Abstract
Background:
C-type natriuretic peptide (CNP) is a protective hormone that is synthesized in the kidney and endothelium and possesses anti-remodeling properties. Urinary and plasma CNP levels are elevated in pathophysiological conditions, however, their regulation and prognostic value in heart failure (HF) is unclear.
Objectives:
We sought to characterize urinary and plasma CNP in acute decompensated HF (ADHF), to define their relationship with clinical variables and to determine whether urinary and plasma CNP together, add prognostic value.
Methods:
Urinary and plasma CNP were measured in 109 healthy subjects and 208 ADHF patients and the 95th percentile of CNP values from healthy subjects established normal contemporary cutoffs. ADHF patients were stratified based on urinary and plasma CNP levels for clinical characterization and the assessment of risk for adverse outcomes.
Results:
In both cohorts, there was no significant correlation between urinary and plasma CNP. Urinary and plasma CNP were significantly elevated in ADHF patients and both increased with disease severity and positively correlated with plasma NT-proBNP. 23% of ADHF patients had elevations in both urinary and plasma CNP, while 24% had normal CNP levels. During a median follow-up of 3 years, patients with elevated urinary and plasma CNP had significantly higher risk of rehospitalization/death (HR = 1.79, P = 0.03) and rehospitalization (HR = 2.16, P = 0.01) after adjusting for age, sex, LVEF, renal function and plasma NT-proBNP. The c-statistic and integrated discrimination analyses further supported that the addition of urinary and plasma CNP to established risk models improved the prediction of adverse outcomes in ADHF patients.
Conclusions:
Urinary and plasma CNP are differentially regulated in ADHF, and elevations in both, provided independent prognostic value for predicting adverse outcomes.
Keywords: C-type natriuretic peptide, Urinary, Plasma, Acute decompensated heart failure, Prognosis, Rehospitalization
INTRODUCTION
C-type natriuretic peptide (CNP) is a hormone belonging to the family of protective cardiorenal hormones that include atrial natriuretic peptide (ANP) and b-type natriuretic peptide (BNP). While ANP and BNP are cardiac-derived hormones, CNP is predominantly produced in the kidney (1,2) and endothelium (3). Indeed, CNP is synthesized in response to a number of stimuli, such as hypoxia, proinflammatory and profibrotic cytokines, and shear stress (4–6), which commonly lead to organ injury, remodeling, and dysfunction. Studies have reported that CNP plays a key compensatory role in maintaining cardiovascular (CV) and renal homeostasis through its salutary actions including the inhibition of fibrosis (7,8), suppression of cardiac hypertrophy (9,10), inhibition of mesangial cell proliferation (11), regulation of vascular tone (12,13), and venodilatation (14). In accordance with these findings are human studies demonstrating that CNP levels are elevated in patients with pathophysiological stress such as that induced by renal diseases, diabetes, and heart failure (HF) (15–19).
A classical feature of HF is the elevation of plasma ANP and BNP, which is thought to be a compensatory response (20,21) as both peptides serve as circulating markers of structure, function, and prognosis in HF. Unlike plasma ANP and BNP, the understanding of CNP and its clinical value in HF remain far less established. Beyond plasma CNP levels, which have been reported to be elevated in HF (16–18), CNP is markedly present in the urine (1). An emerging concept is that the elevation of urinary CNP is a marker of renal injury and structural abnormalities (2,15,19,22). As renal impairment contributes to HF progression and is associated with adverse outcomes (23,24), the evaluation of urinary CNP, together with plasma CNP levels, may provide important pathophysiological insights and aid in risk assessment, which to date, has yet to be investigated.
Thus, the goal of our current study was to advance the understanding of urinary and plasma CNP in human acute decompensated HF (ADHF). We first determined proof-of-principle contemporary normal values of urinary and plasma CNP levels in a healthy cohort, as no such values exist and then examined their relationships to clinical variables under physiological conditions. Additionally, we also sought to define the relationship of urinary and plasma CNP to clinical variables, in comparison to plasma NT-proBNP, in a cohort of ADHF patients. We then determined their clinical phenotype based on whether they had normal or elevated levels of urinary and plasma CNP. We hypothesized that urinary and plasma CNP in ADHF would: 1) be elevated compared to healthy subjects; 2) not be significantly correlated with one another; and 3) together, add prognostic value to established risk models for adverse outcomes.
METHODS
Study populations
This study was approved by the institutional review board at the Mayo Clinic. Healthy subjects were identified, screened, and recruited from the Mayo Clinic Biobank as previously reported (25). Healthy subjects were consented if they met the criteria of being a non-smoker, had no history of CV or systemic diseases, were not taking any CV medications, and were willing to provide a blood and urine sample. ADHF patients hospitalized at Mayo Clinic Hospital (St. Mary’s Campus, Rochester, MN) were identified, enrolled, and followed from an ongoing registry of admissions between August 2009 and September 2017. ADHF patients were consented if they had a clinical diagnosis of HF consistent with the Framingham criteria (26) and were willing to provide a blood and urine sample. A baseline history assessment including comorbidities and medications at admission, a physical examination, and transthoracic echocardiography were conducted as part of routine clinical care. A total of 109 healthy and 208 ADHF non-consecutively enrolled participants who met our criteria and had sufficient urine and plasma sample available for CNP measurements were evaluated in the current study.
Urinary and Plasma Samples
For healthy subjects, urine and blood samples were collected on enrollment. For ADHF patients, 24-hour urine and blood samples were obtained within 72 hours of admission. Blood samples were drawn into EDTA tubes, immediately placed on ice, then centrifuged at 4°C and 2500 rpm for 10 minutes. Plasma samples were aliquoted and stored at −80°C until analysis. Urine samples were collected into containers that were stored on ice and contained acetic acid (30 mL of 1:1 acetic acid; 17.4M). After collection, the total urine volume was recorded, and samples were aliquoted and stored at −80°C until analysis. Plasma creatinine and urinary creatinine and protein were measured by the central clinical laboratory and renal laboratory, respectively, at Mayo Clinic following standard laboratory procedures. Plasma NT-proBNP was measured with the Elecsys NT-proBNP electrochemiluminescence immunoassay (Roche Diagnostics) in the central clinical laboratory at Mayo Clinic. In healthy subjects, the 95th percentile of plasma NT-proBNP was 203 pg/mL. All urinary components were normalized to urinary creatinine to reduce dilutional variation associated with volume difference (27). Plasma creatinine levels were 0.83 [interquartile range (IQR): 0.75–0.96] mg/dL in healthy subjects and 1.3 (IQR: 1.0–1.6) mg/dL in ADHF patients. Urinary creatinine levels were 70 (IQR: 32–130) mg/dL in healthy subjects and 43 (IQR: 28–75) mg/dL in ADHF patients. Estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) equation.
Analysis of Urinary and Plasma CNP
Urinary and plasma CNP was measured using a non-equilibrium radioimmunoassay (RIA) kit (Phoenix Pharmaceutical, Burlingame, CA, Catalog #: RK-012–03) with an antibody that detects human CNP. Stored urine and plasma samples were thawed on the day of the assay. 100 μl of urine sample, plasma sample or standards were incubated with 100 μl of the CNP antibody at 4 °C for 18 hours. 100 μl of 125I-labeled CNP (supplied with the kit) was added to all tubes and incubated at 4 °C for another 18 hours. Normal rabbit serum (100 μl) and goat anti-rabbit serum (100 μl) were then added to all samples to separate the free and bound fractions, and samples were centrifuged, the free fraction was aspirated, and the bound fraction was counted on a gamma counter. The range of the standard curve used in the assay was 1 to 128 pg, with the lowest detection of 1 pg and 4-PL sigmoidal curve was used to extrapolate the unknown concentrations. Inter- and intra-assay coefficient of variation were 9.6% and 6.4%, respectively. There was no detectable cross-reactivity with ANP, urodilatin and BNP below 600 pg/mL (Supplemental Table 1). The cross-reactivity with BNP at 600 pg/mL was 1.3%. This was verified by addition of synthetic ANP, BNP and urodilatin (Phoenix Pharmaceutical, Burlingame, CA) to assay buffer at concentrations ranging from 0 to 600 pg/mL and then measured in the CNP RIA. The fractional CNP excretion was calculated with the formula: (urinary CNP x plasma creatinine) / (plasma CNP x urinary creatinine).
Statistical analysis
Descriptive variables were presented as mean ± SD for those following a normal distribution and as median (IQR) for those that did not follow an approximate normal distribution. Comparisons between healthy subjects and ADHF patients were conducted using appropriate linear or logistic regression methods to allow adjustment for age, sex, and body mass index (BMI). Urinary CNP, plasma CNP, and plasma NT-proBNP were right-skewed distributed in both healthy subjects and ADHF patients, and thus were log-transformed to normalize the distributions. Pearson’s correlation coefficients were used to determine the correlation between urinary CNP and plasma CNP after log transformation. Multivariable regression models were constructed to determine independent characteristics associated with the log transformed plasma CNP and urinary CNP levels, incorporating variables with univariable association significant at P<0.10.
Urinary and plasma CNP concentrations in healthy subjects were referred to set thresholds for the stratification of ADHF patients as previously reported (25). Specifically, patients were classified as having elevated CNP levels if their levels were greater than or equal to the 95th percentile threshold. All-cause rehospitalization and all-cause mortality were ascertained from institutional records, including local primary care data. Patients were otherwise censored at the time of last known clinical follow-up. Event free survival curves were generated using the Kaplan-Meier method based on time to event outcomes and comparisons between groups were performed with log-rank test. Cox proportional hazards regression methods were used to study the association between time to event outcomes and CNP levels while accounting for other important covariates. Results of these analyses were presented with hazard ratio (HR) and corresponding 95% confidence limit. C-statistic and integrated discrimination index (IDI) were calculated to quantify discriminatory ability and to evaluate the improvement in prediction accuracy of the combination of urinary and plasma CNP over known risk factors and plasma NT-proBNP, for outcomes. IDI confidence intervals and comparison P-values were derived based on 1000 bootstrap samples, each having sample size of 208. A sensitivity analysis was performed using the Get With The Guidelines-HF (GTWG-HF) risk score (28) in place of known risk factors. SAS version 9.4 and JMP (Cary, NC) were used for analyses and two-sided P<0.05 was considered statistically significant.
RESULTS
Study Populations
Baseline clinical characteristics are shown in Table 1. Compared to healthy subjects, ADHF patients were older, more male, had higher BMI, and lower eGFR (P<0.001 for all). ADHF patients had comorbidities, were taking CV medications, and the median length of hospital stay (LOHS) was 6 (IQR: 4–10) days. 45% of patients were New York Heart Association (NYHA) class 3 and had a mean left ventricular ejection fraction (LVEF) of 37±17 %. Plasma NT-proBNP levels were significantly elevated in ADHF patients compared to healthy subjects. The urinary protein levels between healthy subjects [0.07 (IQR: 0.05–0.09) mg/mg Cr] and ADHF patients [0.05 (IQR: 0.03–0.12) mg/mg Cr] were not significantly different (P=0.10).
Table 1.
Clinical Characteristics
| Characteristics | Healthy Subjects (n=109) | ADHF Patients (n=208) | Adjusted P Value |
|---|---|---|---|
| Age, years | 56 ± 12 | 69 ± 13 | <0.001 |
| Sex, female (%) | 70% | 32% | <0.001 |
| BMI, kg/m2 | 27 ± 5 | 32 ± 8* | <0.001 |
| LVEF, % | --- | 37 ± 17† | |
| eGFR, mL/min/1.73m2 | 85 ± 13 | 58 ± 24 | <0.001 |
| Length of hospital stay, days | --- | 6 (4, 10) | |
| Comorbidities (at admission) | |||
| Hypertension, n (%) | --- | 150 (72%) | |
| Diabetes, n (%) | --- | 79 (38%) | |
| Atrial fibrillation, n (%) | --- | 125 (60%) | |
| Ischemic heart disease, n (%) | --- | 130 (63%) | |
| Myocardial infarction, n (%) | --- | 47 (23%) | |
| Hyperlipidemia, n (%) | --- | 136 (66%) | |
| Stroke, n (%) | --- | 10 (5%) | |
| Medications (at admission) | |||
| ACEi or ARB, n (%) | --- | 134 (64%) | |
| Beta-blocker, n (%) | --- | 135 (65%) | |
| Loop diuretic, n (%) | --- | 203 (97%) | |
| Statin, n (%) | --- | 124 (59%) | |
| Plasma Variables | |||
| NT-proBNP, pg/mL | 56 (29, 85) | 2791 (1286, 7029) | <0.001 |
| CNP, pg/mL | 11.8 (9.5, 15.0) | 18.2 (10.7, 30.3) | <0.001 |
| Urinary Variable | |||
| CNP, ng/g Cr | 15.8 (12.1, 20.2) | 43.1 (27.9, 74.7) | <0.001 |
Values are presented as mean ± SD, n (%), or median (interquartile range).
n=154
n=198.
P values have been adjusted for age, sex, and BMI.
BMI = body mass index; LVEF = left ventricular ejection fraction; eGFR = estimated glomerular filtration rate; ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; NT-proBNP = N-terminal pro-B type natriuretic peptide; CNP = C-type natriuretic peptide; Cr = creatinine.
Urinary and Plasma CNP Levels
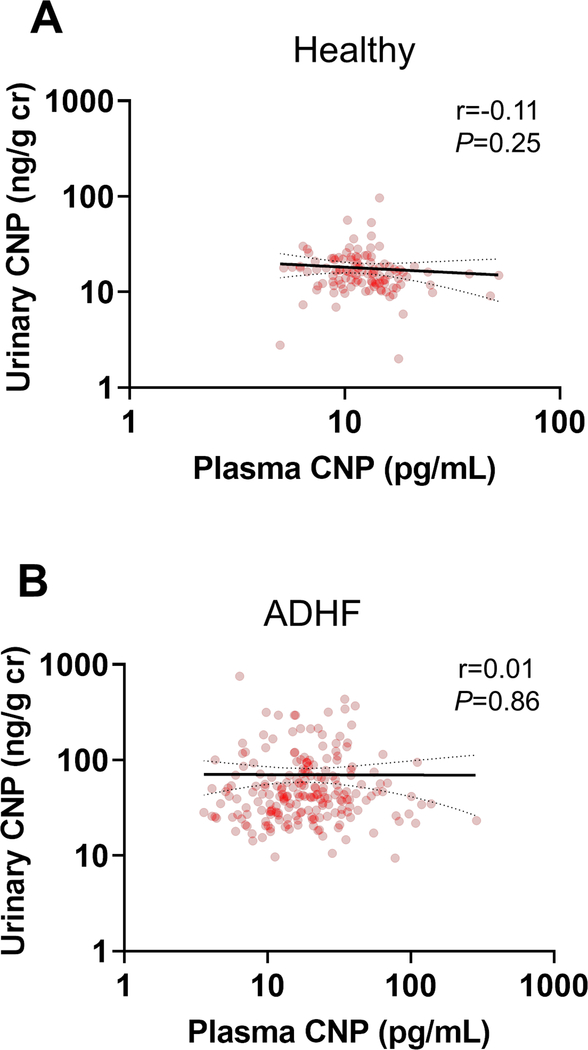
Urinary and plasma CNP levels were significantly elevated in ADHF patients compared to healthy subjects (Table 1). Importantly, there was no significant correlation between urinary and plasma CNP in healthy subjects (Figure 1A) or ADHF patients (Figure 1B). The accuracy of urinary and plasma CNP in diagnosing ADHF was evaluated by area under the curve (AUC) and positive predictive value (PPV). In ADHF patients, the AUC for elevated urinary CNP was 0.92 (95%CI: 0.89–0.95) with a PPV of 93% (95%CI: 89–96%), while the AUC for elevated plasma CNP was 0.69 (95%CI: 0.64–0.75) with a PPV of 93% (95%CI: 86–97%). Furthermore, the fractional CNP excretion in ADHF patients [0.04 (IQR: 0.02–0.07)] was 4 times higher than in healthy subjects [0.01 (IQR: 0.01–0.02)].
Figure 1. CNP Correlations.
Urinary and plasma CNP in healthy subjects (A) and ADHF patients (B). CNP = C-type natriuretic peptide; ADHF = acute decompensated heart failure
Clinical Associations of CNP in Healthy Subjects and ADHF Patients
Univariable and multivariable regression analysis are shown in healthy (Supplemental Table 2, 3) and ADHF cohorts (Table 2, Supplemental Table 4). In healthy subjects, both urinary and plasma CNP were not associated with age, sex, and BMI, while in contrast, plasma NT-proBNP was affected by age and sex after multivariable adjustment. Meanwhile, urinary CNP and plasma NT-proBNP were positively and negatively, respectively, associated with eGFR. In ADHF patients, multivariable analysis revealed that plasma CNP positively correlated with LOHS, while urinary CNP positively correlated with female sex and LVEF. When ADHF patients were separated by LVEF ≤ 40% (reduced EF) and LVEF > 40% (preserved EF) (Supplemental Figure 1), ADHF patients with preserved EF had significantly higher urinary CNP and lower plasma NT-proBNP compared to ADHF patients with reduced EF, while no differences were observed with plasma CNP. Moreover, there were no significant associations between CV medications or comorbidities with urinary and plasma CNP levels. Despite the significant positive correlation of urinary and plasma CNP with plasma NT-proBNP, the clinical associations of urinary and plasma CNP were markedly different from those of plasma NT-proBNP in ADHF patients.
Table 2.
Multivariable Analysis of Urinary CNP, Plasma CNP, and Plasma NT-proBNP in ADHF Patients
| Log10(urinary CNP) | Log10(plasma CNP) | Log10(plasma NT-proBNP) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictors (Multivariable)† | β (SE) | P Value | β (SE) | P Value | β (SE) | P Value |
| Age | −0.0002 (0.002) | 0.85 | --- | --- | 0.003 (0.003) | 0.34 |
| Sex, female | 0.09 (0.03) | 0.005 | --- | --- | --- | --- |
| BMI | −0.002 (0.003) | 0.49 | --- | --- | −0.01 (0.004) | 0.02 |
| LVEF | 0.004 (0.002) | 0.02 | --- | --- | −0.007 (0.002) | 0.002 |
| eGFR | --- | --- | 0.00003 (0.001) | 0.97 | −0.004 (0.002) | 0.01 |
| Length of Hospital Stay | --- | --- | 0.003 (0.001) | 0.04 | --- | --- |
| Hypertension | --- | --- | 0.03 (0.02) | 0.30 | 0.01 (0.04) | 0.79 |
| Atrial fibrillation | 0.05 (0.03) | 0.05 | 0.02 (0.02) | 0.32 | --- | --- |
| Ischemic heart disease | 0.03 (0.03) | 0.31 | 0.02 (0.02) | 0.49 | −0.02 (0.04) | 0.61 |
| Myocardial infarction | --- | --- | --- | --- | 0.05 (0.04) | 0.30 |
| Stroke | --- | --- | --- | --- | 0.02 (0.08) | 0.81 |
| ACEi or ARB | --- | --- | --- | --- | −0.07 (0.04) | 0.07 |
| Loop diuretic | --- | --- | 0.05 (0.02) | 0.42 | --- | --- |
| Statin | −0.02 (0.03) | 0.46 | --- | --- | --- | --- |
| Log10(plasma NT-proBNP) | 0.19 (0.06) | 0.002 | 0.25 (0.05) | <0.001 | --- | --- |
| Log10(plasma CNP) | --- | --- | --- | --- | 0.44 (0.11) | <0.001 |
| Log10(urinary CNP) | --- | --- | --- | --- | 0.35 (0.10) | 0.0008 |
Log10 transformation was performed on urinary CNP, plasma CNP, and plasma NT-proBNP.
Predictors were selected for multivariable analysis based on a cutoff of P<0.10 in univariable analysis (Supplemental Table 3).
In multivariable model for Log10(urinary CNP), R2=0.25; In multivariable model for Log10(plasma CNP), R2=0.23; In multivariable model for Log10(plasma NT-proBNP), R2=0.45.
SE = standard error; other abbreviations as in Table 1.
95th Percentile Values of Urinary and Plasma CNP in Healthy Subjects
The 95th percentile for urinary CNP and plasma CNP in healthy subjects was 33.1 ng/g Cr and 24.7 pg/mL, respectively.
Characterization of ADHF Patients with Normal and Elevated Urinary and Plasma CNP levels
In ADHF, 24% had normal urinary and plasma CNP, 9% had normal urinary CNP and elevated plasma CNP, 44% had elevated urinary CNP and normal plasma CNP, and 23% had elevated urinary and plasma CNP (Supplemental Figure 2). Clinical characteristics among the 4 subgroups are shown in Table 3. LVEF, eGFR, LOHS, the presence of atrial fibrillation (AF), and plasma NT-proBNP levels were found to be different among subgroups (P<0.05 for all). The subgroup with elevated levels of both urinary and plasma CNP was associated with more disease severity as these patients had the highest level of plasma NT-proBNP, lowest eGFR, longest LOHS, and highest proportion of AF. The subgroup with elevated levels of urinary CNP, but normal plasma CNP, had the highest LVEF. The subgroup with elevated plasma and normal urinary CNP had similar clinical characteristics as the subgroup with normal levels of both plasma and urinary CNP (P >0.05 for all, multiple comparisons), except for plasma NT-proBNP levels.
Table 3.
Clinical Characteristics of ADHF Patients Grouped by Urinary and Plasma CNP Levels
| Variable | Normal uCNP Normal pCNP (n=51) |
Normal uCNP Elevated pCNP (n=19) |
Elevated uCNP Normal pCNP (n=91) |
Elevated uCNP Elevated pCNP (n=47) |
P value | Adjusted P value† |
|---|---|---|---|---|---|---|
| Age, years | 66 ± 12 | 67 ± 15 | 68 ± 13 | 73 ± 13 | 0.04 | 0.06 |
| Sex, female (%) | 20% | 21% | 40% | 34% | 0.07 | 0.10 |
| BMI, kg/m2 | 34 ± 9 | 33 ± 9 | 32 ± 8 | 29 ± 9 | 0.05 | 0.14 |
| LVEF, % | 32 ± 15 | 30 ± 15 | 42 ± 18 | 35 ± 18 | 0.002 | 0.01 |
| eGFR, mL/min/1.73m2 | 64 ± 27 | 52 ± 21 | 60 ± 24 | 49 ± 21 | 0.10 | 0.05 |
| Length of hospital stay, days | 6 (4, 9) | 6 (3, 12) | 5 (3, 8) | 7 (4, 17) | 0.16 | 0.005 |
| Comorbidities | ||||||
| Hypertension, n (%) | 37 (76%) | 16 (84%) | 59 (65%) | 37 (79%) | 0.16 | 0.18 |
| Diabetes, n (%) | 17 (35%) | 8 (42%) | 42 (46%) | 12 (26%) | 0.11 | 0.10 |
| Atrial fibrillation, n (%) | 21 (43%) | 11 (58%) | 58 (64%) | 35 (74%) | 0.01 | 0.02 |
| Ischemic heart disease, n (%) | 27 (55%) | 12 (63%) | 57 (63%) | 33 (70%) | 0.50 | 0.63 |
| Myocardial infarction, n (%) | 11 (22%) | 4 (21%) | 18 (20%) | 14 (30%) | 0.61 | 0.76 |
| Hyperlipidemia, n (%) | 32 (65%) | 14 (74%) | 57 (63%) | 33 (70%) | 0.72 | 0.84 |
| Stroke, n (%) | 1 (2%) | 1 (5%) | 3 (3%) | 4 (9%) | 0.41 | 0.59 |
| Medications | ||||||
| ACEi or ARB, n (%) | 32 (65%) | 14 (74%) | 57 (63%) | 33 (70%) | 0.70 | 0.81 |
| Beta-blocker, n (%) | 35 (69%) | 12 (63%) | 57 (63%) | 30 (64%) | 0.62 | 0.84 |
| Loop diuretic, n (%) | 47 (92%) | 19 (100%) | 89 (98%) | 47 (100%) | 0.09 | 0.51 |
| Statin, n (%) | 34 (67%) | 12 (63%) | 48 (53%) | 30 (64%) | 0.35 | 0.65 |
| Plasma variables | ||||||
| NT-proBNP, pg/mL | 1589 (647, 2999) | 4457 (1942, 9869) | 2301 (1292, 5407) | 7441 (3462, 14029) | <0.001 | <0.001 |
| CNP, pg/mL | 11.3 (7.8, 16.3) | 37.5 (31.9, 78.9) | 15.4 (9.6, 19.1) | 35.7 (30.1, 48.9) | <0.001 | <0.001 |
| Urinary variables | ||||||
| CNP, ng/g Cr | 24.5 (20.2, 28.1) | 25.0 (19.5, 28.0) | 55.8 (43.5, 99.5) | 62.6 (42.0, 114.6) | <0.001 | <0.001 |
| Protein, mg/mg Cr | 0.04 (0.02, 0.08) | 0.06 (0.02, 0.09) | 0.06 (0.03, 0.12) | 0.08 (0.03, 0.16) | 0.03 | 0.23 |
Values are presented as mean ± SD, n (%), or median (interquartile range).
Age and sex adjusted.
uCNP = urinary CNP; pCNP = plasma CNP; other abbreviations as in Table 1.
Clinical Outcomes
During a median (IQR) follow-up of 3.0 (1.0, 4.9) years, the composite clinical outcome of all-cause rehospitalization/death occurred in 176 ADHF patients. There were 114 deaths and 131 rehospitalizations. The rates of events at 3 years were 42% for death, 70% for rehospitalization, and 77% for rehospitalization/death.
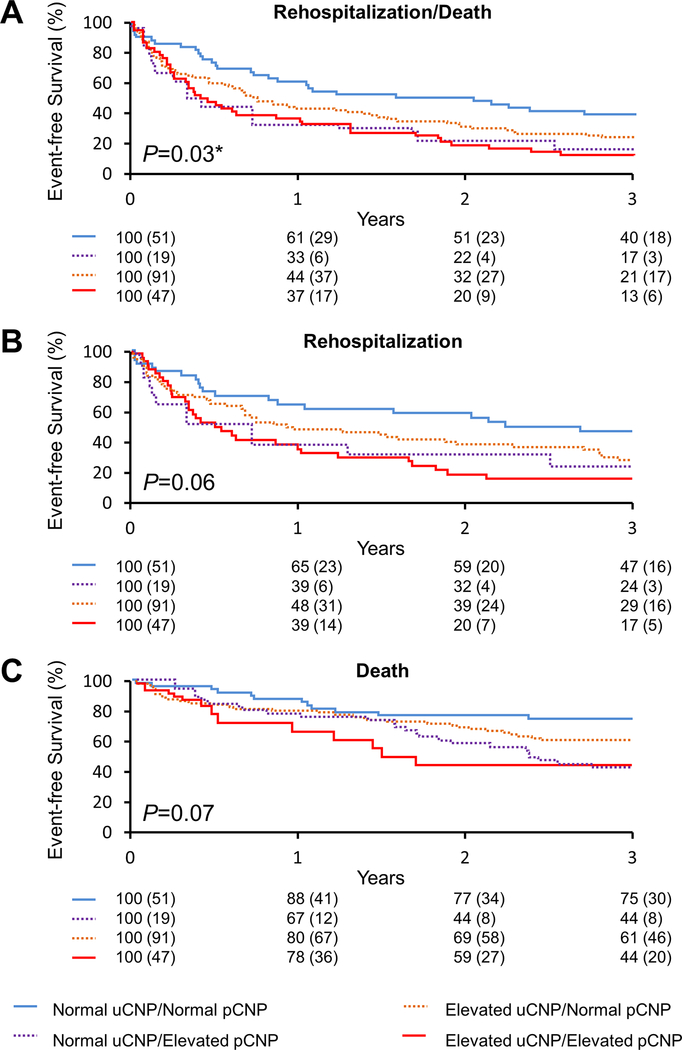
Kaplan Meier analysis revealed persistent divergence of event free survival rates out to 3 years, particularly in those ADHF patients with elevated urinary and plasma CNP (Figure 2). Cox regression analysis illustrated in Table 4 revealed that compared to patients with normal urinary and plasma CNP levels (reference group), only patients with an elevation in both urinary and plasma CNP showed a significantly higher risk of rehospitalization/death and rehospitalization even after adjusting for age, sex, eGFR, urinary protein/creatinine ratio, LVEF and plasma NT-proBNP. The risk of death alone was not different significantly among four subgroups in all adjusted models.
Figure 2. Clinical Outcomes in ADHF Patients Stratified by CNP Levels.
Event-free survival curves for ADHF patients with follow-up to 3 years for (A) rehospitalization/death, (B) rehospitalization, and (C) death. uCNP = urinary C-type natriuretic peptide; pCNP = plasma C-type natriuretic peptide.
Table 4.
Predictive Values of Urinary and Plasma CNP Levels for Clinical Outcomes in ADHF Patients
| Model | Outcome |
|||||
|---|---|---|---|---|---|---|
| Rehospitalization/Death | Rehospitalization | Death | ||||
|
| ||||||
| HR(95% CI) | P value | HR(95% CI) | P value | HR(95% CI) | P value | |
| Normal urinary CNP / Normal plasma CNP | reference | |||||
|
| ||||||
| Normal urinary CNP / Elevated plasma CNP | ||||||
|
| ||||||
| Unadjusted | 1.76(0.98, 3.15) | 0.06 | 1.75(0.90, 3.38) | 0.10 | 1.80(0.84, 3.82) | 0.13 |
| Model 1 | 1.65(0.92, 2.97) | 0.21 | 1.69(0.87, 3.30) | 0.12 | 1.56(0.73, 3.34) | 0.25 |
| Model 2 | 1.67(0.92, 3.03) | 0.09 | 1.72(0.88, 3.38) | 0.12 | 1.64(0.76, 3.55) | 0.21 |
| Model 3 | 1.61(0.87, 2.98) | 0.13 | 1.72(0.85, 3.46) | 0.26 | 1.25(0.55, 2.86) | 0.60 |
|
| ||||||
| Elevated urinary CNP / Normal plasma CNP | ||||||
|
| ||||||
| Unadjusted | 1.46(0.99, 2.14) | 0.05 | 1.42(0.90. 2.22) | 0.13 | 1.57(0.94, 2.62) | 0.08 |
| Model 1 | 1.46(0.98, 2.19) | 0.07 | 1.50(0.92, 2.44) | 0.10 | 1.46(0.86, 2.47) | 0.16 |
| Model 2 | 1.44(0.96, 2.17) | 0.08 | 1.47(0.90, 2.40) | 0.12 | 1.46(0.86, 2.48) | 0.17 |
| Model 3 | 1.41(0.93, 2.15) | 0.13 | 1.49(0.89, 2.48) | 0.13 | 1.29(0.74, 2.23) | 0.37 |
|
| ||||||
| Elevated urinary CNP/ Elevated plasma CNP | ||||||
|
| ||||||
| Unadjusted | 1.91(1.24, 2.94) | 0.004 | 1.97(1.19, 3.27) | 0.009 | 2.08(1.20, 3.10) | 0.009 |
| Model 1 | 1.77(1.11, 2.81) | 0.02 | 1.92(1.11, 3.33) | 0.02 | 1.55(0.88, 2.72) | 0.13 |
| Model 2 | 1.77(1.11, 2.83) | 0.02 | 1.92(1.10, 3.34) | 0.02 | 1.60(0.90, 2.82) | 0.11 |
| Model 3 | 1.79(1.07, 3.02) | 0.03 | 2.16(1.16, 4.03) | 0.01 | 1.26(0.66, 2.38) | 0.49 |
Unadjusted and adjusted proportional hazards regression analysis. Model 1, adjusted for age, sex, eGFR and urinary protein/creatinine ratio; Model 2, adjusted for age, sex, eGFR, urinary protein/creatinine ratio and LVEF; Model 3, adjusted for age, sex, eGFR, urinary protein/creatinine ratio, LVEF and plasma NT-proBNP.
HR = hazard ratio; CI = confidence interval; other abbreviations as in Table 1.
The added predictive value of urinary and plasma CNP over established risk factors in relation to rehospitalization/death and rehospitalization were further assessed by calculating the C-statistic and IDI as illustrated in Table 5. In a sensitivity analysis, the GWTG-HF risk model (28) was used in place of established risk factors (Supplemental Table 5). In both analyses, the addition of urinary and plasma CNP showed a moderate increase in C-statistic for both outcomes. Significant increases in the IDI and relative IDI provide further evidence of the additive value of urinary and plasma CNP to established risk models in predicting the risk of rehospitalization/death and rehospitalization in ADHF patients.
Table 5.
Reclassification Analyses for Prognostic Value of Urinary and Plasma CNP Levels for Clinical Outcomes in ADHF Patients
| Model | C-statistic | IDI (95% CI) | Relative IDI (95% CI) | P value |
|---|---|---|---|---|
| Rehospitalization/Death | ||||
| Base model | 0.59 (0.54, 0.63) | |||
| Base model + uCNP/pCNP groups | 0.60 (0.56, 0.65) | 0.028 (0.003, 0.079)* | 46.5% (0.2%, 174.0%)* | 0.005* |
| Base model + NT-proBNP | 0.60 (0.55, 0.64) | |||
| Base model + NT-proBNP + uCNP/pCNP groups | 0.61 (0.56, 0.65) | 0.022 (0.001, 0.072)# | 30.0% (0.0%, 115.0%)# | 0.01# |
| Rehospitalization | ||||
| Base model | 0.59 (0.53, 0.64) | |||
| Base model + uCNP/pCNP groups | 0.60 (0.55, 0.65) | 0.034 (0.003, 0.095)* | 49.1% (0.2%, 185.0%)* | 0.004* |
| Base model + NT-proBNP | 0.59 (0.54, 0.65) | |||
| Base model + NT-proBNP + uCNP/pCNP groups | 0.61 (0.55, 0.66) | 0.036 (0.004, 0.103)# | 45.8% (0.6%, 164.0%)# | 0.004# |
Base model included age, sex, eGFR, urinary protein/creatinine ratio and LVEF.
uCNP/pCNP groups, the 4 subgroups based on urinary and plasma CNP levels as indicated in Table 3 and Table 4.
IDI analyses compared to base model
IDI analyses compared to base model + NT-proBNP; the 95% CIs and P values were calculated based on 1000 bootstrap samples.
IDI = integrated discrimination index; CI = confident interval.
DISCUSSION
Here we reported, to our knowledge, the largest study that simultaneously investigated urinary and plasma CNP in humans. Specifically, we found urinary and plasma CNP was not significantly correlated which supports, in part, their levels may offer distinct biological insights in both healthy and ADHF populations. In ADHF, both urinary and plasma CNP increased with disease severity and positively correlated with plasma NT-proBNP. Importantly, the elevations in both urinary and plasma CNP, predicted rehospitalization/death and rehospitalization, and had additive predictive value for both endpoints on top of established risk factors in patients with ADHF.
The protective and therapeutic potential of CNP in HF and HF-related CV diseases is growing in recognition (9,10,12,13,29–31). Nonetheless, far less is known regarding the biology and clinical utility of CNP in humans compared to that of ANP and BNP, and in particular, the majority of studies have focused on the plasma CNP (16,18,32,33) which is predominantly of endothelial cell origin. Herein, we extended to also include urinary CNP, as the kidney is a rich source of CNP production and elevations in urinary CNP has been reported to serve as a compensatory marker for renal injury and structural abnormalities (1,2,15,19,22). To underscore the importance of CNP in HF, we first characterized urinary and plasma CNP in a cohort of healthy subjects, where we observed no significant correlation between urinary and plasma CNP. Moreover, physiologic levels of urinary and plasma CNP were found to be unaffected by age, sex, and BMI, while only urinary CNP showed a modest and positive association with eGFR. This observed correlation may reflect, in part, the clearance of CNP via glomerular filtration, in addition to, its secretion from the post-glomerular circulation and thus supports a contribution of plasma CNP to urinary CNP under healthy conditions.
Herein, we observed marked elevations of urinary and plasma CNP in ADHF patients, which are consistent with previous studies in cohorts with cardiorenal disease or injury (15,17,18,32). Furthermore, fractional CNP excretion in ADHF patients was higher than that found in healthy subjects. Notably, the diagnostic accuracy of urinary CNP for ADHF was superior to that of plasma CNP and there was no significant correlation between urinary and plasma CNP as well as between eGFR and urinary CNP. Together, this data highlights the potential importance of CNP in the ADHF, while providing evidence that urinary and plasma CNP are from different sites of origin and that each provide distinct information related to ADHF pathophysiology. This is in contrast to NT-proBNP where previous studies have demonstrated a significant positive correlation between plasma and urinary NT-proBNP, as well as a significant inverse correlation between eGFR and both plasma and urinary NT-proBNP in acute HF (34). Given the presence of the natriuretic peptide clearance receptor and the CNP degrading enzyme neprilysin within the kidney, which is reported to elevated in congestive HF (35), it is reasonable to conclude that the local compensatory activation and secretion of CNP within kidney is the major source that contributes to urinary CNP in ADHF patients.
ADHF is a heterogeneous syndrome, and the differential regulation of urinary and plasma CNP observed within our ADHF cohort is also reflective of this heterogeneity. Using defined cutoff values based on the healthy cohort, we observed that 24% of ADHF patients had normal levels of urinary and plasma CNP, and approximately half of the patients had an elevation in either urinary or plasma CNP. Notably, the remaining 23% of ADHF patients had elevated levels of both urinary and plasma CNP, and these patients had the highest level of plasma NT-proBNP, highest prevalence of AF, lowest eGFR, and longest LOHS, which altogether may serve as an indication of a more severe HF phenotype. While significant correlations between urinary and plasma CNP with plasma NT-proBNP in ADHF patients was observed in multivariable analysis, patients with elevations in both urinary and plasma CNP also had the highest risk for rehospitalization/death and rehospitalization over the 3-year follow-up even after adjusting for established risk factors including plasma NT-proBNP. The improvement in C-statistic and risk reclassification analyses further supports the added prognostic utility of incorporating urinary and plasma CNP in established risk models in ADHF.
Previous studies have reported the significant elevations of plasma CNP and its prognostic value in heart failure (16). Herein, we further corroborate these findings and importantly demonstrate for the first time, the vital prognostic value of urinary CNP together with plasma CNP in ADHF. The mechanism(s) for the increase in CNP in ADHF pathophysiology remains to be elucidated, however it is tempting to speculate that congestion, a hallmark feature in ADHF (36), plays a key role in CNP activation and subsequent elevations of CNP in the plasma and urine. Indeed, congestion induces neurohumoral activation and hypoxia, the release of pro-inflammatory and pro-fibrotic cytokines such as tumor necrosis factor-α, transforming growth factor-β and interleukin-1 and elevates renal interstitial pressure, all of which are known stimuli for CNP production and secretion (4–6). Thus, our data reporting the elevation in urinary and plasma CNP in ADHF could be interpreted as a protective compensatory response to counteract maladaptive effects related to congestion in the kidney and endothelium, as CNP has been reported to increase microvascular arterial vasodilation, promote venodilatation, and inhibit pro-inflammatory and pro-fibrotic processes (5–8,12–14). As ADHF is a challenging syndrome, the use of a multi-marker strategy that provides some insights into dysfunction in the multiple organ systems that contribute ADHF pathophysiology, is potentially advantageous in risk assessment. While there was significant interaction of urinary and plasma CNP with plasma NT-proBNP, the combination of these differentially expressed hormones provided a more accurate assessment of prognosis in ADHF patients. This finding supports the possibility of distinct pathological insults in different organ systems, where elevated urinary CNP, plasma CNP, and plasma NT-proBNP are compensatory markers of renal, endothelial, and cardiac injury and/or dysfunction, respectively. This observation is also consistent with previously reported interactions between the CNP and BNP systems in both human and experimental studies, in which elevations of one, leads to the increase of the other, presumably to optimize the reparative response (18,37). Together, these findings underscore an important cross-talk between the kidney, endothelium, and heart under stress induced by ADHF and further studies are needed to better define the unique role of CNP in ADHF. Moreover, additional studies are warranted to measure urinary, plasma, and tissue CNP in experimental and clinical settings to assess whether ADHF patients with elevated urinary and plasma CNP would benefit from novel therapies to improve clinical outcomes.
Study Limitations
Our study was observational and thus cause and effect cannot be determined. While our study is the largest to simultaneously investigate urinary and plasma CNP in healthy subjects and ADHF patients, the sample sizes of each cohort is relatively small as well as lacked ethnic diversity, thus limiting generalizability. Our contemporary normal thresholds used to determine elevated status of urinary or plasma CNP was based upon a small number of healthy subjects and a larger cohort should be utilized to confirm these normal thresholds. Our inclusion criteria were broad to reflect the heterogeneous spectrum of HF and additional larger studies are needed to determine if our findings are similar or different in patients with HF with reduced or preserved EF. Lastly, due to sample size limitations we could not evaluate the interactions between clinical variables and urinary or plasma CNP with clinical outcomes.
CONCLUSION
Concentrations of urinary and plasma CNP are differentially regulated in ADHF patients and the combination of elevated urinary and plasma CNP predicted the future risk of rehospitalization/death and rehospitalization, even in the context of established risk factors including plasma NT-proBNP. Our findings provide novel pathophysiological insights and highlights the prognostic value of urinary and plasma CNP in ADHF patients.
Supplementary Material
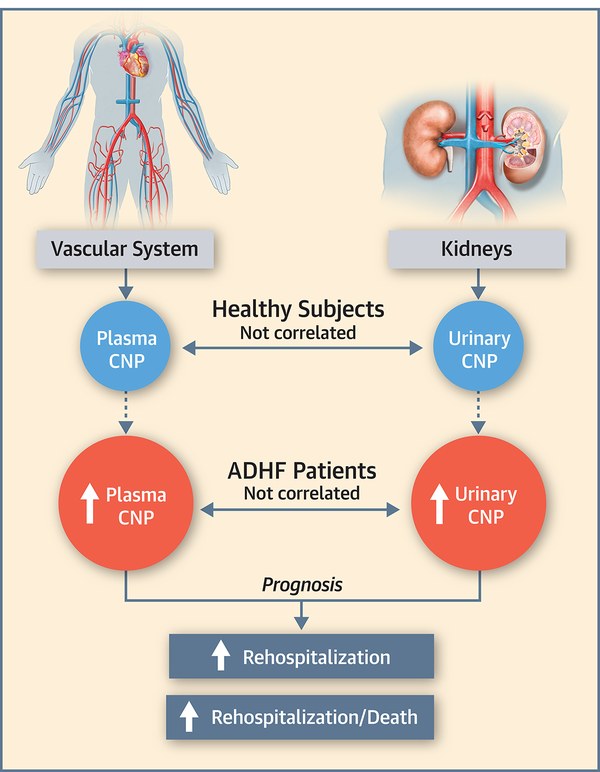
Central Illustration. Renal- and Endothelium-Derived CNP and its Prognostic Value in ADHF Patients.
Urinary and plasma CNP levels are elevated in ADHF patients and elevations in both have prognostic value for adverse outcome.
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
CNP is a renal and endothelium-derived hormone that has anti-remodeling properties. The levels of urinary and plasma CNP are differentially regulated in ADHF patients, and elevations in both, may help identify ADHF patients at high risk for unfavorable outcomes.
Translational Outlook:
Given the growing recognition of the protective role for CNP in ADHF and other cardiorenal disease states, measuring and developing urinary and plasma CNP as a clinical tool deserves further exploration. Additionally, this study lays the foundation for mechanistic studies to further understand the regulation of urinary and plasma CNP under pathophysiological conditions such as ADHF, and to investigate if optimizing CNP activation with novel therapies can improve outcomes.
Acknowledgments
Funding: This work was supported by National Heart, Lung and Blood Institute grants R01 HL36634 (Dr. Burnett) and R01 HL132854 (Dr. Sangaralingham), Division of Circulatory Failure, Department of Cardiovascular Medicine at Mayo Clinic and the Mayo Foundation.
ABBREVIATIONS:
- ADHF
acute decompensated heart failure
- ANP
atrial natriuretic peptide
- BMI
body mass index
- BNP
b-type natriuretic peptide
- CNP
c-type natriuretic peptide
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- IDI
integrated discrimination index
- LVEF
left ventricular ejection fraction
- LOHS
length of hospital stay
Footnotes
Disclosures: Drs. Ma, Y. Chen, H. Chen, Burnett, Sangaralingham and Mayo Foundation have issued or filed patents for assessing the use of CNP as well as for natriuretic peptide-based or particulate guanylyl cyclase enhancing therapeutics. The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Mattingly MT, Brandt RR, Heublein DM, Wei CM, Nir A, Burnett JC Jr. Presence of C-type natriuretic peptide in human kidney and urine. Kidney Int 1994;46:744–7. [DOI] [PubMed] [Google Scholar]
- 2.Sangaralingham SJ, Heublein DM, Grande JP et al. Urinary C-type natriuretic peptide excretion: a potential novel biomarker for renal fibrosis during aging. Am J Physiol Renal Physiol 2011;301:F943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC Jr. Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol 1992;263:H1318–21. [DOI] [PubMed] [Google Scholar]
- 4.Moyes AJ, Hobbs AJ. C-type Natriuretic Peptide: A Multifaceted Paracrine Regulator in the Heart and Vasculature. Int J Mol Sci 2019;20:2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suga S, Itoh H, Komatsu Y et al. Cytokine-induced C-type natriuretic peptide (CNP) secretion from vascular endothelial cells--evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology 1993;133:3038–41. [DOI] [PubMed] [Google Scholar]
- 6.Horio T, Tokudome T, Maki T et al. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology 2003;144:2279–84. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Zheng Y, Iyer SR et al. C53: A novel particulate guanylyl cyclase B receptor activator that has sustained activity in vivo with anti-fibrotic actions in human cardiac and renal fibroblasts. J Mol Cell Cardiol 2019;130:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichiki T, Schirger JA, Huntley BK et al. Cardiac fibrosis in end-stage human heart failure and the cardiac natriuretic peptide guanylyl cyclase system: regulation and therapeutic implications. J Mol Cell Cardiol 2014;75:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soeki T, Kishimoto I, Okumura H et al. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol 2005;45:608–16. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, de Waard MC, Sterner-Kock A et al. Cardiomyocyte-restricted over-expression of C-type natriuretic peptide prevents cardiac hypertrophy induced by myocardial infarction in mice. Eur J Heart Fail 2007;9:548–57. [DOI] [PubMed] [Google Scholar]
- 11.Canaan-Kuhl S, Ostendorf T, Zander K, Koch KM, Floege J. C-type natriuretic peptide inhibits mesangial cell proliferation and matrix accumulation in vivo. Kidney Int 1998;53:1143–51. [DOI] [PubMed] [Google Scholar]
- 12.Spiranec K, Chen W, Werner F et al. Endothelial C-Type Natriuretic Peptide Acts on Pericytes to Regulate Microcirculatory Flow and Blood Pressure. Circulation 2018;138:494–508. [DOI] [PubMed] [Google Scholar]
- 13.Nakao K, Kuwahara K, Nishikimi T et al. Endothelium-Derived C-Type Natriuretic Peptide Contributes to Blood Pressure Regulation by Maintaining Endothelial Integrity. Hypertension 2017;69:286–296. [DOI] [PubMed] [Google Scholar]
- 14.Wei CM, Aarhus LL, Miller VM, Burnett JC Jr. Action of C-type natriuretic peptide in isolated canine arteries and veins. Am J Physiol 1993;264:H71–3. [DOI] [PubMed] [Google Scholar]
- 15.Cataliotti A, Giordano M, De Pascale E et al. CNP production in the kidney and effects of protein intake restriction in nephrotic syndrome. Am J Physiol Renal Physiol 2002;283:F464–72. [DOI] [PubMed] [Google Scholar]
- 16.Lok DJ, Klip IT, Voors AA et al. Prognostic value of N-terminal pro C-type natriuretic peptide in heart failure patients with preserved and reduced ejection fraction. Eur J Heart Fail 2014;16:958–66. [DOI] [PubMed] [Google Scholar]
- 17.Zakeri R, Sangaralingham SJ, Sandberg SM, Heublein DM, Scott CG, Burnett JC Jr. Urinary C-type natriuretic peptide: a new heart failure biomarker. JACC Heart Fail 2013;1:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright SP, Prickett TC, Doughty RN et al. Amino-terminal pro-C-type natriuretic peptide in heart failure. Hypertension 2004;43:94–100. [DOI] [PubMed] [Google Scholar]
- 19.Prickett TCR, Lunt H, Warwick J, Heenan HF, Espiner EA. Urinary Amino-Terminal Pro-C-Type Natriuretic Peptide: A Novel Marker of Chronic Kidney Disease in Diabetes. Clin Chem 2019;65:1248–1257. [DOI] [PubMed] [Google Scholar]
- 20.Burnett JC Jr., Kao PC, Hu DC et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science 1986;231:1145–7. [DOI] [PubMed] [Google Scholar]
- 21.Richards AM, Nicholls MG, Yandle TG et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation 1998;97:1921–9. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Harty GJ, Zheng Y et al. Crrl269. Circ Res 2019;124:1462–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 2014;35:455–69. [DOI] [PubMed] [Google Scholar]
- 24.Mullens W, Damman K, Testani JM et al. Evaluation of kidney function throughout the heart failure trajectory - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020. [DOI] [PubMed] [Google Scholar]
- 25.Reginauld SH, Cannone V, Iyer S et al. Differential Regulation of ANP and BNP in Acute Decompensated Heart Failure: Deficiency of ANP. JACC Heart Fail 2019;7:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmood SS, Wang TJ. The epidemiology of congestive heart failure: the Framingham Heart Study perspective. Glob Heart 2013;8:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaduganathan M, White WB, Charytan DM et al. Relation of Serum and Urine Renal Biomarkers to Cardiovascular Risk in Patients with Type 2 Diabetes Mellitus and Recent Acute Coronary Syndromes (From the EXAMINE Trial). Am J Cardiol 2019;123:382–391. [DOI] [PubMed] [Google Scholar]
- 28.Peterson PN, Rumsfeld JS, Liang L et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes 2010;3:25–32. [DOI] [PubMed] [Google Scholar]
- 29.Langenickel TH, Buttgereit J, Pagel-Langenickel I et al. Cardiac hypertrophy in transgenic rats expressing a dominant-negative mutant of the natriuretic peptide receptor B. Proc Natl Acad Sci U S A 2006;103:4735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyes AJ, Chu SM, Aubdool AA et al. C-type natriuretic peptide co-ordinates cardiac structure and function. Eur Heart J 2020;41:1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W, Werner F, Illerhaus A et al. Stabilization of Perivascular Mast Cells by Endothelial CNP (C-Type Natriuretic Peptide). Arterioscler Thromb Vasc Biol 2020;40:682–696. [DOI] [PubMed] [Google Scholar]
- 32.Palmer SC, Prickett TC, Espiner EA, Yandle TG, Richards AM. Regional release and clearance of C-type natriuretic peptides in the human circulation and relation to cardiac function. Hypertension 2009;54:612–8. [DOI] [PubMed] [Google Scholar]
- 33.Sangaralingham SJ, McKie PM, Ichiki T et al. Circulating C-type natriuretic peptide and its relationship to cardiovascular disease in the general population. Hypertension 2015;65:1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manzano-Fernandez S, Januzzi JL, Boronat-Garcia M et al. Impact of kidney dysfunction on plasma and urinary N-terminal pro-B-type natriuretic peptide in patients with acute heart failure. Congest Heart Fail 2010;16:214–20. [DOI] [PubMed] [Google Scholar]
- 35.Knecht M, Pagel I, Langenickel T et al. Increased expression of renal neutral endopeptidase in severe heart failure. Life Sci 2002;71:2701–12. [DOI] [PubMed] [Google Scholar]
- 36.Mentz RJ, O’Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol 2016;13:28–35. [DOI] [PubMed] [Google Scholar]
- 37.Nazario B, Hu RM, Pedram A, Prins B, Levin ER. Atrial and brain natriuretic peptides stimulate the production and secretion of C-type natriuretic peptide from bovine aortic endothelial cells. J Clin Invest 1995;95:1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.