Abstract
The locus coeruleus (LC) provides the primary noradrenergic input to the forebrain and hippocampus, and may be vulnerable to degeneration and contribute to age-related cognitive decline and neuroinflammation. Additionally, inhibition of noradrenergic transmission by brain-permeable beta-blockers could exacerbate cognitive impairment. This study examined effects of age and acute beta-blocker administration on LC and hippocampus pathology, neuroinflammation and learning and memory behavior in mice. Male mice, 3 and 18 months old, were administered propranolol (beta-blocker) or mabuterol (beta-adrenergic agonist) acutely around behavioral assessment. Terminal inflammatory markers in plasma, hippocampus and LC were assessed alongside histopathology. An increase in hippocampal and LC microgliosis and inflammatory proteins in the hippocampus was detected in aged mice. We report pathological hyperphosphorylation of the postsynaptic NMDA receptor subunit 2B (NR2B) in the hippocampus, suggesting neuronal hyperexcitability. Furthermore, the aged proteome revealed an induction in proteins related to energy metabolism, and mitochondria dysfunction in the LC and hippocampus. In a series of hippocampal dependent behavioral assessment tasks acute beta-adrenergic agonist or beta blocker administration altered learning and memory behavior in both aged and young mice. In Y-maze, propranolol and mabuterol differentially altered time spent in novel versus familiar arms in young and aged mice. Propranolol impaired Novel Object Recognition in both young and aged mice. Mabuterol enhanced trace learning in fear conditioning. Aged mice froze more to context and less to cue. Propranolol impaired contextual recall in aged mice. Concluding, aged mice show LC and hippocampus pathology and heightened effects of beta-adrenergic pharmacology on learning and memory.
Keywords: Locus coeruleus, beta-blocker, aging, proteomics, inflammation, behavior
1. Introduction
The noradrenergic system regulates sympathetic nervous system activation, the stress response, and many aspects of arousal and cognition. Noradrenergic neurons of the locus coeruleus (LC), the primary source of noradrenergic tone in the brain, are active during waking, show increased activity in response to novelty, and there is a well-established role for noradrenergic signaling in attention and arousal-dependent learning and memory (Sara, 2009; Sara and Bouret, 2012; Thomas, 2015). Noradrenergic function declines with age and may underlie cognitive decline in aging (Liu et al., 2020; Mather and Harley, 2016). The LC is vulnerable to degeneration, and loss of noradrenergic neurons of the LC in humans with normal aging is quantifiable with imaging (Liu et al., 2019). Indeed, consequences of loss of noradrenergic tone with aging can be observed in physiological responses such as impaired pupillary dilation reflex and deficits in attention, learning and memory and arousal (Grudzien et al., 2007; Jacobs et al., 2019; Liu et al., 2020; Mather and Harley, 2016). Furthermore, in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease, pathology in the LC precedes more widespread pathological cell death (Betts et al., 2019; Braak and Del, 2011) and may play a contributing role in that more widespread pathology with loss of noradrenergic tone as an important anti-inflammatory regulator in the brain (Feinstein et al., 2016; Heneka et al., 2015; Weinshenker, 2018). An important question is why LC neurons display this early-onset vulnerability to aging and what are the factors that determine the rate and extent of loss of function. One possibility is that monoaminergic neurons may be particularly vulnerable to degeneration due to chronic activation, high metabolic activity and extended activity of the powerful oxidizing enzyme monoamine oxidase and accumulation of free oxygen radicals (Wang et al., 2020). Consequent degeneration of the LC could be an underlying etiological factor in progressive neuroinflammation observed widely in the aging brain and contributing to cognitive decline. Importantly, altered adrenergic signaling with impact on cognitive function may precede loss of noradrenergic neurons as they become dysregulated (Goodman et al., 2021).
Age-related degeneration of noradrenergic systems is further complicated by the clinical use of brain-permeable beta-adrenergic antagonists, beta-blockers, commonly prescribed for hypertension and anxiety, co-morbid with aging. This literature is somewhat complicated as both hypertension and anxiety are each associated with impaired cognitive performance, and effects of beta-blocker use on cognitive performance may be confounded by pro-cognitive effects related to the anti-hypertensive and anti-anxiety effects of beta-blockers (Duron and Hanon, 2010; Hajjar et al., 2005). Despite these confounds, pharmacological impairment of cognitive function and enhanced inflammatory responses as consequences of beta-blocker use have been reported (Evans et al., 2020; Gliebus and Lippa, 2007; Paran et al., 2010). Importantly, large scale epidemiological studies have clearly indicated beta-blocker use as a risk factor for chronic neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases (Cepeda et al., 2019; Mittal et al., 2017). These studies suggest that inflammatory consequences of clinical beta-blocker use in aged cognitively declining subjects need further study.
This study was designed to test the effects of age and the acute effects of a brain-permeable beta-adrenergic agonist and antagonist on 1) learning and memory, 2) inflammation in plasma and the brain and 3) pathology in the LC and hippocampus. Young (3 months) and aged (18 months) male mice were administered the beta-blocker, propranolol or agonist, mabuterol, and behavior was assessed across multiple learning and memory platforms. Inflammatory markers were evaluated in plasma and in the brain and evidence for microgliosis, pathology, and changes in the proteome were evaluated in the LC and hippocampus.
2. Methods
2.1. Mice
Two cohorts of forty C57BL/6J male mice were obtained from the NIA Aging Colony aged ~2 and ~17 months with experiments performed at 3 and 18 months in the behavioral cohort and terminal collection at 4 and 19 months for both cohorts. A main cohort of 40 mice (20 young and 20 aged) was used for behavior, pharmacology, and pathological endpoints (Figure 1, experimental design), and the second age-matched cohort of 40 mice (20 young and 20 aged) was used only for tissue collection for specific pathological endpoints (proteomics, western blot, and hippocampal histopathology) as described in the results. For all studies, mice were group-housed before each experiment under a reversed light-dark cycle with lights off at 8:30 AM and on at 8:30 PM. Mice were handled prior to the experiment for obtaining body weight. All procedures related to animal maintenance and experimentation were approved by the Stanford University Administrative Panel for Laboratory Animal Care and conformed to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize the number of mice used and their suffering.
Figure 1.
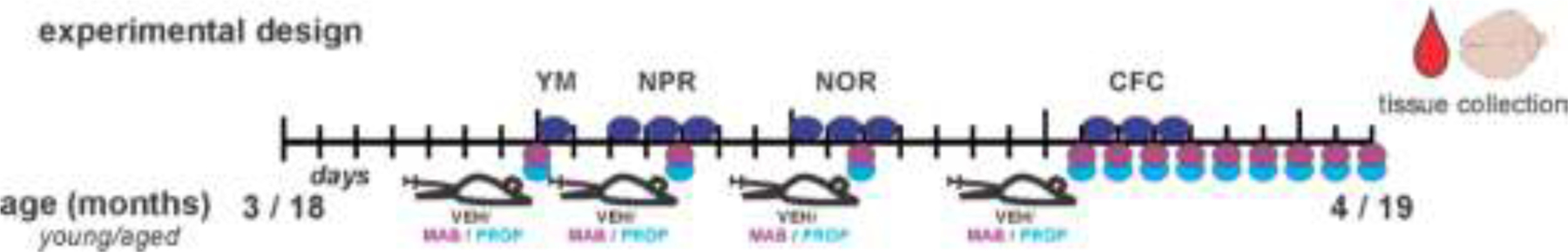
Experimental Design depicts timing of dosing and behavioral testing for young (3–4 months) and aged (18–19 months) mice. Mice were dosed with vehicle, propranolol or mabuterol around each behavioral task as well as daily for 9 days prior to termination. Timing of dosing around each specific behavioral task is indicated in the respective figures. Sample size is 6–7 per group; 2 × 3 factor, age x treatment design.
2.2. Drug administration
For experimental design see Figure 1. The main cohort of 40 mice were dosed acutely with the beta-adrenergic antagonist, propranolol (RS-propranolol; Sigma; 10mg/kg, s.c), beta-adrenergic agonist, mabuterol (synthesized in synthetic chemistry lab; 0.3mg/kg, s.c.), or 0.9% saline vehicle, in single daily doses around respective behavioral assessments (see each Figure for depiction of dosing around each behavior). After the final behavioral session of contextual fear conditioning, all mice were dosed daily for an additional 6 days, with the final dose occurring 30 minutes prior to tissue collection on the 6th day. Final group sizes for the pharmacology cohort were 3M-vehicle (n=6), 3M-propranolol (n=7), 3M-mabuterol (n=7), 18M-vehicle (n=6), 18M-propranolol (n=6), and 18M-mabuterol (n=7).
2.3. Behavioral Testing
2.3.1. Y-maze - Forced Alternation:
The Forced Alternation Y-maze is a two-trial spatial reference memory task that measures the innate tendency of rodents to explore novel environments and tests hippocampal-dependent spatial reference memory (Kraeuter et al., 2019). The symmetrical Y-maze was made of white plastic with three arms separated by a 120-degree angle. Each arm was 40 cm long x 8 cm wide with 15 cm high walls. The testing room was dimly lit (~20 Lux). The Y-Maze test consisted of two trials (training and testing) separated by an Inter-Trial-Interval (ITI) of 1 hour. Each mouse was dosed with propranolol, mabuterol, or vehicle 15 minutes prior to the training. Training: One of the arms was blocked off by a plastic insert, and the mouse was released in one of the open arms and allowed to explore the two open arms for 5 minutes. Testing: After 1 hr ITI, the plastic insert was removed, and the mouse was reintroduced into the maze to explore all 3 arms. The duration and frequency in each arm were recorded using Ethovision XT (Noldus Information Technology, Wageningen, the Netherlands, version 14) tracking system. Y-Maze was cleaned with 1% Virkon solution after each trial. The experiment was conducted when the mice were 3 and 18 months old. The novel arm was pseudorandomized across different age and treatment groups. Time spent in novel versus familiar arms was measured.
2.3.2. Novel Place Recognition
The Novel Place Recognition (NPR) test is used to assess hippocampal function believed to be driven by spatial working memory in rodents. This test is based on the tendency of rodents to preferentially explore a novel location rather than a familiar one. The choice to explore the novel location is thought to reflect the use of hippocampal-dependent spatial learning and recognition memory in rodents. The NPR test was conducted in a black plastic chamber measuring 50 cm by 50 cm. The walls of the plastic arena were black, and the floor was white. A plastic 36 cm (length) x 25 cm (width) blue and white striped sheet (1.5cm vertical stripes) was fixed onto the upper wall as a visual cue. The NPR test was conducted over 3 consecutive days. Habituation: On the first day, each mouse was placed into the center of the empty arena, allowing free exploration for the duration of the 10 minute trial. Each mouse was then returned to its home cage, and the arena was cleaned with a 1% Virkon solution. Training: On the second day, mice were placed into the center of the arena, which contained two identical objects, each placed in the center vertically and 10 cm from the top and bottom walls (the top wall having the cue). The two identical objects used for this test were either a green tower (18cm H x 4cm L x 4cm W) or a white bottle (16cm H x 4cm L x 4cm W). The green tower was made of dark green plastic and had a square base. The white bottle was made of white plastic and had a circular base. Use of either the green tower or while bottle for NPR and introduction of the other for Novel Object Recognition in the following test was pseudorandomized to ensure results were not influenced by an innate preference for either object. NPR training duration was 10 minutes. At the end of the NPR training, mice were dosed with propranolol, mabuterol, or vehicle and returned to the home cage for 24 hours (ITI) followed by NPR testing. Testing: On the third day, the object closer to the cue was moved to the right or left (pseudorandomized) of the vertical center, placed 10 cm distance from the top, and either left or right sidewall. Mice were then placed into the center of the arena and allowed to explore for a 10 minute trial. Exploration was recorded, and mice were tracked with the automated tracking system Ethovision XT 13 (Noldus Information Technology, Wageningen, Netherlands). Interaction with the objects was defined as when the nose of the mouse was within 2 cm from the objects. The interaction time during NPR training was tracked by the Ethovision system, while the interactions during NPR testing were hand scored by the experimenter who was blind to the treatment group. Arena and objects were cleaned with 1% Virkon between each trial.
2.3.3. Novel Object Recognition
The Novel Object Recognition (NOR) test is used to assess long term object memory. This test is based on the spontaneous tendency of rodents to preferentially explore a novel object rather than a familiar one and is thought to reflect the use of learning and recognition memory in rodents controlled by multiple centers in the brain including neocortex and hippocampus. The NOR test was conducted in the same arena as NPR and likewise conducted over 3 consecutive days. The two objects used for this test were the green tower and white bottle described above for NPR. Assignment of one object as the familiar object and the other as the novel object was pseudorandomized to ensure results were not influenced by an innate preference for either object. Habituation: On the first day, mice were each placed into the center of the empty arena, allowing free exploration for the duration of the 10 minute trial. The subject was then returned to its home cage, and the arena was cleaned with a 1% Virkon solution. Training: On the second day, mice were placed into the center of the arena, which contained two identical objects, each placed in the center vertically and 10 cm from the top and bottom walls (the top wall having the cue). NOR training duration was 10 minutes. At the end of the NOR training, mice were dosed with propranolol, mabuterol, or vehicle and returned to the home cage for 24 hours ITI followed by NOR testing. Testing: On the third day, the object closer to the cue was replaced by a novel object but placed in the same location as the original object. Mice were then placed into the center of the arena and allowed to explore for the duration of the 10 minute trial. The objects in NOR were pseudorandomized across subjects. The trials were recorded, and mice were tracked with the automated tracking system Ethovision XT 13 (Noldus Information Technology, Wageningen, Netherlands). An interaction was defined as when the nose of the mouse was within 2 cm from each object. The interaction time during NOR training was tracked by the Ethovision system, while the interaction during NOR testing were hand scored by the experimenter who was blind to the treatment group. Interaction time is presented as both Total Interaction Time as well as Interaction Time (%) which was calculated for each object as amount of interaction time with each object divided by the total (novel+familiar) interaction time. Arena and objects were cleaned with 1% Virkon between each trial.
2.3.4. Fear Conditioning
Effects of aging and beta-adrenergic pharmacology were assessed based on a previously described fear conditioning protocol (Evans et al., 2020; Faizi et al., 2012) with modifications. Coulbourn Instruments (Whitehall, PA) fear conditioning system and FreezeFrame software were used for data acquisition and analysis. A trace fear conditioning protocol was used for the training day, followed by one day of contextual memory retrieval and one day of cued testing. The training and contextual testing chambers were identical; the walls were made of aluminum, the floor of the chamber was a gray metal grid through which the unconditioned stimulus (US) shock was delivered. The training and contextual chambers were scented with mint extract and were under yellow light. The training and contextual testing chambers were cleaned with a 10% simple green solution between each mouse. The cued testing chamber was placed in a different room and consisted of a circular-shaped chamber made of plastic, lit with blue light, and scented with vanilla extract. The cued testing chamber was cleaned with 70% Ethanol between each mouse. Both chambers were mounted within sound-attenuating boxes. Each chamber had speakers mounted on the wall and included an exhaust fan and camera. A detailed protocol follows. Mice were dosed with propranolol, mabuterol, or saline vehicle 15 minutes prior to exposure to the experimental chambers on each day (days 1, 2, and 3). Training: On Day 1, mice were placed individually into a training chamber for a 200-second baseline followed by 3 tone-shock pairings. A tone (20 seconds, 80 dB, 2 kHz) was presented to the mouse, followed by an electrical shock (intensity 0.5mA, 2 seconds duration) 18 seconds after the end of the tone. This procedure was repeated for a total of 3 times with a 60 second interval at the end of the shock to the next tone. The mouse was removed from the chamber and returned to the home cage 60 seconds after the last shock. The 18 second period following each tone and preceding the shock was defined as the TRACE period for analysis of freezing in expectation of the shock. Increased freezing in consecutive TRACE periods was used as an indication of learning. Context: On Day 2, each mouse was returned to the same context of the training chamber without any tone or shock for contextual memory testing for 5 minutes. Cue: On Day 3, each mouse was placed into a novel context in the cued testing chamber (see description above). After 200 seconds of baseline recording for habituation, three tones (20 seconds, 80 dB, 2 kHz) were presented without shocks with ITIs of 60 seconds. An overhead camera was used to record freezing behavior. Freezing during a TRACE period of 20 seconds following the tone was used as an indication of cued recall. Freezing was defined as the complete lack of motion for a minimum of 0.75 seconds, as assessed by FreezeFrame software (Actimetrics, Evanston, IL).
2.4. Tissue collection
For the main cohort of forty mice, thirty minutes following timed terminal dosing, mice were deeply anesthetized with isoflurane. Prior to perfusion, whole blood was collected from the right ventricle via cardiac puncture (23 g needle) into lithium heparin-containing vials (Greiner Bio-One, MiniCollect Tube Reference #450479). For perfusion, the right atrium was opened, and mice were transcardially perfused with ice-cold phosphate-buffered saline (PBS; pH 7.4) through a 23 g needle. The perfused brain was removed. The brain was bisected coronally at the level of the mammillary bodies into a forebrain and hindbrain. The intact hindbrain was post-fixed in a 15ml conical centrifuge tube with 4% paraformaldehyde and held at 4°C for post-fixation (24 hours 4 degrees C). Forebrains were immediately further dissected into the hippocampus, frontal cortex, hypothalamus, and cortex with each dissection flash-frozen on dry ice and stored at −80 for later analysis (multiplex mouse cytokine assay and western blot). Following post-fixation, hindbrains were washed in PBS and then cryoprotected for at least 72 hours (until sunk) in 30% sucrose in phosphate buffer (PB). Fixed, cryoprotected hindbrains were then rapidly frozen in isopentane on dry ice for later analysis (LC histopathology; TH/iba1/AT8). Whole blood was centrifuged (3000 g for 10 minutes) for plasma separation, and plasma was aliquoted and frozen on dry ice. All brain and plasma samples were stored at −80 C prior to further assay. For the second cohort of 40 mice (20 young and 20 aged) brains from half of the subjects were dissected and flash-frozen for protein analyses (n=10; proteomics and western blot) and brains from the other half of the subjects were post-fixed as whole brains for histopathology (n=10; LC histopathology TH/AT180 and hippocampal histopathology AT8/AT180/iba1).
2.5. Multiplex mouse cytokine assay
Multiplex tissue cytokines were analyzed in plasma or brain homogenate from hippocampal dissections using a Luminex 38-plex (Affymetrix) mouse cytokine assay. The Luminex assay was performed in the Human Immune Monitoring Center at Stanford University, following manufacturer instructions. Briefly, hippocampal tissue was homogenized in RIPA buffer containing proteinase inhibitor (1:100) by pulling tissue through a 23 g needle (15x) and then sonicated for 3×3 second pulses. Homogenate was spun at 14,000 g for 10 minutes, and protein concentrations were determined by Pierce BCA assay. Samples were diluted to a common concentration of 6 ug/uL. Plasma samples were diluted 1:3. Plasma and brain homogenate samples were run in duplicate on a 96 well plate alongside standard curve and quality control calibration samples.
2.6. Immunohistochemistry
Multilabel fluorescent immunohistochemistry was done using iba1 as a macrophage marker for neuroinflammation/microgliosis, and using two markers of tau pathology (AT8-immunostaining and AT180-immunostaining) in the locus coeruleus and hippocampus, alongside tyrosine hydroxylase (TH-immunoreactivity), as a marker of noradrenergic neurons in the LC. LC sections were obtained from the behavioral and pharmacology cohort and hippocampal sections were obtained from the second cohort of mice that were not administered adrenergic compounds or administered behavior. A DAPI nuclear stain was included for all immunohistochemical analyses. Fixed brains were serially sectioned (at −18°C using a Microm HM-550 cryostat) in a coronal plane across 6 series through the rostrocaudal extent of the hindbrain (30-micrometer sections; 180 micrometers between sections within each series) and through the hippocampus (40-micrometer sections; 240 micrometers between sections within each series) and stored in cryoprotectant storage buffer (30% ethylene glycol, 20% glycerol in 0.05M PB, pH 7.4). Multilabel fluorescent immunohistochemistry was used to stain one series of LC sections each for AT8/TH double-label, AT180/TH double label, or iba1/TH double label through the rostral to mid-rostrocaudal LC (from −5.35 to −5.8 mm Bregma) (Paxinos and Franklin 2001). Hippocampal sections were stained for AT8/iba1 double label or AT180 staining through the dorsal hippocampus (from −0.82 to −2.54 mm Bregma) (Paxinos and Franklin 2001). For all stainings, free-floating sections were incubated at room temperature in 24-well tissue culture plates gently shaken on an orbital shaker throughout the procedure. All rinses were 15 minutes unless stated otherwise. Sections were rinsed three times in 0.05 M PBS and then preincubated 1 hour in PBS containing 0.3% Triton X-100 (PBST) and 3% bovine serum albumin. Sections were incubated 18 hours with the above-mentioned combination of primary antibodies (See Supplemental Table S1; including mouse anti-AT8 primary antibody, recognizes tau phosphorylated at serine 202 and threonine 205, Invitrogen, MN1020, 1:500; mouse anti-AT180 primary antibody, recognizes tau phosphorylated at threonine 231, Invitrogen, MN1040, 1:500; chicken anti-TH primary antibody, Abcam, ab76442, 1:2000; and goat anti-iba1 primary antibody, Abcam, ab5076, 1:1000) in 0.3% PBST and 3% bovine serum albumin. Following 3 PBS rinses, sections were incubated for 2 hours in IgG or IgY secondary antibodies each diluted 1:250 in PBS (Alexa Fluor 488-conjugated AffiniPure donkey anti-mouse, 715–545-150; Cy5-conjugated AffiniPure donkey anti-chicken, 703–175-155; Cy3-conjugated AffiniPure donkey anti-goat, 705–165-147; Jackson Immunoresearch, Bar Harbor, ME). Sections were then rinsed 2 times prior to incubation for 30 minutes with DAPI (D9542, Sigma-Aldrich, St. Louis, MO) diluted 1:5000 in PBS. Free-floating sections were then rinsed 3 times in PBS, mounted on 0.15% gelatin-treated glass slides, and allowed to air-dry to affix sections to slides immediately prior to coverslipping with polyvinyl alcohol mounting medium with DABCO antifade (10981, Sigma-Aldrich). Slides were stored at 4 °C prior to imaging. Primary antibody controls were included to address autofluorescence in aged tissue. For primary antibody controls tissue was run through the same immunohistochemistry protocol described above, but primary antibodies were left out for the overnight incubation. All other steps including blocking and secondary incubations were performed identical to the main study. Additionally, to address autofluorescence in tissue from aged mice, sections stained with AT180 were incubated in 0.05% Sudan Black for 5 minutes to quench autofluorescence as the final step in the immunohistochemistry protocol.
Image analysis:
Images were quantified by an experimenter blind to treatment groups. Regions of analysis were selected through the rostral to mid-rostrocaudal LC as the midbrain source of noradrenergic projections to the forebrain as well as the dorsal hippocampus, as a projection site of locus coeruleus related to learning and memory (Corcoran et al. 2016; Todd and Bucci 2015; Samuels and Szabadi 2008; Murchison et al. 2004). TH-immunoreactive (-ir) cell bodies were counted across 3 consecutive serial sections throughout the rostrocaudal extent of the LC. Iba1-ir was thresholded using NIH ImageJ version 1.52a and % area was quantified within the LC across 3 consecutive serial sections (180 micrometers apart) and within the hippocampus in CA1, CA3 and DG at one rostrocaudal level. Each image was thresholded for % area 3 times, and an average value (accounting for within-evaluator variation) was obtained for each image. Immunostaining was quantified in both left and right LC at each rostrocaudal level, and an average value across left and right LC was used to quantify immunostaining at each rostrocaudal level for analysis. Images were captured under consistent exposure settings at 10X magnification using a Zeiss Axioscope M2 microscope with Stereo Investigator 2019.1.1 Software (MicroBrightField Bioscience, VT). Cell counts were performed within individual image layers for each immunostaining channel using Adobe Photoshop.
2.7. Western Blot
Flash-frozen hippocampus from one hemisphere was homogenized in 0.15 mL of T-PER (Tissue Protein Extraction Reagent; Life Technologies, Cat: 78510 Carlsbad, CA) with Protease Inhibitor (Life Technologies, Cat: 78429, Carlsbad, CA) and Phosphatase Inhibitor Cocktails (Abcam, Cat: ab201113, ab201112, ab201114, Cambridge, UK) on ice by sonication using Ultrasonic Probe Homogenizer (Omni International, Kennesaw, GA). The homogenate was centrifuged at 12,000 x rpm for 10 min at 4°C. The protein concentration was determined using the BCA protein assay kit (Pierce, Cat: 23227 Rockford, IL). Samples were prepared with Novex Bolt lithium dodecyl sulfate sample buffer and Novex Bolt sample reducing agent (Fisher Scientific) and boiled at 95°C for 5 min. Samples were loaded 40ug/well on 10% Bis-Tris, 1.0mm, Mini Protein Gel, 17-well (Life Technologies, Cat: NW00107BOX Carlsbad, CA). The protein was transferred to a polyvinylidene difluoride membrane (Abcam, Cat: ab133411, Cambridge, UK) and incubated in Intercept® (TBS) Blocking Buffer (Li-cor, Cat: 927–60001, Lincoln, NE) for 1 h at room temperature. The membranes were incubated at 4°C overnight with anti-NR2B (1:1000, Invitrogen, Cat: MA1–2014, Carlsbad, CA), anti-phospho-NR2B Ser1303 (1:1000, Sigma-Aldrich, Cat: 07–398, St. Louis, MO), anti-synaptophysin (1:1000, Sigma-Aldrich, Cat: S5768–100UL, St. Louis, MO), anti-synapsin (1:1000, Invitrogen, Cat: 51–5200, Carlsbad, CA), anti-phospho-synapsin (1:1000, Sigma-Aldrich, Cat: AB9848, St. Louis, MO), anti-TH (1:1000, Invitrogen, Cat: 701949, Carlsbad, CA), anti-AT8 (1:100, Thermo Fisher Scientific, Cat: MN1020 Waltham, MA), anti-Tau (1:500, Abcam, Cat: ab64193, Cambridge, UK), and anti-tubulin (1:10000, Sigma-Aldrich, Cat: T5168–100UL, St. Louis, MO) primary antibodies (see Supplemental Table S1). The following day, membranes were washed (4 × 10 min) with 0.01% Tween-20 in 1x TBS and incubated for 1h at room temperature with IRDye® IgG Secondary Antibody (goat anti-mouse Cat: 926–68070, goat anti-rabbit Cat: 926–32211; 1:10000, goat anti-mouse Cat: 827–08364, goat anti-rabbit Cat: 926–68071, Li-cor, Lincoln, NE). Following secondary antibody incubation, membranes were washed (4 × 10 min) with 0.01% Tween-20 in 1x TBS. Membranes were then scanned with the Sapphire Biomolecular Imager (Azure Biosystems, Dublin, CA) in the appropriate wavelengths. Azurespot Version 2.0 (Azure Biosystems, Dublin, CA) was used for densitometry analysis of target protein levels and normalized to internal level of tubulin for each sample as control. In order to stain membranes with multiple primary antibodies, membranes were stripped by incubating in 1X Restore Fluorescent Western Blot Stripping Buffer (Thermo Fisher Scientific, Cat: 62300 Waltham, MA) for 20 minutes at room temperature on a shaker. Membranes were washed (3 × 5 min) with ultrapure water and (3 × 5 min) with 0.01% Tween-20 in 1x TBS. Then, membranes were incubated in respective primary antibodies overnight as described above.
2.8. Proteomics
Flash-frozen hindbrains from aged and young mice were thick sectioned at 300 micrometers and LC was microdissected bilaterally and pooled across two consecutive sections (4 punches per subject) using a 500 micron diameter tissue punch (Fine Science Tools). Hippocampus from one brain hemisphere was dissected intact and flash-frozen at time of tissue collection. Proteomics analyses were performed at the Stanford University Mass Spectrometry facility. Lysis buffer (2% SDS, 100 mM TrisHCl, 100 mM DTT and 1X Protease and Phosphatase Inhibitors) was added to tissue samples, which were then sonicated for 30 minutes and heated at 95C for 2 hours at 1000 rpm. Proteins were precipitated out with 4X volume of acetone and incubated at −80°C overnight. Air-dried protein pellets were reconstituted in 400 ul of 50 mM ammonium bicarbonate buffer, sonicated, and vortexed to solubilize proteins. The samples were then reduced with 10 mM DTT at 55⁰C for 30 minutes followed by alkylation with 30 mM acrylamide for 30 minutes at room temperature. 1 µg of Trypsin/LysC protease (Promega) was added to each sample for digestion at 37⁰C overnight. After digestion, the reaction was quenched using 1% formic acid and peptides were de-salted on C18 Monospin reversed phase columns (GL Sciences). Peptide quantification was performed with the Pierce Quantitative Fluorometric Peptide Assay kit (Thermo Fisher Scientific). The peptide mixture was dried by speed vac before dissolution in reconstitution buffer (2% acetonitrile with 0.1% formic acid). 1 µg was used for subsequent LC-MS/MS analysis.
Mass spectrometry experiment was performed using an Orbitrap Fusion Tribrid mass spectrometer (Thermo Scientific, San Jose, CA) with liquid chromatography using a Nanoacquity UPLC (Waters Corporation, Milford, MA). A flow rate of 300 nL/min was used, where mobile phase A was 0.2% formic acid in water and mobile phase B was 0.2% formic acid in acetonitrile. Analytical columns were prepared in-house with an I.D. of 100 microns pulled to a nanospray emitter using a P2000 laser puller (Sutter Instrument, Novato, CA). The column was packed using C18 reprosil Pur 1.8 micron stationary phase (Dr. Maisch) to a length of ~25 cm. Peptides were directly injected onto the analytical column using a gradient (2–45% B, followed by a high-B wash) of 80 min. The mass spectrometer was operated in a data dependent fashion using CID fragmentation for MS/MS spectra generation.
For data analysis, the .RAW data files were processed using Byonic (Protein Metrics, Cupertino, CA) to identify peptides and infer proteins. Proteolysis with Trypsin/LysC was assumed to be semi-specific allowing for N-ragged cleavage with up to two missed cleavage sites. Precursor mass accuracies were held within 12 ppm, and 0.4 Da for MS/MS fragments. Cysteine modified with propionamide were set as fixed modifications in the search. Proteins were held to a false discovery rate of 1%, using standard reverse-decoy technique (Elias and Gygi, 2007).
Pathway analysis for proteomics was done with KEGG mouse 2019 analysis (Enrichr). For LC, all proteins that fit criteria of 2-fold up or 2-fold down-regulated with age (186 proteins) were included in pathway analyses. For hippocampus, only 24 proteins fit the 2-fold criteria, so for pathway analyses, all proteins that were significantly up or down regulated (p<.05; 118 proteins) were included in pathway analyses.
2.9. Statistics
Statistical analyses were performed with GraphPad Prism 7.0. Repeated measures, one-way or two-way analyses of variance, were followed by a post-hoc comparison of select treatment groups with correction for multiple comparisons as indicated. Main effects of age were examined between vehicle-treated young versus aged groups, and separate statistical analyses of effects of pharmacology were done in young and old mice, independently. Significance was reported relative to p < .05, but results with effects approaching this threshold are also discussed as relevant trends (Amrhein et al., 2019; Wasserstein et al., 2019).
3. Results
Quantification of tyrosine hydroxylase (TH) and iba1 immunostaining in locus coeruleus (LC) revealed increased iba1-immunoreactivity in aged mice (t-test young vs aged; t(14)=2.736, p = .016; Figure 2), but no differences in TH-immunoreactive cell counts between aged and young mice and no effect of propranolol within aged mice on TH-ir cell counts (one-way ANOVA within each rostrocaudal anatomical level with Sidak’s posthoc adjustment for multiple comparisons; Figure 2). Immunohistochemical analyses were only performed on vehicle-treated young and vehicle- and propranolol-treated aged mice to determine effects of age in vehicle treated mice and effects of propranolol in aged mice. Mabuterol treatment was not included in immunohistochemical studies.
Figure 2.
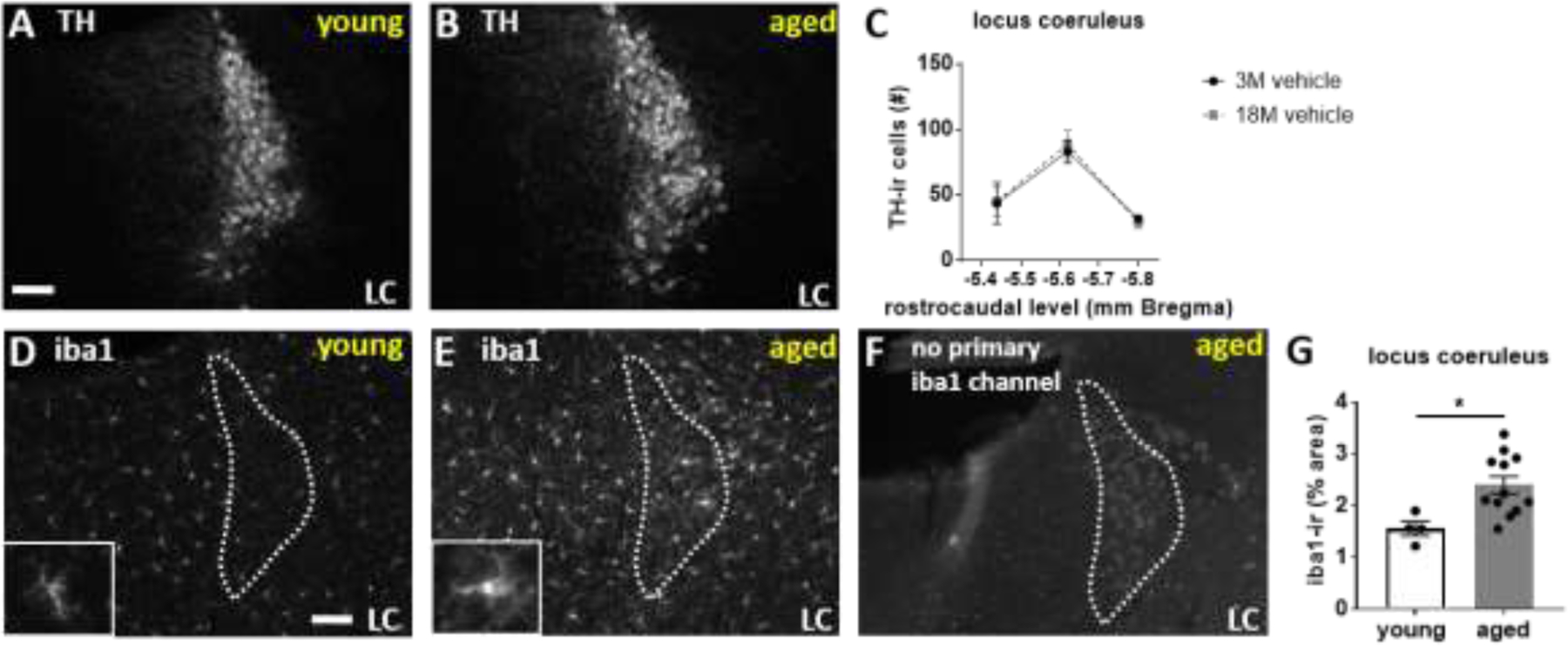
Aged (19 months) and young (4 months) mice have equivalent tyrosine hydroxylase (TH) immunoreactive (-ir) cell counts across the rostrocaudal extent of the locus coeruleus. Aged mice have increased iba1-ir in the locus coeruleus. Photomicrographs depict representative TH-ir in the locus coeruleus at −5.62 mm bregma in A) young versus B) aged mice. White scale bars equal 100 micrometers. C) Line graph shows quantification of TH-ir cell counts. D-F) Photomicrographs show representative iba1-ir staining in D) young versus E) aged mice. Insets in C and D show higher magnification iba-ir staining. F) No primary antibody control for the iba1 channel in aged mice show age-related autofluorescence that is distinct from iba1-ir. Iba1-ir can be thresholded out above age-related background staining. G) Scatter bar graph depicts quantification of thresholded iba-ir % area in locus coeruleus averaged across 3 rostrocaudal levels.
Proteomics analyses of young versus aged locus coeruleus and hippocampus revealed changes in protein expression between young and aged mice in both the locus coeruleus and hippocampus (Figure 3). Analysis of locus coeruleus showed 173 proteins upregulated and 13 proteins downregulated using a criteria of 2-fold up- or down-regulation and p-value of p<.05 (Figure 3A). Pathway analyses from the proteins modulated with age in the locus coeruleus were related to neurodegenerative disease, oxidative phosphorylation, metabolic pathways, and mitochondrial function (Figure 3B). Initial analysis of hippocampus using the same criteria of 2-fold up- or down-regulation and p-value < .05 showed only 12 proteins significantly upregulated and 12 proteins significantly downregulated, so criteria was expanded by removing the 2-fold criteria and including all proteins significantly modulated by age (p<.05; Figure 3C). Pathways implicated in age-related proteomic changes included synaptic plasticity, retrograde endocannabinoid signaling, and oxidative phosphorylation (Figure 3D).
Figure 3.
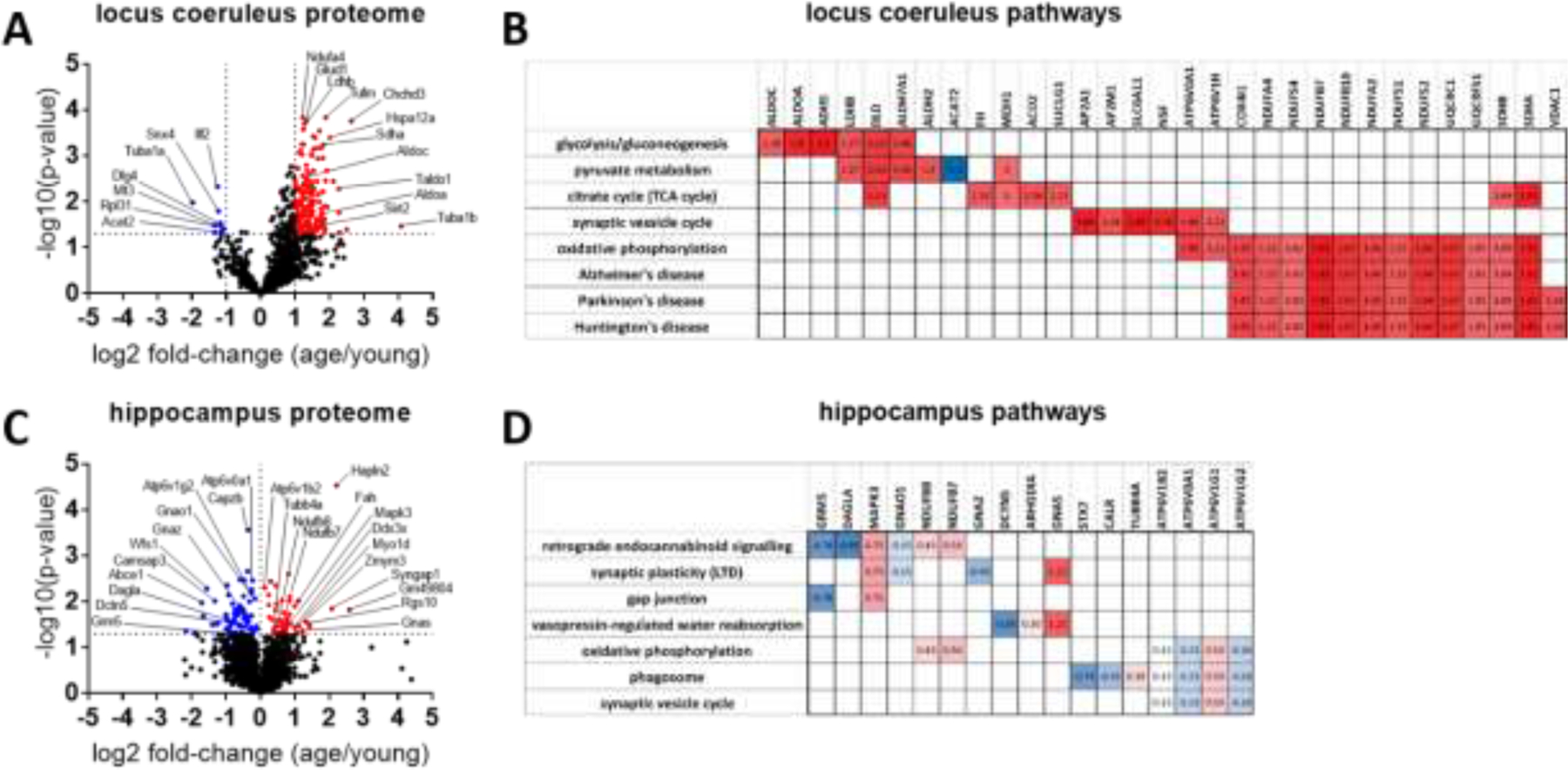
Volcano plots depict age-related fold-change and significance for proteins detected in the A) locus coeruleus and C) hippocampus with proteomics. A,B) In locus coeruleus (1043 proteins detected), 186 proteins were identified to be 2-fold up- (red) or down-regulated (blue) with age (fold change as old/young; p-value cutoff p<.05; -Log10(p-value)>1.3). B) Clustergram analysis for locus coeruleus identifies age-modulated proteins in specific pathways. Locus coeruleus pathways include metabolic pathways, oxidative phosphorylation and neurodegeneration as indicated by KEGG analysis. C,D) In hippocampus (2603 proteins detected), only 24 proteins met the 2-fold change criteria, so for hippocampal pathway analysis criteria was expanded to include all proteins that were significantly up- or down-regulated (p<.05; -Log10(p-value)>1.3; 118 proteins) independent of fold-change. D) Clustergram analysis for hippocampus identifies age-modulated proteins in specific pathways. Hippocampal pathways modulated with aging include retrograde endocannabinoid signaling, synaptic plasticity, oxidative phosphorylation, and phagosomes indicated by KEGG analysis. Blue indicates downregulation with age and red indicates upregulation with age. Numbers in clustergram indicate Log2 fold-change (aged/young) for each protein.
Quantification of iba1-immunoreactivity (-ir) in hippocampus of vehicle-treated young versus aged mice revealed an increase in iba1-immunoreactivity in aged mice in the dentate gyrus (DG; t(17) =3.737, p=.0016), with a trend in CA3 (t(17) =1.975, p=.06), and a non-significant increase in CA1 (t-test for age-effect within each region; Figure 4).
Figure 4.
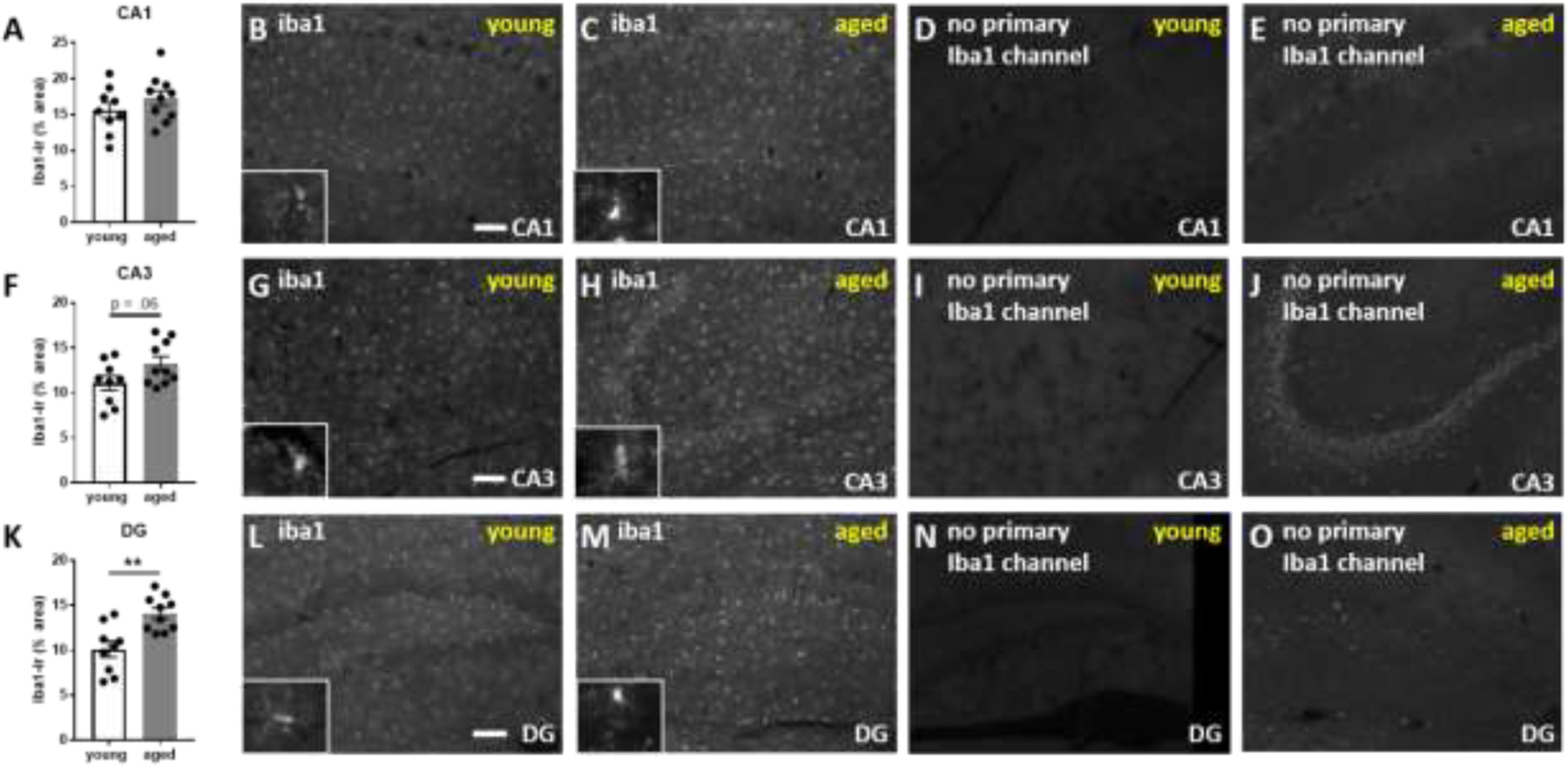
Aged mice have increased iba1-immunoreactivity (-ir) in the hippocampus. A) Scatter bar graph depicts quantification of iba1-ir in CA1. Photomicrographs depict representative immunoreactivity in the iba1 channel for B) young versus C) aged mice in the CA1 region with representative no antibody controls for young D) versus E) old mice. F-J) shows the quantification and representative images for CA3 and K-O) shows the quantification and representative images for dentate gyrus (DG). Iba1-immunoreactivity is morphologically distinct from age-related autofluorescence and can be thresholded out above age-related autofluorescence. White scale bars equal 100 micrometers.
Examination of inflammation-related proteins in young and aged mice in plasma and hippocampus revealed a trend for reduced markers of inflammation in plasma in old mice relative to young mice across a broad spectrum of proteins (Figure 5A), although no individual comparisons reached significance. On the other hand, the hippocampus of aged mice had elevated protein concentrations of BAFF, IL33, IL15, MCP3/CCL7, MIP1A/CCL3, IL22, IP10/CXCL10, and LIF (one-way ANOVA within each protein followed by relevant post-hoc comparisons with Sidak’s adjustment for multiple comparisons; Figure 5B). There were no major modulatory effects of acute propranolol or mabuterol administration on inflammatory markers in plasma or hippocampus aside from an increase in MCSF with propranolol in young mice (see Supplementary Data, Figures S1 and S2).
Figure 5.
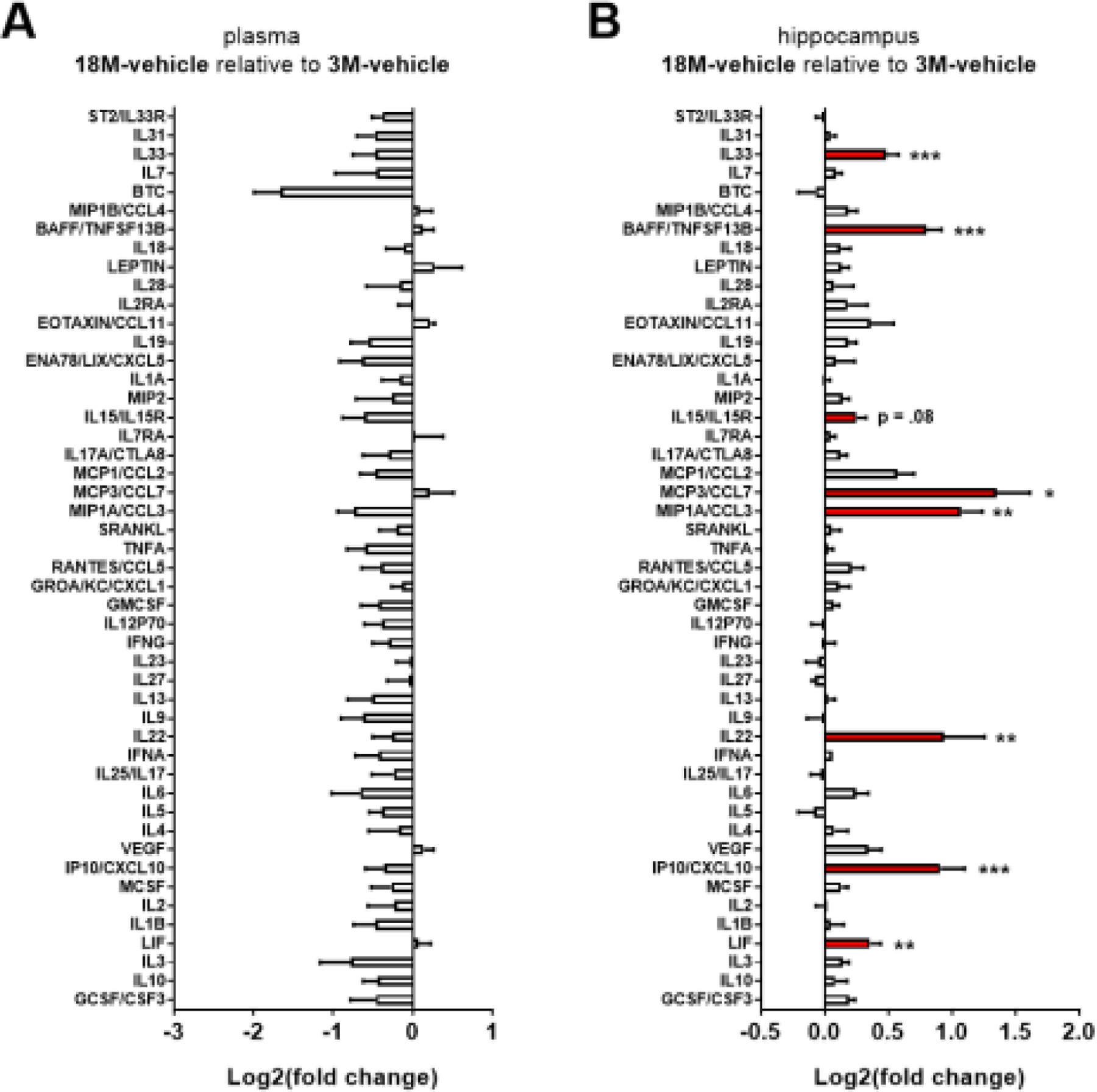
Aged mice have elevated markers of inflammation in hippocampus as compared to young mice. Log2-fold change bar graphs (mean ± SEM, n=6) indicate changes in immune related proteins in plasma and hippocampus. A) Plasma: No effects of age or drug were observed on plasma markers of inflammation. A general trend is observed for 18 month (18M) mice to have lower concentrations of most markers of inflammation measured (40 out of 48), although no individual markers reach significance. B) Hippocampus: 18 month (18M) mice have elevated markers of inflammation in hippocampus as compared to 3M mice. There was no further effect of beta-adrenergic pharmacology on these age-related effect (see related supplementary Figure S1 and Figure S2). * indicates p < .05, **p < .01, ***p < .001; Sidak’s posthoc following one-way ANOVA within each protein.
Due to observed inflammation and microgliosis in the hippocampus, several markers of synaptic physiology were examined in the hippocampus. Quantification of Western blot from hippocampus revealed no change in presynaptic markers, synapsin or synaptophysin (data not shown), but we show an increase in phosphorylation of the postsynaptic NMDA receptor 2B (NR2B) in aged mice (t-test, t(22) = 3.154, p = .005, Figure 6). No changes were observed in tyrosine hydroxylase or a marker of phosphorylated tau, AT8 (Supplementary Data, Figure S3).
Figure 6.
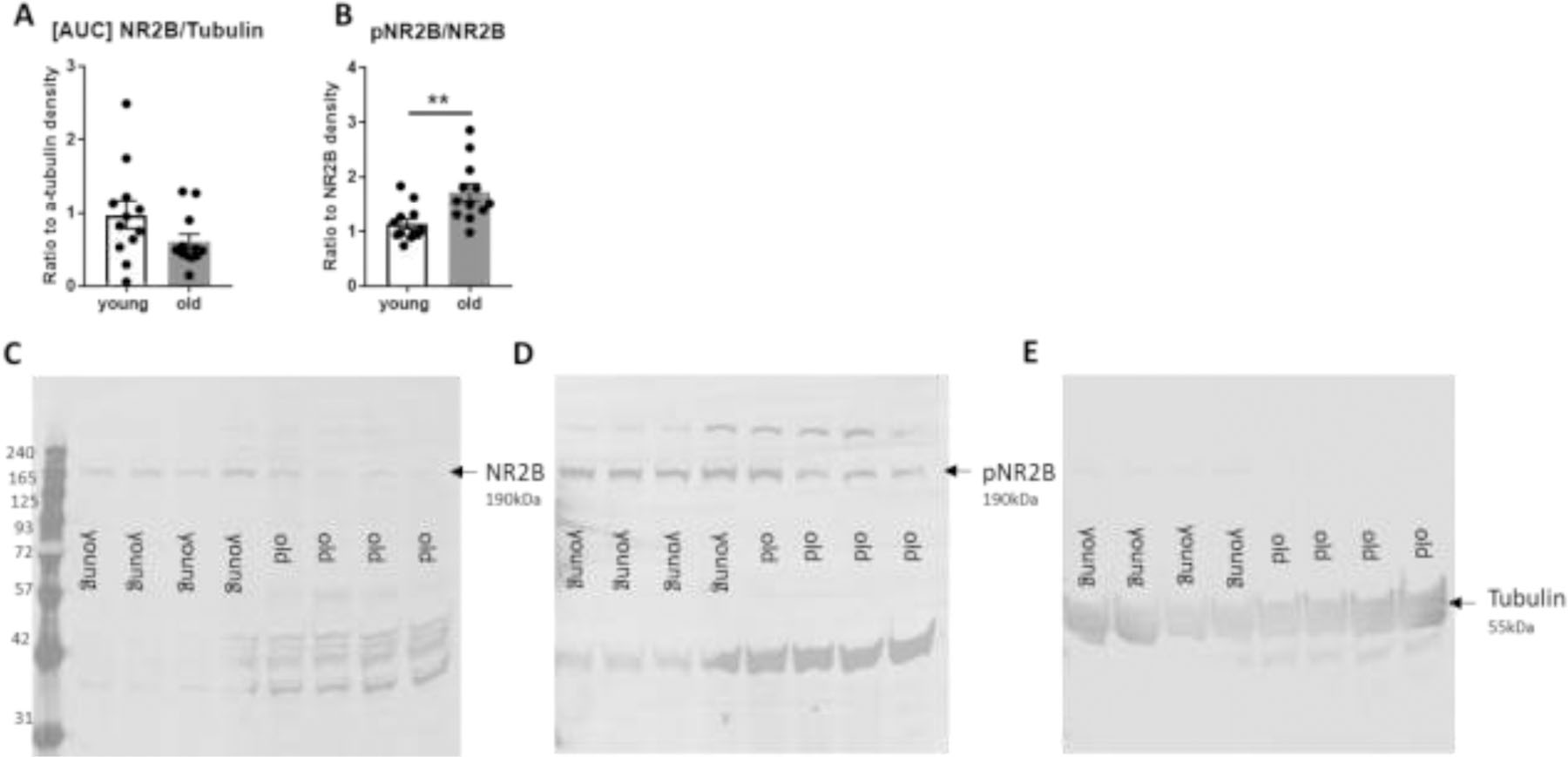
Western blot analysis of young and aged hippocampus shows no change in the expression level of the NMDA receptor 2B (NR2B) but an increase in the phosphorylation state of the NR2B receptor protein at the Ser1303 site in aged mice. A,B) Scatter bar graphs depict quantification of western blot for NR2B relative to tubulin and pNR2B relative to NR2B. C,D,E) Representative western blot images for NR2B, pNR2B, and tubulin. ** indicates p < .01, t-test.
Putative AT8-ir and AT180-ir were observed in some but not all aged mice (2 out of 10 mice) and not in young mice (Figure 7). Notably, there was a high level of age-related autofluorescence in aged mice relative to young mice resembling a neuronal label and interfering with quantification of the AT8-ir or AT180-ir. However, primary antibody controls in which the AT8 or AT180 primary antibody was omitted demonstrated some specificity of AT8 and AT180 immunoreactivity over the autofluoresence in the hippocampus of some aged mice (Figure 7D-F). Clear instances of AT8 and AT180 immunoreactivity were only observed in CA1 and CA3 in 2 out of 10 aged mice, but quantification of label was not possible due to interference from age-related autofluorescence. AT8-ir and AT180-ir was also observed in the locus coeruleus alongside high autofluorescence in the aged LC, although the LC label was not as distinctive over the autofluorescence as the AT8-ir and AT180-ir label observed in the hippocampus. (Figure 7J-O). Qualitative representations of putative AT8-ir and AT180-ir are depicted in Figure 7.
Figure 7.
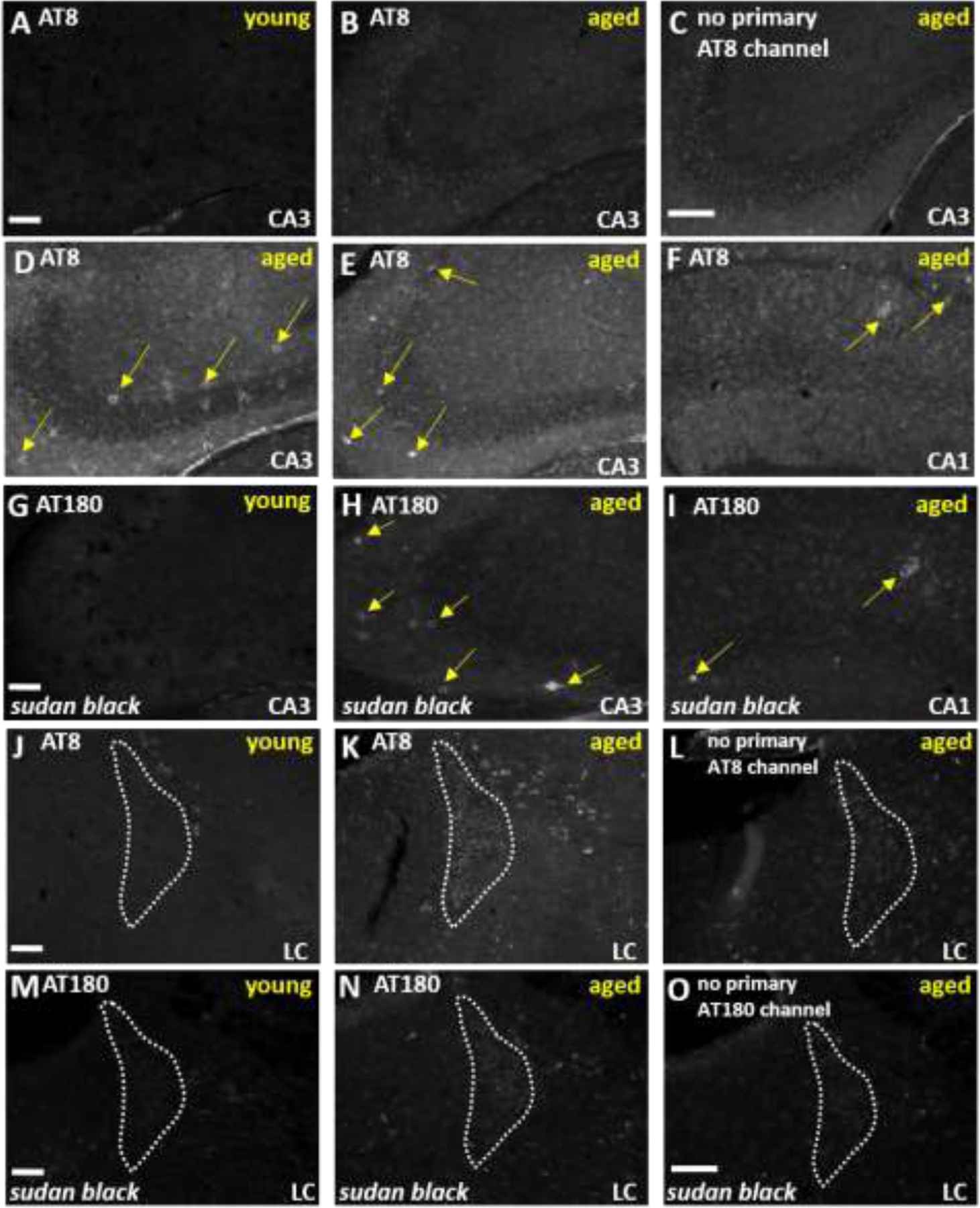
Aged mice have evidence for AT8 and AT180-immunoreactivity in the hippocampus and locus coeruleus in addition to a distinctive age-related autofluorescence. Photomicrographs of the CA3 region of the hippocampus show representative AT8-immunoreactivity in A,B) young and aged mice as compared to C) no primary control in aged mice. D-F) Distinctive AT8-immunoreactivity is depicted in CA3 and CA1 of two independent aged mice (2 out of 10 mice had this type of staining). G-I) AT180 immunoreactivity is depicted in young and aged tissue treated with Sudan Black in order to reduce non-specific background staining. Panels H and I show a similar immunoreactivity pattern with AT180 as observed with the AT8 antibody in the same subjects as panels D-F. Photomicrographs of the locus coeruleus at −5.62 mm Bregma show representative J,K) AT8-immunoreactivity in young versus aged mice alongside L) a no primary control in aged mice. M,N) AT180 immunoreactivity is depicted in young and aged tissue treated with Sudan Black. O) No antibody control for the AT180 immunostaining conditions. White scale bars equal 100 micrometers.
In Y-maze, aged mice traveled less distance overall than young mice, a trend that approached significance (t-test, t(10) = 2.027, p=.07; Figure 8B). Analyses of effects of pharmacology in young and old mice revealed a main effect of treatment in both young (one-way ANOVA, F(2, 17) = 11.79, p<.001; Figure 8C) and old mice (one-way ANOVA, F(2, 16) = 7.598, p=.005; Figure 8D). Post-hoc analyses of effects of propranolol and mabuterol relative to control (Dunnett’s test) revealed that mabuterol decreased distance moved in both young and old mice (Figure 8C, D). While young mice explored both novel and familiar arms, aged mice showed a preference for the familiar arms as shown both with duration in arms (Figure 8E-G) as well as arm entries (Figure 8H-J). Looking at duration spent in arms, aged mice show a familiar arm preference (two-way ANOVA(Age X Novelty), F(Interaction)(1, 10) = 8.036, p=.018, post-hoc comparisons of novelty discrimination at each age with Sidak’s correction for multiple comparisons; Figure 8E) Mabuterol induced a novelty preference for duration in the novel versus familiar arm in young mice (two-way ANOVA(Drug X Novelty), F(Interaction)(2, 17) = 4.044, p=.037, post-hoc comparisons of novelty discrimination within each drug treatment with Sidak’s correction for multiple comparisons; Figure 8F) and mabuterol preserved the familiar preference in aged mice whereas propranolol blocked the preference for the familiar arm in aged mice. (two-way ANOVA(Drug X Novelty), F(Novelty)(1, 16) = 7.947, p=.012, post-hoc comparisons of novelty discrimination within each drug treatment with Sidak’s correction for multiple comparisons; Figure 8G). With number of arm entries as a measure of novelty preference, aged mice also show a familiar arm preference (two-way ANOVA(Age X Novelty), F(Interaction)(1, 10) = 8.378, p=.016, post-hoc comparisons of novelty discrimination at each age with Sidak’s correction for multiple comparisons; Figure 8H). Neither mabuterol nor propranolol had an effect on familiar versus novel arm entries in young mice (Figure 8I). However, as with duration in arms, propranolol blocked the preference for increased familiar arm entries in aged mice (two-way ANOVA(Drug X Novelty), F(Novelty)(1, 16) = 19.02, p<.001, post-hoc comparisons of novelty discrimination within each drug treatment with Sidak’s correction for multiple comparisons; Figure 8J).
Figure 8.
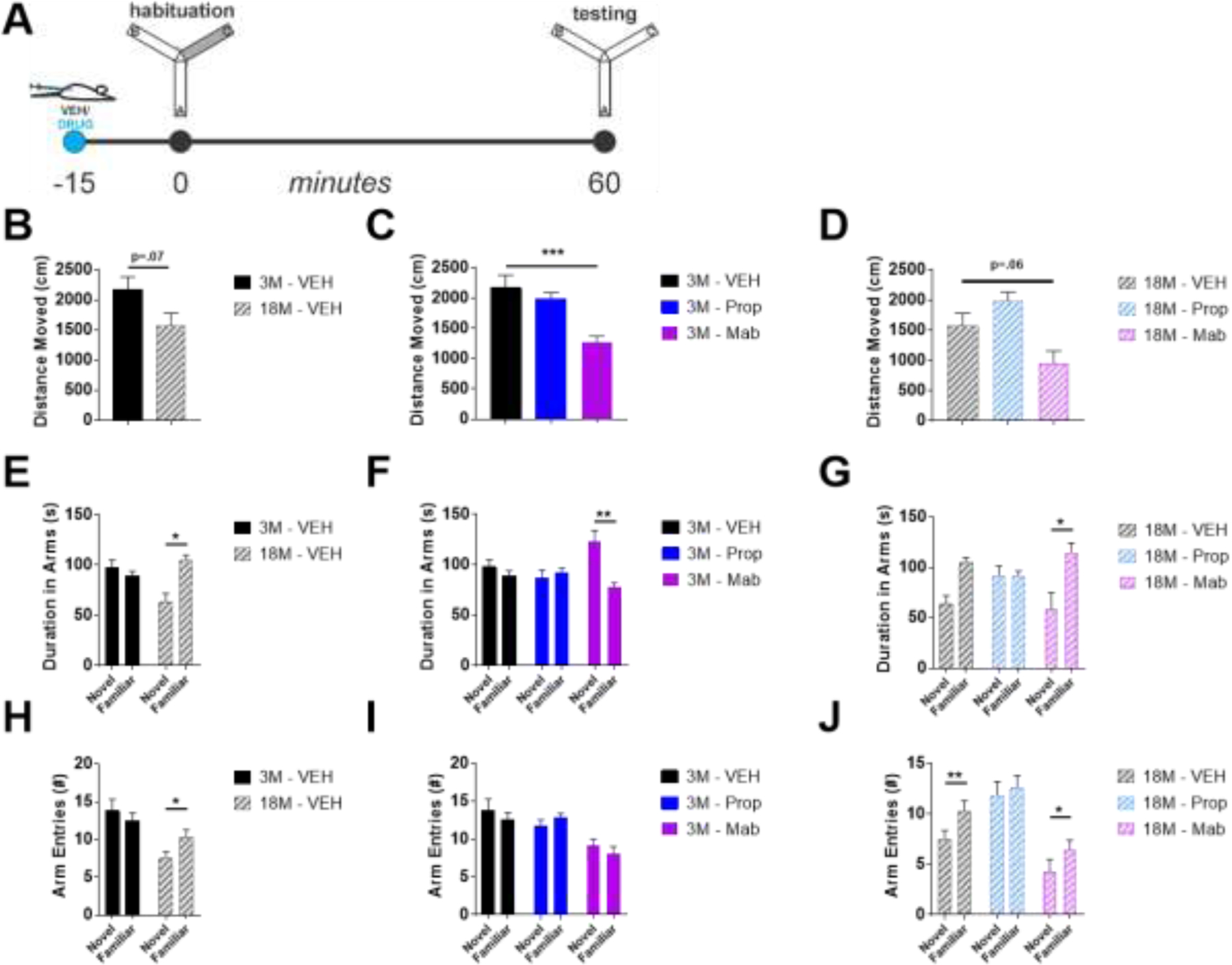
Mabuterol decreased distance moved in Y-maze and increased time spent in novel versus familiar arms in young versus aged mice respectively. A) Y-Maze Forced Alternation experimental design. B) 18 month (18M) mice tended to move less than 3M mice during Y-Maze forced alternation testing. C,D) Mabuterol decreased distance moved in both 3M and 18M mice and D) propranolol tended to increase distance moved in 18M mice, but increase did not reach significance. E) 3M mice explore both novel and familiar arms, while 18M mice show a preference for the familiar arm. F) Mabuterol increases time spent in the novel arm in 3M mice. G) Propranolol decreases preference for familiar arm in 18M mice. H) 18M mice but not 3M mice had more entries into the familiar versus novel arms and J) propranolol blocked the increased number of entries into the familiar arm in 18M mice. * indicates p < .05, **p < .01, ***p < .001; Dunnett’s posthoc after one-way ANOVA (C, D), or Sidak’s posthoc after two-way ANOVA (E-J).
In NPR, aged mice had a tendency to travel less distance (t-test, t(9) = 1.653, p=.13; Figure 9B), but both young and old mice showed accurate novelty discrimination (two-way ANOVA(Age X Novelty), F(Novelty)(1, 20) = 18.68, p<.001, post-hoc comparisons of novelty discrimination at each age with Sidak’s correction for multiple comparisons; Figure 9E). No effect of either drug was observed in young or old mice on either distance moved (Figure 9CD) or on novelty discrimination (Figure 9FG) aside from a main effect of treatment on distance moved in old mice (one-way ANOVA, F(2, 14) = 4.628, p=.029; Figure 9D), but with only a tendency for propranolol to increase distance moved in old mice (p=.105, Dunnett’s posthoc).
Figure 9.
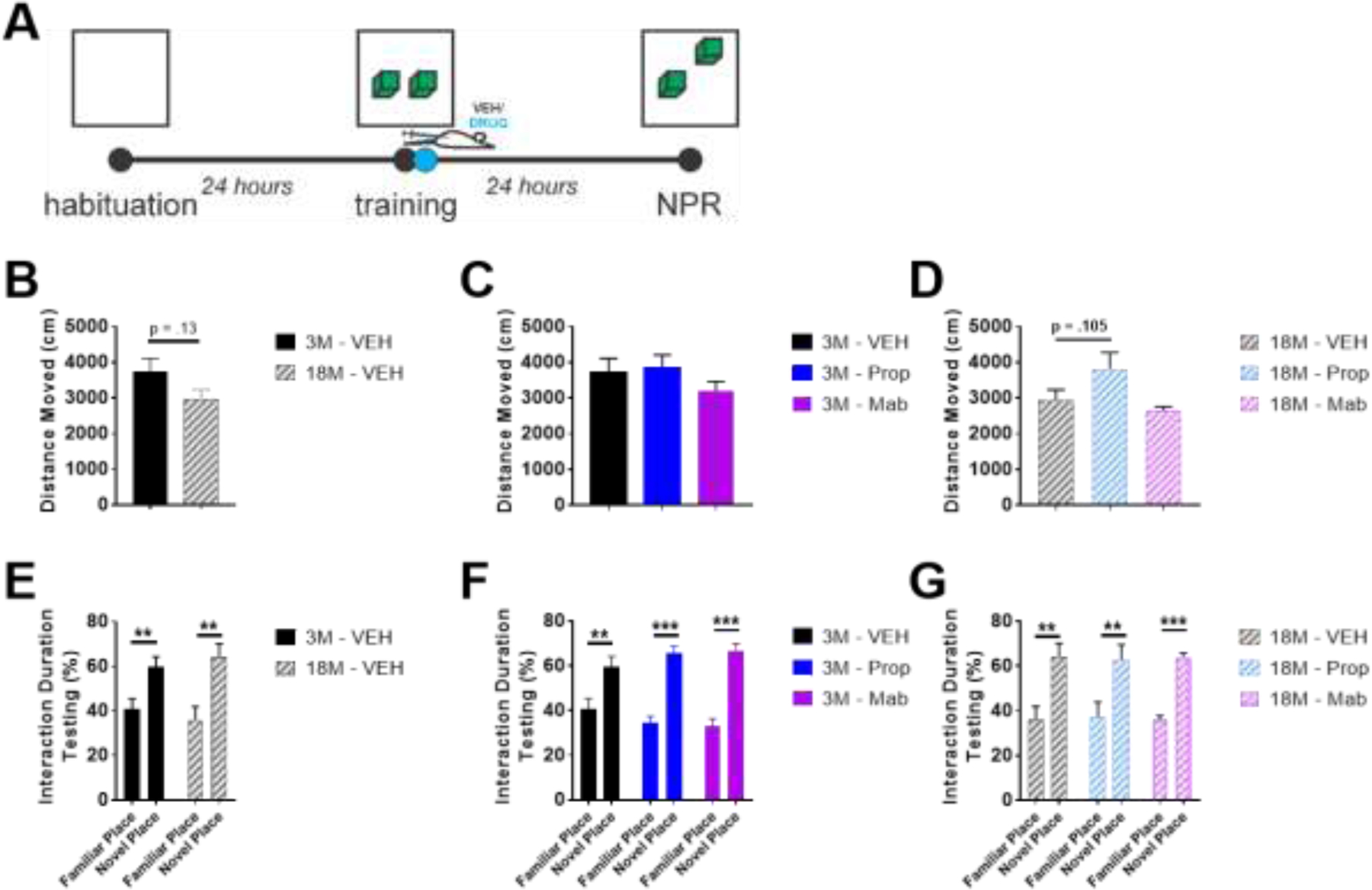
In Novel Place Recognition (NPR), propranolol tends to increase distance moved in old mice, but all groups successfully show novelty discrimination. A) NPR experimental design. B-D) 18 month (18M) mice tend to move less during NPR testing, but E-G) both 3 and 18 month old mice show preference for novel place in terms of % time interaction during testing. ** indicates p < .01, ***p < .001; Dunnett’s posthoc after one-way ANOVA (C, D), or Sidak’s posthoc after two-way ANOVA (E-G).
In NOR, aged mice traveled less distance during testing than young mice (two-way ANOVA(Age X Session), F(Age)(1, 30) = 11.46, p=.002, F(Session)(2, 30) = 3.486, p=.044, post-hoc comparisons of age during each session with Sidak’s correction for multiple comparisons; Figure 10B). No effects of pharmacology were observed on distance moved (Figure 10CD). Both young and aged mice showed novelty discrimination and novelty preference (two-way ANOVA(Age X Novelty), F(Novelty)(1, 16) = 32.24, p<.001, post-hoc comparisons of novelty discrimination at each age with Sidak’s correction for multiple comparisons; Figure 10E). However, propranolol blocked this novelty discrimination in young mice (two-way ANOVA(Drug X Novelty), F(Interaction)(2, 32) = 4.159, p=.025, post-hoc comparisons of novelty discrimination with each drug treatment with Sidak’s correction for multiple comparisons; Figure 10F) and both mabuterol and propranolol impaired the novelty discrimination in old mice (two-way ANOVA(Drug X Novelty), F(Interaction)(2, 30) = 2.259, p=.122, F(Novelty)(1, 30) = 18.26, p<.001, post-hoc comparisons of novelty discrimination with each drug treatment in old mice with Sidak’s correction for multiple comparisons; Figure 10G).
Figure 10.
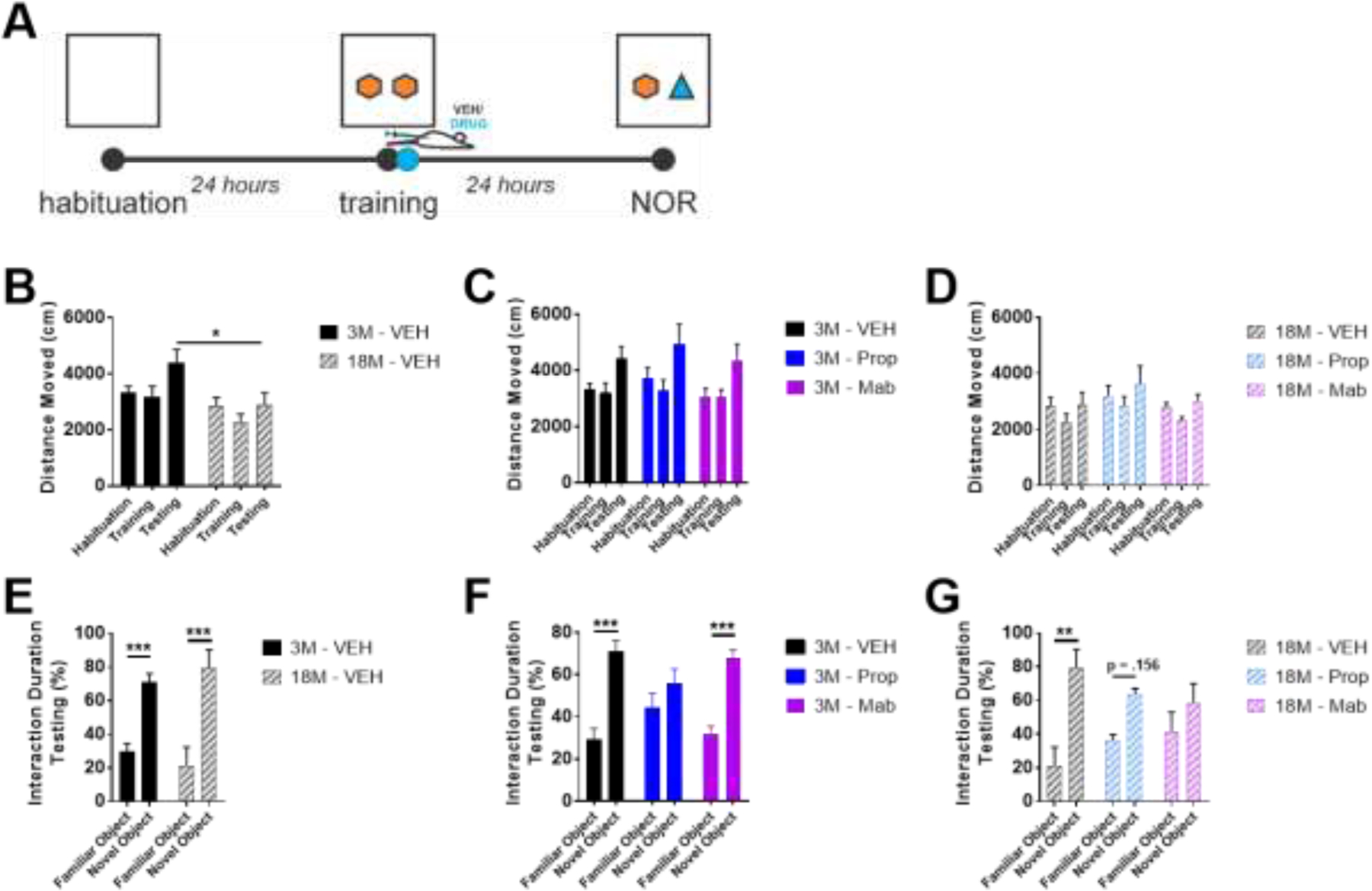
In Novel Object Recognition (NOR), young and old mice show novelty discrimination, but propranolol blocks discrimination in young mice and impairs discrimination in old mice. A) NOR experimental design. B-D) 3 month (3M) but not 18M mice move greater distance during the testing phase of NOR relative to habituation and training. E-G) Both 3M and 18M mice show preference for novel object in terms of % time interaction. F,G) Propranolol impairs novel object preference in 3M and 18M mice, and G) mabuterol impairs novel object preference in 18M mice. * indicates p < .05, ** p < .01, *** p < .001; Sidak’s posthoc after two-way ANOVA.
Young and old mice both learn to associate freezing with a tone, following tone-shock pairings (two-way ANOVA(Drug X Trace), young: F(Trace)(1, 34) = 54.33, p<.001, aged: F(Trace)(1, 32) = 70.67, p<.001; Figure 11B). Post-hoc comparisons of effects of propranolol and mabuterol compared to vehicle (Dunnett’s) during each trace reveal that mabuterol enhances this learning during traces 1 and 2 in young and old mice, but by trace 3, all groups show comparable freezing in response to tone during the trace period (Figure 11C, D). Contextual Recall: Aged mice freeze more in response to context during contextual recall on day 2 (t-test, t(10) = 2.452, p=.034; Figure 11E). Propranolol impairs this freezing in aged mice (one-way ANOVA, F(2, 16) = 11.73, p<.001, Dunnett’s post-hoc; Figure 11G). Cued Recall: Aged mice freeze less than young mice during cued recall of fear (two-way ANOVA(Age X Trace), F(Interaction)(1,20) = 7.889, p=.0108; Sidak’s post-hoc comparisons of effects of age on freezing during baseline or trace; Figure 11H). No effect of either propranolol or mabuterol was observed on cued recall in either young or old mice (Figure 11IJ).
Figure 11.
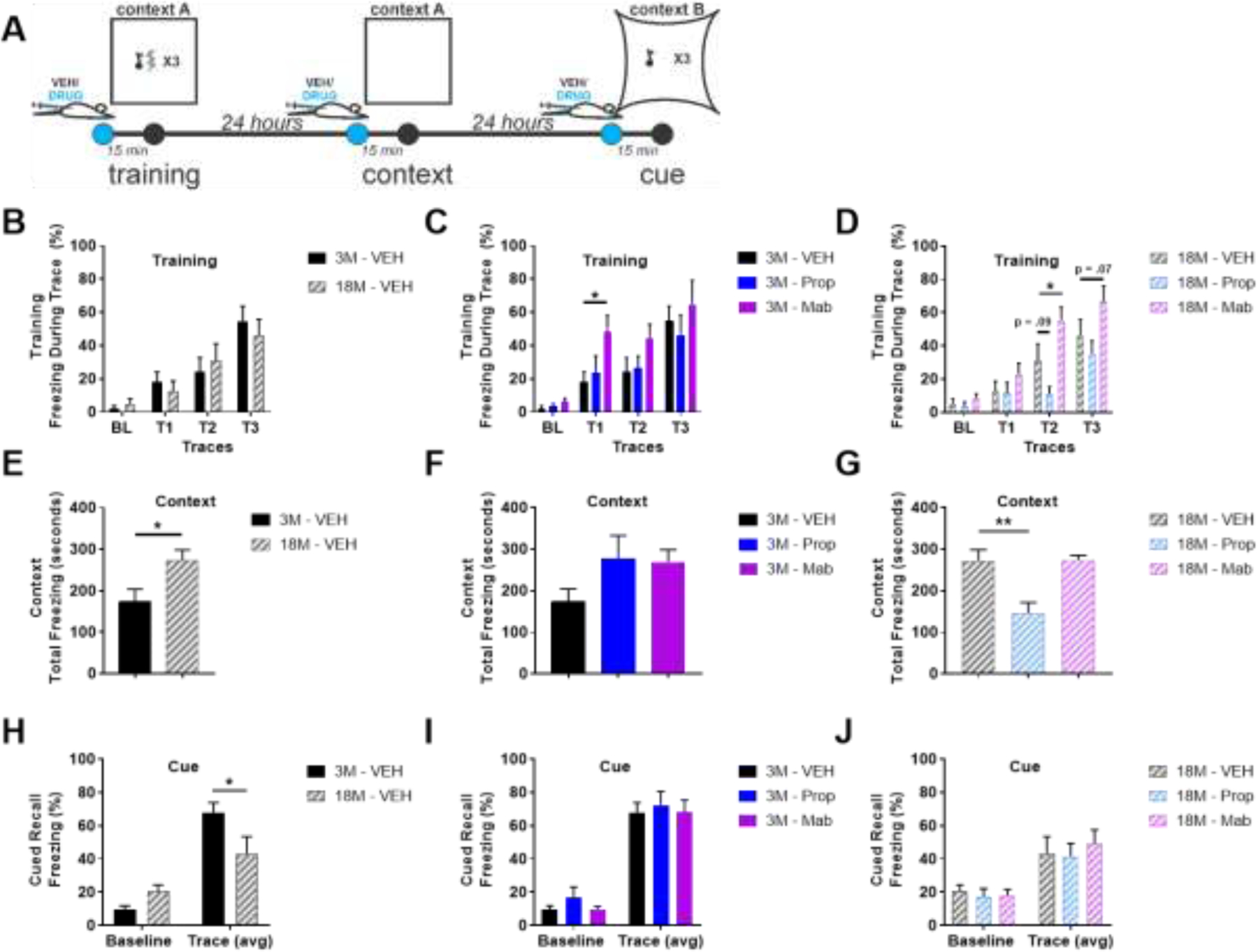
Young and old mice both learn to associate a tone with freezing behavior following tone-shock pairings, but old mice show greater freezing in response to context and less freezing response to cue when tested for recall. A) Fear conditioning experimental design. B-D) Training. B) 3 month (3M) and 18M mice learn to freeze in response to a tone paired with shock. C,D) Mabuterol enhances this freezing during training in both 3M and 18M mice and D) propranol impairs this freezing during training but only in 18M mice. E,F,G) Context. E) 18 M mice freeze more in response to re-exposure to context. F) Propranol and metoprolol increase freezing to context in 3M mice. G) Propranolol reduces freezing to context in 18 M mice. H,I,J) Cue. H) 3M mice freeze more in response to cue, I,J) with no effect of drug in either 3M or 18M. * indicates p < .05, ** p < .01; Dunnett’s after two-way ANOVA (B-D), t-test (E), Dunnett’s after one-way ANOVA (F-G) or Sidak’s posthoc after two-way ANOVA (H-J).
4. Discussion
Data presented here provide evidence for neuroinflammation and metabolic stress in the locus coeruleus and hippocampus in 19 months old (aged) mice relative to 4 months old (young) mice. We show increased microgliosis in the locus coeruleus and hippocampus and elevated inflammatory cytokines in the hippocampus. Aged mice have a proteomic signature of metabolic demand and mitochondrial stress in the LC and for dysregulation of synaptic plasticity and mitochondrial stress in the hippocampus. We show evidence for hyperphosphorylation (Ser-1303) of NR2B extrasynaptic receptors in the hippocampus. Finally, we show that aged mice are more susceptible to behavioral impairment following acute administration of the commonly used beta-blocker, propranolol. Moreover, propranolol impairs behavior in both young and old mice. Acute delivery of the beta-adrenergic agonist, mabuterol, improves cognitive function in both young and old mice.
4.1. Inflammation and metabolic stress in the aged LC
Aged mice show evidence for microgliosis in the LC with increases in iba1-immunoreactivity and show elevated proteomic pathways related to cellular metabolism and mitochondrial stress (oxidative phosphorylation) in the LC. Enhanced microgliosis and primed microglia in aged animals have been previously reported (Fenn et al., 2013; Fonken et al., 2018; Huang et al., 2008; Wendt et al., 2008) although this is a novel report of microgliosis from the LC of aged mice. Primed microglia have been shown to be more responsive with cytokine release following challenge and more likely to potentiate chronic neuroinflammation or to exacerbate neurodegenerative pathways in otherwise compromised neuronal populations. Furthermore, in the context of compromised neuronal populations, we show that the aged LC has increased expression of many proteins also known to be elevated in neurodegenerative disorders such as related to oxidative stress and mitochondrial dysfunction. This evidence for an aged LC with neuroinflammation and mitochondrial stress is in line with evidence that the locus coeruleus is specifically vulnerable to degeneration (Matchett et al., 2021). Metabolic activity and specifically overactivation of noradrenergic neurons such as occurs in response to chronic stress or sleep deprivation, has been associated with LC degeneration. Previous studies have shown LC neurons to be vulnerable to neurodegeneration, with mechanisms such as NOS induction and oxidative stress proposed to underlie selective vulnerability (Wang et al., 2020; Zhan et al., 2005; Zhang et al., 2014). In the current study, there was no evidence for degeneration of TH-ir cells in the LC despite evidence for metabolic stress and inflammation. It is possible that with further aging, beyond 19 months, degeneration of TH-ir cells would be observed. We did observe limited evidence for tau pathology in the LC as evidenced by immunoreactivity with two different markers of phosphorylated tau, although it is possible that this would also have been more prominent with further aging. In humans, the LC is the first site of pre-tangle hyperphosphorylated tau accumulation and thought to precede pathology in other brain areas (Braak et al., 2011; Theofilas et al., 2017; Weinshenker, 2018). Additionally, there was no exacerbation of inflammation with acute administration of beta-blockers, although this may be due to the acute dosing protocol used for this study. The effects of chronic beta-blocker exposure on LC pathology remains an important question which needs further investigation. In these current studies, we were only able to investigate the neuroinflammatory cytokine profile in the hippocampus and not the LC due to the limited size of the tissue available from the LC in mice. We are currently working on a method that will allow us to investigate the inflammatory fingerprint of this structure with its limited size.
4.2. Inflammation, mitochondrial stress, and evidence for extra-synaptic pathology in the aged hippocampus
Aged mice show evidence for microgliosis in the hippocampus with increases in iba1-immunoreactivity and show elevated proteomic pathways related to synaptic plasticity and mitochondrial stress (oxidative phosphorylation) in the hippocampus. As discussed above, microgliosis and primed microglia in aged animals has been previously reported, and specifically in hippocampus of aged mice and may contribute to chronic neuroinflammation and synaptic degeneration. Indeed, we show elevated markers of inflammation in the hippocampus of aged mice. We report increases in B-cell activating factor (BAFF), a lymphocyte recruitment factor in the TNF family released by activated astrocytes (Krumbholz et al., 2005). Additionally, we report increases in CXCL10, CCL3, and CCL7, all positive modulators of lymphocyte trafficking and chemotaxis released by macrophages. Increases in IL33, as reported here in the hippocampus, have been reported previously in aged mice and suggested to be important for protection against tau pathology and behavioral deficits in aged mice (Carlock et al., 2017).
Alongside an increase in inflammatory cytokines in the aged hippocampus, we detected increased phosphorylation of the NR2B receptor at the Ser1303 site. This hyperphosphorylation of the NR2B subunit of the NMDA receptor can be caused by excessive glutamate release and activation of the extrasynaptic NMDA receptors and be mediated by death-associated protein kinase 1 (DAPK1) (Li et al., 2018) which is a Ca2+/calmodulin (CaM)-regulated serine/threonine kinase that has been implicated in the pathophysiology of neurodegeneration, brain injury, neuronal cell death, neuroinflammation, and depression, as well as aging (Bialik and Kimchi, 2006; Deiss et al., 1995; Farag and Roh, 2019; Gade et al., 2014; Hainsworth et al., 2010). The phosphorylation of the NR2B subunit of NMDA receptor in the hippocampus of aged mice suggests a shift to extrasynaptic localization and activation of NMDA receptors (predominantly NR2B containing) (Martin and Wang, 2010). This pathological activation of the NMDA receptor and subsequent activation of the DAPK can lead to detrimental downstream signaling resulting in a neuronal injury and neurodegeneration in the aged brain. Furthermore, the activation of the DAPK could lead to modulation of the neuroinflammatory response detected in our study. Further investigation of NR2B signaling and DAPK activation in aged brains is warranted and currently ongoing in our laboratory.
A broad trend for reduction in plasma inflammatory markers in aged mice alongside an enhanced neuroinflammation in the brain was unexpected, suggesting an independent modulation of central neuroinflammation compared to systemic inflammation. Otherwise systemic inflammation has been suggested to prime neuroinflammation the CNS (Perry et al., 2007), but here we report CNS inflammation in aged mice in the absence of systemic inflammation.
4.3. Beta-blockers impair learning and memory in aged mice
Data presented here support previous evidence for behavioral differences in learning and memory for aged versus young mice. However, age-related behavioral effects were somewhat limited in scope to reduced activity across multiple tasks, novelty aversion in forced alternation YM, and deficits in cued fear conditioning. Beta-blockers impacted behavior in old mice in 3 out of 4 tests performed (YM, NOR, FC context) and in young mice only in NOR. In both young and old mice, mabuterol enhanced discrimination behavior in YM and enhanced FC training. Preference for the familiar arm in forced alternation Y-maze in aged mice was particularly interesting as mabuterol improved a divergent preference for novelty versus familiarity in young versus old mice, respectively, whereas propranolol impaired this preference in aged mice. While young and aged mice had different strategies (novelty seeking vs. avoidance) in this test, mabuterol independently enhanced the strategy in each young and aged, while propranolol impaired the preferred strategy in aged mice.
4.4. Conclusions
In summary, our data provide evidence for age-related neuroinflammation in the locus coeruleus and hippocampus alongside evidence for metabolic challenge in the locus coeruleus and extrasynaptic deficiency of NMDA receptor signaling in the hippocampus (see Figure 12). We present a hypothetical model whereby oxidative stress and metabolic demand from neurons in the LC and hippocampus accompany an increase in inflammatory signaling from astrocytes and microglia. An excessive release of glutamate leads to activation of extrasynaptic NMDA receptors predominantly containing NR2B subunits leading to impairment of neuronal function and behavioral impairment observed in this study. This cognitive impairment in aged mice is exacerbated with acute beta-blocker delivery. It is possible that our study captures the LC at a pre-degeneration but compromised stage, as is evident by microgliosis and a proteome pointing towards oxidative stress and metabolic demand in the absence of either reduction in TH-positive cell counts in the LC or reduced TH expression in projection regions in the hippocampus. Further studies are needed to understand mechanisms linking metabolic stress, neuroinflammatory signaling from astrocytes and microglia, and the extra-synaptic pathology. Additional studies need to examine further consequences of chronic beta-blocker administration for cognitive deficits, pathological progression, and enhanced neuroinflammation in the LC as well as other key regions in the aged brain. Future studies will be needed to explore the impact of long-term exposure to beta-blockers on neuroinflammation, brain pathology, and behavioral function.
Figure 12.
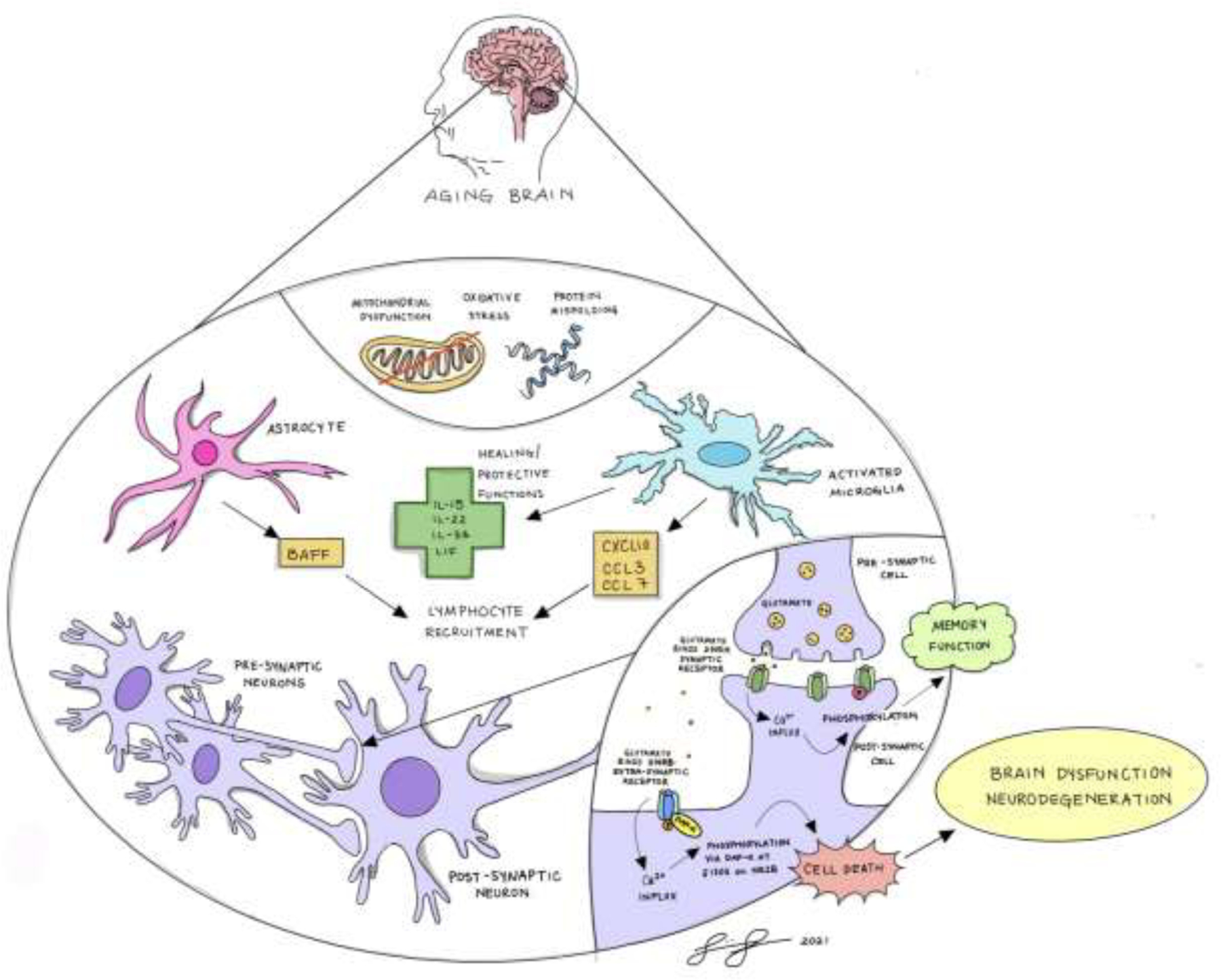
Summary schematic illustrates hypothetical model in which oxidative stress and metabolic demand from neurons in the LC and hippocampus accompany an increase in inflammatory signaling from astrocytes and microglia. Excessive glutamate release in aging brain leads to activation of extrasynaptic NMDA receptors predominantly containing NR2B subunits leading to impairment of neuronal function and behavioral impairment.
Supplementary Material
Highlights.
Aged mice have inflammation in the locus coeruleus and hippocampus.
Aged mice proteome reveals metabolic stress in the locus coeruleus and hippocampus.
Beta-blockers impair learning and memory in aged mice.
Acknowledgments
This work was partially supported by the National Institutes of Health (NIH R21 NS097945) (NIH R01 AG054533 and 1R56 AG068394–01; thanks to NIA for mice from aged colony related to these grants) and (NINDS P30 NS069375) awarded to MS. This work was supported in part by NIH P30 CA124435 utilizing the Stanford Cancer Institute Proteomics/Mass Spectrometry Shared Resource. Proteomics was done at Vincent Coates Foundation Mass Spectrometry Laboratory, Stanford University Mass Spectrometry, RRID:SCR_017801. We would also like to thank Ms. Shirin Shamloo (BA) for her contribution to the graphic conceptualization in Figure 12.
Abbreviations
- CNS
central nervous system
- -ir
immunoreactivity
- ITI
inter-trial intervals
- FC
fear conditioning
- LC
locus coeruleus
- NE
norepinephrine, noradrenaline
- NOR
Novel Object Recognition
- NPR
Novel Place Recognition
- NR2B
NMDA receptor 2B
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- TH
tyrosine hydroxylase
- US
unconditioned stimulus
- YM
Y-maze
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
None declared
References
- Amrhein V, et al. , 2019. Scientists rise up against statistical significance. Nature. 567, 305–307. [DOI] [PubMed] [Google Scholar]
- Betts MJ, et al. , 2019. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain. 142, 2558–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialik S, Kimchi A, 2006. The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem. 75, 189–210. [DOI] [PubMed] [Google Scholar]
- Braak H, Del TK, 2011. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 121, 171–181. [DOI] [PubMed] [Google Scholar]
- Braak H, et al. , 2011. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 70, 960–969. [DOI] [PubMed] [Google Scholar]
- Carlock C, et al. , 2017. Interleukin33 deficiency causes tau abnormality and neurodegeneration with Alzheimer-like symptoms in aged mice. Transl Psychiatry. 7, e1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda MS, et al. , 2019. Comprehensive Real-World Assessment of Marketed Medications to Guide Parkinson’s Drug Discovery. Clin Drug Investig. [DOI] [PMC free article] [PubMed]
- Deiss LP, et al. , 1995. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 9, 15–30. [DOI] [PubMed] [Google Scholar]
- Duron E, Hanon O, 2010. Antihypertensive treatments, cognitive decline, and dementia. J Alzheimers Dis. 20, 903–14. [DOI] [PubMed] [Google Scholar]
- Elias JE, Gygi SP, 2007. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 4, 207–14. [DOI] [PubMed] [Google Scholar]
- Evans AK, et al. , 2020. Beta-adrenergic receptor antagonism is proinflammatory and exacerbates neuroinflammation in a mouse model of Alzheimer’s Disease. Neurobiol Dis. 146, 105089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faizi M, et al. , 2012. Thy1-hAPP(Lond/Swe+) mouse model of Alzheimer’s disease displays broad behavioral deficits in sensorimotor, cognitive and social function. Brain Behav. 2, 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag AK, Roh EJ, 2019. Death-associated protein kinase (DAPK) family modulators: Current and future therapeutic outcomes. Med Res Rev. 39, 349–385. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, et al. , 2016. Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: noradrenergic signaling system. J Neurochem. 139Suppl 2, 154–178. [DOI] [PubMed] [Google Scholar]
- Fenn AM, et al. , 2013. Increased micro-RNA 29b in the aged brain correlates with the reduction of insulin-like growth factor-1 and fractalkine ligand. Neurobiol. Aging. 34, 2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, et al. , 2018. Stress and aging act through common mechanisms to elicit neuroinflammatory priming. Brain Behav Immun. 73, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade P, et al. , 2014. Regulation of the death-associated protein kinase 1 expression and autophagy via ATF6 requires apoptosis signal-regulating kinase 1. Mol Cell Biol. 34, 4033–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliebus G, Lippa CF, 2007. The influence of beta-blockers on delayed memory function in people with cognitive impairment. Am. J. Alzheimers. Dis. Other Demen. 22, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, et al. , 2021. Heightened hippocampal beta-adrenergic receptor function drives synaptic potentiation and supports learning and memory in the TgF344-AD rat model during prodromal Alzheimer’s disease. J Neurosci. [DOI] [PMC free article] [PubMed]
- Grudzien A, et al. , 2007. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging. 28, 327–35. [DOI] [PubMed] [Google Scholar]
- Hainsworth AH, et al. , 2010. Death-associated protein kinase (DAPK1) in cerebral cortex of late-onset Alzheimer’s disease patients and aged controls. Neuropathol Appl Neurobiol. 36, 17–24. [DOI] [PubMed] [Google Scholar]
- Hajjar I, et al. , 2005. Cross-sectional and longitudinal association between antihypertensive medications and cognitive impairment in an elderly population. J Gerontol A Biol Sci Med Sci. 60, 67–73. [DOI] [PubMed] [Google Scholar]
- Heneka MT, et al. , 2015. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, et al. , 2008. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol. Aging. 29, 1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, et al. , 2019. Alzheimer’s disease pathology: pathways between central norepinephrine activity, memory, and neuropsychiatric symptoms. Mol Psychiatry. [DOI] [PubMed]
- Kraeuter AK, et al. , 2019. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol Biol. 1916, 105–111. [DOI] [PubMed] [Google Scholar]
- Krumbholz M, et al. , 2005. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med. 201, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, et al. , 2018. Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects. Mol Psychiatry. 23, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, et al. , 2019. In vivo visualization of age-related differences in the locus coeruleus. Neurobiol Aging. 74, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, et al. , 2020. Noradrenergic-dependent functions are associated with age-related locus coeruleus signal intensity differences. Nat Commun. 11, 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HG, Wang YT, 2010. Blocking the deadly effects of the NMDA receptor in stroke. Cell. 140, 174–6. [DOI] [PubMed] [Google Scholar]
- Matchett BJ, et al. , 2021. The mechanistic link between selective vulnerability of the locus coeruleus and neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 141, 631–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Harley CW, 2016. The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn Sci. 20, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal S, et al. , 2017. beta2-Adrenoreceptor is a regulator of the alpha-synuclein gene driving risk of Parkinson’s disease. Science. 357, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paran E, et al. , 2010. Cognitive function and antihypertensive treatment in the elderly: a 6-year follow-up study. Am. J. Ther. 17, 358–364. [DOI] [PubMed] [Google Scholar]
- Perry VH, et al. , 2007. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 7, 161–167. [DOI] [PubMed] [Google Scholar]
- Sara SJ, 2009. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 10, 211–223. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S, 2012. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 76, 130–141. [DOI] [PubMed] [Google Scholar]
- Theofilas P, et al. , 2017. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 13, 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, 2015. Neuromodulatory signaling in hippocampus-dependent memory retrieval. Hippocampus. 25, 415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, et al. , 2020. Locus coeruleus neurons are most sensitive to chronic neuroinflammation-induced neurodegeneration. Brain Behav Immun. 87, 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstein RL, et al. , 2019. Moving to a World Beyond “p < 0.05”. American Statistician. 73, 1–19. [Google Scholar]
- Weinshenker D, 2018. Long Road to Ruin: Noradrenergic Dysfunction in Neurodegenerative Disease. Trends Neurosci. 41, 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt W, et al. , 2008. Upregulation of cathepsin S in the aging and pathological nervous system of mice. Brain Res. 1232, 7–20. [DOI] [PubMed] [Google Scholar]
- Zhan G, et al. , 2005. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 172, 921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. , 2014. Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons. J Neurosci. 34, 4418–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


