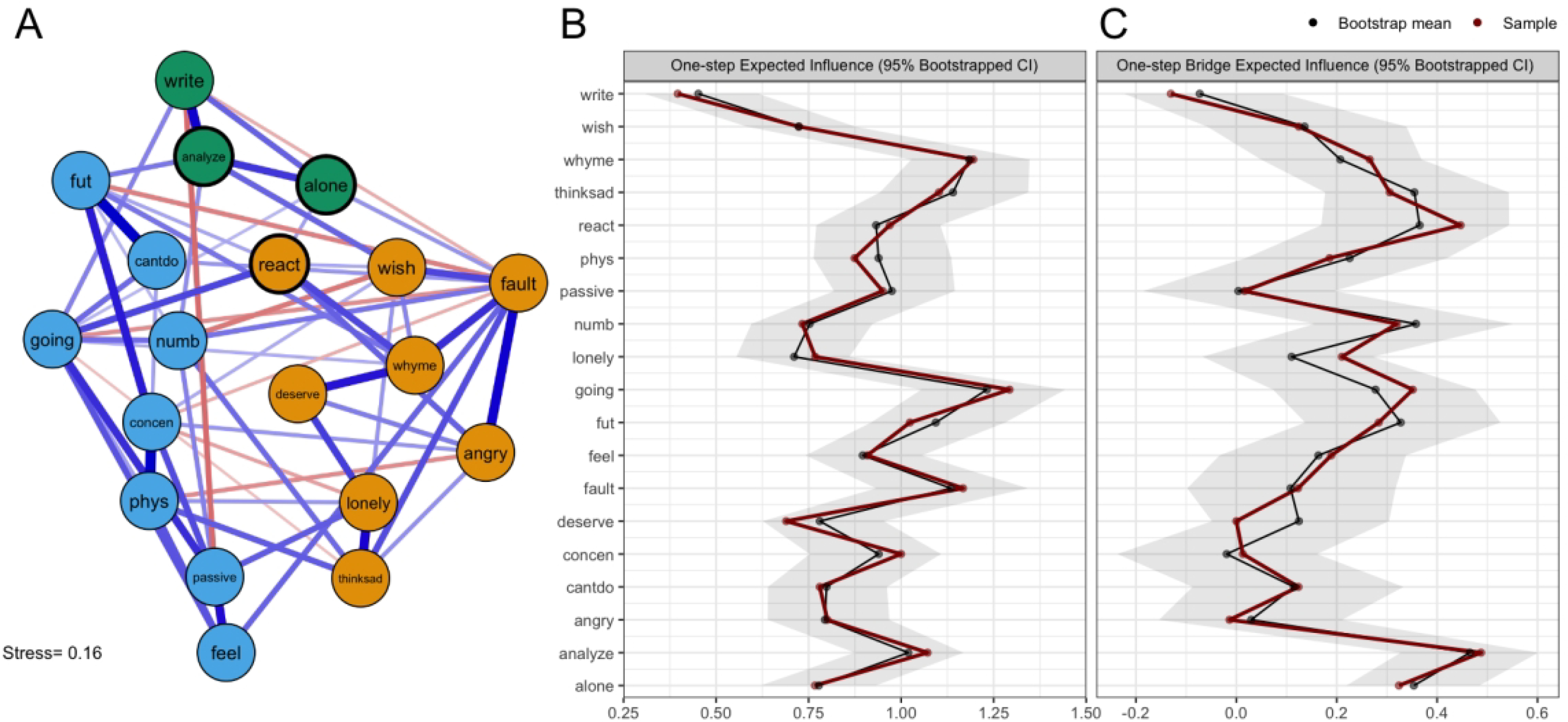
Note.(A) Network graph arranged using multidimensional scaling. Edge thickness reflects partial correlation strength (thickest edge, rp=0.346), with blue lines representing positive correlations and red lines representing negative correlations. Nodes are grouped into three communities: (1) rumination about emotions and situations (orange), (2) reflective pondering (blue), and (3) rumination about neurovegetative symptoms (green). Item content for each node is presented in Table 2. (B) Raw one-step expected influence values for each node, along with 95% bootstrapped confidence intervals. (C) Raw one-step bridge expected influence values for each node, along with 95% bootstrapped confidence intervals.
Figure 2. Network of Ruminative Responses Scale (RRS) and Beck Depression Inventory–II Items and Expected Influence Metrics.
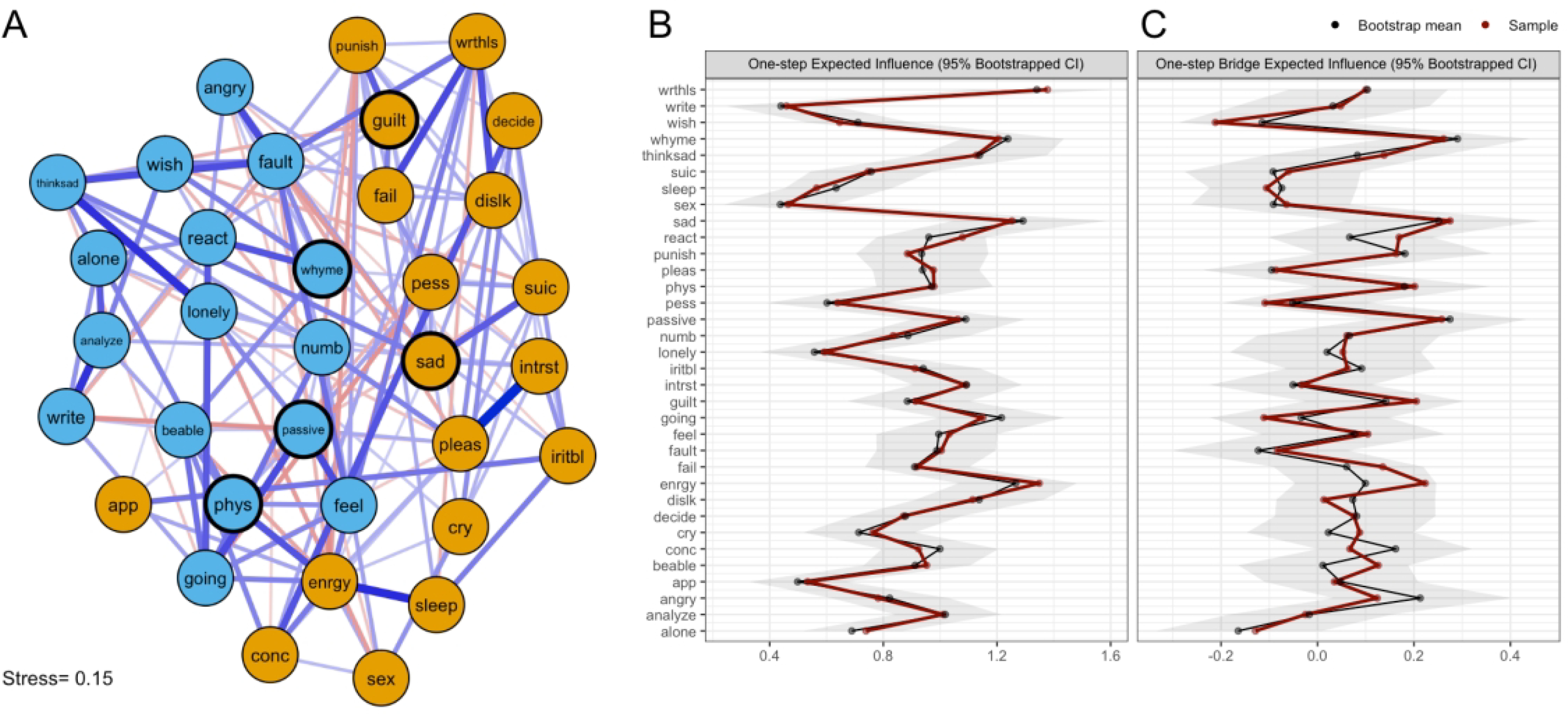
Note.(A) Network graph arranged using multidimensional scaling. Edge thickness reflects partial correlation strength (thickest edge, rp=0.362), with blue lines representing positive correlations and red lines representing negative correlations. The proximity of two nodes is proportional to their degree of association with one another. Nodes derived from the RRS are presented in blue, while nodes derived from the BDI-II are presented in orange. Item content for each node is presented in Table 2. (B) Raw one-step expected influence values for each node, along with 95% bootstrapped confidence intervals. (C) Raw one-step bridge expected influence values for each node, along with 95% bootstrapped confidence intervals.
One- and two-step expected influence metrics were calculated for each network to determine the most influential nodes (Figure 1B; Figure 2B; Supplemental Figure S1–2). Within the RRS network, the most influential nodes were (a) wondering why one cannot start tasks (going; EI1=1.29, EI2=2.32), (b) questioning why one has so many problems (whyme; EI1=1.20, EI2=2.33), and self-critical rumination (fault; EI1=1.17, EI2=2.17). Within the combined RRS-BDI network, the most influential symptoms were (a) lack of energy (enrgy; EI1=1.42, EI2=2.59), (b) sadness (sad; EI1=1.15, EI2=2.23), (c) questioning why one has so many problems (whyme; EI1=1.25, EI2=2.42), (d) feelings of worthlessness (wrthls; EI1=1.25, EI2=2.56), (e) rumination about one’s sadness (thinksad; EI1=1.17, EI2=2.15), (f) thinking about one’s inability to start tasks (going; EI1=1.15, EI2=2.33), and lack of interest (intrst; EI1=1.14, EI2=2.24). Sensitivity analyses indicated that node centrality estimates in both networks were not meaningfully confounded by differential variance (RRS: r=−0.189, CI95% [−0.598,0.282]; RRS-BDI: r=−0.099, CI95% [−0.423,0.247]), and the case-dropping bootstrap indicated that EI1 estimates were highly stable (RRS: CSEI1=0.666; RRS-BDI: CSEI1=0.745; Supplemental Figure S3), supporting the validity of these centrality estimates.
Community Detection and Bridge Centrality
Within the RRS network, the spin glass algorithm detected three communities of nodes (Figure 2A), which we interpreted as (1) “rumination about emotions and situations” (8 items), (2) “reflective pondering” (3 items), and (3) “rumination about neurovegetative symptoms” (8 items). Bridge centrality estimates (Figure 1C; Supplemental Figure S4) indicated that three nodes met our criteria for “bridge nodes” (i.e., BEI1 or BEI2 Z-score ≥ 1), including (a) analyzing recent events and oneself to understand why one is depressed (analyze [community 2]; BEI1=0.49, BEI2=0.85), wondering why one always reacts this way (react [community 1]; BEI1=0.45, BEI2=0.94), and going away by oneself to think about one’s feelings (alone [community 2]; BEI1=0.32, BEI2=0.75). Bootstrap analyses indicated that these estimates were highly stable (CSBEI1=0.589). Influential bridge nodes in the RRS network are highlighted in Figure 2A.
In the combined RRS-BDI network, the spin glass algorithm detected two communities of nodes, corresponding exactly to the RRS items and BDI-II items, respectively, and were thus interpreted as “rumination” and “depression” (Figure 2A). Based on bridge expected influence criteria, five nodes were classified as bridge nodes (Figure 2C; Supplemental Figure S5). Within the depression cluster, sadness (sad; BEI1=0.30, BEI2=0.50) and guilty feelings (guilt; BEI1=0.12, BEI2=0.34) had the highest overall bridge expected influence. The RRS items with the greatest influence on depressive symptoms were (a) questioning why one has so many problems (whyme; BEI1=0.31, BEI2=0.57), (b) ruminating about fatigue and achiness (phys; BEI1=0.15, BEI2=0.42), and (c) ruminating about avolition (passive; BEI1=0.19, BEI2=0.38). Bootstrap analyses indicated that these estimates were very stable (CSBEI1=0.512). Influential bridge nodes in the RRS-BDI network are highlighted in Figure 2A.
Network Comparison Testing
The NCT examining sex differences in the RRS network found no differences in overall network structure (MSex=0.344, p=0.437), global strength (SSex=2.09, p=0.228), or global expected influence metrics (E1Sex=0.405, p=0.228; E2Sex=0.881, p=0.190), and thus we did not test for group differences in node EI1/EI2 or individual edge weights. Furthermore, permutation tests revealed no significant sex differences in BEI1/BEI2 for the four RRS bridge nodes after correcting for multiple comparisons (BEI1: all pFDR>0.610; BEI2: all pFDR>0.443). Additionally, there were no significant differences in any of the tested parameters between individuals diagnosed with autism before or after age 18 (MDxAge=0.350, p=0.309; SDxAge=0.363, p=0.830; E1DxAge=0.302, p=0.337; E2Sex=0.779, p=0.212; BEI1: all pFDR>0.858; BEI2: all pFDR>0.739) or individuals with SRS-2 T-scores above or below 70 (MSRS=0.279, p=0.868; SSRS=0.832, p=0.641; E1SRS=0.458, p=0.148; E2SRS=0.589, p=0.315; BEI1: all pFDR>0.126; BEI2: all pFDR>0.108). As with RRS network structures, RRS-BDI networks did not significantly differ on any metric according to sex (MSex=0.389, p=0.196; SSex=6.80, p=0.409; E1Sex=0.395, p=0.559; E2Sex=1.35, p=0.277; BEI1: all pFDR>0.687; BEI2: all pFDR>0.545), age of diagnosis (MDxAge=0.344, p=0.333; SDxAge=5.24, p=0.397; E1DxAge=0.205, p=0.884; E2DxAge=0.884, p=0.431; BEI1: all pFDR>0.494; BEI2: all pFDR>0.639) or autistic trait level (MSRS=0.317, p=0.627; SSRS=0.6.93, p=0.289; E1SRS=0.708, p=0.269; E2SRS=0.192, p=0.858; BEI1: all pFDR>0.233; BEI2: all pFDR>0.427).
Discussion
This is the first study to apply network analysis to examine the architecture of rumination and its symptom-level connections with depression in autistic adults, as well as the first study using any method to analyze individual rumination symptoms in this population. We first examined the network structure of trait rumination as operationalized by the RRS, further investigating the specific nodes with the highest expected influence, the manner in which nodes clustered into distinct communities, and the “bridge” nodes that had the strongest connections between these various communities. These procedures were then repeated for the combined rumination and depression symptom network, which included items from both the RRS and BDI-II. Lastly, we examined the ways in which network structures differed between subsets of autistic adults grouped by sex, age of autism diagnosis, and autistic trait levels, finding that these networks did not significantly differ across any tested contrast. Overall, these analyses produced clinically interpretable findings that allow us to better understand the complex relationships between various types of ruminative thinking and depressive symptomatology in the autistic adult population. Furthermore, by highlighting potentially influential symptoms within the network of rumination and depression, the current study sets the stage for future work attempting to specifically assess the causal relationships of these symptoms and determine their suitability as targets of psychological interventions.
Influential nodes within the rumination network included wondering why one cannot start tasks (going), (b) questioning why one has so many problems (whyme), and self-critical rumination such as pointing out faults and mistakes (fault), all of which are self-directed negative cognitions. Self-critical thought is both negatively valenced and may be reflective of broader negative self-related beliefs. Prior research in non-autistic populations suggests that negative self-related beliefs may increase the risk of undesirable outcomes from repetitive thinking, especially when that repetition is negatively focused (Watkins, 2008). Other researchers have also suggested that autistic individuals may engage in more rumination related to mistakes or rejection in social spheres, perhaps particularly tied to a sense of not-belonging and rejection sensitivity (Jordan et al., 2020; Keenan et al., 2018). Notably, the RRS nodes considered most central in the current study only partially overlapped with those reported in a previous study of the measure’s network structure (i.e., passive, fut, person [a part of the analyze node], and handle [a part of the whyme node]; Bernstein et al., 2019). However, that prior study did not collapse nodes together based on particularly strong edge weights, and thus the high centrality of nodes such as person, handle, and fut in that study may be largely due to one single very strong edge between nodes with similar wording. Thus, as these differences in centrality rankings are likely due to methodological differences, future studies that utilize the same estimation methods in both populations are required to determine whether the network structures of rumination in autistic and non-autistic adults are comparable.
Within the rumination network, the spin glass community finding algorithm identified three RRS node clusters, which reflected (a) rumination about emotions and situations (containing all “brooding” items and a number of RRS “depression” items that reflect the cognitive-affective symptoms of depression), (b) reflective pondering (containing all items from the “reflective pondering” subscale), and (c) rumination about neurovegetative symptoms (containing the RRS “depression” items specifically referring to somatic-vegetative depressive symptoms). Bridge symptoms in this network included the react node in the “emotions/situations” community and the analyze and alone nodes in the “reflective pondering” community. In our rumination network, we found largely positive connections between nodes, including between the seemingly adaptive “reflective pondering” items and the more clearly maladaptive “emotions/situations” and “neurovegetative” items. Specifically, items such as alone and analyze demonstrated multiple positive connections with more maladaptive behaviors such as dwelling on a counterfactual situation (wish), ruminating about one’s emotional numbness (numb), and dwelling on one’s own faults and mistakes (fault). The positive relationship between “adaptive” and “maladaptive” dimensions of rumination on the RRS replicates prior findings in non-autistic adults, which demonstrate moderate positive correlations between the “brooding” and “reflective pondering” subscales of the RRS (Raes & Hermans, 2008; Treynor et al., 2003), as well as the almost exclusively positive edge weights seen in a previous network study of the RRS (Bernstein et al., 2019). Thus, while the lack of a non-autistic control group prevented us from testing differences in network structure in the autistic population, these data suggest that the relationships between RRS items in the autistic population are generally similar to those seen in the general population.
The reasons for “adaptive” and “maladaptive” forms of rumination covarying within individuals are likely multiple, but we believe that the structure of the RRS in particular contributes greatly to this overlap. In particular, the RRS presents (a) item content on the reflection scale that could be construed as positive or negative (i.e., items like “Go someplace alone to think about your feelings” could be endorsed by individuals who are fixated on negative emotions in an unproductive and maladaptive manner), as well as (b) instructions that participants should respond regarding their patterns of behavior when they “feel down, sad, or depressed” (i.e., individuals who feel depressed infrequently will not endorse any of the items, regardless of whether they engage in reflection or other “adaptive” repetitive thinking at other times). Notably, correlations between “rumination” and “reflection” dimensions of other repetitive thinking measures such as the Rumination-Reflection Questionnaire (RRQ; Trapnell & Campbell, 1999) have been reported as much smaller in some studies (e.g., Harrington & Loffredo, 2010), further supporting the idea that at least some of the positive relationship between these dimensions is due to methodological artifact. Future work in this area should attempt to measure both positive and negative dimensions of repetitive thinking with alternative measures, determining whether networks of these symptoms remain positively associated.
Expanding upon previous work studying the network structure of negative repetitive thinking and depression (Everaert & Joormann, 2019), we also conducted network analyses on a combined network of RRS and BDI-II items with the goal of identifying the “bridge” symptoms that were most strongly associated with symptoms in the other community. Of the BDI-II nodes, sadness and guilty feelings showed the highest bridge expected influence. Notably, these BDI-II symptoms were among those most strongly connected to the “Repetitive Negative Thinking” node in a previous study by Everaert & Joormann (2019), indicating that the “bridge” status of these nodes may not differ across autistic and non-autistic populations. Bridge items from the RRS were drawn from both the “neurovegetative” and “emotions/situations” sections of the RRS, potentially indicating that both cognitive-affective and somatic-vegetative symptoms are linked to rumination, although the causality of these relationships has yet to be established in autistic individuals. Notably, it is likely that in some cases such as the link between anergia/fatigue and ruminating about fatigue and achiness, the symptom is present before an individual begins to ruminate about it, and thus rumination is (Granger) caused by depressive symptoms rather than the other way around. Future studies should collect longitudinal data on both of these variables to gain a better understanding of the complex and likely bidirectional links between ruminative and depressive symptoms in the autistic population, additionally determining whether interventions targeting rumination can protect non-depressed autistic adults from developing depression in the future.
Network comparisons across groups based on sex, age of diagnosis, and autistic trait levels indicated that overall RRS and RRS-BDI network structures did not significantly differ between subgroups. These findings appear consistent with prior work demonstrating that the relationships between rumination and depression do not appear to be moderated by sex/gender (Nolen-Hoeksema, 2012) or autistic traits/autism diagnosis (Bos et al., 2018; Keenan et al., 2018; Rieffe et al., 2014). However, there are many other potential moderators of this relationship not tested in the current study, and future studies are necessary to determine whether these relationships are generalizable to individuals outside of the current study’s age range (e.g., adolescents and older adults), autistic individuals with intellectual disability, and other clinically-defined subgroups.
This study notably has a number of limitations, many of which are suggestive of future directions in the study of repetitive thinking in autism. First, as mentioned previously, the cross-sectional nature of the data precludes us from making causal claims regarding the relationships between various network nodes. Additional longitudinal studies will be particularly helpful in clarifying the temporal precedence of one symptom over another, which is more suggestive of a potentially beneficial effect of targeted interventions. Second, as noted in previous studies of this cohort (Williams et al., 2020; Williams & Gotham, 2020b), this sample of autistic adults is overwhelmingly female, highly educated, and later-diagnosed, and thus less representative of the adult autistic population encountered in typical clinical research samples. However, given the high stability of the centrality coefficients and minimal differences in network structure according to either sex or age of diagnosis, it is unlikely that our findings would have differed meaningfully in a sample of earlier-diagnosed male participants. Nevertheless, additional studies of repetitive thinking in autistic adults should attempt to include more representative samples in order to assess these differences empirically. Lastly, as seen in other psychological network studies (e.g., Rodebaugh et al., 2018), findings regarding the centrality of specific nodes may be heavily influenced by the measures used to operationalize the construct of interest. Thus, additional network analyses using other rumination and depression questionnaires should be conducted in the autistic population to determine the degree to which the current findings are limited to the RRS and/or BDI-II. Despite these limitations, the current study substantially advances our understanding of ruminative cognitions in autistic adults and their relationships with depressive symptomatology. We hope that others in the field of autism research will see the appeal of network technique for assessing the complex interactions between symptoms, thoughts, and behaviors.
Conclusion
In the current study, we employed psychological network analysis to gain insights into the phenomenology of rumination in autism, as well as the connections between different facets of rumination and depressive symptoms. Our analyses found strong positive relationships between all subtypes of ruminative thinking in autistic adults, which were generally in line with previous results from general population samples. The most influential nodes in the rumination network all included some form of self-referential cognition, potentially indicating that the self-focused nature of passive repetitive thinking may be particularly important in sustaining this particular symptom network. Furthermore, in examining the relationships between ruminative and depressive symptoms, we found a number of “bridge” symptoms on both measures that may play outsized roles in the overlap between rumination and depression. Although these findings are preliminary and purely correlational, they have highlighted a number of symptom interactions that warrant further study in the autistic population, potentially leading to the development of targeted interventions for mental health concerns in autistic individuals.
Supplementary Material
Acknowledgements
The authors are grateful to all of the individuals and families enrolled in SPARK, the SPARK clinical sites and SPARK staff. They appreciate obtaining access to demographic and phenotypic data on SFARI Base. Approved researchers can obtain the SPARK population dataset described in this study by applying at https://base.sfari.org.
Funding
This work was supported by National Institute of Mental Health grant R01-MH113576 (KG); National Institute of General Medical Sciences grant T32-GM007347 (ZJW); National Institute Deafness and Other Communication Disorders grant F30-DC019510 (ZJW); and the Nancy Lurie Marks Family Foundation (ZJW). Content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. No funding body or source of support had a role in the study design, data collection, analysis, or interpretation, decision to publish, or preparation of this manuscript.
Footnotes
The terms ‘autistic person’ and ‘person on the autism spectrum’ are the preferred language of the majority of people diagnosed as autistic (Bottema-Beutel et al., 2020; Bury et al., 2020; Kenny et al., 2016). Out of respect for these preferences, we use these terms to refer to individuals on the spectrum rather than exclusively using person-first language.
Notably, the ggmModSelect algorithm becomes computationally burdensome with greater than 30 nodes, and thus bootstrapped and permuted networks based on the combined RRS-BDI network did not undergo the full stepwise model-selection procedures during resampling procedures (i.e., the “stepwise” argument was set to “FALSE”). However, as ggmModSelect results with and without the stepwise algorithm resulted in almost identical centrality estimates in the full sample (rs>0.972), we believe that CS coefficients, centrality confidence limits, and permutation test results based on these resampled networks are very good approximations of those derived from networks implementing the full stepwise algorithm.
Declaration of Conflicting Interests
ZJW serves as a consultant to Roche and owns stock/stock options in Axsome Therapeutics, Editas Medicine, CRISPR Therapeutics, and Fulgent Genetics. ZJW also serves on the family advisory committee of the Autism Speaks Autism Learning Health Network Vanderbilt site and the autistic researcher review board of the Autism Intervention Network for Physical Health (AIR-P). The other authors declare no competing interests.
References
- Barlow DH, Sauer-Zavala S, Carl JR, Bullis JR, & Ellard KK (2014). The nature, diagnosis, and treatment of neuroticism: Back to the future. Clinical Psychological Science, 2(3), 344–365. 10.1177/2167702613505532 [DOI] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). BDI-II, Beck Depression Inventory: Manual (2nd ed). Psychological Corporation. [Google Scholar]
- Benevides TW, Shore SM, Andresen M-L, Caplan R, Cook B, Gassner DL, Erves JM, Hazlewood TM, King MC, Morgan L, Murphy LE, Purkis Y, Rankowski B, Rutledge SM, Welch SP, & Wittig K (2020). Interventions to address health outcomes among autistic adults: A systematic review. Autism, 24(6), 1345–1359. 10.1177/1362361320913664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 57(1), 289–300. 10.2307/2346101 [DOI] [Google Scholar]
- Bernstein EE, Heeren A, & McNally RJ (2019). Reexamining trait rumination as a system of repetitive negative thoughts: A network analysis. Journal of Behavior Therapy and Experimental Psychiatry, 63, 21–27. 10.1016/j.jbtep.2018.12.005 [DOI] [PubMed] [Google Scholar]
- Bezemer ML, Blijd-Hoogewys EMA, & Meek-Heekelaar M (2020). The Predictive Value of the AQ and the SRS-A in the Diagnosis of ASD in Adults in Clinical Practice. Journal of Autism and Developmental Disorders. 10.1007/s10803-020-04699-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MGN, Diamantopoulou S, Stockmann L, Begeer S, & Rieffe C (2018). Emotion Control Predicts Internalizing and Externalizing Behavior Problems in Boys With and Without an Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 48(8), 2727–2739. 10.1007/s10803-018-3519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottema-Beutel K, Kapp SK, Lester JN, Sasson NJ, & Hand BN (2020). Avoiding ableist language: Suggestions for autism researchers. Autism in Adulthood. 10.1089/aut.2020.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, & Thayer JF (2006). The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60(2), 113–124. 10.1016/j.jpsychores.2005.06.074 [DOI] [PubMed] [Google Scholar]
- Brouwer D, Meijer RR, & Zevalkink J (2013). On the factor structure of the Beck Depression Inventory-II: G is the key. Psychological Assessment, 25(1), 136–145. 10.1037/a0029228 [DOI] [PubMed] [Google Scholar]
- Bruni TP (2014). Test Review: Social Responsiveness Scale–Second Edition (SRS-2). Journal of Psychoeducational Assessment, 32(4), 365–369. 10.1177/0734282913517525 [DOI] [Google Scholar]
- Burns A, Irvine M, & Woodcock K (2019). Self-Focused Attention and Depressive Symptoms in Adults with Autistic Spectrum Disorder (ASD). Journal of Autism and Developmental Disorders, 49(2), 692–703. 10.1007/s10803-018-3732-5 [DOI] [PubMed] [Google Scholar]
- Bury SM, Jellett R, Spoor JR, & Hedley D (2020). “It defines who I am” or “It’s something I have”: What language do [autistic] Australian adults [on the autism spectrum] prefer? Journal of Autism and Developmental Disorders. 10.1007/s10803-020-04425-3 [DOI] [PubMed] [Google Scholar]
- Cadman T, Eklund H, Howley D, Hayward H, Clarke H, Findon J, Xenitidis K, Murphy D, Asherson P, & Glaser K (2012). Caregiver Burden as People With Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder Transition into Adolescence and Adulthood in the United Kingdom. Journal of the American Academy of Child & Adolescent Psychiatry, 51(9), 879–888. 10.1016/j.jaac.2012.06.017 [DOI] [PubMed] [Google Scholar]
- Cassidy S, Bradley L, Shaw R, & Baron-Cohen S (2018). Risk markers for suicidality in autistic adults. Molecular Autism, 9(1), 42. 10.1186/s13229-018-0226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AP, Golino H, & Silvia PJ (2020). A Psychometric Network Perspective on the Validity and Validation of Personality Trait Questionnaires. European Journal of Personality, 34(6), 1095–1108. 10.1002/per.2265 [DOI] [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social Responsiveness Scale–Second Edition (SRS-2): Manual (2nd ed.). Western Psychological Services. [Google Scholar]
- Contreras A, Nieto I, Valiente C, Espinosa R, & Vazquez C (2019). The study of psychopathology from the network analysis perspective: A systematic review. Psychotherapy and Psychosomatics, 88(2), 71–83. 10.1159/000497425 [DOI] [PubMed] [Google Scholar]
- Crane L, Adams F, Harper G, Welch J, & Pellicano E (2019). ‘Something needs to change’: Mental health experiences of young autistic adults in England. Autism, 23(2), 477–493. 10.1177/1362361318757048 [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L, & Pring L (2013). Autobiographical memory in adults with autism spectrum disorder: The role of depressed mood, rumination, working memory and theory of mind. Autism, 17(2), 205–219. 10.1177/1362361311418690 [DOI] [PubMed] [Google Scholar]
- Csárdi G, & Nepusz T (2006). The igraph software package for complex network research. InterJournal, Complex Systems, 1695. [Google Scholar]
- Dablander F, & Hinne M (2019). Node centrality measures are a poor substitute for causal inference. Scientific Reports, 9(1), 6846. 10.1038/s41598-019-43033-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels AM, Rosenberg RE, Anderson C, Law JK, Marvin AR, & Law PA (2012). Verification of parent-report of child autism spectrum disorder diagnosis to a web-based autism registry. Journal of Autism and Developmental Disorders, 42(2), 257–265. 10.1007/s10803-011-1236-7 [DOI] [PubMed] [Google Scholar]
- Epskamp S (2020). bootnet: Bootstrap Methods for Various Network Estimation Routines. https://CRAN.R-project.org/package=bootnet [Google Scholar]
- Epskamp S, Borsboom D, & Fried EI (2018). Estimating psychological networks and their accuracy: A tutorial paper. Behavior Research Methods, 50(1), 195–212. 10.3758/s13428-017-0862-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epskamp S, Cramer AOJ, Waldorp LJ, Schmittmann VD, & Borsboom D (2012). qgraph: Network Visualizations of Relationships in Psychometric Data. Journal of Statistical Software, 48(4). 10.18637/jss.v048.i04 [DOI] [Google Scholar]
- Epskamp S, & Fried EI (2018). A tutorial on regularized partial correlation networks. Psychological Methods, 23(4), 617–634. 10.1037/met0000167 [DOI] [PubMed] [Google Scholar]
- Everaert J, & Joormann J (2019). Emotion regulation difficulties related to depression and anxiety: A network approach to model relations among symptoms, positive reappraisal, and repetitive negative thinking. Clinical Psychological Science, 7(6), 1304–1318. 10.1177/2167702619859342 [DOI] [Google Scholar]
- Feliciano P, Daniels AM, Snyder LG, Beaumont A, Camba A, Esler A, Gulsrud AG, Mason A, Gutierrez A, Nicholson A, Paolicelli AM, McKenzie AP, Rachubinski AL, Stephens AN, Simon AR, Stedman A, Shocklee AD, Swanson A, Finucane B, … Chung WK (2018). SPARK: A US Cohort of 50,000 Families to Accelerate Autism Research. Neuron, 97(3), 488–493. 10.1016/j.neuron.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, Eidhof MB, Palic S, Costantini G, Huisman-van Dijk HM, Bockting CLH, Engelhard I, Armour C, Nielsen ABS, & Karstoft K-I (2018). Replicability and generalizability of posttraumatic stress disorder (PTSD) networks: A cross-cultural multisite study of PTSD symptoms in four trauma patient samples. Clinical Psychological Science, 6(3), 335–351. 10.1177/2167702617745092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham KO, Bishop SL, Brunwasser S, & Lord C (2014). Rumination and perceived impairment associated with depressive symptoms in a verbal adolescent-adult ASD sample. Autism Research, 7(3), 381–391. 10.1002/aur.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham KO, Siegle GJ, Han GT, Tomarken AJ, Crist RN, Simon DM, & Bodfish JW (2018). Pupil response to social-emotional material is associated with rumination and depressive symptoms in adults with autism spectrum disorder. PLOS ONE, 13(8), e0200340. 10.1371/journal.pone.0200340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysi DM, Voigt A, Fragoso T. de M., Almaas E, & Nowick K (2018). wTO: An R package for computing weighted topological overlap and a consensus network with integrated visualization tool. BMC Bioinformatics, 19(1), 392. 10.1186/s12859-018-2351-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Wright AGC, & Molenaar PCM (2019). Problems with centrality measures in psychopathology symptom networks: Why network psychometrics cannot escape psychometric theory. Multivariate Behavioral Research, 1–25. 10.1080/00273171.2019.1640103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington R, & Loffredo DA (2010). Insight, rumination, and self-reflection as predictors of well-being. The Journal of Psychology, 145(1), 39–57. 10.1080/00223980.2010.528072 [DOI] [PubMed] [Google Scholar]
- Ho RYF, Zhang D, Chan SKC, Gao TT, Lee EKP, Lo HHM, Au Yeung P, Lai KYC, Bögels SM, de Bruin EI, & Wong SYS (2021). Brief Report: Mindfulness Training for Chinese Adolescents with Autism Spectrum Disorder and Their Parents in Hong Kong. Journal of Autism and Developmental Disorders. 10.1007/s10803-020-04729-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks MJ, Lerh JW, Magiati I, Meiser-Stedman R, & Brugha TS (2019). Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychological Medicine, 49(4), 559–572. 10.1017/s0033291718002283 [DOI] [PubMed] [Google Scholar]
- Huang C, & Chen J-H (2015). Meta-analysis of the factor structures of the Beck Depression Inventory-II. Assessment, 22(4), 459–472. 10.1177/1073191114548873 [DOI] [PubMed] [Google Scholar]
- Hudson CC, Hall L, & Harkness KL (2019). Prevalence of Depressive Disorders in Individuals with Autism Spectrum Disorder: A Meta-Analysis. Journal of Abnormal Child Psychology, 47(1), 165–175. 10.1007/s10802-018-0402-1 [DOI] [PubMed] [Google Scholar]
- Isvoranu A-M, & Epskamp S (2021). Continuous and Ordered Categorical Data in Network Psychometrics: Which Estimation Method to Choose? Deriving Guidelines for Applied Researchers. In PsyArXiv 10.31234/osf.io/mbycn [DOI] [PubMed] [Google Scholar]
- Iverson GL, Jones PJ, Karr JE, Maxwell B, Zafonte R, Berkner PD, & McNally RJ (2020). Network structure of physical, cognitive, and emotional symptoms at preseason baseline in student athletes with attention-deficit/hyperactivity disorder. Archives of Clinical Neuropsychology, 35(7), 1109–1122. 10.1093/arclin/acaa030 [DOI] [PubMed] [Google Scholar]
- Jones PJ (2020). networktools: Tools for Identifying Important Nodes in Networks (1.2.3) [R Package]. https://CRAN.R-project.org/package=networktools
- Jones PJ, Ma R, & McNally RJ (2019). Bridge centrality: A network approach to understanding comorbidity. Multivariate Behavioral Research, 1–15. 10.1080/00273171.2019.1614898 [DOI] [PubMed] [Google Scholar]
- Jones PJ, Mair P, & McNally RJ (2018). Visualizing psychological networks: A tutorial in R. Frontiers in Psychology, 9, 1742. 10.3389/fpsyg.2018.01742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan AL, Marczak M, & Knibbs J (2020). ‘I felt like I was floating in space’: Autistic adults’ experiences of low mood and depression. Journal of Autism and Developmental Disorders. 10.1007/s10803-020-04638-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G, Wozniak J, Petty C, Martelon MK, Fried R, Bolfek A, Kotte A, Stevens J, Furtak SL, Bourgeois M, Caruso J, Caron A, & Biederman J (2013). Psychiatric Comorbidity and Functioning in a Clinically Referred Population of Adults with Autism Spectrum Disorders: A Comparative Study. Journal of Autism and Developmental Disorders, 43(6), 1314–1325. 10.1007/s10803-012-1679-5 [DOI] [PubMed] [Google Scholar]
- Keenan EG, Gotham K, & Lerner MD (2018). Hooked on a feeling: Repetitive cognition and internalizing symptomatology in relation to autism spectrum symptomatology. Autism, 22(7), 814–824. 10.1177/1362361317709603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny L, Hattersley C, Molins B, Buckley C, Povey C, & Pellicano E (2016). Which terms should be used to describe autism? Perspectives from the UK autism community: Autism, 20(4), 442–462. 10.1177/1362361315588200 [DOI] [PubMed] [Google Scholar]
- Knijnenburg TA, Wessels LFA, Reinders MJT, & Shmulevich I (2009). Fewer permutations, more accurate P-values. Bioinformatics, 25(12), i161–i168. 10.1093/bioinformatics/btp211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LP, Richdale AL, Haschek A, Flower RL, Vartuli J, Arnold SR, & Trollor JN (2020). Cross-sectional and longitudinal predictors of quality of life in autistic individuals from adolescence to adulthood: The role of mental health and sleep quality. Autism, 24(4), 954–967. 10.1177/1362361320908107 [DOI] [PubMed] [Google Scholar]
- McNally RJ (2021). Network Analysis of Psychopathology: Controversies and Challenges. Annual Review of Clinical Psychology, 17(1). 10.1146/annurev-clinpsy-081219-092850 [DOI] [PubMed] [Google Scholar]
- Michl LC, McLaughlin KA, Shepherd K, & Nolen-Hoeksema S (2013). Rumination as a mechanism linking stressful life events to symptoms of depression and anxiety: Longitudinal evidence in early adolescents and adults. Journal of Abnormal Psychology, 122(2), 339–352. 10.1037/a0031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri F, de Bildt A, Dekker V, & Anderson GM (2020). Network Analysis of Behaviors in the Depression and Autism Realms: Inter-Relationships and Clinical Implications. Journal of Autism and Developmental Disorders, 50(5), 1580–1595. 10.1007/s10803-019-03914-4 [DOI] [PubMed] [Google Scholar]
- Moseley RL, Druce T, & Turner-Cobb JM (2020). ‘When my autism broke’: A qualitative study spotlighting autistic voices on menopause. Autism, 24(6), 1423–1437. 10.1177/1362361319901184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100(4), 569–582. 10.1037/0021-843X.100.4.569 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2000). The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology, 109(3), 504–511. 10.1037/0021-843X.109.3.504 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2012). Emotion Regulation and Psychopathology: The Role of Gender. Annual Review of Clinical Psychology, 8(1), 161–187. 10.1146/annurev-clinpsy-032511-143109 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Morrow J (1991). A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology, 61(1), 115–121. 10.1037/0022-3514.61.1.115 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, & Lyubomirsky S (2008a). Rethinking Rumination. Perspectives on Psychological Science, 3(5), 400–424. 10.1111/j.1745-6924.2008.00088.x [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, & Lyubomirsky S (2008b). Rethinking rumination. Perspectives on Psychological Science, 3(5), 400–424. 10.1111/j.1745-6924.2008.00088.x [DOI] [PubMed] [Google Scholar]
- Park SH, Song YJC, Demetriou EA, Pepper KL, Norton A, Thomas EE, Hickie IB, Hermens DF, Glozier N, & Guastella AJ (2019). Disability, functioning, and quality of life among treatment-seeking young autistic adults and its relation to depression, anxiety, and stress. Autism, 23(7), 1675–1686. 10.1177/1362361318823925 [DOI] [PubMed] [Google Scholar]
- Pellicano E, Dinsmore A, & Charman T (2014). What should autism research focus upon? Community views and priorities from the United Kingdom. Autism, 18(7), 756–770. 10.1177/1362361314529627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing (4.0.2) [Computer software].R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Raes F, & Hermans D (2008). On the mediating role of subtypes of rumination in the relationship between childhood emotional abuse and depressed mood: Brooding versus reflection. Depression and Anxiety, 25(12), 1067–1070. 10.1002/da.20447 [DOI] [PubMed] [Google Scholar]
- Reichardt J, & Bornholdt S (2006). Statistical mechanics of community detection. Physical Review E, 74(1), 016110. 10.1103/PhysRevE.74.016110 [DOI] [PubMed] [Google Scholar]
- Rieffe C, De Bruine M, De Rooij M, & Stockmann L (2014). Approach and avoidant emotion regulation prevent depressive symptoms in children with an Autism Spectrum Disorder. International Journal of Developmental Neuroscience, 39, 37–43. 10.1016/j.ijdevneu.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Robinaugh DJ, Hoekstra RHA, Toner ER, & Borsboom D (2020). The network approach to psychopathology: A review of the literature 2008–2018 and an agenda for future research. Psychological Medicine, 50(3), 353–366. 10.1017/S0033291719003404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinaugh DJ, Millner AJ, & McNally RJ (2016). Identifying highly influential nodes in the complicated grief network. Journal of Abnormal Psychology, 125(6), 747–757. 10.1037/abn0000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodebaugh TL, Tonge NA, Piccirillo ML, Fried E, Horenstein A, Morrison AS, Goldin P, Gross JJ, Lim MH, Fernandez KC, Blanco C, Schneier FR, Bogdan R, Thompson RJ, & Heimberg RG (2018). Does centrality in a cross-sectional network suggest intervention targets for social anxiety disorder? Journal of Consulting and Clinical Psychology, 86(10), 831–844. 10.1037/ccp0000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin S, Abela J, & Auerbach R (2005). The response styles theory of depression: A test of specificity and causal mediation. Cognition and Emotion, 19(5), 751–761. 10.1080/02699930441000463 [DOI] [Google Scholar]
- Schwarz G (1978). Estimating the Dimension of a Model. Annals of Statistics, 6(2), 461–464. 10.1214/aos/1176344136 [DOI] [Google Scholar]
- Smith IC, & White SW (2020). Socio-emotional determinants of depressive symptoms in adolescents and adults with autism spectrum disorder: A systematic review. Autism, 24(4), 995–1010. 10.1177/1362361320908101 [DOI] [PubMed] [Google Scholar]
- Terluin B, de Boer MR, & de Vet HCW (2016). Differences in connection strength between mental symptoms might be explained by differences in variance: Reanalysis of network data did not confirm staging. PLOS ONE, 11(11), e0155205. 10.1371/journal.pone.0155205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell PD, & Campbell JD (1999). Private self-consciousness and the five-factor model of personality: Distinguishing rumination from reflection. Journal of Personality and Social Psychology, 76(2), 284–304. 10.1037/0022-3514.76.2.284 [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, & Nolen-Hoeksema S (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27(3), 247–259. 10.1023/A:1023910315561 [DOI] [Google Scholar]
- Unruh KE, Bodfish JW, & Gotham KO (2020). Adults with autism and adults with depression show similar attentional biases to social-affective images. Journal of Autism and Developmental Disorders, 50(7), 2336–2347. 10.1007/s10803-018-3627-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Borkulo C, Boschloo L, Borsboom D, Penninx BWJH, Waldorp LJ, & Schoevers RA (2015). Association of Symptom Network Structure With the Course of Depression. JAMA Psychiatry, 72(12), 1219. 10.1001/jamapsychiatry.2015.2079 [DOI] [PubMed] [Google Scholar]
- van Borkulo C, Boschloo L, Kossakowski J, Tio P, Schoevers R, Borsboom D, & Waldorp L (2017). Comparing network structures on three aspects: A permutation test. ResearchGate Preprint. 10.13140/RG.2.2.29455.38569 [DOI] [PubMed] [Google Scholar]
- van Borkulo C, Haslbeck J, Epskamp S, Jones PJ, & Millner A (2019). NetworkComparisonTest: Statistical comparison of two networks based on three invariance measures. (2.2.1) [R Package]. https://cran.r-project.org/web/packages/NetworkComparisonTest/index.html
- Van Hees V, Moyson T, & Roeyers H (2015). Higher Education Experiences of Students with Autism Spectrum Disorder: Challenges, Benefits and Support Needs. Journal of Autism and Developmental Disorders, 45(6), 1673–1688. 10.1007/s10803-014-2324-2 [DOI] [PubMed] [Google Scholar]
- van Heijst BF, Deserno MK, Rhebergen D, & Geurts HM (2020). Autism and depression are connected: A report of two complimentary network studies. Autism, 24(3), 680–692. 10.1177/1362361319872373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins ER (2008). Constructive and unconstructive repetitive thought. Psychological Bulletin, 134(2). 10.1037/0033-2909.134.2.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZJ (2021). network_extra: Additional functions to conduct psychological network analysis in R [R]. 10.13140/RG.2.2.10226.04803/1 [DOI]
- Williams ZJ, Everaert J, & Gotham KO (2020). Measuring Depression in Autistic Adults: Psychometric Validation of the Beck Depression Inventory–II. Assessment, 107319112095288. 10.1177/1073191120952889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZJ, & Gotham KO (2020a). Current and lifetime somatic symptom burden among transition-aged young adults on the autism spectrum. Manuscript Submitted for Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZJ, & Gotham KO (2020b). Improving the Measurement of Alexithymia in Autistic Adults: A Psychometric Investigation and Refinement of the Twenty-item Toronto Alexithymia Scale. Research Square. 10.21203/rs.3.rs-96364/v1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Williams ZJ, & Gotham KO (2021). Assessing global and autism-relevant quality of life in autistic adults: A psychometric investigation using item response theory. Manuscript Submitted for Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, & Horvath S (2005). A General Framework for Weighted Gene Co-Expression Network Analysis. Statistical Applications in Genetics and Molecular Biology, 4(1). 10.2202/1544-6115.1128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


