Abstract
Background:
Hispanics in South Texas have high rates of HCC and NAFLD. Liver fibrosis severity is the strongest predictive factor of NAFLD progression to HCC. We examined the association between free fatty acids (FAs) and advanced liver fibrosis or HCC in this population.
Methods:
We quantified 45 FAs in plasma of 116 subjects of the Cameron County Hispanic Cohort, 15 Hispanics with HCC and 56 first/second-degree relatives of Hispanics with HCC. Liver fibrosis was assessed by FibroScan.
Results:
Advanced liver fibrosis was significantly associated with low expression of very long chain (VLC) saturated FAs (SFAs), odd chain SFAs and VLC n-3 polyunsaturated FAs (PUFAs) (AOR [95% CI]: 10.4 [3.7-29.6], p<0.001; 5.7 [2.2-15.2], p<0.001; and 3.7 [1.5-9.3], p=0.005). VLC n3-PUFAs significantly improved the performance of the non-invasive markers for advanced fibrosis - APRI, FIB-4 and NFS. Plasma concentrations of VLC SFAs and VLC n-3 PUFAs were further reduced in patients with HCC. Low concentrations of these FAs were also observed in relatives of HCC patients and in subjects with the PNPLA3 rs738409 homozygous genotype.
Conclusions:
Low plasma concentrations of VLC n-3 PUFAs and VLC SFAs were strongly associated with advanced liver fibrosis and HCC in this population. Genetic factors were associated with low concentrations of these FAs as well.
Impact:
These results have implications in identifying those at risk for liver fibrosis progression to HCC and in screening this population for advanced fibrosis. They also prompt the evaluation of VLC n-3 PUFAs or VLC SFAs supplementation to prevent cirrhosis and HCC.
Keywords: lipid metabolism, non-alcoholic fatty liver disease, cancer disparity, liver fibrosis, hepatocellular carcinoma
Introduction
Liver cancer is the second leading cause of cancer death globally (1–3). Non-alcoholic fatty liver disease (NAFLD) constitutes an increasingly important risk for hepatocellular carcinoma (HCC). Because of the epidemics of obesity and type 2 diabetes, NAFLD prevalence has increased steadily (4, 5). In patients with NAFLD, the degree of liver fibrosis is the strongest predictive factor for life-threatening complications including HCC (6–8). It is therefore of utmost importance to detect liver fibrosis and prevent its progression.
In the United States (US), Hispanics have the highest prevalence of NAFLD (9) and Hispanics in South Texas have the highest age-adjusted rate of HCC (10). The Cameron County Hispanic Cohort (CCHC) is a population-based cohort of Hispanics in South Texas at the US-Mexico border region, with high prevalence of obesity (51%), diabetes (28%) and NAFLD (49%) (11–13). Liver cancer ranked third among cancers in males and sixth in females based on self-reported data (14). We also reported a 4-fold higher prevalence of advanced liver fibrosis and cirrhosis in this population compared to the general US population, primarily attributable to central obesity and diabetes (15, 16).
Levels of circulating free fatty acids (FAs) are closely connected to lipid metabolism (17) and insulin resistance (18). FAs are important sources of lipotoxic metabolites that induce mitochondrial dysfunction and oxidative stress, and are involved in NAFLD progression (19, 20). We reported that in mice with NAFLD-associated HCC, hepatocarcinogenesis is accompanied by important changes in circulating and hepatic FAs (21). We further demonstrated the utility of selected FAs in HCC risk prediction in patients with cirrhosis (22). We also identified a panel of FAs associated with NAFLD severity (23). Herein, we aimed to determine whether circulating FAs are associated with advanced liver fibrosis and HCC in Hispanics of South Texas and could serve as risk prediction biomarkers or therapeutic targets.
Materials and Methods
Study participants
The study includes 116 participants from the CCHC (11), recruited between March 2016 and June 2018. Liver imaging, fasting blood collection and extensive clinical interview were performed on day of recruitment. Vibration-controlled transient elastography (FibroScan® 502 Touch or 530 Compact, Echosens) with automatic probe selection, was used to assess liver steatosis measured by controlled attenuation parameter (CAP) and liver fibrosis measured by stiffness (LSM) in kiloPascals (kPa). The following criteria were used: CAP≥281 for steatosis and LSM≥8.8kPa for advanced liver fibrosis, as previously established (24, 25). Among the 116 participants, 39 were selected for having advanced liver fibrosis and 77 participants without advanced liver fibrosis were randomly selected from the cohort (Supplementary Table S1). The study also included 15 Hispanics with HCC and advanced liver fibrosis, and 56 first- and second-degree relatives of Hispanics with HCC, all enrolled from August 2016 to January 2018, at the Doctors Hospital at Renaissance, Edinburg, South Texas. To reduce variability, fasting blood collection and clinical interview were performed prior to treatment, by CCHC personnel (Supplementary Tables S1-2). Subjects positive for hepatitis B or C virus were excluded from the study. The following criteria were used as categorical or diagnostic definitions: obesity (BMI≥30), pre-diabetes (no history of diabetic medication, plus either fasting blood glucose (FBG) of 100-125mg/dl or HbA1c of 5.7-6.4 %), diabetes (FBG≥126mg/dl, HbA1c≥6.5% or history of diabetic medication), abnormal aspartate aminotransferase (AST) (>33U/L), abnormal alanine aminotransferase (ALT) (>40U/L for males; >31U/L for females), heavy drinking (>14 drinks for men and >7 for women, weekly), moderate drinking (non-zero consumption but below criteria for heavy drinking), former smoking (lifetime cigarette consumption ≥100, plus no smoking at time of survey), current smoking (lifetime cigarette consumption ≥100, plus smoking at time of survey) (26).
Quantification of plasma FAs
FA profiling was performed blinded to clinical data, at the Metabolomics Core at MD Anderson Cancer Center. The research-grade assay used a methanol extraction approach derived from Mok et al (27) and a chemical derivatization approach derived from Li et al (28). Internal standard (32μL) consisting of (1, 2, 3, 4, 5, 6-13C6) 22:0 (12.5μg/mL) and 13C-labeled 14:0, 16:1n7c, 16:0, 17:0, 18:2n6, 18:1n9c, 18:1n9t, and 18:0 (25μg/mL) (Cambridge Isotope Laboratories, MA, USA) and extraction solvent (1mL) were added to 20μL of plasma. Following centrifugation at 4,122g at 4°C for 10min, supernatants were transferred to 2mL vials with Teflon caps and dried using a centrifugal vacuum concentrator. Extracted FAs were converted to acyl chloride using 200μL of 2 molar oxalyl chloride in dichloromethane at 65°C for 5min. Samples were dried and then derivatized by adding 150μL of 1% (v/v) 3-picolylamine in acetonitrile. Finally, samples were dried and stored at −80°C. Derivatization products were reconstituted in 100μL ethanol, transferred to auto-sampler vials, dried, and reconstituted in 15μL ethanol. Injection volume was 5μL. Mobile phase A (MPA) was 0.1% formic acid in water, and mobile phase B (MPB) was 0.1% formic acid in acetonitrile. The chromatographic method included a Thermo Fisher Scientific Accucore C30 column (2.6μm, 150 x 2.1mm) and the following gradient elution: 0-5min, 65% MPB; 5-5.1min, 65-90% MPB; 5.1-55min, 90% MPB; 55-55.1min, 90-65% MPB; 55.1-60min, 65% MPB. A Thermo Fisher Scientific Orbitrap Fusion Tribrid mass spectrometer with heated electrospray ionization source was operated in data dependent acquisition mode with a scan range of 150-550m/z.
SNP genotyping
Patatin-like phospholipase domain-containing protein 3 (PNPLA3) rs738409 and transmembrane 6 superfamily member 2 (TM6SF2) rs58542926 were genotyped by TaqMan 5’-nuclease assays using predesigned TaqMan probes (Applied Biosystems, Foster City, CA), on a ViiA7 Real time PCR system (Applied Biosystems, Foster City, CA). Both SNPs were in concordance with Hardy-Weinberg equilibrium, based on chi-squared, exact and likelihood ratio tests (Supplementary Table S3).
Statistics
Demographic and clinical parameters were compared between subject groups using two-tailed student’s t-test for continuous variables and Fisher tests for categorical variables. Differences in FA concentrations were tested using Mann-Whitney U or Kruskal-Wallis tests. P-values were adjusted using the Benjamini-Hochberg method to reduce the likelihood of false positives (29). Logistic regression was performed to predict sample status (control vs. case) based on continuous biomarker concentrations. For each logistic regression model, the area under the receiver operating characteristic (ROC) curve (AUC) was calculated. A likelihood ratio test was conducted to compare models. Correlations between FA concentrations and LSMs or diagnostic markers for advanced liver fibrosis were assessed using Spearman correlation analysis. Redundancy analysis (RDA) was performed to evaluate effects of clinical or demographic parameters on FA profiles, using the RDA function in the Vegan package for R. Log10-transformed abundances of FAs were the response variables; log10-transformed BMI, waist circumference, advanced fibrosis and steatosis were the explanatory variables. Analysis of variance-like, permutation-based tests were used to assess the significance (two-tailed p values <0.05) of the model and of each constrained axis, as well as the marginal effects of each explanatory variable. Logistic regression was performed using SPSS to estimate odds ratio (OR) or adjusted OR (AOR) and 95% confidence interval (CI) for association of FAs with disease status or family history.
Results
Changes in plasma FA concentrations associated with advanced liver fibrosis in Hispanics of South Texas
We selected 39 CCHC subjects with advanced liver fibrosis (LSM average = 21.9 [8.8-75.0] kPa). We also randomly selected 77 CCHC subjects without advanced liver fibrosis (LSM average = 5.2 [1.7-8.7] kPa). Subjects with advanced liver fibrosis were more likely to have elevated AST levels (56.4% vs 7.8%, p<0.001), lower platelet counts (180.6x109/L vs 238.4x109/L, p<0.001) and higher alkaline phosphatase levels (115.6U/L vs 85.2U/L, p<0.001). Subjects with advanced liver fibrosis also had higher waist circumference (115.1cm vs 106.1cm, p=0.009) and BMI (35.1 vs 31.8, p=0.029). Importantly, there was no difference in gender, age, diabetes, steatosis, alcohol consumption and smoking, between the two groups (Supplementary Table S1).
A total of 45 FAs [14 saturated FAs (SFAs), 13 monounsaturated FAs (MUFAs) and 18 polyunsaturated FAs (PUFAs)] were quantified by mass spectrometry in plasma of the 116 study participants. We compared the absolute concentrations of the 45 FAs between subjects with advanced liver fibrosis and subjects without advanced fibrosis. Subjects with advanced fibrosis had significantly lower concentrations of odd chain SFAs (45.0μM vs 75.0μM, fold change (FC)=−1.7, p<0.001), very long chain (VLC) even chain SFAs (176.6μM vs 322.5μM, FC=−1.8, p<0.001), VLC n-3 PUFAs (73.9μM vs 160.0μM, FC=−2.2, p<0.001) and VLC n-6 PUFAs (57.2μM vs 116.3μM, FC=−2.0, p<0.001) (Fig. 1A). Individual odd chain SFAs included 17:0 (10.1μM vs 15.5μM, FC=−1.5, p=0.008), 19:0 (0.4μM vs 1.0μM, FC=−2.3, p<0.001), 21:0 (2.1μM vs 4.7μM, FC=−2.2, p<0.001), 23:0 (25.4μM vs 47.0μM, FC=−1.8, p<0.001) and 25:0 (0.7μM vs 1.2μM, FC=−1.9, p<0.001) (Fig. 1B). Individual VLC SFAs included 20:0 (28.9μM vs 54.6μM, FC=−1.9, p<0.001), 22:0 (102.7μM vs 179.7μM, FC=−1.7, p<0.001) and 24:0 (45.0μM vs 88.1μM, FC=−2.0, p<0.001) (Fig. 1C). Individual VLC PUFAs included for n-3: 20:5n3 (2.9μM vs 6.9μM, FC=−2.4, p<0.001), 22:5n3 (4.6μM vs 10.1μM, FC=−2.2, p=0.001) and 22:6n3 (66.5μM vs 143.0μM, FC=−2.2, p=0.001) (Fig. 1D); and for n-6: 20:4n6 (35.0μM vs 74.8μM, FC=−2.1, p<0.001), 22:4n6 (17.6μM vs 31.9μM, FC=−1.8, p=0.002) and 24:2n6 (4.5μM vs 9.6μM, FC=−2.1, p<0.001) (Fig. 1D). Significance remained for all FAs and FAs groups after adjusting p-values using the Benjamini-Hochberg method for multiple test correction. Significance also remained after exclusion of heavy drinkers. Finally, at the exception of 17:0, all FAs and FA groups remained significant in never drinkers and in subjects with liver steatosis (Supplementary Table S4A).
Figure 1.
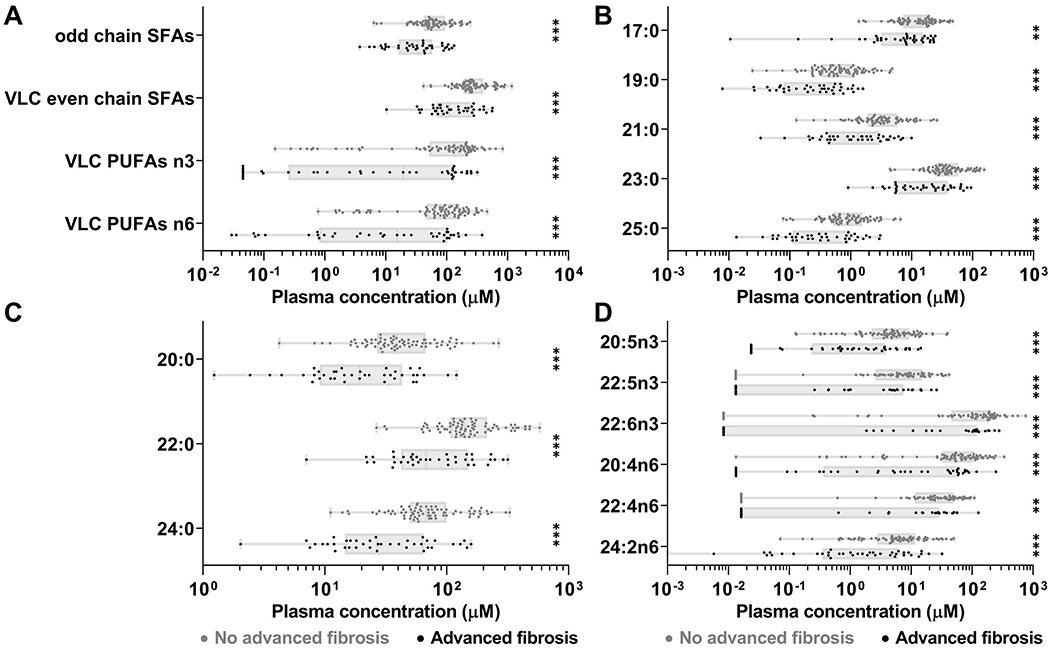
Subjects with advanced liver fibrosis had lower concentrations of odd chain SFAs, VLC even chain SFAs, VLC n-3 PUFAs and VLC n-6 PUFAs. (A) Concentrations of these FA groups. (B) Individual odd chain SFAs. (C) Individual VLC even chain SFAs. (D) Individual VLC n-3 PUFAs and VLC n-6 PUFAs. **p≤0.01, ***p≤0.001. Boxes: range between first and third quartiles; Lines: median values; Whiskers: minimum and maximum values.
In logistic regression analysis, low levels (quartile Q1) of odd chain SFAs, VLC even chain SFAs and VLC n-3 PUFAs were strongly associated with advanced liver fibrosis (OR [95% CI]: 5.1 [2.1-12.6], p<0.001; 10.1 [3.8-26.4], p<0.001; and 4.2 [1.7-10.1], p=0.001, respectively). Among individual FAs in these groups, 23:0 and 25:0, 24:0 and 20:5n3 had the strongest associations (OR [95% CI]: 8.0 [3.1-20.3], p<0.001; 8.0 [3.1-20.3], p<0.001; 12.9 [4.8-35.3], p<0.001; and 6.4 [2.6-15.9], p<0.001; respectively). After adjustment for age, gender, BMI and alcohol intake (g/day), low levels (Q1) of odd chain SFAs, VLC even chain SFAs and VLC n-3 PUFAs remained strongly associated with advanced fibrosis (AOR [95% CI]: 5.7 [2.2-15.2], p<0.001; 10.4 [3.5-29.6], p<0.001; and 3.7 [1.5-9.3], p=0.005) with again the strongest associations observed for 25:0, 24:0 and 20:5n3 (AOR [95% CI]: 8.7 [3.1-24.2], p<0.001; 15.0 [4.9-46.3], p<0.001; and 6.2 [2.4-16.0], p<0.001) (Fig. 2).
Figure 2.
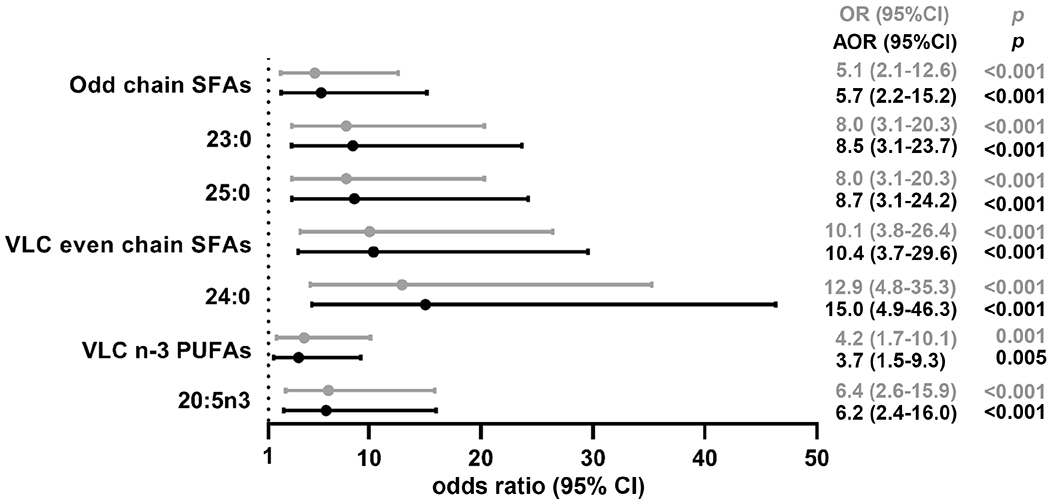
Forest plot of associations between low plasma concentrations (quartile Q1) of selected FAs and advanced liver fibrosis. AOR: adjusted for age, gender, BMI and alcohol intake.
RDA further confirmed the strong relationship between advanced liver fibrosis and abundance of the identified FAs. In this analysis, the FAs described in Fig. 1 were used as response variables and advanced liver fibrosis, steatosis, BMI and waist circumference as explanatory variables. The model was statistically significant (p=0.003) with 9.8% of the FA profiles explained by the presence of advanced liver fibrosis (p=0.001) (Supplementary Fig. S1). No contribution of steatosis, BMI, nor waist circumference was observed.
VLC n-3 PUFAs improve the performance of non-invasive biomarkers for the diagnosis of advanced liver fibrosis in this population
To evaluate whether addition of selected FAs could improve the performance of current non-invasive markers for advanced liver fibrosis, we first performed Spearman correlation analysis of the identified FAs with aspartate aminotransferase-to-Platelet Ratio Index (APRI), fibrosis 4 index (FIB-4) and NAFLD Fibrosis Score (NFS). While APRI did not correlate with any selected FAs groups, FIB-4 and NFS negatively correlated with odd chain SFAs (r=−0.21, p=0.026 and −0.29, p=0.001) and VLC even chain SFAs (r=−0.27, p=0.004 and −0.37, p<0.001) (Supplementary Table S5). Since no correlation was observed between VLC n-3 PUFAs and APRI, FIB-4 or NFS, we tested whether VLC n-3 PUFAs could improve the performance of these three tests. Among them, APRI had the highest AUC for advanced liver fibrosis (0.84 [95% CI: 0.76-0.92]), followed by FIB-4 (0.78 [95% CI: 0.68-0.87]) and NFS (0.73 [95% CI: 0.63-0.83]). The addition of VLC n3-PUFAs significantly improved their performance, reaching 0.90 [95% CI: 0.85-0.96] for the combination with APRI (p=0.03), 0.84 [95% CI: 0.76-0.91) for the combination with FIB-4 (p=0.12), and 0.78 [95% CI: 0.69-0.87] for the combination with NFS (p=0.08) (Fig. 3). At 85% specificity, the addition of VLC n-3 PUFAs increased the sensitivity of APRI from 70% to 80%. At 85% sensitivity, the addition of VLC n-3 PUFAs increased the specificity of APRI from 54% to 82%.
Figure 3.
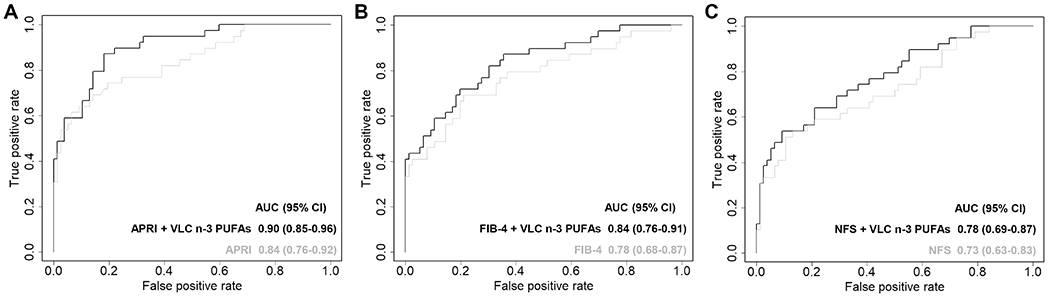
ROC curves for the diagnosis of advanced liver fibrosis in CCHC subjects. (A) APRI and combination APRI + VLC n-3 PUFAs; (B) FIB-4 and combination FIB-4 + VLC n-3 PUFAs; (C) NFS and combination NFS + VLC n-3 PUFAs.
Plasma FAs concentrations in Hispanics with HCC in the context of advanced liver fibrosis: a pilot study
We also investigated in a pilot study whether the concentrations of the identified FAs further decreased in plasma of Hispanics with HCC in the context of advanced liver fibrosis. We quantified FAs in 15 Hispanics in South Texas diagnosed with HCC. All had advanced liver fibrosis measured by FibroScan. Compared to the 39 CCHC subjects with advanced liver fibrosis but no HCC, subjects with HCC were more likely to be male (86.7% vs 30.8%, p=0.001) and to have higher alkaline phosphatase levels (167.2U/L vs 115.6U/L, p=0.025), lower albumin levels (3.2g/dL vs 3.7g/dL, p<0.001), lower platelet counts (129.6x109/L vs 180.6x109/L, p=0.008) and lower steatosis (CAP=253.4dB/m vs 297.3dB/m, p=0.022) (Supplementary Table S1). VLC n-3 PUFAs concentrations were significantly lower in HCC subjects compared to CCHC subjects with advanced liver fibrosis but no HCC. These included 20:5n3 (0.33μM vs 2.87μM, FC=−8.6, p=0.002), 22:5n3 (0.86μM vs 4.57μM, FC=−5.3, p=0.020), and 22:6n3 (17.7μM vs 66.5μM, FC=−3.8, p=0.030) (Fig. 4A). Abundance of VLC SFAs 24:0 (20.1μM vs 45.0μM, FC=−2.2, p=0.034), 23:0 (11.4μM vs 25.7μM, FC=−2.3, p=0.024) and 25:0 (0.21μM vs 0.65μM, FC=−3.1, p=0.017) were also significantly lower in subjects with HCC compared to subjects with advanced liver fibrosis but no HCC (Fig. 4B). The strongest association was observed for 20:5n3 when evaluating risk of HCC among subjects with advanced liver fibrosis (OR [95% CI]: 5.2 [1.4-19.2], p=0.013; AOR/age and gender [95% CI]: 5.3 [1.0-27.9], p=0.05) or among all study participants (OR [95% CI]: 11.8 [3.4-40.4, p<0.001; AOR/age and gender [95% CI]: 11.2 [2.9-42.3], p<0.001).
Figure 4.
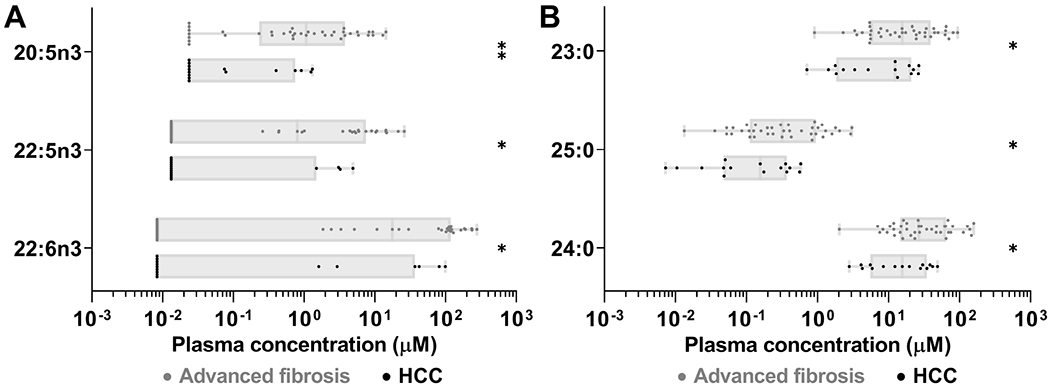
HCC subjects with advanced fibrosis had lower concentrations of VLC n-3 PUFAs and VLC SFAs. (A) Concentrations of individual VLC n-3 PUFAs (A) and individual VLC SFAs (B). *p≤0.05, **p≤0.01. Boxes: range between first and third quartiles; Lines: median values; Whiskers: minimum and maximum values.
Potential genetic contribution to low plasma concentrations of VLC n-3 PUFAs and VLC SFAs
We also profiled FAs in 56 first- and second-degree relatives of Hispanics diagnosed with HCC in South Texas. We confirmed that none of these subjects had liver fibrosis by FibroScan. The 56 relatives of patients with HCC were significantly younger (43.8 vs 57.6, p<0.001) and less likely to be diabetic (17.9% vs 42.6%, p<0.001) than 61 CCHC study participants without liver fibrosis nor family history of HCC (Supplementary Table S2). Remarkably, VLC n-3 PUFAs and VLC SFAs were significantly lower in relatives of HCC patients than in CCHC subjects without family history of HCC. These included 20:5n3 (3.6μM vs 6.7μM, FC=−1.8, p<0.001), 22:5n3 (6.1μM vs 9.7μM, FC=−1.6, p=0.013), 22:6n3 (85.7μM vs 141.1μM, FC=−1.6, p=0.005) (Fig. 5A) as well as 24:0 (50.5μM vs 89.4μM, FC=−1.8, p<0.001), 23:0 (31.1μM vs 47.7μM, FC=−1.5, p=0.009) and 25:0 (0.67μM vs 1.28μM, FC=−1.9, p=0.001) (Fig. 5B). Significance remained after adjusting p-values using the Benjamini-Hochberg method (Supplementary Table S4C). In logistic regression analysis, low concentrations (Q1) of 20:5n3 and 24:0 had the strongest associations with family history of HCC (OR [95% CI]: 9.0 [3.1-26.0], p<0.001 and 3.5 [1.4-8.5], p=0.006). After adjustment by age, gender, diabetes and alcohol intake (g/day), the association with family history of HCC remained strong for both 20:5n3 and 24:0 (AOR [95% CI]: 7.6 [2.3-24.4], p=0.001 and 4.5 [1.6-13.0], p=0.005) (Fig. 5C).
Figure 5.
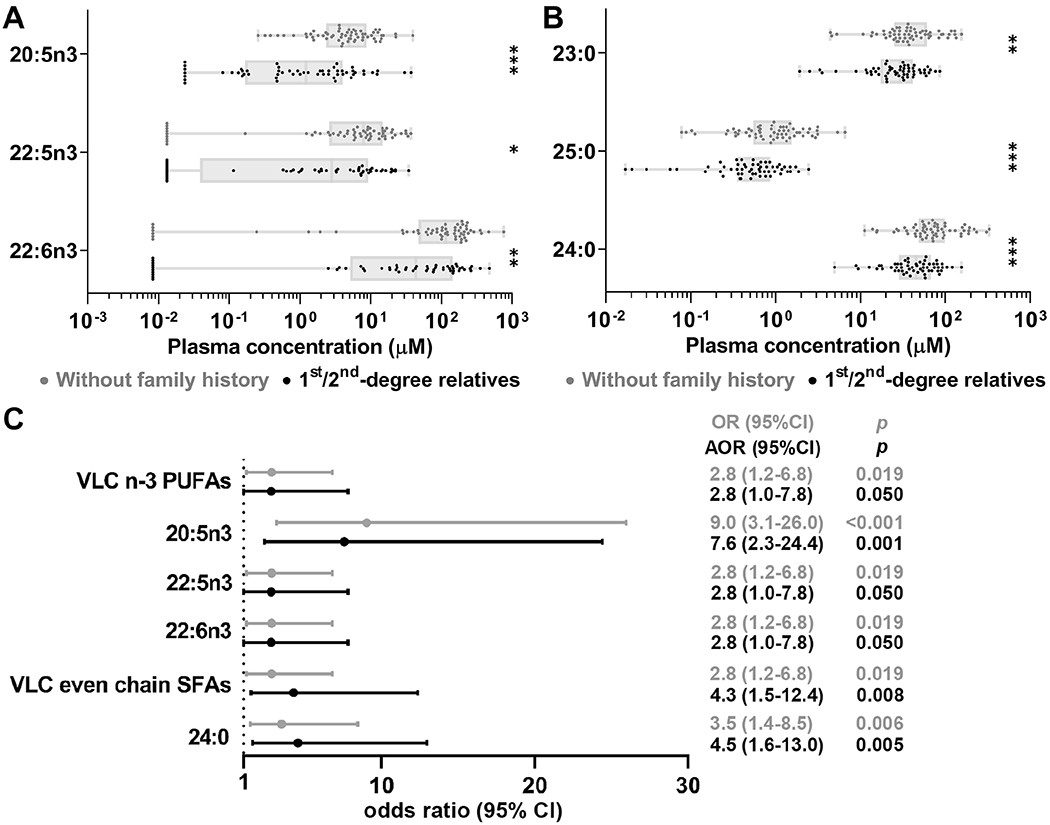
Non-fibrotic 1st/2nd -degree relatives of HCC patients had lower concentrations of VLC n-3 PUFAs and VLC SFAs. Concentrations of individual VLC n-3 PUFAs (A) and individual VLC SFAs (B). (C) Forest plot of significant associations between being 1st/2nd-degree relatives of HCC patients and selected low FAs abundance (quartile Q1) among non-fibrotic subjects. Boxes: range between first and third quartiles; Lines: median values; Whiskers: minimum and maximum values. AOR: adjusted for age, gender, diabetes and alcohol intake. *p≤0.05, **p≤0.01,***p≤0.001.
We then evaluated whether polymorphisms in PNPLA3 and TM6SF2 previously associated with liver fibrosis and HCC, were associated with low concentrations of VLC SFAs and VLC n-3 PUFAs. We genotyped PNPLA3 rs738409 and TM6SF2 rs58542926 in over 900 CCHC subjects as well as in all study participants. As anticipated, the frequency of PNPLA3 rs738409 homozygous GG genotype in CCHC (27.8%) was significantly higher than in other populations (4.0% in Caucasians and 9.3% in all from the 1000 Genomes Project) and similar to reported frequency in Hispanics in California (34.4%) (30) (Supplementary Fig. S2A). The frequency of TM6SF2 rs58542926 heterozygous CT genotype in CCHC (7.6%) was similar to other populations ranging from 12.1% in all from the 1000 Genomes Project to 16.2% in Caucasians (Supplementary Fig. S2A). In our study participants, the frequency of PNPLA3 rs738409 GG was 31%, 39% and 40% in subjects without advanced liver fibrosis, with advanced fibrosis or with HCC, respectively (Supplementary Fig. S2B). The frequency of TM6SF2 rs58542926 CT significantly increased from subjects without advanced fibrosis, to subjects with advanced fibrosis and to subjects with HCC (3%, 16% and 27%, p=0.016) (Supplementary Fig. S2B). In logistic regression analysis, presence of TM6SF2 rs58542926 CT was associated with increased risk for advanced liver fibrosis (OR [95% CI]: 3.3 [1.0-10.2], p=0.042; AOR/age and gender [95% CI]: 3.2 [1.0-10.1], p=0.045) and HCC (OR [95% CI]: 3.4 [0.9-12.4], p=0.067; AOR [95% CI]: 4.9 [1.0-23.9], p=0.050). Lower concentrations of VLC SFAs, 24:0 in particular, and of VLC n-3 PUFAs were observed in subjects with PNPLA3 variant (Table 1). No difference was observed with TM6SF2 heterozygous CT genotype (Table 1).
Table 1.
Concentrations of FAs in PNPLA3 rs738409 and TM6SF2 rs58542926 genotypes in CCHC subjects. MXL, Mexican Ancestry from Los Angeles; CEU, Utah Residents with Northern and Western European Ancestry; ALL, all individuals from 1000 Genomes Project. AF: advanced fibrosis; w/o AF: without advanced fibrosis. Data are presented as mean (range).
| PNPLA3 CC | PNPLA3 CG | PNPLA3 GG | P& | TM6SF2 CC | TM6SF2 CT | P | |
|---|---|---|---|---|---|---|---|
| VLC SFAs (μM) | 320.8 (35.4-910.4) | 280.3 (55.0-1179.8) | 237.0 (10.3-882.0) | 0.131 | 274.8 (10.3-1179.8) | 280.9 (71.9-570.5) | 0.532 |
| 24:0 (μM) | 83.8 (7.1-192.5) | 76.3 (10.5-329.8) | 63.9 (2.0-305.5) | 0.107 | 74.3 (2.0-329.8) | 72.8 (10.5-158.8) | 0.775 |
| VLC n-3 PUFAs (μM) | 135.5 (0.0-405.5) | 142.5 (0.0-643.9) | 110.3 (0.0-838.0) | 0.045 | 131.7 (0.0-838.0) | 137.4 (0.0-381.7) | 0.805 |
| 20:5n3 (μM) | 5.8 (0.0-22.6) | 5.9 (0.0-38.2) | 4.8 (0.0-39.7) | 0.116 | 5.6 (0.0-39.7) | 5.4 (0.0-17.7) | 0.927 |
| 22:5n3 (μM) | 7.4 (0.0-27.3) | 9.7 (0.0-42.1) | 6.4 (0.0-37.0) | 0.034 | 8.4 (0.0-42.1) | 8.2 (0.0-32.6) | 0.996 |
| 22:6n3 (μM) | 122.4 (0.0-355.6) | 127.0 (0.0-563.6) | 99.1 (0.0-761.3) | 0.052 | 117.7 (0.0-761.3) | 123.8 (0.0-343.6) | 0.730 |
P comparing PNPLA3 GG versus PNPLA3 CC+CG.
Discussion
In this study, we first observed decreased concentrations of odd chain SFAs, VLC SFAs, VLC n-3 PUFAs and VLC n-6 PUFAs with advanced liver fibrosis in Hispanics from South Texas. We further showed that VLC n-3 PUFAs could have utility in the diagnosis of advanced fibrosis in this population. Indeed, VLC n-3 PUFAs significantly improved the performance of APRI, FIB-4 and NFS, non-invasive markers currently used for the diagnosis of advanced fibrosis. There are very few studies of fatty acid profiling focusing on advanced liver fibrosis, and none in population studies focusing on communities disproportionally affected by liver fibrosis and HCC. It was previously reported that cirrhotic patients had a higher ratio of n-6/n-3 PUFAs, which correlated with disease severity and oxidative stress markers (31). However, decreased concentrations of VLC even chain SFAs and odd chain SFAs in subjects with advanced fibrosis had not been reported to date. VLC SFAs, fatty acids with backbones containing 20 or more carbon atoms, are constituents of sphingolipids in outer plasma membranes (32). Population-based studies reported inverse correlations of VLC SFAs concentrations with diabetes, favorable profiles of blood lipids and coronary heart disease risk (33–36). In healthy subjects, 20:0 is positively associated with circulating adiponectin, a molecule with putative anti-inflammatory properties (37). The most studied odd chain SFAs, 15:0 and 17:0, are biomarkers for dairy intake and are inversely associated with lower metabolic risk (38), including type 2 diabetes (39, 40), and gestational diabetes (41). Odd chain SFAs can also be endogenously synthesized using gut-derived propionate (42) and were recently identified as markers for fiber intake (43). It was also reported that 15:0 is inversely associated with alcohol consumption in a cohort of subjects over 60 years old (44). We previously showed that 15:0 and 17:0 negatively correlated with NAFLD severity and hepatocyte ballooning in two independent NAFLD cohorts (23). In that study, serum levels of 15:0 and 17:0 also negatively correlated with fasting glucose and AST (23).
Next, we showed that concentrations of VLC n-3 PUFAs n3 and VLC SFAs further decreased during progression from advanced fibrosis to HCC in Hispanics in South Texas. Consumption of n-3 PUFAs has been inversely associated with HCC risk (45, 46). Furthermore, in vitro and in vivo studies have suggested a potential therapeutic effect of 22:6n3 and 20:5n3 for HCC. The addition of 22:6n3 and 20:5n3 had anti-HCC effect in cell lines by inhibition of COX-2 and beta-catenin (26) and by induction of apoptosis (47). Delivery of 22:6n3 by low-density lipoprotein-based nanoparticle selectively induced tumor specific necrosis and ferroptosis, and reduced growth of orthotopic liver tumors in rats (48, 49). Treatment with 20:5n3 also reduced development of NASH-related HCC in liver specific Pten-deficient mice (50) and in carcinogen- and diet-induced mouse models (51) by suppression of the pro-tumorigenic IL-6 effector STAT3. Several VLC SFAs have been linked to cancer, including liver cancer. In particular, 24:0 was found absent in plasma of HCC patients (52). Ceramide synthase 2-deficiency in mice resulted in a strong reduction of sphingolipids containing 24:0 and increased rates of hepatocyte proliferation, regenerative hepatocellular hyperplasia and HCC (53, 54). Prediagnostic levels of 24:0 were also inversely associated with risk of prostate cancer (55) and pancreatic cancer (56).
Prior studies reported low abundances of VLC n-3 PUFAs in Hispanic ethnicity (57). Genome wide and exome wide association studies have also identified SNPs related to the susceptibility of liver fibrosis and HCC, in the context of NAFLD in particular. PNPLA3 I148M variant (rs738409 [G]) and TM6SF2 E167K variant (rs58542926 [T]) were associated with liver fibrosis progression and HCC development (58). Frequency of PNPLA3 rs738409 minor alleles is significantly higher in Hispanics as confirmed in our CCHC analysis. The association of TM6SF2 rs58542926 SNP with advanced fibrosis and HCC was also confirmed in our study population. Subjects with PNPLA3 rs738409 minor alleles or mice overexpressing mutant alleles had relative depletion of VLC n-3 PUFAs (59, 60). Subjects with rs738409 minor alleles also had reduced VLC SFAs 20:0 and 24:0 in liver triglycerides (61). Our study further shows that plasma concentrations of VLC SFAs, 24:0 in particular, and VLC n-3 PUFAs, were also lower in subjects with this PNPLA3 variant in our study population, warranting further validation in larger population-based cohorts.
A major limitation of this study is the small sample size, particularly for HCC cases. Additional studies are needed to further determine the role of the identified FAs in liver fibrosis progression and HCC development in Hispanics. Among them, 24:0 and VLC n-3 PUFAs showed the strongest association with advanced liver fibrosis and HCC, and their plasma concentrations may be affected by genetic polymorphisms, including in PNPLA3. Remarkably, the addition of VLC n-3 PUFAs to APRI strongly improved the diagnostic performance of APRI for advanced fibrosis in our cohort. The utility of 24:0 and VLC n-3 PUFAs in prediction of fibrosis progression and HCC development should be evaluated in prospective cohorts. The therapeutic potential of 24:0 and VLC n-3 PUFAs to prevent progression to advanced fibrosis and HCC in this population should also be evaluated.
Supplementary Material
Acknowledgements
We thank Rocío Uribe, Ivana Zavla and Dr. Monica Betancourt-Garcia for the enrollment of CCHC participants and HCC patients; Marcela Morris and Hugo Soriano for data support; and the participants from the Lower Rio Grande Valley who so willingly participated in this study.
Financial support:
This research was supported in part by the MD Anderson Cancer Center SPORE in Hepatocellular Carcinoma Grant P50 CA217674 from the National Cancer Institute (NCI), and by NIH/NCI grants R01CA204665 and R01CA195524. The Metabolomics Core Facility was supported by Cancer Prevention and Research Institute of Texas grant RP130397 and NIH grants S10OD012304-01 and P30CA016672. The cohort was supported by a NIH/Center for Clinical and Translational Sciences, grant UL1 TR000371.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol 2017;3(12):1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soeijomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Cancer C, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2018;4(11):1553–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011;9(6):524–30 e1; quiz e60. [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 6.Hagstrom H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. Journal of hepatology 2017;67(6):1265–73. [DOI] [PubMed] [Google Scholar]
- 7.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65(5): 1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018;155(2):443–57 e17. [DOI] [PubMed] [Google Scholar]
- 9.Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16(2):198–210 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017;152(4):812–20 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher-Hoch SP, Rentfro AR, Salinas JJ, Perez A, Brown HS, Reininger BM, et al. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004-2007. Preventing chronic disease 2010;7(3):A53. [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher-Hoch SP, Vatcheva KP, Rahbar MH, McCormick JB. Undiagnosed Diabetes and Pre-Diabetes in Health Disparities. PLoS One 2015;10(7):e0133135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill C, Vatcheva KP, Pan JJ, Smulevitz B, McPherson DD, Fallon M, et al. Frequency of Nonalcoholic Fatty Liver Disease and Subclinical Atherosclerosis Among Young Mexican Americans. Am J Cardiol 2017;119(11):1717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garza AL, Vatcheva KP, Pan JJ, Rahbar MH, Fallon MB, McCormick JB, et al. Liver and Other Gastrointestinal Cancers Are Frequent in Mexican Americans. J Racial Ethn Health Disparities 2016;3(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watt GP, Lee M, Pan JJ, Fallon MB, Loomba R, Beretta L, et al. High Prevalence of Hepatic Fibrosis, Measured by Elastography, in a Population-Based Study of Mexican Americans. Clin Gastroenterol Hepatol 2019;17(5):968–75 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao J, Watt GP, Lee M, Rahbar MH, Vatcheva KP, Pan JJ, et al. Cirrhosis and Advanced Fibrosis in Hispanics in Texas: The Dominant Contribution of Central Obesity. PLoS One 2016;11(3):e0150978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jump DB. Fatty acid regulation of hepatic lipid metabolism. Current opinion in clinical nutrition and metabolic care 2011;14(2):115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefan N, Haring HU. Circulating fetuin-A and free fatty acids interact to predict insulin resistance in humans. Nature medicine 2013;19(4):394–5. [DOI] [PubMed] [Google Scholar]
- 19.Neuschwander-Tetri BA. Nontriglyceride hepatic lipotoxicity: the new paradigm for the pathogenesis of NASH. Curr Gastroenterol Rep 2010;12(1):49–56. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology 2008;47(5):1495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muir K, Hazim A, He Y, Peyressatre M, Kim DY, Song X, et al. Proteomic and lipidomic signatures of lipid metabolism in NASH-associated hepatocellular carcinoma. Cancer research 2013;73(15):4722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan IM, Gjuka D, Jiao J, Song X, Wang Y, Wang J, et al. A Novel Biomarker Panel for the Early Detection and Risk Assessment of Hepatocellular Carcinoma in Patients with Cirrhosis. Cancer Prev Res (Phila) 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo W, Gjuka D, Stevenson HL, Song X, Shen H, Yoo SY, et al. Fatty acids in non-alcoholic steatohepatitis: Focus on pentadecanoic acid. PLoS One 2017;12(12):e0189965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Ledinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. Journal of hepatology 2017;66(5):1022–30. [DOI] [PubMed] [Google Scholar]
- 25.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51(2):454–62. [DOI] [PubMed] [Google Scholar]
- 26.Lim K, Han C, Dai Y, Shen M, Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol Cancer Ther 2009;8(11):3046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mok HJ, Lee JW, Bandu R, Kang HS, Kim KH, Kim KP. A rapid and sensitive profiling of free fatty acids using liquid chromatography electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS) after chemical derivatization. Rsc Adv 2016;6(38):32130–9. [Google Scholar]
- 28.Li X, Franke AA. Improved LC-MS method for the determination of fatty acids in red blood cells by LC-orbitrap MS. Anal Chem 2011;83(8):3192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc B 1995;57(1):289–300. [Google Scholar]
- 30.Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature 2015;526(7571):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basili S, Raparelli V, Napoleone L, Del Ben M, Merli M, Riggio O, et al. Polyunsaturated fatty acids balance affects platelet NOX2 activity in patients with liver cirrhosis. Dig Liver Dis 2014;46(7):632–8. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet 2012;44(7):760–4. [DOI] [PubMed] [Google Scholar]
- 33.Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. The lancet Diabetes & endocrinology 2014;2(10):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemaitre RN, Fretts AM, Sitlani CM, Biggs ML, Mukamal K, King IB, et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr 2015;101(5):1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik VS, Chiuve SE, Campos H, Rimm EB, Mozaffarian D, Hu FB, et al. Circulating Very-Long-Chain Saturated Fatty Acids and Incident Coronary Heart Disease in US Men and Women. Circulation 2015;132(4):260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papandreou C, Sala-Vila A, Galie S, Muralidharan J, Estruch R, Fito M, et al. Association Between Fatty Acids of Blood Cell Membranes and Incidence of Coronary Heart Disease. Arterioscler Thromb Vasc Biol 2019;39(4):819–25. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Real JM, Vendrell J, Ricart W. Circulating adiponectin and plasma fatty acid profile. Clin Chem 2005;51(3):603–9. [DOI] [PubMed] [Google Scholar]
- 38.Zheng JS, Sharp SJ, Imamura F, Koulman A, Schulze MB, Ye Z, et al. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: a cross-sectional analysis in the EPIC-InterAct study. BMC Med 2017;15(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santaren ID, Watkins SM, Liese AD, Wagenknecht LE, Rewers MJ, Haffner SM, et al. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am J Clin Nutr 2014;100(6):1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kratz M, Marcovina S, Nelson JE, Yeh MM, Kowdley KV, Callahan HS, et al. Dairy fat intake is associated with glucose tolerance, hepatic and systemic insulin sensitivity, and liver fat but not beta-cell function in humans. Am J Clin Nutr 2014;99(6): 1385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Tsai MY, Sun Q, Hinkle SN, Rawal S, Mendola P, et al. A prospective and longitudinal study of plasma phospholipid saturated fatty acid profile in relation to cardiometabolic biomarkers and the risk of gestational diabetes. Am J Clin Nutr 2018;107(6):1017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins BJ, Seyssel K, Chiu S, Pan PH, Lin SY, Stanley E, et al. Odd Chain Fatty Acids; New Insights of the Relationship Between the Gut Microbiota, Dietary Intake, Biosynthesis and Glucose Intolerance. Scientific reports 2017;7:44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weitkunat K, Schumann S, Nickel D, Hornemann S, Petzke KJ, Schulze MB, et al. Odd-chain fatty acids as a biomarker for dietary fiber intake: a novel pathway for endogenous production from propionate. Am J Clin Nutr 2017;105(6): 1544–51. [DOI] [PubMed] [Google Scholar]
- 44.Laguzzi F, Riserus U, Marklund M, Vikstrom M, Sjogren P, Gigante B, et al. Circulating fatty acids in relation to alcohol consumption: Cross-sectional results from a cohort of 60-year-old men and women. Clinical nutrition 2018;37(6 Pt A):2001–10. [DOI] [PubMed] [Google Scholar]
- 45.Sawada N, Inoue M, Iwasaki M, Sasazuki S, Shimazu T, Yamaji T, et al. Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology 2012;142(7):1468–75. [DOI] [PubMed] [Google Scholar]
- 46.Gao M, Sun K, Guo M, Gao H, Liu K, Yang C, et al. Fish consumption and n-3 polyunsaturated fatty acids, and risk of hepatocellular carcinoma: systematic review and meta-analysis. Cancer Causes Control 2015;26(3):367–76. [DOI] [PubMed] [Google Scholar]
- 47.Sun SN, Jia WD, Chen H, Ma JL, Ge YS, Yu JH, et al. Docosahexaenoic acid (DHA) induces apoptosis in human hepatocellular carcinoma cells. International journal of clinical and experimental pathology 2013;6(2):281–9. [PMC free article] [PubMed] [Google Scholar]
- 48.Ou W, Mulik RS, Anwar A, McDonald JG, He X, Corbin IR. Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma. Free Radic Biol Med 2017;112:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen X, Reynolds L, Mulik RS, Kim SY, Van Treuren T, Nguyen LH, et al. Hepatic Arterial Infusion of Low-Density Lipoprotein Docosahexaenoic Acid Nanoparticles Selectively Disrupts Redox Balance in Hepatoma Cells and Reduces Growth of Orthotopic Liver Tumors in Rats. Gastroenterology 2016;150(2):488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishii H, Horie Y, Ohshima S, Anezaki Y, Kinoshita N, Dohmen T, et al. Eicosapentaenoic acid ameliorates steatohepatitis and hepatocellular carcinoma in hepatocyte-specific Pten-deficient mice. Journal of hepatology 2009;50(3):562–71. [DOI] [PubMed] [Google Scholar]
- 51.Inoue-Yamauchi A, Itagaki H, Oda H. Eicosapentaenoic acid attenuates obesity-related hepatocellular carcinogenesis. Carcinogenesis 2018;39(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patterson AD, Maurhofer O, Beyoglu D, Lanz C, Krausz KW, Pabst T, et al. Aberrant lipid metabolism in hepatocellular carcinoma revealed by plasma metabolomics and lipid profiling. Cancer research 2011;71(21):6590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imgrund S, Hartmann D, Farwanah H, Eckhardt M, Sandhoff R, Degen J, et al. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. The Journal of biological chemistry 2009;284(48):33549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pewzner-Jung Y, Brenner O, Braun S, Laviad EL, Ben-Dor S, Feldmesser E, et al. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. The Journal of biological chemistry 2010;285(14): 10911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. International journal of cancer 1997;71(4):545–51. [DOI] [PubMed] [Google Scholar]
- 56.Shu X, Zheng W, Yu D, Li HL, Lan Q, Yang G, et al. Prospective metabolomics study identifies potential novel blood metabolites associated with pancreatic cancer risk. International journal of cancer 2018;143(9):2161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy RA, Yu EA, Ciappio ED, Mehta S, McBurney MI. Suboptimal Plasma Long Chain n-3 Concentrations are Common among Adults in the United States, NHANES 2003-2004. Nutrients 2015;7(12):10282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol 2014;109(3):325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luukkonen PK, Nick A, Holtta-Vuori M, Thiele C, Isokuortti E, Lallukka-Bruck S, et al. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. JCI Insight 2019;4(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitsche MA, Hobbs HH, Cohen JC. Patatin-like phospholipase domain-containing protein 3 promotes transfer of essential fatty acids from triglycerides to phospholipids in hepatic lipid droplets. The Journal of biological chemistry 2018;293(18):6958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peter A, Kovarova M, Nadalin S, Cermak T, Konigsrainer A, Machicao F, et al. PNPLA3 variant I148M is associated with altered hepatic lipid composition in humans. Diabetologia 2014;57(10):2103–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


