Abstract
Inhibition of mTORC1 signaling has been shown to diminish growth of meningiomas and schwannomas in preclinical studies, and clinical data suggest that everolimus, an orally administered mTORC1 inhibitor, may slow tumor progression in a subset of NF2 patients with vestibular schwannoma (VS). To assess the pharmacokinetics, pharmacodynamics and potential mechanisms of treatment resistance, we performed a pre-surgical (phase 0) clinical trial of everolimus in patients undergoing elective surgery for VS or meningiomas.
Eligible patients with meningioma or VS requiring tumor resection enrolled on study received everolimus 10 mg daily for 10 days immediately prior to surgery. Everolimus blood levels were determined immediately prior to and after surgery. Tumor samples were collected intraoperatively.
Ten patients completed protocol therapy. Median pre- and post-operative blood levels of everolimus were found to be in a high therapeutic range (17.4 ng/ml and 9.4 ng/ml, respectively). Median tumor tissue drug concentration determined by mass spectrometry was 24.3 pg/mg (range 9.2–169.2). We observed only partial inhibition of phospho-S6 in the treated tumors, indicating incomplete target inhibition compared to control tissues from untreated patients (p=0.025).
Everolimus led to incomplete inhibition of mTORC1 and downstream signaling. These data may explain the limited anti-tumor effect of everolimus observed in clinical studies for NF2 patients and will inform the design of future pre-clinical and clinical studies targeting mTORC1 in meningiomas and schwannomas.
Keywords: Vestibular schwannoma, meningioma, everolimus, pharmacokinetics, pharmacodynamics
INTRODUCTION
Meningiomas and vestibular schwannomas (VS) are among the most common intracranial neoplasms. According to the most recent Central Brain Tumor Registry of the United States (CBTRUS) report (1), meningioma represents the most common histology for this population, accounting for 37.8% of all tumors, with an annual incidence of more than 31,000 and an average annual age-adjusted incidence rate (AAAIR) of 8.58 per 100,000. For VS, the histology-based annual incidence is over 6,000, with an AAAIR of 1.90 per 100,000. Given that a large proportion of patients with meningioma and schwannoma are diagnosed based on imaging and primary therapy is often non-surgical (e.g., radiation therapy), the true incidence and prevalence of meningioma and VS are substantially higher (2,3).
Depending on the size and location, meningiomas cause neurological symptoms including seizures and focal neurological deficits. VS typically cause hearing loss, and may also lead to facial nerve dysfunction, dysphagia and brainstem compression. Primary treatment of meningiomas and VS typically involves surgery and/or radiation therapy, which may cause further neurological deficits and other complications, as well as late effects.
While VS and meningioma most often occur sporadically, they are also associated with the inheritable tumor predisposition syndrome Neurofibromatosis type 2 (NF2), an autosomal dominant genetic disorder with an incidence of approximately 1/40,000. NF2 is caused by inactivation of the NF2 gene located on chromosome 22q (4,5), that codes for the NF2 gene product, Merlin. NF2 patients are predisposed to develop cranial nerve schwannomas, most commonly involving the vestibular branch of the eighth cranial nerve (i.e., VS), as well as meningiomas, ependymomas and peripheral schwannomas. The majority of NF2 patients develop progressive hearing loss in adolescence or young adulthood due to bilateral VS, and additional progressive neurological deficits from multiple meningiomas, schwannomas and/or ependymomas, which are often inoperable. As a result, NF2 patients suffer from significant morbidity and reduced life expectancy (6).
Tumor formation in NF2 patients is thought to involve biallelic loss of NF2 through mutations and loss of heterozygosity. Biallelic somatic mutations in NF2 are also found in sporadic schwannomas and meningiomas (7) and thought to represent a primary oncogenic driver in these tumors. Biallelic inactivation of NF2 is present in approximately 40% of sporadic meningiomas (8), and both sporadic and NF2-related VS consistently lack expression of detectable Merlin (9).
Prior studies have revealed that inactivation of Merlin activates the mTORC1 (mammalian target of rapamycin complex-1) signaling pathway (10–12). Accordingly, the mTOR pathway was also identified as a priority therapeutic target at an NF2 Clinical Trials Consensus Workshop (12). The AKT effector mTORC1 is a key mediator of RTK signaling and a major sensor of metabolic fitness. mTOR regulates essential signal transduction pathways, linking growth stimuli to cell-cycle progression and integrates signals involving nutrient availability, energy status, and stress (13,14). Phosphorylation of 4E-BP1 and S6K downstream of mTORC1 promotes translation of mRNAs encoding proteins that regulate cell growth, survival and cell cycle progression (15). Additionally, mTORC1 suppresses autophagy and activates transcription factors, which promote lipid biosynthesis and mitochondrial function (13,14). The mechanism by which Merlin inhibits mTORC1 signaling has not yet been fully elucidated. Current evidence, however, suggests that Merlin acts at or below the level of TSC2/1, because loss of Merlin does not activate AKT or ERK, and silencing of TSC2 abrogates the growth inhibitory effect of Merlin (10). Interestingly, unless phosphorylated by AKT, TSC2 accumulates in the nucleus(16). Furthermore, genetic analysis in Drosophila suggests that a CRL4 ligase ubiquitylates and targets TSC for degradation (17). These observations raise the possibility that CRL4DCAF1 is the ligase that targets TSC2 for degradation. Alternatively, since CRL4DCAF1 regulates a relatively broad program of oncogenic gene expression (18), it may regulate one or more genes, which in turn affect mTORC1 signaling. Importantly, inhibition of mTORC1 by rapamycin exerted a selective cytostatic effect, but did not induce apoptosis in Merlin-deficient arachnoidal, meningioma and schwannoma cells in vitro (10,11), with similar observations made in schwannomas in vivo (19). In mouse models of meningioma, mTORC1 inhibitors suppressed tumor growth in both NF2 deficient and non-deficient models (20,21). Preclinical evidence also suggests that everolimus may inhibit VEGF production and therefore reduce tumor angiogenesis (22). This mechanism of action may be relevant in VS, because prospective clinical trials have demonstrated that inhibition of VEGF has therapeutic efficacy in this tumor (23,24).
Taken together, these data suggested that treatment with mTORC1 inhibitors, such as everolimus, should be explored clinically in the treatment of vestibular schwannomas and meningiomas. While bevacizumab has emerged as an useful treatment option for a subset of NF2 patients with progressive VS (23,24), there remains an urgent need for novel, effective and less toxic therapies for patients with VS and meningioma.
Everolimus is FDA approved for the treatment of renal cell carcinoma (25) and for the treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis (TS) in patients who are not candidates for curative surgical resection (26). A pre-requisite for efficacy of any molecular targeted anti-tumor therapy is target inhibition in the tumor. This is particularly true for brain tumors, which often exist in a protected environment behind the blood brain barrier (BBB), blood-cerebrospinal fluid barrier or blood-nerve barrier (27,28). There is a paucity of data regarding the delivery of systemic drugs to VS or meningioma tissue. VS may be protected by the BBB, blood nerve barrier or cerebrospinal fluid brain barrier. Meningiomas typically derive their blood supply from extracranial arteries and therefore considered outside the BBB (29), but it is unknown if systemic drugs achieve adequate tissue concentration within meningiomas. Everolimus has been proposed to cross the BBB, however, the drug concentration achieved in these target tissues is unknown (30).
Our study was conceived as a phase 0 (or pharmacodynamic and biomarker endpoint driven) study, to inform interpretation of results from advanced stage clinical trials of everolimus in brain tumors, identify potential resistance mechanisms, and devise novel strategies to enhance therapeutic efficacy.
PATIENTS AND METHODS
Patient Eligibility and Enrollment
Adult patients (age ≥18 years) requiring surgical resection of a meningioma or vestibular schwannoma (VS) were eligible for this study. Histological confirmation was not required prior to study entry. Additional key eligibility criteria included Karnofsky performance score ≥60%, absolute neutrophil count ≥1,000/mm³ (unsupported), platelet count ≥100,000/mm³ (unsupported), hemoglobin ≥8 g/dl (transfusion support allowed), creatinine ≤1.5 times upper limit of normal (ULN) or corrected glomerular filtration rate ≥70 ml/min, total bilirubin ≤1.5 times ULN, ALT ≤2.5 times ULN, serum albumin ≥2 g/dl, INR <1.3 (or <3 on anticoagulants), fasting serum cholesterol ≤300 mg/dl, and fasting triglycerides ≤2.5 times ULN. Key exclusion criteria included prior therapy with mTOR inhibitors, known hypersensitivity to everolimus or other rapalogs, concurrent therapy with cytochrome 3A4 inducers or inhibitors, and chronic hepatitis B or C infection (due to the risk of disease reactivation with rapalogs).
All baseline evaluations were required within 14 days prior to starting everolimus and included: history and physical including neuro exam, hepatitis screening, Karnofsky performance score (KPS), complete blood count, PT/INR, serum sodium, potassium, chloride, bicarbonate, blood urea nitrogen, creatinine, albumin, fasting glucose and serum lipid profile (triglycerides, total cholesterol, HDL and LDL). For patients with positive hepatitis screening history, blood testing for HBsAb, HBsAg, HBcAb, HBV-DNA and HCV-DNA was required. In addition, a serum pregnancy test was required for all females of childbearing age.
Ethics and Study Oversight
The study was conducted under a protocol approved by the Institutional Review Boards of all institutions enrolling patients on the clinical trial (NYU Langone, Johns Hopkins, Piedmont Healthcare, Massachusetts General Hospital) and registered at ClinicalTrials.gov (NCT01880749). Informed consent was obtained from the patients in accordance with institutional policies. General oversight of the trial was by the principal investigator (M.A.K.) and the Data and Safety Monitoring Board (DSMB) of NYU Langone Health.
Study Design
This study was a prospective, open label, phase 0 study. Given that only a single surgery is generally performed to resect meningiomas and schwannomas without prior biopsy, obtaining matched pre and post treatment samples from the same tumor was not feasible. We therefore relied on matched archival control tissue for comparisons.
The primary and secondary study endpoints were molecular target inhibition and intratumoral drug concentration, respectively. According to phase I trial data in cancer patients, terminal half-life of RAD001 administered daily is 30 hours and steady-state levels are reached within one week. Trough levels we shown to be stable thereafter, averaging 13.2 ng/ml with 10 mg daily dosing (31). Subsequent clinical studies established the safety and efficacy of RAD001 administered on a 10 mg daily oral dosing schedule in renal cell carcinoma patients (25).
The primary endpoint for each patient was the complete inhibition of phospho-S6 in tumor tissue after at least 10 days of exposure to everolimus at a daily dose of 10 mg, as determined by immunohistochemistry (IHC). This endpoint was chosen based on prior pharmacodynamic data from a published trial, showing complete loss of phospho-S6 expression by IHC in solid tumor tissue of patients treated with everolimus (32).
Secondary endpoint was to assess the delivery of everolimus to tumor tissue along with pre– and post-operative blood concentrations. Exploratory endpoints included assessment of downstream pharmacodynamic biomarkers.
Only patients enrolled on study who completed at least 10 days of everolimus at the full dose of 10 mg daily immediately prior to surgery and who had adequate tumor tissue available after clinical pathology were considered evaluable for analysis. Ten days was chosen as minimum treatment duration since steady-state is reached at that time point(33) and S6-kinase inhibition reaches a plateau with daily dosing, based on modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data (34). Based on preclinical data, it was considered likely that everolimus will reach measurable concentrations in tumor tissue and it was expected that all of the samples will have measurable drug. Everolimus levels in tumor tissue and blood (immediately prior and after surgery) were assessed for each patient. Exploratory analysis was performed to assess this PK data in conjunction with the primary endpoint of inhibition of phospho-S6.
Treatment
Everolimus was supplied by Novartis, Inc. and prescribed at a dose of 10 mg daily at bedtime by mouth for 10 days, immediately preceding the planned tumor resection. If necessary, patients were allowed to take everolimus for a maximum of seven additional days to allow for unexpected delays in the surgical procedure. Patients were supplied with a study drug diary and asked to document the time of each dose of everolimus taken.
Any patient who received at least one dose of everolimus was considered evaluable for safety and for inhibition of phospho-S6; however, to evaluate the full potential of the drug to inhibit phospho-S6 in tumor tissue, the primary analysis was based on evaluable patients who complete at least 10 days of everolimus at the full dose of 10 mg daily immediately prior to surgery and who had adequate tumor tissue available after clinical pathology was completed.
Sample Collection and Analysis
Everolimus blood levels:
Prior to start of surgery and after completion of surgery, whole venous blood samples were collected in tubes containing K2 EDTA and sent to the participating hospital’s clinical laboratory. Time of blood collections was recorded. Everolimus blood level testing was performed using a standard commercial laboratory assay (Quantitative Liquid Chromatography-Tandem Mass Spectrometry) approved for clinical use to monitor everolimus whole blood levels.
Tumor tissue collection and processing:
After obtaining tissue required for diagnostic pathology, additional tumor samples were collected for study purposes and snap frozen in liquid nitrogen. Time of sample collection was recorded. Care was taken to exclude tissue samples that have come in direct contact with heat (cauterization). Frozen samples were stored in liquid nitrogen until shipment and analysis. 15 unstained slides with freshly cut standard 4 μm tissue sections from formalin-fixed, paraffin-embedded tissue was also requested from each tumor sample for immunohistochemical analysis.
Mass Spectrometry:
Snap frozen samples were thawed and homogenized with 80% methanol after weighing. Control samples were spiked in with standard at 14 different amounts from 0 to 1,280 ng/ml. Three technical replicates were set for each point, and the standard curves were created. Each sample was analyzed in triplicate by liquid chromatography/tandem mass spectrometry using a 4.6 × 100 mm Acclaim120 C18 column (Thermo Scientific) and gradient of 0.1% (v/v) formic acid in 2mM ammonium acetate to 100% methanol in 0.1% formic acid and 2 mM ammonium acetate over 10 min at 600 μL/min flow rate. The solvents were delivered by a Vanquish UHPLC coupled directly to a Q Exactive HF-X (Thermo Scientific). Data were acquired by parallel reaction monitoring (PRM) with a 2 Th window around 975.6 and a normalized collision energy of 25. Everolimus amount was calculated based on a linear standard curve using ion intensities of ammonium ion adducts of intact everolimus (m/z 975.6152) and fragment ions of m/z 908.5499 and 890.5396. Values were then normalized to pg everolimus/mg tissue based on the weight of each tissue sample.
Immunohistochemistry:
Immunohistochemistry (IHC) was performed on formalin-fixed, paraffin-embedded, 4 μm tissue sections using unconjugated rabbit anti-human phospho-S6 ribosomal protein (pS6), clone 91B2 (Cell Signaling Technology, Catalog #4857, AB_2181035), unconjugated rabbit anti-human phospho-AKT-1 (pAKT), clone S473/D9E (Cell Signaling Technology, Catalog #4060, AB_2315049) and unconjugated rabbit anti-human phospho-mitogen activated protein kinase (pERK) clone D13.14.4E (Cell Signaling Technology, Catalog #4370, AB_2315112). IHC was performed on a Ventana Medical Systems Discovery XT instrument using Ventana’s reagents and detection kits unless otherwise noted. Antibody was detected with Ventana biotinylated goat anti-rabbit followed by application of streptavidin-horseradish-peroxidase conjugate. The complex was visualized with 3,3 diaminobenzidene and enhanced with copper sulfate. Appropriate positive and negative controls with known expression levels of antigen were included with the study sections. Immunohistochemistry results were scored using a histoscore, as previously described (32).
Immunoblotting:
Tumor samples collected for study purposes were snap-frozen in liquid nitrogen until analysis. To prepare samples for immunoblotting, the tumor samples were lysed in RIPA buffer (Thermo Fisher Scientific) with protease and phosphatase inhibitors (Cell Signaling Technology) and quantified with Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). PVDF Membranes (Bio-rad) were incubated in blocking buffer (5% skim milk in TBS with 0.1% Tween, Cell Signaling Technology) for 1 hour at room temperature and then with primary antibodies diluted in blocking buffer for overnight at 4°C. After three washes, the membranes were incubated with goat anti-Rabbit HRP-conjugated antibody (Thermo Fisher Scientific) at room temperature for 1 hour and subjected to chemiluminescence using ECL (Thermo Fisher Scientific). Densitometry analysis was performed using Image Lab Software (Bio-rad) (25).
Statistical Considerations
Initial Statistical Analysis Plan and Accrual Goals:
With 21 evaluable patients in each of the two tumor types under study, an optimum 2-stage clinical trial design for each tumor type will test the null hypothesis that the proportion of patients with complete phospho-S6 inhibition in tumor (i.e., histoscore of 0 by IHC) post treatment is ≤0.65, versus the alternative that this proportion is ≥0.90. If everolimus is actually ineffective in inhibiting phospho-S6, there is a 0.031 probability of concluding that it is effective (target α = 0.05); if the drug is actually effective, there is a 0.17 probability of concluding that it is not effective (power = 83%). For each tumor type, the trial will terminate after 10 patients are tested in the first stage if 7 or more patients have complete inhibition of phospho-S6. If the trial continues to a second stage, a total of up to 21 patients will be tested; if 17 or fewer patients have complete inhibition of phospho-S6, the drug will not be considered biologically effective in that tumor type [Calculations from PASS, NCSS, 2008, J. Hintze, Kaysville, UT].
Modification of accrual goals due to slow subject accrual:
Given significantly slower than expected accrual, we performed an early interim analysis and tested the available specimens as of October, 2016, i.e. eight meningiomas and one VS, for phospho-S6 expression (primary endpoint). We found that 5/9 samples had an incomplete p-S6 inhibition (i.e. histoscore >0), which meant that the requirement for opening Stage 2 was not going to be met for meningioma, and that timely completion of Stage 1 accrual for VS was unrealistic. Following DSMB recommendations, the protocol was amended to combine meningiomas and VS into a single analytical stratum, and eventually closed after a total of 10 patients (eight with meningiomas, two with VS) were accrued.
Exploratory endpoints:
Exploratory analysis was performed to assess the relationship of everolimus tissue concentrations to blood levels and in conjunction with the primary endpoint of inhibition of p-S6, as well as downstream signaling effectors. Levels of p-S6, p-ERK and p-AKT as determined by IHC (histoscore) were compared to 10 controls with matching distribution of tumor types (meningioma or VS) and NF2 status (sporadic or NF2-related). Correlations of blood and tumor drug levels were estimated using a Spearman correlation. Distributions of biomarkers between study patients and controls were compared using a Mann-Whitney-Wilcoxon test.
RESULTS
Patients
Thirteen eligible patients were enrolled on study between 12/16/2013 and 10/11/2016. There were nine females (69%) and four males (31%), with a median age of 39 years of age at enrollment (range 21–69 years). Three patients did not complete the planned protocol therapy prior to surgery and were therefore considered non-evaluable; two patients discontinued drug early due to toxicities that were anticipated (mucositis and nausea), and one patient required urgent surgical intervention for clinically progressive disease. Of the remaining ten evaluable patients, seven patients had a clinical diagnosis of NF2, five with meningiomas and two with VS requiring surgery. The remaining three patients had sporadic meningiomas. All meningiomas were WHO grade I by histology, and only one tumor (M-10) was recurrent. Detailed clinical and demographic information for all evaluable study patients is provided in Table 1. All patients provided written informed consent for study participation prior to enrollment.
Table 1.
Summary of general patient characteristics at enrollment (evaluable patients only)
| Patient | Age [years] | Sex | Tumor Type | WHO Grade | NF2 Status |
|---|---|---|---|---|---|
| M-01 | 21 | M | meningioma | I | NF2 |
| M-02 | 64 | M | meningioma | I | NF2 |
| M-03 | 25 | F | meningioma | I | NF2 |
| M-05 | 67 | F | meningioma | I | sporadic |
| M-06 | 23 | F | meningioma | I | NF2 |
| M-07 | 37 | M | meningioma | I | sporadic |
| M-09 | 39 | F | meningioma | I | NF2 |
| M-10 | 69 | F | meningioma | I | sporadic |
| S-01 | 49 | F | VS | I | NF2 |
| S-03 | 34 | F | VS | I | NF2 |
Abbreviations: F = female; M = male; VS = vestibular schwannoma
Treatment and Toxicity
All ten evaluable study patients completed a 10-day course of everolimus immediately prior to scheduled surgery. The three non-evaluable patients received at least one dose of everolimus. All observed toxicity at least possibly related to everolimus was expected and minor (CTCAE 4.0 grades 1 and 2), including mucositis (30%), nausea (15%), fatigue (8%) and rash (8%).
Everolimus blood levels and tissue concentrations
The suggested therapeutic range for the treatment of SEGA is 5–15 ng/ml, based on a predose (trough) specimen. Everolimus blood levels in the ten study patients ranged between 6.9–49.6 ng/ml (median 17.4) pre-operatively and 6.3–43.4 ng/ml (median 9.4 ng/ml) post-operatively. Frozen tumor tissue of sufficient quantity and quality for mass spectrometry was available in 9/10 study patients, with everolimus concentrations in tumor tissue ranging between 9.2–169.2 pg/mg (median 24.2). Detailed results for all patients are shown in Table 2 and tissue drug levels are displayed in Figure 1.
Table 2.
Summary of pharmacokinetic and pharmacodynamic assessments in study patients. “Time since last dose” refers to time difference between final dose of everolimus and sample acquisition.
| Patient | Everolimus blood level (pre-OP) [ng/ml] | Time since last dose [h] | Everolimus blood level (post-OP) [ng/ml] | Time since last dose [h] | Everolimus tumor tissue concentration [pg/mg]* | Time since last dose [h] | p-S6 histoscore | p-ERK histoscore | p-AKT histoscore |
|---|---|---|---|---|---|---|---|---|---|
| M-01 | 11.3 | NA | 10.3 | 13.5 | NA | 12.0 | 220 | 210 | 100 |
| M-02 | 6.9 | 14.7 | 6.3 | 18.8 | 18.6 | 14.5 | 0 | 120 | 5 |
| M-03 | 17.6 | 15.1 | 8.5 | 17.8 | 17.3 | 17.3 | 85 | 190 | 40 |
| M-05 | 28.6 | 7.4 | 14.8 | 16.7 | 40.2 | 12.0 | 110 | 180 | 10 |
| M-06 | 13.8 | 11.8 | 7.7 | 22.2 | 26.5 | NA | 65 | 145 | 50 |
| M-07 | 35.3 | NA | 19.3 | NA | 61.9 | NA | 100 | 180 | 5 |
| M-09 | 9.4 | 10.5 | 7.5 | 17.0 | 9.2 | 13.1 | 40 | 40 | 15 |
| M-10 | 49.6 | 13.9 | 43.4 | 20.9 | 169.2 | 15.8 | 110 | 180 | 5 |
| S-01 | 17.2 | NA | 12.1 | NA | 15.3 | NA | 95 | 220 | 60 |
| S-03 | 21.5 | 11.5 | 7.8 | 26.0 | 24.2 | 15.5 | 80 | 170 | 0 |
mean from 3 independent measurements, NA: not available
Figure 1. Everolimus concentrations in tumor tissue from study patients.

Measured by mass spectrometry (all measurements performed in triplicate).
Pre-operative blood levels were higher compared to post-operative (Wilcoxon signed rank test p = 0.002), and both values were strongly correlated, Spearman correlation = 0.85 (Figure 2A). Pre-operative blood levels and tumor tissue concentrations were strongly correlated, Spearman correlation = 0.77 (Figure 2B). Post-operative bood levels and tumor tissue concentrations were moderately correlated, Spearman correlation = 0.67 (Figure 2C).
Figure 2. Blood pharmacokinetics and tumor tissue concentrations of everolimus.
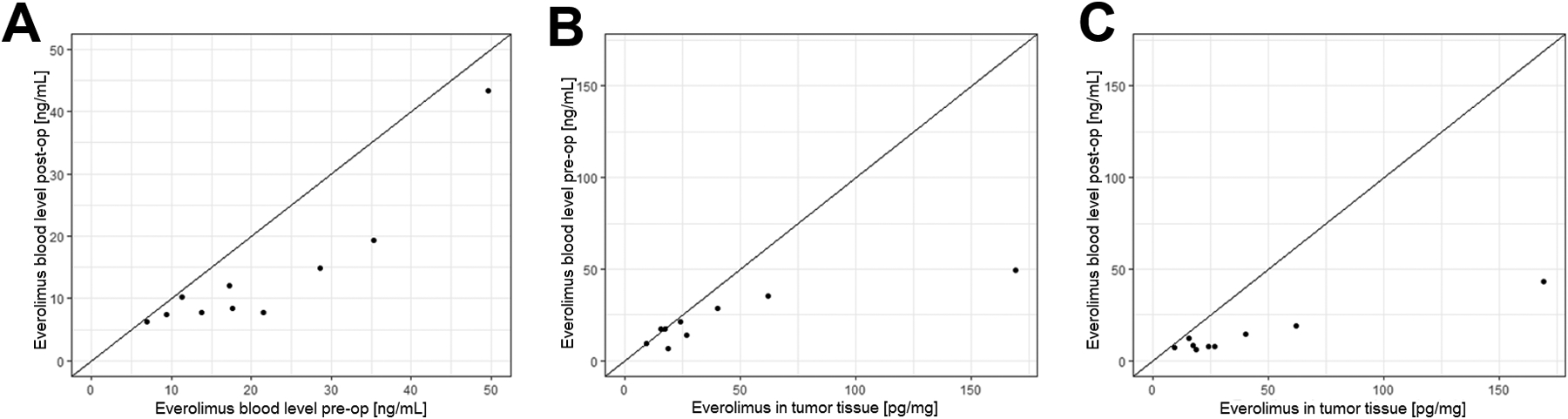
A: Pre-operative blood levels of everolimus were higher compared to post-operative (Wilcoxon signed rank test p = 0.002), and both values were strongly correlated (Spearman correlation = 0.85). B: Comparison of pre-operative blood levels and tumor tissue levels of everolimus. The drug concentrations in blood and tumor tissue were strongly correlated (Spearman correlation = 0.77). C: The drug concentrations in blood post-op and in tumor tissue were moderately correlated (Spearman correlation = 0.67).
Molecular biomarker and signaling response assessment
Detailed IHC results for all patients and controls are shown in Tables 2 and 3, respectively. Levels of p-S6 as determined by histoscore were lower in subjects (median = 90, range 0–220) compared to controls (median = 122, range 60–265), Mann-Whitney-Wilcoxon test p = 0.025 (Figure 3A). There was no evidence of a difference between levels of p-ERK in subjects (median = 180, range = 40–220) and controls (median = 135, range 100–240), Mann-Whitney-Wilcoxon test p = 0.27 (Figure 3B). There was no evidence of a difference in value of p-AKT between controls (median = 42, range 5–100) and subjects (median = 12, range 0–100), Mann-Whitney-Wilcoxon test p = 0.18 (Figure 3C).
Table 3.
Summary of Control Samples with IHC results
| Tumor Type | NF2 Status | p-S6 histoscore | p-ERK histoscore | p-AKT histoscore |
|---|---|---|---|---|
| meningioma | NF2 | 105 | 150 | 70 |
| meningioma | NF2 | 120 | 180 | 15 |
| meningioma | NF2 | 265 | 240 | 15 |
| meningioma | NF2 | 110 | 110 | 5 |
| meningioma | sporadic | 60 | 140 | 35 |
| meningioma | sporadic | 125 | 100 | 70 |
| meningioma | NF2 | 260 | 130 | 50 |
| meningioma | sporadic | 95 | 100 | 90 |
| VS | NF2 | 220 | 130 | 100 |
| VS | NF2 | 225 | 190 | 10 |
Figure 3. Expression levels of p-S6, p-ERK, and pAkt based immunohistochemistry (histoscore).
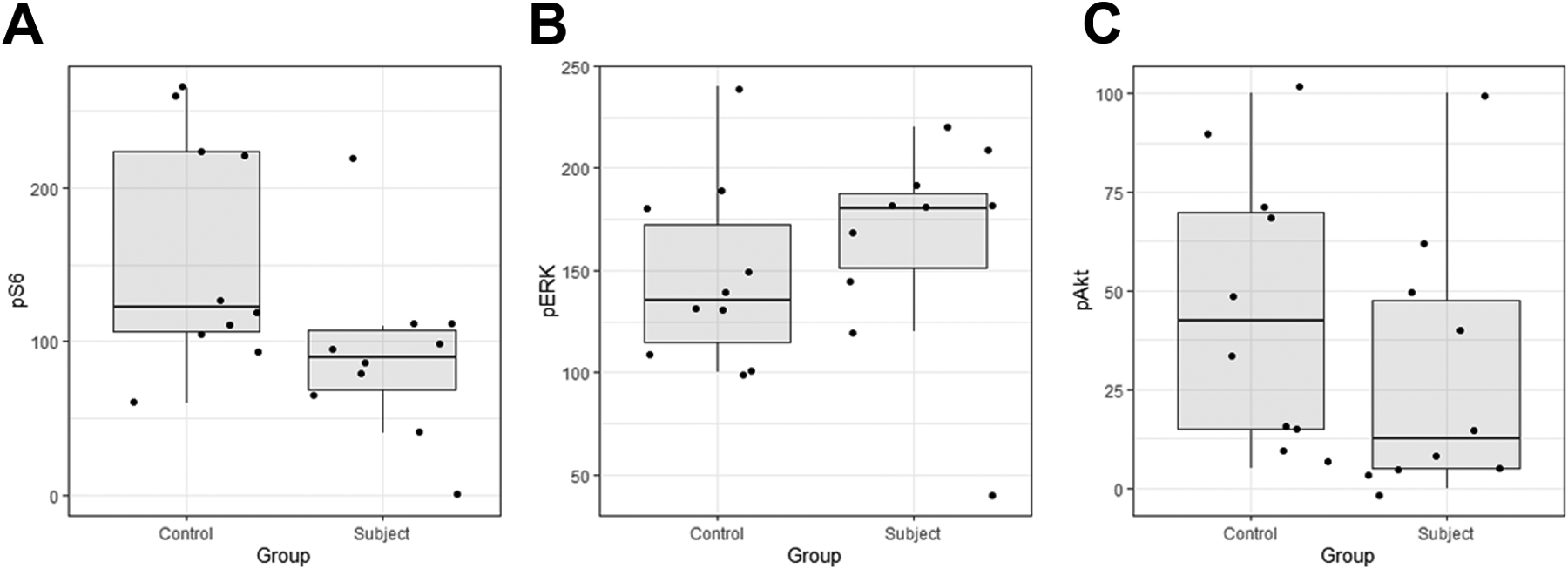
A: Subjects had lower values of p-S6 (median = 90) than controls (median = 122, Mann-Whitney-Wilcoxon test p = 0.025). B: There was no evidence of a difference in value of p-ERK between subjects (median = 180) and controls (median = 135), Mann-Whitney-Wilcoxon test p = 0.27). C: There was no evidence of a difference in value of p-AKT between subjects (median = 12) and controls (median = 42, Mann-Whitney-Wilcoxon test p = 0.18).
We further examined mTORC1 signaling in patients with sufficient remaining frozen tumor samples by immunoblot analysis. Detailed immunoblotting results for everolimus-treated patients and non-treated controls are shown in Figure 4A. In keeping with IHC results, the p-S6/S6 ratio was decreased in subjects compared to controls (P<0.005) (Figure 4B), while the p-ERK/ERK ratio did not show a significant difference between the two groups (Figure 4C).
Figure 4. mTORC1 signaling pathway was inhibited in tumor tissues from Everolimus treated patients.

A: Tumor lysates from non-treated (control) or everolimus-treated (subject) patients (M = meningioma, S = schwannoma) were subjected to immunoblotting with antibodies as indicated. RhoGDI was used as a loading control. B, C: p-S6/S6 and p-ERK/ERK levels shown in (A) were quantified, and the ratios for each patient were plotted. N.S., not statistically significant.
DISCUSSION
Despite many efforts, there remains a paucity of effective treatment options for patients with unresectable or recurrent meningiomas and schwannomas, with surgery and radiation therapy remaining the main therapeutic modalities for local tumor control. Recent prospective clinical trials have identified bevacizumab to be effective in a subset of patients with NF2-related and even sporadic vestibular schwannomas (23,24), however the treatment benefit can only be sustained with continued drug administration in the majority of patients. Although biallelic loss of NF2 is considered a key molecular driver in the majority of schwannomas and approximately 40% of meningiomas (35), development of molecular targeted therapies has been challenging, and predominantly focused on signaling pathways that are aberrantly activated in NF2 mutant tumors, such as the ErbB, PDGFR and mTOR signaling pathways (36). Based on preclinical in vitro data available at the time, we set out to assess the ability of everolimus to penetrate tumor tissue in human patients and abrogate mTORC1 signaling in a phase 0 study in preparation for subsequent phase 2 studies. Due to the study design that relied on volunteer subjects to assume additional risks without a reasonable likelihood of direct benefit, we encountered slower than anticipated approval, leading to early study termination. Nevertheless, we were able to acquire a unique patient-derived dataset shedding light on the results of published phase 2 studies of mTORC1 inhibitors in NF2-related tumors and informing future molecular targeted therapy studies in this patient population.
The first key finding from our study is the detection of measurable drug levels of everolimus in human meningioma and schwannoma tissue at steady-state. Median tumor tissue drug concentrations determined by mass spectrometry corresponded to the therapeutic range previously established in blood, and were strongly correlated with the pre-operative blood levels. To our knowledge, this represents the first published data on everolimus drug levels in human tumor tissue. Tissue levels of temsirolimus, another rapalog and mTORC1 inhibitor, were assessed in two studies including malignant glioma patients (37,38), with no qualitative reduction in tissue p-S6 levels observed based on very limited sample analysis.
The measurement of total drug levels in tissue is an inherent limitation of our study and similar clinical trials. While measuring unbound (free) drug in the extracellular fluid (ECF) using microdialysis remains the gold standard of assessing drug penetration though the BBB in vivo (39), it is generally not feasible in a clinical trial setting such as ours. Another limitation of our study, which is inherent to phase 0 studies where the target tumor is removed with a single surgery, is the reliance on matched control tumor tissue from different subjects that were untreated.
In our study, we observed only partial inhibition of phospho-S6 in the treated tumors compared to controls, indicating incomplete target inhibition, despite reaching trough blood levels in all patients meeting or exceeding the therapeutic range for successful treatment of SEGA. The failure to meet this primary endpoint of phospho-S6 inhibition in our study is in contrast with published data in breast cancer patients treated with everolimus at the same dose and schedule (32). Pharmacological inhibition of mTORC1 may result in MAPK pathway activation through a PI3K-dependent feedback loop (40), and while primary target inhibition in our study was incomplete, we did not observe statistically significant elevation of p-ERK in tumors treated with everolimus. Taken together, our observations may explain the limited anti-tumor effect of everolimus as a single agent seen in clinical studies for NF2 patients to date (41,42). More recently, combination therapy of everolimus and octreotide has shown encouraging clinical activity in a prospective clinical trial (43). Novel pharmacological strategies to enhance mTORC1 inhibition in schwannomas and meningiomas, for example using different drugs, drug combinations and/or delivery systems, merit investigation in future studies.
ACKNOWLEDGEMENTS
We are grateful to the patients participating in this study and the clinical teams of the participating institutions for excellent study-related patient care. Results from this study were presented in part at the 2018 Joint Global Neurofibromatosis Conference, Paris, France, November 2018 and the Society of Neuro-Oncology 24th Annual Meeting, Scottsdale, AZ, November 2019.
Funding:
This study was supported by the National Institutes of Health/National Cancer Institute grant R01CA164295 to M.A.K., and Novartis, Inc. grant CRAD001CUS205. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30CA008748 to Memorial Sloan Kettering Cancer Center and NIH/NCI R01CA191222 to P.G.G. The NYU Langone Experimental Pathology Immunohistochemistry Core Laboratory was supported in part by the Laura and Isaac Perlmutter Cancer Center Support Grant; NIH/NCI P30CA016087 and the National Institutes of Health S10 Grants; NIH/ORIP S10OD01058 and S10OD018338. The NYU Mass Spectrometry Core for Neuroscience was supported by NIH grant S10OD023659 to T.A.N.
Conflicts of Interest/Competing Interests:
MAK received funding for this study from Novartis, Inc. under institutional agreements, and discloses consultant agreements with AstraZeneca, Bayer, CereXis, QED Therapeutics and Recursion Pharma (personal fees received). All other authors declare that they have no conflicts of interest or competing interests related to the subject matter of this paper.
REFERENCES
- 1.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol 2019;21(Suppl 5):v1–v100 doi 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol 2010;99(3):307–14 doi 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinelli JP, Grossardt BR, Lohse CM, Carlson ML. Prevalence of Sporadic Vestibular Schwannoma: Reconciling Temporal Bone, Radiologic, and Population-based Studies. Otol Neurotol 2019;40(3):384–90 doi 10.1097/MAO.0000000000002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 1993;75(4):826. [DOI] [PubMed] [Google Scholar]
- 5.Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 1993;363(6429):515–21. [DOI] [PubMed] [Google Scholar]
- 6.Forde C, King AT, Rutherford SA, Hammerbeck-Ward C, Lloyd SK, Freeman SR, et al. Disease course of Neurofibromatosis Type 2; a 30-year follow-up study of 353 patients seen at a single institution. Neuro Oncol 2020. doi 10.1093/neuonc/noaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahronowitz I, Xin W, Kiely R, Sims K, MacCollin M, Nunes FP. Mutational spectrum of the NF2 gene: a meta-analysis of 12 years of research and diagnostic laboratory findings. Hum Mutat 2007;28(1):1–12 doi 10.1002/humu.20393. [DOI] [PubMed] [Google Scholar]
- 8.Ruttledge MH, Sarrazin J, Rangaratnam S, Phelan CM, Twist E, Merel P, et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet 1994;6(2):180–4 doi 10.1038/ng0294-180. [DOI] [PubMed] [Google Scholar]
- 9.Roche PH, Bouvier C, Chinot O, Figarella-Branger D. Genesis and biology of vestibular schwannomas. Prog Neurol Surg 2008;21:24–31 doi 10.1159/000156556 [pii] 10.1159/000156556. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol 2009;29(15):4235–49 doi MCB.01578–08 [pii] 10.1128/MCB.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol 2009;29(15):4250–61 doi MCB.01581–08 [pii] 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans DG, Kalamarides M, Hunter-Schaedle K, Blakeley J, Allen J, Babovic-Vuskanovic D, et al. Consensus recommendations to accelerate clinical trials for neurofibromatosis type 2. Clin Cancer Res 2009;15(16):5032–9 doi 1078–0432.CCR-08–3011 [pii] 10.1158/1078-0432.CCR-08-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006;124(3):471–84 doi 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature reviews Molecular cell biology 2011;12(1):21–35 doi 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nature reviews Molecular cell biology 2009;10(5):307–18 doi 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 16.Rosner M, Freilinger A, Hengstschlager M. Akt regulates nuclear/cytoplasmic localization of tuberin. Oncogene 2007;26(4):521–31 doi 1209812 [pii] 10.1038/sj.onc.1209812. [DOI] [PubMed] [Google Scholar]
- 17.Hu J, Zacharek S, He YJ, Lee H, Shumway S, Duronio RJ, et al. WD40 protein FBW5 promotes ubiquitination of tumor suppressor TSC2 by DDB1-CUL4-ROC1 ligase. Genes Dev 2008;22(7):866–71 doi 22/7/866 [pii] 10.1101/gad.1624008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell 2010;140(4):477–90 doi 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovannini M, Bonne NX, Vitte J, Chareyre F, Tanaka K, Adams R, et al. mTORC1 inhibition delays growth of neurofibromatosis type 2 schwannoma. Neuro Oncol 2014;16(4):493–504 doi 10.1093/neuonc/not242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pachow D, Andrae N, Kliese N, Angenstein F, Stork O, Wilisch-Neumann A, et al. mTORC1 inhibitors suppress meningioma growth in mouse models. Clin Cancer Res 2013;19(5):1180–9 doi 10.1158/1078-0432.CCR-12-1904. [DOI] [PubMed] [Google Scholar]
- 21.von Spreckelsen N, Waldt N, Poetschke R, Kesseler C, Dohmen H, Jiao HK, et al. KLF4(K409Q)-mutated meningiomas show enhanced hypoxia signaling and respond to mTORC1 inhibitor treatment. Acta Neuropathol Commun 2020;8(1):41 doi 10.1186/s40478-020-00912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane HA, Wood JM, McSheehy PM, Allegrini PR, Boulay A, Brueggen J, et al. mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res 2009;15(5):1612–22 doi 1078–0432.CCR-08–2057 [pii] 10.1158/1078-0432.CCR-08-2057. [DOI] [PubMed] [Google Scholar]
- 23.Blakeley JO, Ye X, Duda DG, Halpin CF, Bergner AL, Muzikansky A, et al. Efficacy and Biomarker Study of Bevacizumab for Hearing Loss Resulting From Neurofibromatosis Type 2-Associated Vestibular Schwannomas. J Clin Oncol 2016;34(14):1669–75 doi 10.1200/JCO.2015.64.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotkin SR, Duda DG, Muzikansky A, Allen J, Blakeley J, Rosser T, et al. Multicenter, Prospective, Phase II and Biomarker Study of High-Dose Bevacizumab as Induction Therapy in Patients With Neurofibromatosis Type 2 and Progressive Vestibular Schwannoma. J Clin Oncol 2019;37(35):3446–54 doi 10.1200/JCO.19.01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372(9637):449–56 doi S0140–6736(08)61039–9 [pii] 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 26.Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013;381(9861):125–32 doi 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 27.Ubogu EE. Biology of the human blood-nerve barrier in health and disease. Exp Neurol 2020;328:113272 doi 10.1016/j.expneurol.2020.113272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueno M, Chiba Y, Murakami R, Matsumoto K, Kawauchi M, Fujihara R. Blood-brain barrier and blood-cerebrospinal fluid barrier in normal and pathological conditions. Brain Tumor Pathol 2016;33(2):89–96 doi 10.1007/s10014-016-0255-7. [DOI] [PubMed] [Google Scholar]
- 29.Haldemann AR, Rosler H, Barth A, Waser B, Geiger L, Godoy N, et al. Somatostatin receptor scintigraphy in central nervous system tumors: role of blood-brain barrier permeability. J Nucl Med 1995;36(3):403–10. [PubMed] [Google Scholar]
- 30.O’Reilly T, McSheehy PM, Kawai R, Kretz O, McMahon L, Brueggen J, et al. Comparative pharmacokinetics of RAD001 (everolimus) in normal and tumor-bearing rodents. Cancer Chemother Pharmacol 2010;65(4):625–39 doi 10.1007/s00280-009-1068-8. [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell A, Faivre S, Burris HA 3rd, Rea D, Papadimitrakopoulou V, Shand N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 2008;26(10):1588–95 doi JCO.2007.14.0988 [pii] 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 32.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 2008;26(10):1603–10 doi JCO.2007.14.5482 [pii] 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 33.Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet 2004;43(2):83–95 doi 4322 [pii]. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka C, O’Reilly T, Kovarik JM, Shand N, Hazell K, Judson I, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol 2008;26(10):1596–602 doi JCO.2007.14.1127 [pii] 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 35.Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013;339(6123):1077–80 doi 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karajannis MA, Ferner RE. Neurofibromatosis-related tumors: emerging biology and therapies. Curr Opin Pediatr 2015;27(1):26–33 doi 10.1097/MOP.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen PY, Chang SM, Lamborn KR, Kuhn JG, Norden AD, Cloughesy TF, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04–02. Neuro Oncol 2014;16(4):567–78 doi 10.1093/neuonc/not247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhn JG, Chang SM, Wen PY, Cloughesy TF, Greenberg H, Schiff D, et al. Pharmacokinetic and tumor distribution characteristics of temsirolimus in patients with recurrent malignant glioma. Clin Cancer Res 2007;13(24):7401–6 doi 10.1158/1078-0432.CCR-07-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shannon RJ, Carpenter KL, Guilfoyle MR, Helmy A, Hutchinson PJ. Cerebral microdialysis in clinical studies of drugs: pharmacokinetic applications. J Pharmacokinet Pharmacodyn 2013;40(3):343–58 doi 10.1007/s10928-013-9306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 2008;118(9):3065–74 doi 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karajannis MA, Legault G, Hagiwara M, Giancotti FG, Filatov A, Derman A, et al. Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol 2014;16(2):292–7 doi 10.1093/neuonc/not150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goutagny S, Raymond E, Esposito-Farese M, Trunet S, Mawrin C, Bernardeschi D, et al. Phase II study of mTORC1 inhibition by everolimus in neurofibromatosis type 2 patients with growing vestibular schwannomas. J Neurooncol 2015;122(2):313–20 doi 10.1007/s11060-014-1710-0. [DOI] [PubMed] [Google Scholar]
- 43.Graillon T, Sanson M, Campello C, Idbaih A, Peyre M, Peyriere H, et al. Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial. Clin Cancer Res 2020;26(3):552–7 doi 10.1158/1078-0432.CCR-19-2109. [DOI] [PubMed] [Google Scholar]


