Abstract
Introduction
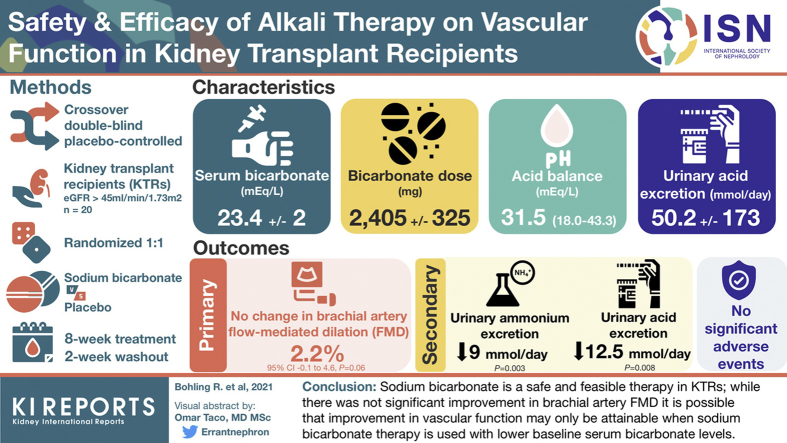
Metabolic acidosis is associated with cardiovascular events, graft function, and mortality in kidney transplant recipients (KTRs). We examined the effect of alkali therapy on vascular endothelial function in KTRs.
Methods
We performed an 18-week, randomized, double-blind, placebo-controlled crossover pilot study examining the effect of sodium bicarbonate therapy versus placebo on vascular function in 20 adult KTRs at least 1 year from transplant with an estimated glomerular filtration rate (eGFR) ≥45 ml/min per 1.73 m2 and a serum bicarbonate level of 20 to 26 mEq/L. Each treatment period was 8 weeks in duration with a 2-week washout period between treatments. The primary outcome was change in brachial artery flow-mediated dilation (FMD) between sodium bicarbonate treatment and placebo.
Results
Twenty patients completed the study and were included in the primary analysis. The mean (SD) baseline eGFR of participants was 75 (22) ml/min per 1.73 m2, respectively. Serum bicarbonate levels did not increase significantly with treatment (0.3 [1.5] mEq/L, P = 0.37). Sodium bicarbonate therapy was not associated with worsening blood pressure, weight gain, or hypokalemia. There was no significant increase in FMD after 8 weeks of sodium bicarbonate therapy compared to placebo (mean change in FMD 2.2%, 95% CI –0.1 to 4.6, P = 0.06). There were no significant changes in high-sensitivity C-reactive protein, interleukin-6, eGFR, or urinary albumin-to-creatinine ratio during treatment. Urinary ammonium excretion decreased by 9 mmol/d (P=0.003), with sodium bicarbonate.
Conclusions
Sodium bicarbonate therapy is safe and feasible in KTRs, and our results strengthen the need for a larger randomized controlled trial.
Keywords: alkali therapy, kidney transplantation, metabolic acidosis, serum bicarbonate, sodium bicarbonate, vascular endothelial function
Graphical abstract
Cardiovascular disease (CVD) is the leading cause of death in KTRs, accounting for 35% to 50% of all-cause mortality.1,2 KTRs have elevated arterial stiffness and endothelial dysfunction, both of which are associated with CVD.3, 4, 5 Additionally, endothelial dysfunction is associated with graft loss in KTRs.6 Metabolic acidosis, as reflected by a low serum bicarbonate level, has been associated with endothelial dysfunction, arterial stiffening, and hypertension in individuals both with and without kidney disease.7, 8, 9, 10, 11, 12, 13 Acid retention is common in KTRs and can be attributed to multiple factors.14 KTRs have a single kidney and a decreased number of nephrons impairing the ability to eliminate the daily hydrogen load.15, 16, 17 Additionally, KTRs receive several medications that can result in metabolic acidosis, including calcineurin inhibitors.15, 16, 17 Transplant recipients with lower serum bicarbonate levels have an increased risk of graft loss, cardiovascular events, and mortality.14,18, 19, 20, 21
Several small interventional trials have shown that bicarbonate administration slows the rate of kidney disease progression, even in individuals with normal serum bicarbonate levels.22, 23, 24 Additionally, a prior pilot study of 20 participants with chronic kidney disease stage 3 or 4 with metabolic acidosis (mean [SD] serum bicarbonate level 19.5 [2.3] mEq/L) found that bicarbonate administration resulted in improved vascular endothelial function as measured by brachial-artery FMD.13 Yet, the effect of alkali therapy on vascular endothelial function in KTRs remains unknown. Accordingly, we performed a prospective, randomized, double-blind, placebo-controlled 18-week crossover pilot study in 20 KTRs to test the hypothesis that sodium bicarbonate therapy is safe and feasible and improves vascular endothelial function, as measured by FMD, in KTRs compared with placebo.
Methods
Study Population
Twenty KTRs were enrolled between December 2018 and November 2019 at the University of Colorado Anschutz Medical Campus. Participants were included if they were at least 1 year from kidney transplantation with an eGFR of ≥45 ml/min per 1.73 m2 and a serum bicarbonate level of 20 to 26 mEq/L. Other eligibility criteria included a body mass index of <40 and a stable immunosuppression and antihypertensive treatment regimen for at least 4 weeks prior to randomization. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Exclusion criteria included significant comorbid conditions that lead the investigator to conclude that life expectancy is less than 1 year, chronic use of daily oral alkali therapy within the past 3 months, uncontrolled hypertension, known ejection fraction 30%, New York Heart Association class 3 or 4 heart failure symptoms, chronic use of supplemental oxygen, and serum potassium <3.3 or ≥5.5 mEq/L, and for female participants, pregnancy, breast feeding, or inconsistent use of birth control. All study participants provided written informed consent before study entry. The study protocol and informed written consent were approved by the Colorado Multiple Institutional Review Board (Aurora, CO) and was registered at ClinicalTrials.gov (NCT03428464).
Study Design
The study was an 18-week double-blind, placebo-controlled crossover study. Each participant served as his or her own control. Eligible participants were randomized 1:1 to either start with placebo or sodium bicarbonate treatment per a randomization schedule generated by a blinded statistician. Study medication (sodium bicarbonate and matched placebo) was prepared by Green Mountain Pharmaceutical (Denver, CO) and was identical in size, color, and shape. Each treatment period lasted 8 weeks in duration with a 2-week washout period in between. Outcome measures (FMD, 24-hour urine collections, venous blood gases, interleukin-6, high-sensitivity C-reactive protein) were obtained at the beginning and end of each treatment period. Serum bicarbonate levels were checked every 4 weeks throughout the study. All participants, study investigators, and the statistician were blinded to treatment assignment.
Study Drug Dosing
Each sodium bicarbonate capsule contained 7.7 mEq of bicarbonate and 178 mg of sodium. The matching placebo contained cornstarch. Participants received study medication at 0.5 mEq/kg of lean body weight per day for the entire treatment period. Participants took half the daily dose in the morning and the other half in the evening. The number of capsules was rounded to the nearest whole capsule. To increase compliance, the maximum number of pills per day was 6.
Study End Points
The primary endpoint was change in brachial artery FMD between the placebo and sodium bicarbonate treatment conditions. Secondary endpoints were used to identify potential mechanisms by which bicarbonate may affect FMD and included serum interleukin-6 and high-sensitivity C-reactive protein. Additionally, urine ammonium excretion, pH, net acid excretion, and albumin-to-creatinine ratio were collected.
Brachial Artery FMD
Conduit artery endothelial-dependent dilation was determined by brachial artery FMD high-resolution ultrasonography (Vivid I, GE, Aurora, CO) by a trained technician as described originally by Celermajer et al.25 and more recently by our group.26 Measurements were performed at least 12 hours after ingesting food and tobacco and 24 hours from ingestion of alcohol, caffeine, or exercise. Electrocardiogram-gated end-diastolic ultrasonographic images were acquired during baseline and FMD conditions. For FMD, reactive hyperemia was produced by inflating a pediatric forearm cuff around the forearm to 250 mm Hg for 5 minutes followed by rapid deflation. Commercially available software (Vascular Analysis Tools 5.10.10; Medical Imaging Applications, LLC, Iowa City, IA) was used to simultaneously obtain brachial artery diameters. Brachial artery dilation was determined as percentage change from baseline.
Data Collection
Fasting blood samples were collected at the beginning and end of each treatment period. Serum high-sensitivity C-reactive protein was measured on a Beckman Coulter analyzer (Beckman Coulter, Indianapolis, IN). Interleukin-6 was measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). A serum basic metabolic panel was also performed at 4 weeks during each treatment period for safety. At the beginning and end of each treatment period, 24-hour urine was collected and was analyzed for urine ammonium, pH, creatinine, sodium, and citrate at Litholink in Chicago, IL. Urinary acid excretion was calculated as the sum of urine ammonium and titratable acid.27 Titratable acid was expressed in millimoles per liter and calculated from total urine phosphorus and urine pH using the Henderson-Hasselbalch equation, pH = pKa + log ([A–]/[HA]), using pKa for phosphate at 6.8.28 Ideal body weight was calculated using previously published equations.28 Dietary protein intake was estimated from 24-hour urine urea nitrogen excretion using the Maroni equation.29 Dietary potassium intake was estimated as the total 24-hour urine potassium excretion. Net endogenous acid production was estimated from these intakes as previously described30: –10.2 + 54.5(protein intake [g/d] / potassium intake [mEq/d]). Acid balance was calculated as net endogenous acid production – urinary acid excretion as previously described.30 Urine albumin and creatinine were measured from spot morning samples. Serum venous blood gases were performed at the beginning and end of each treatment period using ion selective electrode.
Participant Safety Visits
Participants underwent monitoring of vital signs, serum bicarbonate, and electrolyte concentrations and adverse events every 4 weeks during each of the treatment periods for safety. Arterial blood pressure was measured in triplicate while participants were seated at rest using an automated oscillometric machine (Dinamap) and a pill count was performed to ensure medication adherence at all in-person study visits. If the serum bicarbonate was >28 mEq/L, the dose of serum bicarbonate was reduced by 50%, and the participant was given instructions to return in 1 week for a repeat serum bicarbonate measurement. If concentrations remained >28 mEq/L, the study drug would be discontinued.
Statistical Analyses
Baseline characteristics are reported as mean ± SD or median (interquartile range) as appropriate for continuous variables and as a number and percentage for categorical variables. Efficacy analyses were conducted for all randomized participants. Time points used for the analyses included the start and end of each 8-week study period. Mixed effect models were used to examine changes in endpoints between treatment and control conditions with the consideration of repeated measure. Mixed linear models were also used to test for an order effect to ensure the washout worked. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Participant Characteristics
Twenty participants were enrolled and completed the study. Baseline characteristics of participants are shown in Table 1. The mean (SD) age and eGFR of participants was 52 (17) years and 75 (22) ml/min per 1.73 m2, respectively. The mean serum bicarbonate level at baseline was 23.4 (2.0) mEq/L. The majority of the participants were male and non-Hispanic White. Fifty percent of the participants had received a deceased donor kidney transplant, and the median (interquartile range) from transplant was 1.4 (1.1–2.1) years. All participants were on an immunosuppression regimen that included mycophenolate (Myfortic) and tacrolimus. All but 1 participant was also on treatment with prednisone. The mean (SD) dose of bicarbonate was 3.7 (0.5) tablets per day (2405 [325] mg of sodium bicarbonate per day as each tablet was 650 mg). At baseline, the mean (SD) net endogenous acid production was 73.9 (23.5) mEq/L and the mean (SD) urinary acid excretion was 50.2 (173) mmol/d. Overall, the KTR study participants were in a positive acid balance by a median (interquartile range) of 31.5 (18.0–43.3) mEq/d. Serum bicarbonate did not significantly change with oral sodium bicarbonate treatment (+0.3 [1.5] mEq/L; Table 3). Study drug compliance was 94.6% during sodium bicarbonate treatment and 95.6% during placebo.
Table 1.
Baseline characteristics of study participants
| Characteristic | |
|---|---|
| Age (yr) | 52 ± 17 |
| Sex, n (%) | |
| Male | 16 (80.0) |
| Female | 4 (20.0) |
| Race/ethnicity, n (%) | |
| Non-Hispanic White | 15 (75.0) |
| Non-Hispanic Black | 0 (0.0) |
| American Indian | 1 (5.0) |
| Asian | 1 (5.0) |
| Multiple races | 1 (5.0) |
| Unknown/not provided | 2 (10.0) |
| Etiology of kidney disease, n (%) | |
| Diabetes | 3 (15.0) |
| Hypertension | 4 (20.0) |
| Glomerulonephritis | 2 (10.0) |
| Polycystic kidney disease | 4 (20.0) |
| Other | 7 (35.0) |
| Type of transplant, n (%) | |
| Deceased | 10 (50.0) |
| Living related | 5 (25.0) |
| Living unrelated | 5 (25.0) |
| Time from transplant, yr, median (IQR) | 1.4 (1.1–2.1) |
| Diabetes, n (%) | 6 (30.0) |
| Hypertension, n (%) | 20 (100) |
| Cardiovascular disease, n (%) | 2 (10.0) |
| Obstructive sleep apnea, n (%) | 5 (25.0) |
| Smoking, n (%) | |
| Never | 13 (65.0) |
| Former | 7 (35.0) |
| Current | 0 (0.0) |
| Immunosuppression, n (%) | |
| Prednisone | 19 (95.0) |
| Mycophenolate (Myfortic) | 20 (100.0) |
| Tacrolimus | 20 (100.0) |
| Cyclosporine | 0 (0.0) |
| Sirolimus | 0 (0.0) |
| Blood pressure medication, n (%) | |
| ACEi/ARB | 4 (20.0) |
| Diuretic | 1 (5.0) |
| Calcium channel blocker | 6 (30.0) |
| Weight, kg | 79.5 ± 12.5 |
| eGFR, ml/min per 1.73 m2 | 75± 22 |
| Serum bicarbonate, mEq/L | 23.4 ± 2.0 |
| Urinary acid excretion, mmol/d | 50.2 ± 17.3 |
| Net endogenous acid production, mEq/d | 73.9 ± 23.5 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
All values are mean ± SD unless otherwise specified.
Table 3.
Changes in serum bicarbonate, potassium, markers of inflammation, and kidney function during placebo and treatment periods
| Placebo |
Sodium bicarbonate treatment |
P value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 8 wk | P value | Baseline | 8 wk | P value | ||
| Bicarbonate (mEq/L) | 23.4 ± 2.1 | 23.6 ± 1.7 | 0.59 | 24.1 ± 1.5 | 24.4 ± 1.6 | 0.37 | 0.93 |
| Potassium (mEq/L) | 4.0 ± 0.2 | 3.9 ± 0.2 | 0.13 | 4.0 ± 0.3 | 3.9 ± 0.3 | 0.27 | 0.95 |
| hs-CRP (mg/dL) | 4.9 (0.4–2.6) | 4.5 (0.4–1.8) | 0.93 | 4.1 (0.4–3.2) | 1.8 (0.8–2.7) | 0.31 | 0.67 |
| IL-6 (mg/dL) | 4.2 (1.7–4.7) | 3.3 (1.6–3.9) | 0.42 | 3.6 (1.6–4.8) | 3.3 (1.8–4.6) | 0.57 | 0.70 |
| GFR (ml/min per 1.73 m2) | 72 ± 23 | 71 ± 22 | 0.61 | 75 ± 21 | 73 ± 21 | 0.23 | 0.54 |
GFR, glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6.
Values are expressed as mean ± SD or median (interquartile range). To examine whether treatment by sodium bicarbonate has an effect on these secondary outcomes, a study with crossover design was conducted and a mixed effects model was used for data analysis. The P values were calculated from the mixed effects model in testing the null hypothesis that the treatment does not have an effect on the secondary outcome.
Effects of Sodium Bicarbonate on Vascular Endothelial Function
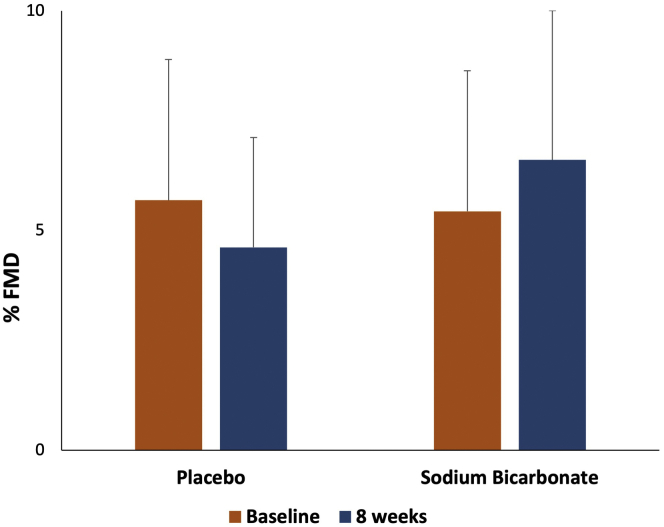
There was no significant increase in FMD after 8 weeks of sodium bicarbonate therapy compared with placebo (change in FMD of 2.2%, 95% CI –0.1 to 4.6, P = 0.06 in sodium bicarbonate vs. placebo; Figure 1, Table 2). We also performed a paired analysis comparing the change in FMD within each participant and found the same results. The order in which participants received treatment did not affect FMD. Sodium bicarbonate treatment did not result in increased systolic or diastolic blood pressure (Table 2).
Figure 1.
Percentage flow-mediated dilation (FMD) at baseline and 8 weeks after sodium bicarbonate therapy or placebo.
Table 2.
Change from baseline in vascular function following treatment with sodium bicarbonate versus placebo
| Placebo | Sodium bicarbonate treatment | P value | |
|---|---|---|---|
| FMD (%) | |||
| Baseline | 5.7 ± 3.2 | 5.4 ± 3.2 | |
| 8 wk | 4.6 ± 3.4 | 6.6 ± 5.4 | |
| Absolute change from baseline | −1.1 ± 3.2 | 1.2 ± 4.3 | 0.06 |
| Systolic blood pressure (mm Hg) | |||
| Baseline | 125 ± 10 | 129 ± 19 | |
| 8 wk | 127 ± 16 | 124 ± 14 | |
| Absolute change from baseline | 2.1 ± 11 | −4.4 ± 14 | 0.17 |
| Diastolic blood pressure (mm Hg) | |||
| Baseline | 72 ± 7 | 75 ± 9 | |
| 8 wk | 77 ± 9 | 73 ± 9 | |
| Absolute change from baseline | 4.6 ± 8 | −1.6 ± 9 | 0.02 |
FMD, flow-mediated dilation.
Values are expressed as mean change from baseline ± SD. Change in brachial artery FMD is expressed as percentage. To examine whether treatment by sodium bicarbonate has an effect on vascular function (i.e., results in change in FMD, systolic blood pressure, and diastolic blood pressure), a study with crossover design was conducted and a mixed effects model was used for data analysis. The P values were calculated from the mixed effects model in testing the null hypothesis that the treatment does not have an effect on FMD, systolic blood pressure, and diastolic blood pressure, respectively.
Effects of Sodium Bicarbonate Therapy Versus Placebo on Secondary Endpoints
The effects of sodium bicarbonate therapy on secondary endpoints are shown in Table 3. There were no significant changes in serum levels of high-sensitivity C-reactive protein, interleukin-6, or potassium with treatment. There were no significant changes in eGFR or urine albumin-to-creatinine ratio following treatment with sodium bicarbonate. Sodium bicarbonate treatment was effective at reducing urinary ammonium excretion and urinary acid excretion (Table 4). Sodium bicarbonate treatment decreased urinary ammonium by 9 mmol/d (P=0.003) and urinary acid excretion by 12.5 mmol/d (P=0.008). Additionally, urine pH increased significantly with sodium bicarbonate treatment (P=0.03). There were no changes in serum venous pH with treatment. Urine sodium excretion also did not change significantly with sodium bicarbonate treatment.
Table 4.
Changes in urine and serum acid base parameters and urine albumin-to-creatinine ratio following treatment with sodium bicarbonate versus placebo
| Placebo |
Sodium bicarbonate treatment |
P value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 8 wk | P value | Baseline | 8 wk | P value | ||
| Urinary ammonium excretion (mmol/d) | 33.2 ± 13.0 | 34.8 ± 11.4 | 0.56 | 32.7 ± 11.2 | 23.6 ± 10.6 | 0.003 | 0.01 |
| Net acid excretion (mmol/d) | 49.8 ± 16.9 | 52.5 ± 14.1 | 0.48 | 49.0 ± 15.1 | 36.4 ± 17.1 | 0.008 | 0.38 |
| Urine pH | 5.99 ± 0.7 | 5.82 ± 0.5 | 0.30 | 5.93 ± 0.6 | 6.31 ± 0.5 | 0.03 | 0.07 |
| Urine sodium (mmol/d) | 176 ± 67 | 167 ± 99 | 0.69 | 158 ± 67 | 166 ± 72 | 0.74 | 0.65 |
| Urine citrate (mg/d) | 295 ± 212 | 255 ± 198 | 0.26 | 274 ± 191 | 310 ± 226 | 0.55 | 0.25 |
| Urine albumin-to-creatinine ratio (mg/g) | 14.0 (6.5–36.0) | 10.0 (6.0–29.5) | 0.25 | 11.0 (7.0–32.0) | 11.5 (5.5–23.0) | 0.59 | 0.60 |
| Serum pH | 7.38 ± 0.04 | 7.38 ± 0.04 | 0.84 | 7.38 ± 0.04 | 7.39 ± 0.04 | 0.56 | 0.88 |
Values are expressed as mean ± SD. To examine whether treatment by sodium bicarbonate has an effect on these secondary outcomes, a study with crossover design was conducted and a mixed effects model was used for data analysis. The P values were calculated from the mixed effects model in testing the null hypothesis that the treatment does not have an effect on any of the secondary outcomes.
For the primary outcome, that is, the pre-post change in FMD, we found a significant interaction between baseline urine citrate level and treatment (P=0.04). As baseline urine citrate increased, the difference between the 2 groups in the change in FMD increased (a difference of 0.012 between the 2 slopes, P=0.04). There were no significant interactions among baseline urine ammonium (difference of –0.033 between the 2 slopes, P=0.75) or urinary acid excretion (difference of –0.05 between the 2 slopes, P=0.52) and change in FMD.
Adverse Effects
All participants completed the study. There were no significant adverse events in either group. The most common adverse events are shown in Table 5. A single participant developed a serum bicarbonate level >28 mEq/L; per protocol, dose was reduced by 50% and serum bicarbonate decreased back to <28 mEq/L. One patient developed mild diarrhea with sodium bicarbonate therapy. The most reported side effect was bloating, which occurred in 40% of participants during sodium bicarbonate treatment and 40% during placebo. More participants reported mild nausea while on sodium bicarbonate treatment compared with placebo (25% compared to 15%). Study medication was not stopped in any participant because of side effects. Lower extremity edema was rare, only occurring in 2 participants during treatment with sodium bicarbonate and 3 participants during placebo treatment. Only 1 participant was on a diuretic at baseline and remained on a stable dose for the duration of the study. Systolic blood pressure did not change during treatment and diastolic blood pressure decreased following sodium bicarbonate treatment (Table 2).
Table 5.
Adverse events
| Adverse event | Sodium bicarbonate | Placebo |
|---|---|---|
| Nausea | ||
| Occasional (<2 times per week) | 2 (10) | 2 (10) |
| Often (3–4 times per week) | 3 (15) | 0 (0) |
| Regularly (daily) | 0 (0) | 1 (5) |
| Bloating | ||
| Occasional (<2 times per week) | 4 (20) | 5 (25) |
| Often (3–4 times per week) | 0 (0) | 3 (15) |
| Regularly (daily) | 4 (20) | 0 (0) |
| Edema | 2 (10) | 3 (15) |
| High serum bicarbonate level (>28 mEq/L) | 1 (5) | 0 (0) |
Values are expressed as n (%).
Discussion
In this randomized, double-blind, placebo-controlled crossover pilot study, we found that there was no significant improvement in vascular endothelial function as measured by FMD with oral sodium bicarbonate therapy in KTRs. Serum bicarbonate did not increase significantly with treatment, but this is consistent with other studies of sodium bicarbonate administration in participants with baseline serum bicarbonate levels in the normal range.31 Similar to other studies examining the effectiveness of bicarbonate therapy, we also observed a decrease in 24-hour urinary acid excretion and ammonium excretion, and significant increase in urine pH with sodium bicarbonate therapy.31, 32, 33 The use of sodium bicarbonate therapy was safe and was not associated with elevated blood pressure, edema, weight gain, hypokalemia, or development of metabolic alkalosis as demonstrated by no changes to serum venous pH. To our knowledge, this is the first interventional trial examining the safety and efficacy of alkali therapy on vascular endothelial function in KTRs.
Vascular endothelial dysfunction is a triggering event for CVD.3 FMD is the gold standard noninvasive assessment of conduit artery endothelial dysfunction, as well as a well-recognized preclinical marker of CVD.34,35 FMD is a known independent predictor of all-cause and cardiovascular mortality in patients with chronic kidney disease.34,35 Impaired endothelial function measured by FMD has been associated with renal allograft loss in KTRs.6 Thus, therapeutic interventions that improve vascular endothelial function in KTRs have the potential to improve kidney transplant outcomes and reduce late graft loss.
Acid retention is common in KTRs.14 The prevalence of serum bicarbonate levels <22 mEq/L ranges from 30% to 70% in those with eGFR <30 ml/min per 1.73 m2.14 In KTRs with higher eGFRs, the prevalence ranges from 11% to 58%.36 KTRs develop metabolic acidosis for several reasons. With a low eGFR, as seen in chronic kidney disease, there is attenuated capacity of the kidney to eliminate the daily hydrogen load.14 Given that low serum bicarbonate levels are still present at higher eGFRs in KTRs, there are other mechanisms besides impaired kidney function contributing to acid retention. KTRs receive several medications that can result in metabolic acidosis, including calcineurin inhibitors, which cause renal tubular acidosis.15, 16, 17 We found that KTRs demonstrated a positive acid balance despite having relatively preserved kidney function and a mean bicarbonate level in the normal range. Serum bicarbonate levels <24 mEq/L have been found to be a significant risk factor for graft failure, cardiovascular events, and mortality in KTRs.14,18, 19, 20, 21 This suggests that KTRs may benefit from alkali therapy to correct the acid retention.
There was no significant improvement in FMD, but it is possible that significant improvement in vascular function may only be attainable when sodium bicarbonate therapy is used in KTRs with lower baseline serum bicarbonate levels. Given the incidence of metabolic acidosis in KTRs and known association with increased risk of CVD, mortality, and graft failure, it may be beneficial to focus on KTRs with lower serum bicarbonate levels in future trials. Additionally, given the small number of participants in our pilot study, we were likely underpowered to detect a significant difference in FMD. Nonetheless, importantly, we observed a change in FMD of 1.2% from baseline following bicarbonate therapy, and a change of 1% unit difference in FMD is associated with an 8% to 13% reduction in CVD risk.37,38
The mechanism by which sodium bicarbonate therapy may improve vascular endothelial function is unclear. Metabolic acidosis results in increased oxidative stress and inflammation,39, 40, 41 both of which result in vascular dysfunction.42,43 However, we found no changes in serum levels of inflammatory markers with sodium bicarbonate treatment. Similarly, a study of sodium bicarbonate therapy in patients with chronic kidney disease also found no changes in inflammatory markers with sodium bicarbonate treatment.15 Experimental and human studies have shown that acid retention results in increased levels of ammonia, angiotensin II, aldosterone, and endothelin-1,44, 45, 46, 47, 48 and each of these factors both directly and indirectly induce endothelial dysfunction.49, 50, 51 Although we did not measure these factors in our current pilot and feasibility study, it will be important to evaluate other potential mechanisms that may explain how metabolic acidosis contributes to vascular dysfunction in future studies.
Our study does have some limitations, including the small sample size, which limited power to detect changes in FMD. Additionally, systolic blood pressure is known to be an important determinant of endothelial function and could therefore be a theoretical confounder. This risk was minimized by requirement of study participants to have their blood pressure controlled before entering the study. We did not measure angiotensin, aldosterone, or endothelin-1 concentrations in this study, which could improve our understanding of potential mechanisms of the effects of sodium bicarbonate therapy on endothelial function. Additionally, we did not collect dietary information from the participants. Although different immunosuppression regimens may affect the results, all of our participants remained on a stable regimen consisting of mycophenolate (Myfortic) and tacrolimus throughout the study. Additionally, all but 1 participant also remained on a stable dose of prednisone. Additional strengths include the study design of a randomized, double-blind, placebo-controlled, crossover study.
In conclusion, our data support sodium bicarbonate as a safe and feasible therapy in KTRs. Although we did not find a significant improvement in FMD with sodium bicarbonate, our results strengthen the need for a larger, randomized, placebo-controlled trial.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Support for this study was provided by the National Institutes of Health (NIH) / National Heart, Lung and Blood Institute (R01HL132868 [JK]); NIH / National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK007135-43 [KLT], DK116720 [PB], and DK114886 [PB]); International Society for Pediatric and Adolescent Diabetes–Juvenile Diabetes Research Foundation (ISPAD-JDRF) Research Fellowship (KLT); and NIH / National Center for Advancing Translational Sciences (NCATS) Colorado Clinical and Translational Science Awards program (CTSA grant UL1TR002535).
Authors Contributions
RB, MG, KM, ZY, and JK were responsible for the conception, design, collection, analysis, interpretation of data, drafting, and final approval of the article. EA and LRR were involved with the collection and interpretation of data, drafting of article, and final approval of the article. KT, PB, and ES were involved in the analysis and interpretation of data, drafting of article and final approval of the article. JK as corresponding author had full access to the data in the study and final responsibility for the decision to submit for publication.
References
- 1.Yeo F.E., Villines T.C., Bucci J.R., Taylor A.J., Abbott K.C. Cardiovascular risk in stage 4 and 5 nephropathy. Adv Chronic Kidney Dis. 2004;11:116–133. doi: 10.1053/j.arrt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Ojo A.O. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006;15:603–611. doi: 10.1097/01.tp.0000235527.81917.fe. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 4.Hausberg M., Kisters K., Kosch M., Rahn K.H., Barenbrock M. Flow-mediated vasodilation and distensibility of the brachial artery in renal allograft recipients. Kidney Int. 1999;55:1104–1110. doi: 10.1046/j.1523-1755.1999.0550031104.x. [DOI] [PubMed] [Google Scholar]
- 5.Morris S.T., McMurray J.J., Rodger R.S., Farmer R., Jardine A.G. Endothelial dysfunction in renal transplant recipients maintained on cyclosporine. Kidney Int. 2000;57:1100–1106. doi: 10.1046/j.1523-1755.2000.00937.x. [DOI] [PubMed] [Google Scholar]
- 6.Dahle D.O., Midtvedt K., Harmann A. Endothelial dysfunction is associated with graft loss in renal transplant recipients. Transplantation. 2013;95:733–739. doi: 10.1097/TP.0b013e31827d6312. [DOI] [PubMed] [Google Scholar]
- 7.Mandel E., Forman J.P., Curhan G.C., Taylor E.N. Plasma bicarbonate and odds of incident hypertension. Am J Hypertens. 2013;26:1405–1412. doi: 10.1093/ajh/hpt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman J.P., Rifas-Shiman S.L., Taylor E.N., Lane K., Gillman M.W. Association between the serum anion gap and blood pressure among patients at Harvard Vanguard Medical Associates. J Hum Hypertens. 2008;22:122–125. doi: 10.1038/sj.jhh.1002286. [DOI] [PubMed] [Google Scholar]
- 9.Taylor E.N., Forman J.P., Farwell W.R. Serum anion gap and blood pressure in the National Health and Nutrition Examination Survey. Hypertension. 2007;50:320–324. doi: 10.1161/HYPERTENSIONAHA.107.092643. [DOI] [PubMed] [Google Scholar]
- 10.Abramowitz M.K., Hostetter T.H., Melamed M.L. Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney Int. 2012;81:1033–1042. doi: 10.1038/ki.2011.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farwell W.R., Taylor E.N. Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabet Med. 2008;25:798–804. doi: 10.1111/j.1464-5491.2008.02471.x. [DOI] [PubMed] [Google Scholar]
- 12.Hanley A.J., Williams K., Stern M.P., Haffner S.M. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: The San Antonio Heart Study. Diabetes Care. 2002;25:1177–1184. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- 13.Kendrick J., Shah P., Andrews E. Effect of treatment metabolic acidosis on endothelial function in patients with CKD. Clin J Am Soc Nephrol. 2018;13:1463–1470. doi: 10.2215/CJN.00380118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S., Kang E., Park S. Metabolic acidosis and long-term clinical outcomes in kidney transplant recipients. J Am Soc Nephrol. 2017;28:1886–1897. doi: 10.1681/ASN.2016070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe S., Tsuruoka S., Vijayakumar S. Cyclosporin A produces distal renal tubular acidosis by blocking peptidyl prolyl cis-trans isomerase activity of cyclophilin. Am J Physiol Renal Physiol. 2005;288:F40–F47. doi: 10.1152/ajprenal.00218.2004. [DOI] [PubMed] [Google Scholar]
- 16.Mohebbi N., Mihailova M., Wagner C.A. The calcineurin inhibitor FK506 (tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport proteins. Am J Physiol Renal Physiol. 2009;297:F499–F509. doi: 10.1152/ajprenal.90489.2008. [DOI] [PubMed] [Google Scholar]
- 17.Ambuhl P.M. Posttransplant metabolic acidosis: a neglected factor in renal transplantation? Curr Opin Nephrol Hypertens. 2007;16:379–387. doi: 10.1097/MNH.0b013e3281bd8860. [DOI] [PubMed] [Google Scholar]
- 18.Brazier F., Jouffroy J., Martinez F. Association of blood bicarbonate and pH with mineral metabolism disturbance and outcome after kidney transplantation. Am J Transplant. 2020;20:1063–1075. doi: 10.1111/ajt.15686. [DOI] [PubMed] [Google Scholar]
- 19.Gojowy D., Skiba K., Bartmanska M., Kolonko A., Wiecek A., Adamczak M. Is metabolic acidosis a novel risk factor for a long-term graft survival in patients after kidney transplantation? Kidney Blood Press Res. 2020;45:702–712. doi: 10.1159/000508476. [DOI] [PubMed] [Google Scholar]
- 20.Wiegand A., Graf N., Bonani M., Frey D., Wüthrich R.P., Mohebbi N. Relationship of serum bicarbonate levels with 1-year graft function in kidney transplant recipients in Switzerland. Kidney Blood Press Res. 2019;44:1179–1188. doi: 10.1159/000502527. [DOI] [PubMed] [Google Scholar]
- 21.Djamali A., Sing T., Melamed M.L. Metabolic acidosis 1 year following kidney transplantation and subsequent cardiovascular events and mortality: an observational cohort study. Am J Kidney Dis. 2019;73:476–485. doi: 10.1053/j.ajkd.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Phisitkul S., Khanna A., Simoni J. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010;77:617–623. doi: 10.1038/ki.2009.519. [DOI] [PubMed] [Google Scholar]
- 23.de Brito-Ashurst I., Varagunam M., Raftery M.J., Yaqoob M.M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahajan A., Simoni J., Sheather S.J., Broglio K.R., Rajab M.H., Wesson D.E. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78:303–309. doi: 10.1038/ki.2010.129. [DOI] [PubMed] [Google Scholar]
- 25.Celermajer D.S., Sorensen K.E., Gooch V.M. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 26.Jablonski K.L., Decker E., Perrenoud L. Assessment of vascular function in patients with chronic kidney disease. J Vis Exp. 2014;16:51478. doi: 10.3791/51478. https://doi.org/3791/51478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menezes C.J., Worcester E.M., Coe F.L., Asplin J., Bergsland K.J., Ko B. Mechanisms for falling urine pH with age in stone formers. Am J Physiol Renal Physiol. 2019;317:F65–F72. doi: 10.1152/ajprenal.00066.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai M.P., Paloucek F.P. The origin of the “ideal” body weight equations. Ann Pharmacother. 2000;34:1066–1069. doi: 10.1345/aph.19381. [DOI] [PubMed] [Google Scholar]
- 29.Maroni B.J., Steinman T.I., Mitch W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27:58–65. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 30.Scialla J.J., Asplin J., Dobre M. Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int. 2017;91:204–215. doi: 10.1016/j.kint.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raphael K.L., Isakova T., Ix J.H. A randomized trial comparing the safety, adherence, and pharmacodynamics profiles of two doses of sodium bicarbonate in CKD: the BASE Pilot Trial. J Am Soc Nephrol. 2020;31:161–174. doi: 10.1681/ASN.2019030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goraya N., Simoni J., Jo C.H., Wesson D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014;85:1031–1038. doi: 10.1038/ki.2014.83. [DOI] [PubMed] [Google Scholar]
- 33.Goraya N., Simoni J., Jo C., Wesson D.E. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012;81:86–93. doi: 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 34.Karras A., Haymann J.P., Bozec E. Nephro Test Study Group: Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60:1451–1457. doi: 10.1161/HYPERTENSIONAHA.112.197210. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz M.I., Stenvinkel P., Sonmez A. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant. 2011;26:3537–3543. doi: 10.1093/ndt/gfr081. [DOI] [PubMed] [Google Scholar]
- 36.Messa P.G., Alfieri C., Vettoretti S. Metabolic acidosis in renal transplantation: neglected but of potential clinical relevance. Nephrol Dial Transplant. 2016;31:730–736. doi: 10.1093/ndt/gfv098. [DOI] [PubMed] [Google Scholar]
- 37.Ras R.T., Streppel M.T., Draijer R., Zock P.L. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168:344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 38.Inaba Y., Chen J.A., Bergmann S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26:631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 39.Kopple J.D., Kalantar-Zadeh K., Mehrotra R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int Suppl. 2005;67(95):S21–S27. doi: 10.1111/j.1523-1755.2005.09503.x. [DOI] [PubMed] [Google Scholar]
- 40.Mehrotra R., Kopple J.D., Wolfson M. Metabolic acidosis in maintenance dialysis patients: Clinical considerations. Kidney Int Suppl. 2003;88:S13–S25. doi: 10.1046/j.1523-1755.2003.08802.x. [DOI] [PubMed] [Google Scholar]
- 41.Pickering W.P., Price S.R., Bircher G. Nutrition in CAPD: serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int. 2002;61:1286–1292. doi: 10.1046/j.1523-1755.2002.00276.x. [DOI] [PubMed] [Google Scholar]
- 42.Vila E., Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1016–H1021. doi: 10.1152/ajpheart.00779.2004. [DOI] [PubMed] [Google Scholar]
- 43.Csanyi G., Miller F.J. Oxidative stress in cardiovascular disease. Int J Mol Sci. 2014;15:6002–6008. doi: 10.3390/ijms15046002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wesson D.E., Simoni J., Broglio K., Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol. 2011;300:F830–F837. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 45.Wesson D.E., Jo C.H., Simoni J. Angiotensin II receptors mediate increased distal nephron acidification caused by acid retention. Kidney Int. 2012;82:1184–1194. doi: 10.1038/ki.2012.267. [DOI] [PubMed] [Google Scholar]
- 46.Wesson D.E., Jo C.H., Simoni J. Angiotensin II-mediated GFR decline in subtotal nephrectomy is due to acid retention associated with reduced GFR. Nephrol Dial Transplant. 2015;30:762–770. doi: 10.1093/ndt/gfu388. [DOI] [PubMed] [Google Scholar]
- 47.Wesson D.E. Regulation of kidney acid excretion by endothelins. Kidney Int. 2006;70:2066–2073. doi: 10.1038/sj.ki.5001905. [DOI] [PubMed] [Google Scholar]
- 48.Wagner C.A. Effect of mineralocorticoids on acid-base balance. Nephron Physiol. 2014;128:26–34. doi: 10.1159/000368266. [DOI] [PubMed] [Google Scholar]
- 49.Montezano A.C., Nguyen Dinh Cat A., Rios F.J., Touyz R.M. Angiotensin II and vascular injury. Curr Hypertens Rep. 2014;16:431. doi: 10.1007/s11906-014-0431-2. [DOI] [PubMed] [Google Scholar]
- 50.Bohm F., Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8–18. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Hashikabe Y., Suzuki K., Jojima T., Uchida K., Hattori Y. Aldosterone impairs vascular endothelial cell function. J Cardiovasc Pharmacol. 2006;47:609–613. doi: 10.1097/01.fjc.0000211738.63207.c3. [DOI] [PubMed] [Google Scholar]