Abstract
Insufficient vascularization during tissue repair is often associated with poor clinical outcomes. This is a concern especially when patients have critical-sized injuries, where the size of the defect restricts vascularity, or even in small defects that have to be treated under special conditions, such as after radiation therapy (relevant to tumor resection) that hinders vascularity. In fact, poor vascularization is one of the major obstacles for clinical application of tissue engineering methods in soft tissue repair. As a key issue, lack of graft integration, caused by inadequate vascularization after implantation, can lead to graft failure. Moreover, poor vascularization compromises the viability of cells seeded in deep portions of scaffolds/graft materials, due to hypoxia and insufficient nutrient supply. In this article we aim to review vascularization strategies employed in tissue engineering techniques to repair soft tissues. For this purpose, we start by providing a brief overview of the main events during the physiological wound healing process in soft tissues. Then, we discuss how tissue repair can be achieved through tissue engineering, and considerations with regards to the choice of scaffold materials, culture conditions, and vascularization techniques. Next, we highlight the importance of vascularization, along with strategies and methods of prevascularization of soft tissue equivalents, particularly cell-based prevascularization. Lastly, we present a summary of commonly used in vitro methods during the vascularization of tissue-engineered soft tissue constructs.
Keywords: angiogenesis, prevascularization, regenerative medicine, soft tissue repair, tissue engineering, vascularization, wound healing
1. INTRODUCTION
Wound healing is a vital biological process that aims to repair tissue injuries caused by trauma, surgery, burn, and pathological conditions (e.g., cancer, infections), among others. When the process does not proceed as expected, due to underlying conditions or severity of the wound, patients will potentially develop a chronic wound with an increased risk for complications, such as infection, associated with pain, discomfort, psychological stress, and likely high medical costs (Gouin & Kiecolt-Glaser, 2011).
When a patient presents a large tissue defect, the intrinsic physiological ability to repair it is limited and often times requires surgical intervention, for instance by using autologous grafts (Oryan & Sahvieh, 2017). However, in many cases, grafts might not be the recommended/appropriate intervention due to limitations in the tissue amount to be grafted, and the need for multiple harvesting and transplanting surgical procedures, often associated with concomitant donor site morbidity (Khmaladze et al., 2013; Oryan & Sahvieh, 2017). Therefore, to overcome these issues, many researchers and clinicians are constantly investigating strategies that will enhance the intrinsic wound healing ability to restore tissue structure and function. The development of artificial tissue equivalents using tissue engineering is one of these strategies.
Tissue engineering has become a fundamental tool in regenerative medicine with regards to the development of tissue substitutes that will replace or reestablish the normal tissue by promoting healing. Tissue engineering components such as scaffolds, cells, and/or growth factors are usually combined in three-dimensional structures in an attempt to replicate and restore the injured tissue.
However, one of the crucial problems associated with tissue-engineered equivalents, especially large-sized ones, is the possible lack or delayed blood vessel supply after their implantation in vivo (Heller et al., 2020). Small tissue equivalents will likely be easily vascularized from the ingrowth of blood vessels from the surrounding host tissue after implantation in vivo and it is enough to maintain cell viability. Nevertheless, tissue-engineered equivalents are usually created in clinically relevant sizes (0.1–10 cm). The diffusion of oxygen and nutrients, which has been reported to be approximately 100–200 μm, from the pre-existing vasculature will likely not be enough to reach and keep the cells viable in the core of these equivalents. Thus, vascularization might be compromised leading to insufficient oxygen and nutrient supply, and potentially to necrosis and failure of the implant (Liew, Zhang, & Yilei, 2017; Tomasina, Bodet, Mota, Moroni, & Camarero-Espinosa, 2019; Yang, Mahadik, Choi, & Fisher, 2020).
In order to improve the engineered construct’s viability, and enhance integration and vascularization post-implantation, many approaches have been investigated to promote vascularization in tissue engineering applications, including growth factor delivery, cell sheet technology, optimization of scaffold properties, in vivo and in vitro prevascularization (Baiguera & Ribatti, 2013; Costa-Almeida, Granja, Soares, & Guerreiro, 2014; Min, Ko, & Yoo, 2019; Sarker, Chen, & Schreyer, 2015).
This review starts with a summary of the main biological events during the wound healing process in soft tissues, followed by an overview of considerations in tissue engineering/regenerative medicine for tissue repair. Next, we highlight the relevance of vascularization and strategies to vascularize soft tissue constructs, particularly cell-based (in vitro) prevascularization, and provide a summary of commonly used in vitro methods during the vascularization of tissue-engineered constructs.
2. TISSUE REPAIR: WOUND HEALING PROCESS OF SOFT TISSUE
When a tissue is injured (e.g., by physical, chemical, thermal, microbial, or immunological insults or pathological conditions), there is a disruption of its cellular, anatomical, and functional continuity. The epithelial integrity is compromised, and the structure and function of the underlying normal tissue might also be impaired (Gonzalez, Costa, Andrade, & Medrado, 2016; Masson-Meyers et al., 2020). To restore tissue integrity, a complex, well-regulated wound healing process consisting of biological processes involving several cell types and their microenvironment must occur (Karppinen, Heljasvaara, Gullberg, Tasanen, & Pihlajaniemi, 2019).
The wound healing process can be schematically divided into three overlapping phases: inflammation, proliferation, and remodeling (Figure 1). Each phase has specific physiological functions and is characterized by molecular, cellular, and extracellular matrix (ECM) level events that occur sequentially aiming at closing the wound (Gonzalez et al., 2016; Masson-Meyers et al., 2020; Rieger, Zhao, Martin, Abe, & Lisse, 2015).
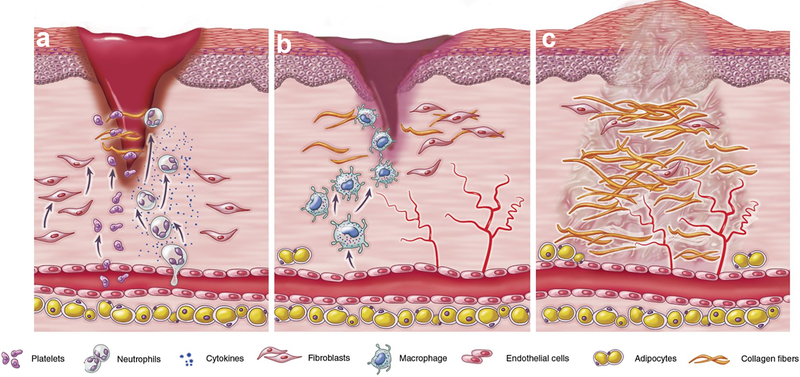
FIGURE 1.
Schematic representation of the overlapping phases during the wound healing process: (a) inflammation, (b) proliferation, and (c) remodeling. Adapted from Foster et al., 2018 (Foster, Jones, Ransom, Longaker, & Norton, 2018), which is under the terms of the Creative Commons Attribution (CC BY 4.0)
The first phase of wound repair, the inflammatory phase, occurs immediately after tissue damage and involves homeostasis and inflammation (Politis, Schoenaers, Jacobs, & Agbaje, 2016). It starts with the formation of a blood clot to close the wound, and vasoconstriction to stop the bleeding. Platelets are activated and will secrete cytokines that will attract inflammatory cells and local cell populations to the site of injury. The fibrin-fibronectin clot or provisional ECM functions as a scaffold for migrating vascular cells, leukocytes, and fibroblasts (Karppinen et al., 2019; Politis et al., 2016). Infiltrating inflammatory cells, such as neutrophils and macrophages, help in eliminating debris, microorganisms, and necrotic tissue, bringing the inflammatory phase to an end. These cells also secrete growth factors, cytokines, and chemokines that activate the proliferation phase (Aghaloo & Hadaya, 2017; Karppinen et al., 2019; Politis et al., 2016; Sculean, Gruber, & Bosshardt, 2014; Shah, Domah, Shah, & Domah, 2020).
During the proliferation phase, important processes such as angiogenesis, reepithelialization, and the formation of a new connective tissue matrix (collagen synthesis) occur (Aghaloo & Hadaya, 2017; Politis et al., 2016; Wang & Xu, 2020). Angiogenesis is known as the formation of new blood vessels from an existing vascular network. For this, specific proteases initiate the degradation of the ECM, enabling endothelial cells (ECs) to change polarization, proliferate, invade, and remodel the ECM towards the avascular tissue and form a functional vasculature (Costa-Almeida et al., 2014; Minor & Coulombe, 2020).
Under normal conditions, blood vessels and ECs are in a quiescent state, but ECs have the dynamic capability to quickly respond to pro-angiogenic signals, for example, in case of injury (Unterleuthner et al., 2017). Several growth factors can activate angiogenesis, such as: vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), transforming growth factor β−1 (TGF-β1), basic fibroblast growth factor (bFGF), insulin-like growth factor (IGF), Interleukin-8 (IL-8), tumor necrosis factor (TNF-α), angiopoietin, hypoxia-inducible factor 1(HIF), and epidermal growth factor (EGF) (Aghaloo & Hadaya, 2017; Heller et al., 2020; Karppinen et al., 2019; Unterleuthner et al., 2017; Wang & Xu, 2020). Angiogenesis is an extremely important event during repair, since the injured tissue and newly formed tissue are deprived of oxygen and nutrients. The new blood vessels and sprouts of capillaries, along with the production of ECM components by fibroblasts, will replace the provisional matrix with granulation tissue and form a substrate for migration of keratinocytes during reepithelization (Aghaloo & Hadaya, 2017; Karppinen et al., 2019).
During reepithelialization, basal keratinocytes migrate from the wound edges along the granulation tissue to restore the barrier function of the epithelium (Karppinen et al., 2019). ECM formation is also initiated at the wound edges, and gradually progresses to the center of the wound. During this phase, angiogenesis decreases and is followed by collagen formation and wound contraction by differentiated fibroblasts (myofibroblasts), leading to the tissue remodeling (Politis et al., 2016).
In the remodeling or maturation phase, production of ECM stops, fibroblasts are degraded, myofibroblasts and other cells enter apoptosis, and gradually, blood vessels become mature (Shah et al., 2020). The connective tissue is remodeled, for example, by matrix metalloproteinases (MMPs), the collagen fibers are rearranged and aligned, and the epithelium is restored (Aghaloo & Hadaya, 2017; Karppinen et al., 2019).
Any disruptions to the wound healing process can result in a delay in healing or in a wound that fails to heal and becomes chronic (Shah et al., 2020). Finding approaches to improve the healing of tissue defects is still a challenge for clinicians and researchers in regenerative medicine (Oryan & Sahvieh, 2017). Small defects can easily be healed by primary intention, i.e., surgical closing or by secondary intention (left open for granulation tissue formation, reepithelialization and remodeling). However, large or critical-sized defects may need tissue engineering techniques including for instance, the use of scaffolds and cells that can be optimized to stimulate the repair process (Oryan & Sahvieh, 2017).
3. REGENERATIVE MEDICINE & TISSUE ENGINEERING: REPAIR AND VASCULARIZATION
In regenerative medicine, tissue engineering is a major component with regards to the development and optimization of biological substitutes, aiming at replacing or restoring injured tissues or maintaining and improving tissue functionality. Tissue repair through tissue engineering techniques can be achieved by implanting biomaterials for in vivo regeneration, or by constructing substitutes in vitro for posterior clinical implantation (Costa-Almeida et al., 2014; Kirkpatrick, Fuchs, & Unger, 2011; Larsson et al., 2016; Samal et al., 2015; Um Min Allah, Berahim, Ahmad, & Kannan, 2017).
Tissue engineering strategies in regenerative medicine include: 1) the injection of cells coming from the same individual (autologous), from a different individual (allogeneic), or from animals (xenogeneic), 2) the application of engineering on matrices/scaffolds to initiate tissue repair, and 3) the development of cell seeded-matrices/scaffolds, including cells such as stem cells, fibroblasts, osteoblasts, chondrocytes, and keratinocytes (Dzobo et al., 2018; Oryan & Sahvieh, 2017). Whichever strategy is chosen, scaffold materials, cells, or a combination of both should be able to replace the injured tissue, restore function as the original tissue, or stimulate repair (Dzobo et al., 2018).
When thick artificial tissue equivalents are needed, either as an experimental model or as a graft to reconstruct large defects, lack of proper vascularization could lead to failure in their function for tissue repair in vivo, and the stability of in vitro constructs (W. C. Liu, Chen, Zheng, & Qin, 2017).
The attainment of functional vascularized tissue constructs is still challenging, and the combination and optimization of biomaterials and tissue engineering techniques are necessary to overcome the current limitations during the development these constructs in order to obtain a well-distributed, interconnected, and stable vascular network that will provide appropriate in vivo vascularization during tissue repair (Dos Santos et al., 2019; Minor & Coulombe, 2020; Sarker et al., 2015; Xie, Zheng, Guan, Ai, & Liang, 2020).
3.1. Relevance of vascularization in tissue engineering
In the course of tissue repair, the presence of an appropriate vascular system is vital for the influx of cells, growth factors, signal molecules, nutrients, oxygen, and drugs to the site of injury or implanted tissue to support the healing process (Chandra & Atala, 2019; Rizwan et al., 2019).
Physiologically, the formation of vascular networks begins early during embryonic development, and changes in vascularization happen throughout life, for example in response to injury. Vascularization occurs through vasculogenesis and angiogenesis. In tissue engineering applications, the formation of new blood vessels in materials or tissue constructs through angiogenesis is believed to be the mechanism of vascularization (Chandra & Atala, 2019; W. C. Liu et al., 2017). Angiogenesis plays a vital role in the integration of implanted scaffolds with the existing tissue, and is required for tissue-engineered constructs to be functional (Rizwan et al., 2019). The goal of the vascularization of tissue engineered constructs should focus not only on developing blood vessels in them, but to ensure that they are mature and organized (Mastrullo, Cathery, Velliou, Madeddu, & Campagnolo, 2020).
There are cases where the prevascularization of tissue constructs is not necessary. These include repair of thin or avascular tissues such as skin, cartilage, cornea, and bladder, since the implants in thin tissues can be quickly vascularized through diffusion from the host vasculature (Auger, Gibot, & Lacroix, 2013; Baiguera & Ribatti, 2013; Costa-Almeida et al., 2014; Datta, Ayan, & Ozbolat, 2017). When an epidermal construct is applied, it does not need to be vascularized since the human epidermis is not vascularized. Also, if the construct includes a dermal layer, it is usually still thin and the host vascular system is enough to supply oxygen and nutrients to the reconstructed dermal layer and keep grafts viable for approximately 14 days. Cartilage and cornea are physiologically avascular and the implantation of vascularized tissue-engineered substitutes could cause inflammatory reactions (Auger et al., 2013; Datta et al., 2017). However, thicker and large-sized constructs to repair tissues or organs with a more complex three-dimensional structure will need an integrated vascular network to sustain cell survival (Costa-Almeida et al., 2014; Min et al., 2019).
Clinical applications of tissue replacement with tissue-engineered equivalents are still scarce (e.g., avascular tissues such as skin and cartilage). The major challenge of using them to repair thick/large defects still depends on the appropriate integration of a vascular network capable of providing adequate diffusion of oxygen and nutrients (Mastrullo et al., 2020).
Following in vivo implantation of a construct, vascularization occurs as a result of the physiological inflammatory phase of the healing process, along with the expression of pro-angiogenic factors from the implanted cells that are stimulated by a hypoxic wound environment. When proper functional vasculature is lacking, the cells seeded in the constructs are subjected not only to hypoxia, but to an insufficient nutrient supply until blood vessels from the surrounding host tissue grow towards the construct (Tomasina et al., 2019; Yang et al., 2020). However, angiogenesis is a relatively slow process, at a rate of several tenths of micrometers per day and therefore, it takes a considerable amount of time for a large implant to be vascularized - during which, the nutrient-starving cells could lose their phenotype, functionality, or potentially go to apoptosis. When thin or avascular tissues are implanted, this timing might be enough, since the distance to capillaries is sufficient to assure nutrition and gas/metabolite exchange by diffusion. However, it might be detrimental for large tissue constructs that could face the risk of ischemia even before implantation, which emphasizes the importance of vascularizing them prior to implantation (Chandra & Atala, 2019; Min et al., 2019; Sharma et al., 2019; Takei, Sakai, & Yoshida, 2016; Tomasina et al., 2019; Wong et al., 2016; Yang et al., 2020).
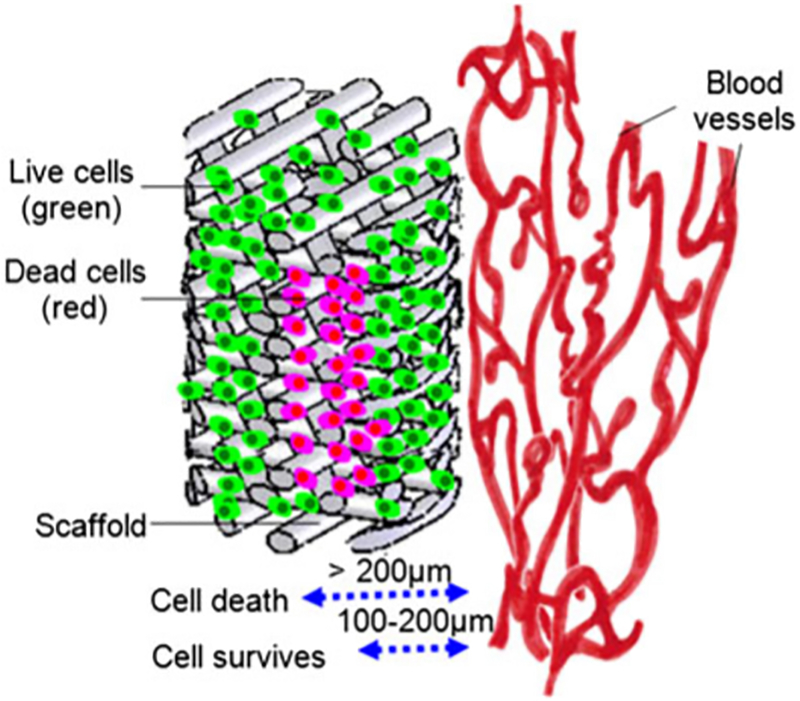
Cell survival, homeostasis, and consequently the successful survival of any engineered tissue upon implantation depends on the effective diffusion of oxygen and nutrients, as well as the removal of metabolic waste by blood vessels near the implant (Samal et al., 2015; Tomasina et al., 2019). Cells in the surrounding tissue and cells inside a scaffold should be in close proximity to a capillary, i.e., approximately 100–200 μm, to ensure long-term survival and function (Chandra & Atala, 2019; Datta et al., 2017; Herrmann et al., 2014; Kniebs et al., 2020; X. Liu et al., 2017; Min et al., 2019; Rouwkema & Khademhosseini, 2016; Sarker et al., 2015; Takei et al., 2016; Tomasina et al., 2019; Xiao et al., 2015; Xie et al., 2020; Yang et al., 2020) (Figure 2).
FIGURE 2.
Representation of cell viability in a thick scaffold with regards to the distance to a microvascular network. Reproduced from Liu et al., 2012 (Y. Liu et al., 2012) with permission from John Wiley and Sons
When a vascular network is formed in the implant, the time necessary to form a new functional vasculature after implantation will decrease, since the construct will already have blood vessels, which will provide them with faster perfusion and better integration in vivo, preventing hypoxia and cellular necrosis (Auger et al., 2013; Herrmann et al., 2014; Kniebs et al., 2020; W. C. Liu et al., 2017; Min et al., 2019; Rouwkema & Khademhosseini, 2016; Sarker et al., 2015).
Several vascularization strategies have been investigated, however, further development and optimization of protocols that can yield engineered constructs with stable, functional, and perfusable microvasculature are still to be attained (Sharma et al., 2019).
3.2. Vascularization strategies in tissue engineering
When a construct is implanted to restore a tissue defect, it will slowly get vascularized as part of the body’s response to a foreign material. One approach to shorten the time required to generate vasculature within the constructs is to promote the ingrowth of blood vessels from the host after construct implantation through the optimization of scaffold properties or the incorporation growth factor delivery systems (Tomasina et al., 2019). However, these approaches will depend on the host response for fast microvasculature formation, and in case of a large construct, the time to complete vascularization could still be a problem. Another approach is to add a microvascular network to the constructs, i.e., prevascularize them in vitro prior to implantation to speed the process of perfusion and provide cells with oxygen and nutrients shortly after surgery (Chandra & Atala, 2019; Rouwkema & Khademhosseini, 2016; Samal et al., 2015; Takei et al., 2016; Weinandy et al., 2014; Yang et al., 2020). According to Heller et al. (Heller et al., 2016), the prevascularization of constructs is a promising technique to facilitate blood vessel formation in vivo after transplantation.
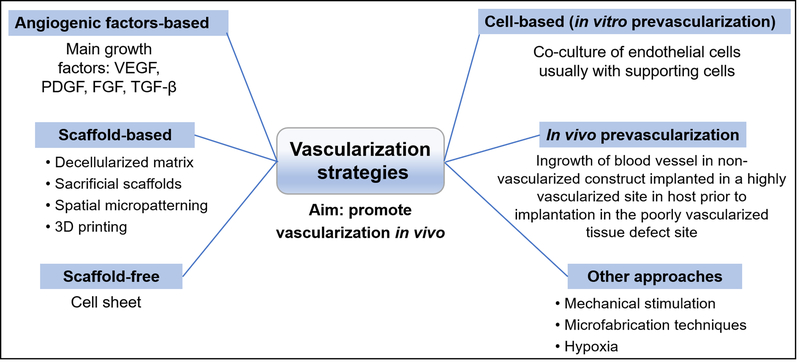
The several strategies that have been investigated to promote vascularization in tissue engineering applications can be broadly classified as: (a) angiogenic factor-based or growth factor delivery, (b) scaffold-based, (c) scaffold-free, (d) in vivo prevascularization, and (e) cell-based (in vitro prevascularization) (Baiguera & Ribatti, 2013; Costa-Almeida et al., 2014; Min et al., 2019; Sarker et al., 2015). Other strategies include direct injection of endothelial cells into the implantation site (Heller et al., 2016), mechanical stimulation, the use of microfabrication and microfluidic techniques (Heller et al., 2016; Nguyen et al., 2012; Sarker et al., 2015; Sharma et al., 2019), and induction of vascularization by hypoxia (Park & Gerecht, 2014; Perez-Amodio, Tra, Rakhorst, Hovius, & van Neck, 2011; Yang et al., 2020; Zhang et al., 2012) (Figure 3).
FIGURE 3.
Overview of common vascularization strategies in tissue engineering. VEGF: vascular endothelial growth factor; PDGF: platelet-derived growth factor; FGF: fibroblast growth factor; TGF-β: transforming growth factor beta
Hypoxia, i.e., low oxygen concentration, regulates angiogenesis by promoting the proliferation and sprouting of ECs, and by the recruitment of pericytes to reach the deficient tissue (Mastrullo et al., 2020; Perez-Amodio et al., 2011; Yang et al., 2020). Hypoxia-induced vascularization is also associated with the expression of hypoxia inducible factor (HIF), which can induce the expression of several pro-angiogenic factors such as VEGF, PDGF and FGF (Chandra & Atala, 2019; Mastrullo et al., 2020). Hypoxia is also known to support the differentiation of progenitor cells and stem cells into the endothelial lineage (Chandra & Atala, 2019). Perez-Amodio et al. investigated how hypoxia preconditioning of a tissue engineered oral mucosa medium would affect endothelial cells. They found that exposing the constructs to hypoxia improved their ability to support endothelial cell proliferation and migration, suggesting that preconditioning tissue engineered constructs in a hypoxic environment could potentially improve angiogenic responses for in vivo implantation (Perez-Amodio et al., 2011). However, presence of hypoxia for a long period of time can lead to opposite effects rather than the stimulation of healing responses observed in acute hypoxia: it could cause dysfunction of fibroblasts, decrease migration and proliferation of keratinocytes, and potentially tissue loss (Handral, 2017). Thus, hypoxic conditions must be controlled if it is a condition that a study may consider using to improve angiogenesis (Perez-Amodio et al., 2011).
In the angiogenic factor-based or growth factor delivery strategy, angiogenic growth factors (e.g., VEGF, PDGF) can be incorporated in the scaffolds or be directly injected into ischemic tissues to improve vascularization (Boccardo et al., 2016; Sarker et al., 2015). The main drawbacks associated with these strategies include limited half-life due to fast degradation and possible imprecise amounts of delivered growth factors, where their overexpression could lead to the development of improper vascularization with immature and unstable blood vessels, or even an increased risk of tumorigenesis due to the formation of vascular tumors (Mastrullo et al., 2020; Min et al., 2019; Omidi, Almeida, & Tayebi, 2020; Rizwan et al., 2019; Tien, 2019). Another disadvantage of this strategy is that a successful outcome, i.e., vascularization, will depend on the patients’ health and the site of implantation (Saberianpour et al., 2018).
To obtain therapeutic efficacy, usually large doses (e.g. 100 μg, as shown in small animal studies) will have to be used to overcome the loss due to fast degradation (Tien, 2019). In an effort to reduce the use of large doses, some strategies have been proposed such as gene therapy (e.g., transfection of seeded cells) to obtain sustained release of growth factors and achieve concentrations in the desired range, use of porous biomaterials as carriers for the local slow release of growth factors, and use of nanoparticles as delivery systems (Boccardo et al., 2016; Gaudiello et al., 2017; Omidi et al., 2020; Sacchi et al., 2014; Sarker et al., 2015; Tien, 2019). In a study by Sacchi et al. 2014 (Sacchi et al., 2014), for instance, an optimized fibrin gel was developed as a platform for controlled delivery of recombinant VEGF and was shown to induce stable and functional angiogenesis and improve tissue perfusion and healing rate, with a tunable and controlled delivery of VEGF.
Scaffold-based approaches involve the optimization of scaffold properties, including the fabrication of tissue equivalents with decellularized matrices, sacrificial scaffolds, spatial micropatterning, biomimetic scaffolds, and 3D printing techniques (Min et al., 2019; Sharma et al., 2019). Scaffolds can be designed with specific porosities to support the in vivo growth of formed blood vessels, and/or be functionalized with platelet-rich fibrin, angiogenic growth factors, cytokines, and proteins and peptide sequences (surface immobilization) that will promote in vivo angiogenesis (Blatt et al., 2020; Costa-Almeida et al., 2014; Nguyen et al., 2012; Novosel, Kleinhans, & Kluger, 2011; Sarker et al., 2015; Tomasina et al., 2019).
The scaffold-free or cell sheet technique consists of growing cells in sheets without a scaffold, where the cells will produce ECM in culture and will be implanted as a cell-ECM construct. This procedure allows for the cells to maintain an intact cell matrix, which provides a good microenvironment for vascularization (Min et al., 2019; Sarker et al., 2015). Ren et al. developed a prevascularized 3D cell sheet construct, consisting of human umbilical vein endothelial cells (HUVECs) seeded on human bone marrow-derived mesenchymal stem cells (hMSCs) sheets to investigate the vascularization in vitro and after subcutaneous implantation in mice. The hMSCs cell sheet stimulated the HUVECs migration to form capillary networks in vitro, and blood vessel formation was observed in vivo (Ren et al., 2014). Lee et al. showed that a prevascularized oral mucosal cell sheet, consisting of oral mucosal keratinocytes and a mixture of fibrin, mucosal fibroblasts, and endothelial progenitor cells (EPCs) promoted healing of cutaneous burn wounds in rats (Lee, Shin, & Roh, 2018).
The in vivo prevascularization strategy is the generation of vascularized tissue through the ingrowth of blood vessels from the host into constructs (Novosel et al., 2011; Weinandy et al., 2014). The non-vascularized constructs are temporarily implanted at well-vascularized host sites before its transplantation into a targeted site (Baiguera & Ribatti, 2013; Y. Liu, Chan, & Teoh, 2012; Sarker et al., 2015; Weigand et al., 2016). Once prevascularized tissue is obtained, the construct is explanted, transplanted into the defect site, and microsurgically connected to local blood vessels, providing rapid blood supply and integration of the construct (Sarker et al., 2015; Weigand et al., 2016; Yang et al., 2020). One disadvantage of this approach that limits its clinical relevance is the need for at least three surgeries: implantation of the scaffold, its removal, and insertion/implant of the prevascularized biomaterial (Y. Liu et al., 2012; Novosel et al., 2011).
Cell-based approaches, or in vitro prevascularization, are one of the key vascularization strategies in tissue engineering, which involve the culture of endothelial cells, usually with other cell types, that will support their growth, directly on a biomaterial. Once the construct is vascularized in vitro, its implantation in vivo will likely provide a beneficial environment for prompt integration with the tissue and further vascularization (Baldwin et al., 2014; Costa-Almeida et al., 2014; Heller et al., 2016; Um Min Allah et al., 2017; Weinandy et al., 2014).
3.2.1. Prevascularization of soft tissue constructs
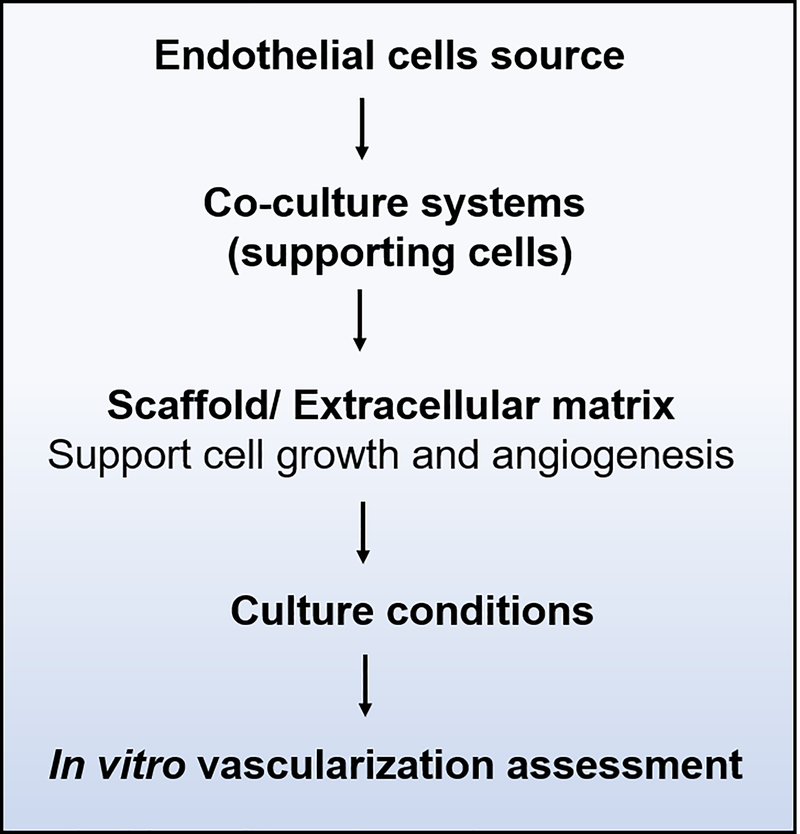
Several approaches have been made with the goal of prevascularizing tissue-engineered constructs in vitro and maintaining their survival after implantation in vivo. These strategies are based on culturing endothelial cells, and their capability of pre-forming capillary-like structures either on scaffolds, in extracellular matrix analogs, or in cellular aggregates (cell sheets), and still remain a challenge in the field (X. Liu et al., 2017; Rouwkema & Khademhosseini, 2016; Um Min Allah et al., 2017). In summary, in vitro prevascularization studies involve: the choice of ECs source, use of supporting cells, selection of scaffold materials, appropriate culture conditions, and finally the choice of assays to characterize vascularization (Figure 4).
FIGURE 4.
Overview of workflow during in vitro prevascularization studies
3.2.1.1. Cell source selection
Several EC types have been used for promoting angiogenesis and vasculogenesis in vitro and in vivo. Among the mature EC types, human umbilical vein ECs (HUVECs) are the most used cells, followed by human dermal microvascular ECs (HDMECs), since these cell types are relatively easy to isolate and keep in culture (Morin & Tranquillo, 2013; Sarker et al., 2015; Um Min Allah et al., 2017). The selection of ECs from macro or microvasculature would be based on whether a study would aim to mimic vessels with large lumens or capillaries, respectively (Guerreiro et al., 2020). HUVECs are commonly used for engineering blood vessels due to their low immunogenicity and are good cell models for studies on endothelial cell biology (Haro Durand et al., 2017). Primary ECs can be isolated from different arteries and blood vessels from different regions of the body and their function and morphology might differ accordingly. For example, HDMECs are elongated, HUVECs are polygonal, artery-derived ECs are long and narrow, while vein-derived ECs are short and wide (dela Paz & D’Amore, 2009; Sarker et al., 2015). The heterogeneity of ECs could lead to differences in functional outcomes of the formed vascular network in prevascularized constructs, including for example the secretion of vasoactive substances, response to endothelial mitogens (Lang et al., 2003), growth factors production and its association with vessel sprouting and maturation (Guerreiro et al., 2020). In a study by Lang et al. 2003 (Lang et al., 2003), the authors suggest that data from experiments using macrovascular ECs, such as HUVECs, should not be extrapolated if the interest of a study is on microvascular bed.
Besides these differences in EC populations, it should also be considered that ECs might present heterogeneity depending on donor-to-donor variations (Morin & Tranquillo, 2013; Zucchelli, Majid, & Foldes, 2019). Such differences must be taken into account when choosing the cell source for specific in vitro and in vivo applications (Sarker et al., 2015; Zucchelli et al., 2019).
Alternatives for overcoming these potential limitations are the use of pluripotent stem cells (PSCs), including embryonic (ESC) and induced pluripotent stem cells (iPSC), for their capability to self-renew indefinitely in culture and differentiate into different types of cells. Mesenchymal stem cells (MSCs) have shown vascular regeneration properties both in vitro and in vivo, either by differentiation into smooth muscle cells (SMC) and endothelial cells, or by the secretion of paracrine factors (Zucchelli et al., 2019). Another alternative to mature ECs is the use of endothelial progenitor cells (EPCs). These cells can be easily harvested from the bone marrow or peripheral blood and rapidly expanded in culture (Lee, Kim, Shin, & Roh, 2017; Rademakers, Horvath, van Blitterswijk, & LaPointe, 2019).
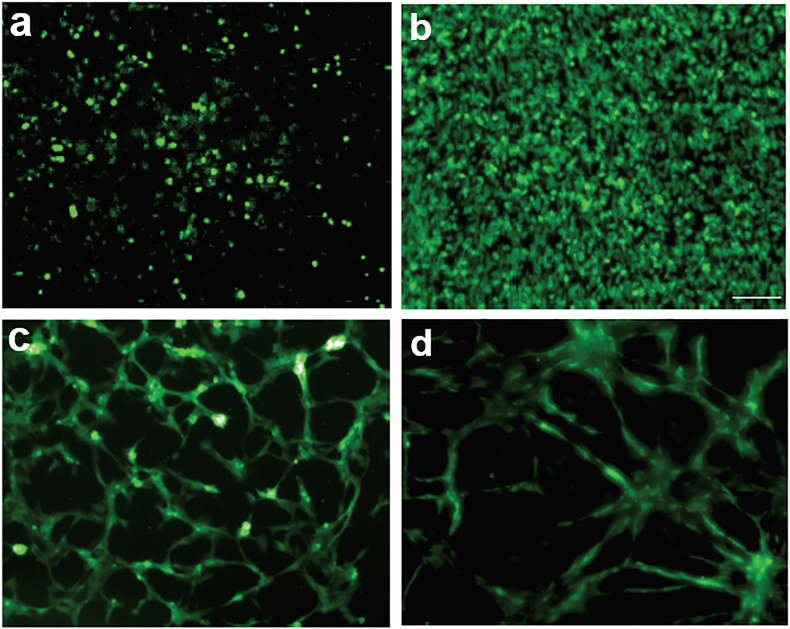
Although ECs are the main cell type involved in angiogenesis, their survival in monocultures in vitro does not seem to be adequate in mimicking physiological angiogenesis in tissue engineering applications, since the vascular structures formed in monocultures are usually rudimentary and unstable in the long term (Costa-Almeida et al., 2015; Costa-Almeida et al., 2014; X. Liu et al., 2017). In monocultures, ECs lose their ability to self-assemble into tube-like structures and are not sufficient to constitute a prolonged, self-sustaining, and functional cell-based vasculature (X. Liu et al., 2017; Morin & Tranquillo, 2013; Um Min Allah et al., 2017; Wan, Bovornchutichai, Cui, O’Neill, & Ye, 2017; Yang et al., 2020). In a study by Ren et al., for instance, they found that no vascular networks were formed when HUVECs were seeded on tissue culture plates, as opposed to rich networks being formed when HUVECs were seeded on hMSCs cell sheet (Ren et al., 2014) (Figure 5).
FIGURE 5.
Fluorescence images showing that HUVECs cultured on tissue culture plates proliferated but didn’t form capillary networks after 2 hours (a) and 7 days (b). When HUVECs were cultured on hMSCs sheets, capillary networks were formed after 1 day (c) and 7 days (d). Scale bar represents 100 𝜇m. Adapted from Ren et al., 2014 (Ren et al., 2014), which is under the terms of the Creative Commons Attribution (CC BY 3.0)
3.2.1.2. Co-culture systems
Angiogenesis in vivo involves a complex signaling pathway and since some pro-angiogenic factors required for ECs migration and formation of microcapillaries cannot be generated by ECs alone, a combination of ECs with supporting pro-angiogenic cells, i.e., co-culture systems, is a common approach to promote scaffolds vascularization (Costa-Almeida et al., 2014; Mastrullo et al., 2020). Cells that are usually cultured with ECs include: fibroblasts, pericytes, human embryonic stem cells (hESCs), human mesenchymal stem cells (MSCs), smooth muscle cells (SMCs), human adult adipose-tissue derived stromal cells (ASCs), retinal cells, etc (Andrée et al., 2019; Baiguera & Ribatti, 2013; Costa-Almeida et al., 2015; Costa-Almeida et al., 2014; Deb, Mandegaran, & Di Silvio, 2010; Irvin, Zijlstra, Wikswo, & Pozzi, 2014; Kniebs et al., 2020; Y. Liu et al., 2012; Mastrullo et al., 2020; Morin & Tranquillo, 2013; Nguyen et al., 2012; Samal et al., 2015; Shi, Andrukhov, Berner, Schedle, & Rausch-Fan, 2014; Um Min Allah et al., 2017; Yang et al., 2020).
In co-culture systems, angiogenesis is controlled by a crosstalk between ECs and supporting cells in different pathways such as through diffusible signaling molecules or by cell-cell contact (Costa-Almeida et al., 2015; Kirkpatrick et al., 2011). Co-culture systems in the prevascularization of tissue constructs can be performed by two (2D) or three-dimensional (3D) culture models. For many years, the standard protocol to grow cells and investigate their interaction in mono and co-cultures consisted of 2D-systems. However, in vitro angiogenesis in 2D-cell culture models doesn’t seem to perfectly resemble the tissue architecture and physiological conditions in vivo. In 3D-systems, the heterotypic cell-cell and cell-matrix interactions seem to better represent the physiological environment in vivo, controlling proliferation, differentiation, and signaling mechanisms (Unterleuthner et al., 2017; Zucchelli et al., 2019). During optimization and fabrication of prevascularized soft tissue-engineered constructs based on 3D models, cells can be incorporated in scaffold materials such as hydrogels, or be assembled in organoids, spheroids, or 3D-printed in an appropriate matrix (Zucchelli et al., 2019). Kolesky et al. 2016 (Kolesky, Homan, Skylar-Scott, & Lewis, 2016) reported a multimaterial 3D bioprinting method to fabricate thick vascularized tissues (≥1 cm) that were actively perfused for over 6 weeks, resembling physiologically 3D tissue microenvironments.
Among the supporting cells that can be co-cultured with ECs, fibroblasts are one of the most common cell types since their activation and migration are associated with the normal physiological processes during angiogenesis, supporting ECs survival, migration, and proliferation, contributing towards the in vitro capillary-like networks formation (Costa-Almeida, Soares, & Granja, 2017; Um Min Allah et al., 2017). In co-culture systems, fibroblasts improve the mechanical properties of the extracellular microenvironment by matrix deposition, which provides a scaffold for other cells to grow and migrate (Costa-Almeida et al., 2015; Guerreiro, Oliveira, Barbosa, Soares, & Granja, 2014; Guerreiro et al., 2020; Kniebs et al., 2020).
By secretion and modification of ECM components for tissue maintenance and repair - their primary role - they promote tubulogenesis, provide structural support for blood vessel formation and long-term stability, and also regulate the activation and propagation of ECs by secreting angiogenic factors such as: VEGF, PDGF, TGF-β1, FGF-2, and nitric oxide (Cheung, Jain, McCulloch, & Santerre, 2015; Costa-Almeida et al., 2015; Costa-Almeida et al., 2017; Guerreiro et al., 2012; Guerreiro et al., 2014; Guerreiro et al., 2020; Kniebs et al., 2020; Min et al., 2019; Samal et al., 2015; Sarker et al., 2015; Um Min Allah et al., 2017; Unterleuthner et al., 2017). Fibroblasts have been shown to modulate the angiogenic process for instance, when embedded in Matrigel plug implanted in mice. Their presence recruited endothelial cells from the mice and promoted vascularization of the implant (Guerreiro et al., 2012).
Potential applications of fibroblasts in tissue engineering and regenerative medicine, including their versatile roles, fibroblast-based therapies and their potential to promote angiogenesis by supporting vascularization have been comprehensively reviewed by Costa-Almeida et al. (Costa-Almeida et al., 2017). Co-culture of fibroblasts and endothelial cells has been shown to stimulate angiogenesis. For example, in a study by Guerreiro et al., (Guerreiro et al., 2014), where HUVECs and human neonatal dermal foreskin fibroblasts immobilized in arginine-glycine-aspartic acid (RGD)-alginate microspheres were co-cultured, fibroblasts created a microenvironment in the scaffolds that contributed to the formation of capillary-like structures. In a recent study by the same group (Guerreiro et al., 2020), where human dermal fibroblasts and HUVECs or HDMECs were co-cultured, fibroblasts stimulated the formation of well-defined microvessel-like structures and collagen production in co-culture with either HUVECs or HDMECs.
3.2.1.3. Biomaterial/Scaffold selection
During ECs proliferation and survival, the ECM functions as a substrate for the organization of ECs into microvessels, and also as a reservoir for growth factors involved in angiogenesis, wound healing, and other physiological processes (Costa-Almeida et al., 2014). The interaction of cells and the ECM is a very important factor to consider during the design of functionalized artificial matrices and should mimic the natural ECM (Costa-Almeida et al., 2015; Mastrullo et al., 2020). The cell-ECM interaction is mediated by transmembrane proteins, e.g., integrins, present on the cell surface, that will initiate an intracellular cascade and promote cell attachment, migration, or differentiation. Some proteins (e.g. collagen, laminin, fibronectin) have been used as a coating for scaffolds to stimulate this process (Mastrullo et al., 2020).
However, the ECM is a complex matrix and many biomaterial characteristics should be considered to reproduce it in vitro, such a mechanical properties, cell adhesion, in vitro culture conditions, etc. (Mastrullo et al., 2020). The biomaterial should mimic the natural ECM by facilitating ECs-biomaterial interactions and activating signaling pathways to stimulate vascular cell survival, attachment to the scaffold, proliferation, differentiation, and migration, as well as support rapid and stable neovascularization within the scaffold (Sarker et al., 2015). Moreover, the scaffolds should present certain parameters such as biodegradability, oxygen permeability, biocompatibility, mechanical strength, water vapor permeability, and appropriate porous architecture and interconnectivity (Baiguera & Ribatti, 2013; Mastrullo et al., 2020; Samal et al., 2015).
If the scaffolds don’t have appropriate porous structures, the capability of ECs to promote angiogenesis will be limited (Xiao et al., 2015). The porous architecture of a scaffold includes pore size, shape, porosity, and surface topography of the pores. Proper porosity allows cell migration, proliferation, cell-cell, and cell-matrix interactions, which will facilitate the formation of a vascular network in vitro and in vivo. Furthermore, the transport of oxygen and nutrients to the cells are promoted through the appropriate porosity of the scaffolds. However, excessive porosity might result in reduced mechanical strength (Sarker et al., 2015).
Different biomaterials, including synthetic, natural, and hybrid polymers have been investigated for the development of prevascularized tissue constructs. However, there is still a need to develop suitable and stable biomaterial scaffolds that can induce ECs proliferation and migration, as well as promote angiogenesis at the wound site (Rizwan et al., 2019). Several biodegradable, biocompatible, and non-toxic synthetic polymers have been investigated: polyglycolic acid (PGA), polylactic acid (PLA), polylactic-co-glycolic acid (PLGA), poly-L-lactic acid (PLLA), poly-ε-caprolactone (PCL), polyethylene glycol (PEG), poly(vinyl alcohol) (PVA), and polyhydroxyalkanoates (PHAs) (Cheung et al., 2015; Handral, 2017; Sarker et al., 2015; Tien, 2019; Xie et al., 2020; Yang et al., 2020). Biopolymers, or naturally derived polymers, investigated for tissue vascularization can be classified into two categories: protein-based and polysaccharidic polymers. Among protein-based polymers are collagen, fibrin, fibronectin, elastin, silk fibroin, and Matrigel; and among polysaccharide polymers, are mostly hyaluronic acid (HA), alginate, agarose, and chitosan (Handral, 2017; Kniebs et al., 2020; Saberianpour et al., 2018; Samal et al., 2015; Sarker et al., 2015; Takei et al., 2016; Tien, 2019; Um Min Allah et al., 2017; Xie et al., 2020; Yang et al., 2020).
Natural polymers would be the preferred choice due to their potential to mimic the natural ECM, however, most of them present poor mechanical properties that should be improved during the design of a construct (Mastrullo et al., 2020). Some of the strategies to improve mechanical properties and the structure of scaffolds include cross-linking of collagen-based scaffolds (e.g., using N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide or N-hydroxysuccinimide), use of compound collagen-based scaffolds (e.g. collagen-chitosan, collagen-glycosaminoglycan, collagen-glycosaminoglycan-chitosan, collagen-chondroitin sulfate, collagen-agarose), and a combination of natural and synthetic polymers [e.g. fibrin-PEG, gelatin-methacrylate (GelMA) - which can form hydrogels within seconds by UV cross-linking] (Handral, 2017; Kreimendahl et al., 2017; Minor & Coulombe, 2020; Suzuki et al., 2020; Xie et al., 2020).
Hydrogels are well-known for providing a 3D microenvironment that supports angiogenesis. Strategies have been on the rise to make them more similar to the ECM, improving ECs survival, attachment, proliferation and migration, and hence, enhancing vascularization. One of these strategies is a customization of their biochemical and biomechanical properties by creating micro-scale hydrogels (microgels) with multicellular aggregates. In this context, the development of injectable cell-delivery microgel-based system has been reported, for instance, by Torres et al. (Torres et al., 2018) and Torres et al. (Torres et al., 2020), where they cultured MSCs co-entrapped with outgrowth endothelial cells (OECs) in soft modified alginate microgels and observed in vitro formation of prevascularized microtissues and improved angiogenic response when the microgels were implanted in vivo.
3.2.1.4. Culture conditions
Along with co-cultures of ECs and supporting cells, as well as use of suitable and stable scaffolds that resemble the natural ECM, an appropriate selection of culture conditions, such as cell seeding ratio, density and techniques, and the choice of culture medium (with or without supplements and growth factors), is critical for cell-based vascularization strategies in tissue engineering and angiogenesis assays (Baldwin et al., 2014; Kirkpatrick et al., 2011; Zucchelli et al., 2019). To prevascularize the scaffold in vitro, controlled culture conditions and environment must be maintained (Sarker et al., 2015). However, no consensus exists on specific culture conditions, especially when using co-culture systems (Um Min Allah et al., 2017).
The influence of specific biomaterials on co-cultures and culture conditions should be determined according to cell sources and the biomaterial (Cheung et al., 2015). To culture endothelial cells, for example, some studies have used cell culture media supplemented with angiogenic components including VEGF, FGF-2, EGF and IGF1 (insulin-like growth factor 1), while others have used media supplemented with bovine-derived endothelial cell growth supplements, hydrocortisone, L-glutamine, heparin, or ascorbic acid (Zucchelli et al., 2019). In a co-culture system, the more sensitive cell type will usually have priority in the formulation of the final culture medium to be used (Kirkpatrick et al., 2011).
While some culture conditions could work for one cell type, it might not work, or adversely affect another cell type, which may make it difficult to manage different cell types cultured in the same system, for example during prevascularization studies using ECs co-cultured with supporting cells. It is important to look for a balance of conditions required by each cell type in the construct, as well as its purpose and applications in order to optimize and standardize culture conditions and to choose an appropriate scaffold material (Baldwin et al., 2014; Zucchelli et al., 2019).
3.3. In vitro methods to assess and characterize vascularization in tissue-engineered constructs
Cells need to be in an appropriate environment (which includes scaffold material and culture conditions) to survive, proliferate and migrate. To investigate the best conditions and characterize the cell-seeded constructs and vascularization in prevascularized equivalents, the selection of pertinent in vitro assays is critical.
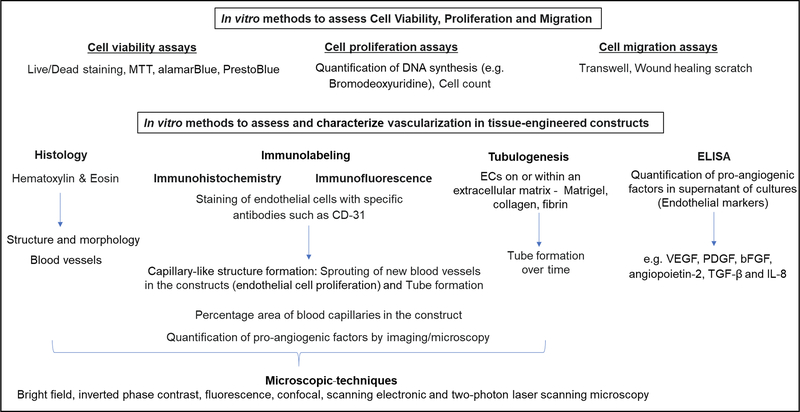
During the fabrication of cell-seeded scaffolds, several preliminary assays of viability, proliferation, and migration are usually performed to assess if the environment is propitious for the cells. Examples of these assays include PrestoBlue, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Live/Dead, Bromodeoxyuridine, cell count, and the wound healing scratch assay. Once it is determined that the cells are healthy and the scaffold material doesn’t present any negative effect on them, more specific assays are performed according to the purpose of the study (Figure 6). Since the focus of our review is on prevascularized soft tissue constructs, we will not discuss preliminary in vitro assays, but focus on an overview of most common in vitro assays used to assess blood vessel formation in 3D constructs.
FIGURE 6.
Overview of commonly used in vitro methods during vascularization of tissue-engineered constructs
Some of the most common parameters to characterize and assess vascularization in tissue-engineered equivalents include sprouting of new blood vessels in the constructs, tube formation, quantification of percentage area of blood capillaries in the constructs, assessment of the vessel-like structures formed in vitro, e.g., if they are hollow and closely resemble the vascular lumen, and quantification of pro-angiogenic factors by imaging or from culture supernatants. These parameters can be assessed by a variety of in vitro methods and visualized by different microscopic techniques that are helpful in providing cell morphology and their spatial distribution.
3.3.1. Histology
Once a soft tissue equivalent is produced and after appropriate incubation period, its structure/morphology (e.g., layers, collagen content, basement membrane) and blood vessel formation in vascularized constructs can be visualized by basic histology (Almela, Brook, & Moharamzadeh, 2016; Chan et al., 2016; Lee et al., 2018). The tissue equivalents can be snap frozen or fixed in 10% neutral buffered formalin, embedded and processed for staining with Hematoxylin & Eosin (H&E), collagen staining, or processed for immunohistochemistry (Chan et al., 2016; Cheung et al., 2015; Lee et al., 2017; Nishiyama, Akagi, Iwai, & Akashi, 2019).
In the past, blood vessels in tissue sections used to be identified by simple histological techniques. Although this protocol provides information on the overall morphology and structure of the constructs, more detailed and accurate information on capillary formation can be obtained using specific endothelial markers such as CD-31 through immunohistochemistry (IHC) or immunofluorescence (IF), for example. Also, the use of a confocal microscope instead of a bright field microscope (during imaging of H&E sections), can provide a 3D-distribution of these endothelial (and other) markers in vascularized tissue equivalents.
3.3.2. Immunolabeling
Endothelial cells express specific markers such as platelet endothelial cell adhesion molecule-1 (PECAM-1 or CD31), von Willebrand factor (vWF), and vascular endothelial (VE)-cadherin (Cheung et al., 2015; Costa-Almeida et al., 2014; Heller et al., 2020; Nishiyama et al., 2019; Unterleuthner et al., 2017). Immunohistochemical or immunofluorescence methods can detect the presence of ECs by binding antibodies to the cells expressing one or more of the above markers, thus providing important information with regards to vascularization in tissue constructs.
Immunohistochemical/immunofluorescence staining of angiogenic sprouting can be assessed by bright field microscopy or fluorescence microscopy depending on the secondary antibody. Samples for immunostaining can be snap frozen or fixed in formaldehyde or ice-cold methanol. After processing and staining with a primary antibody (e.g., anti-CD-31), a chromogenic or fluorescent secondary antibody is added and the blood vessels will be visualized by a brownish color or fluorescence, respectively, depending on the secondary antibody (Heller et al., 2016; Kniebs et al., 2020; Kreimendahl et al., 2017; Nishiyama et al., 2019; Samal et al., 2015; Unterleuthner et al., 2017).
Analysis of the microvascular structures marked by an endothelial marker such as CD-31 can be performed through fluorescence microscopy (Costa-Almeida et al., 2015), epifluorescence microscopy (Weinandy et al., 2014), confocal laser scanning microscopy (Costa-Almeida et al., 2015; Dos Santos et al., 2019; Heller et al., 2020; Heller et al., 2016; Ren et al., 2014; Unterleuthner et al., 2017; Xiao et al., 2015), two-photon laser scanning microscopy (TPLSM) (Kniebs et al., 2020; Kreimendahl et al., 2017; Samal et al., 2015; Weinandy et al., 2014), or scanning electron microscopy (SEM) (Lu et al., 2019; Samal et al., 2015; Shen, Li, Wang, & Meng, 2017).
A fluorescent microscope usually provides visualization of ECs expressing CD-31, for example, while confocal and two-photon microscopes provide deep tissue imagining, and thus 3D images of the microvascular structures can be observed. The images can be further analyzed and quantified by image analysis software (e.g., ImageJ), through the counting of capillary-like structures, and measuring their length and diameter. To determine the average number of microvessels per area, for example, the number of microvessels are counted and divided by the area (mm2) of the image, usually using several areas per sample in replicate (Chen et al., 2017; Costa-Almeida et al., 2015).
In a study by Weinandy et al. constructs consisting of human foreskin fibroblasts and HUVECs were seeded in biofunctionalized poly-(L-lactic acid) fibers and embedded in fibrin gels that were immunostained with CD-31 to assess the development of capillary-like structures. After 9–21 days in culture, the whole gel along with cryosection samples were stained and analyzed. Thawed cryosection samples were fixed with paraformaldehyde, washed, blocked (nonspecific binding sites), and incubated with anti-CD-31 primary antibody. Sections were then incubated with Alexa Fluor® 594-conjugated secondary antibody, and DAPI was added to counterstain the cell nuclei. Microscopic analyses were performed in an epifluorescence microscope or a two-photon laser scanning microscope (Weinandy et al., 2014).
Kreimendahl et al. evaluated angiogenesis in HUVECs (co-cultured with dermal fibroblasts in agarose-type I collagen hydrogel blends) stained with anti-CD-31 and imaged with a TPLSM. Volume, cell surface area, length, and branching points, characteristic parameters during the assessment of capillary-like network formation in hydrogels, were analyzed from 3D stacked images using AutoQuant X3 deconvolution, followed by ImagePro-Analyzer 7.0 software, both Media Cybernetics (Kreimendahl et al., 2017).
Scanning electron microscopy has also been reported in morphological analysis of HUVECs and capillary-like structures, for example, on interpenetrating network carboxybetaine-gelatin hydrogels (Shen et al., 2017), silk fibroin (Samal et al., 2015) and hyaluronic acid hydrogels (Lu et al., 2019).
3.3.3. Tube formation
After proliferation and migration of ECs in 3D constructs, it is expected that they will assemble into tubes with lumen structures, and eventually form new vessels (Simons et al., 2015). Tube formation assay, or tubulogenesis, can be mimicked in vitro by incorporating ECs on or within an ECM, usually in a serum free medium, and by monitoring tube formation over time (Irvin et al., 2014; W. C. Liu et al., 2017). Some of the commonly used matrices for tube formation assay are collagen, fibrin, and Matrigel, with the latter being the most used due to its similarity with the natural ECM and basement membrane proteins components (W. C. Liu et al., 2017). Tube formation assays can be two-dimensional (cells seeded on top of a thin layer of ECM) or three-dimensional (cells seeded within an ECM) (Irvin et al., 2014). Tube formation can be visualized by a phase contrast inverted microscope over 4–24 hours with maximum tube formation at 4–6 hours (DeCicco-Skinner et al., 2014). The captured images can be analyzed by commercially available software to quantify different parameters representing the degree of tube formation, such as the length and/or number of the formed tubes, number of branch points, and loops/meshes (Heller et al., 2020; Irvin et al., 2014; W. C. Liu et al., 2017). Tube formation can also be visualized by fluorescence microscopy (when ECs are stained with calcein AM, for instance) (Heller et al., 2020; W. C. Liu et al., 2017), confocal, and transmission electron microscopy (Irvin et al., 2014; Simons et al., 2015). Once an endothelial cell network is assembled, it is important to assess if the structures are hollow, which can be performed for example, by incubating 3D constructs with Texas red-labeled dextran, followed by confocal microscopy imaging (Andrée et al., 2019).
3.3.4. Quantification of pro-angiogenic growth factors
Along with the labeling/staining of endothelial cells for microscopic analysis, it is important to investigate pro-angiogenic growth factors secreted in the supernatant of cell-seeded scaffold cultures. There are several growth factors involved in blood vessel formation and stabilization. Among them, VEGF, the angiopoietin (Ang) family of growth factors (e.g. Ang 2), PDGF, fibroblast growth factor (FGF), epidermal growth factor (EGF), transforming growth factor-β (TGF-β), IL-8, and hypoxia-inducible factor 1 (HIF), have been often described in scaffold vascularization studies in tissue engineering (Blatt et al., 2020; Chandra & Atala, 2019; Chen et al., 2017; Cheung et al., 2015; Haro Durand et al., 2017; Heller et al., 2020; Heller et al., 2016; Kniebs et al., 2020; Perez-Amodio et al., 2011; Samal et al., 2015; Sarker et al., 2015; Xiao et al., 2015; Zhang et al., 2012). The quantification of these pro-angiogenic markers is commonly performed by ELISA, however, other methods such as IHC, IF, flow cytometry, transcription analysis (PCR) (Shi et al., 2014) and proteomics (e.g., Western blot) (Unterleuthner et al., 2017) have also been used.
Kniebbs et al. used a multiplex ELISA to detect secreted pro-angiogenic factors in the culture medium to investigate the effect of the different co-cultures (HUVECs with MSCs on fibrin gels and agarose–collagen hydrogels scaffolds) on vascularization. This kit detected different proangiogenic factors including tumor necrosis factor alpha (TNF-α), insulin-like growth factor 1 (IGF-1), VEGF, interleukin 6 (IL-6), basic fibroblast growth factor (bFGF), TGF-β, epidermal growth factor (EGF), and leptin (Kniebs et al., 2020).
Heller et al. used ELISA kits to quantify the concentration of each cytokine (VEGF, IL-8, and bFGF) in the supernatants of monocultures (oral fibroblasts or dermal microvascular ECs cells) and co-cultures of these cells seeded in a collagen membrane). It is worth noting that the culture medium was replaced with a serum-free medium 24 hours before collecting the supernatant (Heller et al., 2020).
In another study from the same group (Heller et al., 2016), PDGF-BB, IL-8, Angiopoietin-2, and VEGF were quantified in the supernatants of co-cultures of oral fibroblasts and dermal microvascular ECs seeded in collagen membranes using a bio-plex angiogenesis assay. Briefly, this is a bead-based sandwich immunoassay that consists of adding antibody-bound beads to the culture supernatant, and after incubation with the secondary antibody, the secreted growth factors from cells are quantified using a Luminex-100 reader.
3.3.5. Summary
Different in vitro assays can be performed to investigate angiogenesis in prevascularized tissue constructs, ranging from a variety of imaging techniques for visualization of formed capillary-like networks, to the quantification of growth factors expressed by endothelial and supporting cells seeded in the tissue equivalents. These assays are valuable to gain insights into the basic mechanisms of vessel growth, to demonstrate cell viability and migration within the scaffolds, and to examine the influence of cell-cell (ECs and supporting cells) and cell-matrix interactions.
However, in vitro models could fail in fully representing the process of blood vessel formation under normal or pathological conditions in vivo, for example, during revascularization and tissue repair, thus a combination of more than one in vitro assay and in vivo assays is recommended. In in vitro assays variables that influence angiogenesis can be controlled, but the environment does not truly represent the physiological conditions. On the other hand, in vivo models provide more information on the understanding of cellular and molecular interactions that contribute to the formation of functional blood vessels, but not all variables can be experimentally controlled.
Ideally, studies in tissue engineering and regenerative medicine aiming at the prevascularization of constructs should consist of initial in vitro assays to determine appropriate culture conditions, including scaffold material characteristics, that will allow the formation of stable capillaries, followed by implantation in vivo for subsequent analysis of integration, stimulation of angiogenesis, and contribution to repair a damaged tissue (X. Liu et al., 2017; Simons et al., 2015). Since the main focus of this review was on vascularization strategies in tissue engineering approaches, the discussion of methods to assess vascularized constructs was focus only on in vitro assays. In vitro assays provide important preliminary information (e.g., culture conditions, scaffolds characteristics that allow vascularization) to be a starting point in vascularization studies to be followed by in vivo studies.
4. CONCLUSION
Major advances have been made in tissue engineering over the years, however, the number of tissue constructs that have gained clinical use is still limited and this is due, in part, to lack a of vascularization especially when these equivalents present large sizes and/or are intended to repair large-sized tissue injuries. The development of tissue equivalents that more closely mimic the injured tissue to be repaired, i.e., vascularized constructs, will certainly make them more clinically relevant in terms of potentially providing a better integration after in vivo implantation and overcome the ischemia present in the defect site. Clinically, well-designed prevascularized constructs will also reduce donor site morbidity and shorten surgery time since multiple surgeries to harvest and transplant tissues/cells would not be needed. Moreover, by being vascularized, they will promote faster angiogenesis, enhance cellular survival in the constructs, and promote faster tissue repair.
Despite successes in many approaches to develop and obtain vascularized tissue constructs, there is no consensus on the best procedures for vascularization in vitro. The multitude of scaffold production techniques, scaffold materials, different cell sources, culture conditions, among others make this process very challenging. One single method/protocol might not work for all tissues for which vascularization is necessary, instead, an optimal protocol to add a well-organized vascular network will likely combine multiple approaches to provide an implantable tissue with a mature vascular network.
In this review, the authors aimed at providing an overview of vascularization strategies in tissue engineering approaches for soft tissue repair, based on articles published during the last 10 years. Several aspects were discussed from the wound healing process in soft tissue, the relevance of vascularization in tissue engineering and regenerative medicine, to strategies during the development and assessment of vascularized soft tissue constructs. Thus, this article can certainly assist other researchers in the field during the design and execution of their experiments.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Dental & Craniofacial Research/National Institutes of Health (NIDCR/NIH), award numbers R15DE027533 and 3R15DE027533-01A1. The content of this manuscript is solely the responsibility of the authors and does not represent the official views of NIH.
Footnotes
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
References
- Aghaloo TL, & Hadaya D (2017). Basic Principles of Bioengineering and Regeneration. Oral Maxillofac Surg Clin North Am, 29(1), 1–7. doi: 10.1016/j.coms.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almela T, Brook IM, & Moharamzadeh K (2016). Development of three-dimensional tissue engineered bone-oral mucosal composite models. J Mater Sci Mater Med, 27(4), 65. doi: 10.1007/s10856-016-5676-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrée B, Ichanti H, Kalies S, Heisterkamp A, Strauß S, Vogt P-M, … Hilfiker A (2019). Formation of three-dimensional tubular endothelial cell networks under defined serum-free cell culture conditions in human collagen hydrogels. Scientific Reports, 9(1), 5437. doi: 10.1038/s41598-019-41985-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger FA, Gibot L, & Lacroix D (2013). The pivotal role of vascularization in tissue engineering. Annu Rev Biomed Eng, 15, 177–200. doi: 10.1146/annurev-bioeng-071812-152428 [DOI] [PubMed] [Google Scholar]
- Baiguera S, & Ribatti D (2013). Endothelialization approaches for viable engineered tissues. Angiogenesis, 16(1), 1–14. doi: 10.1007/s10456-012-9307-8 [DOI] [PubMed] [Google Scholar]
- Baldwin J, Antille M, Bonda U, De-Juan-Pardo EM, Khosrotehrani K, Ivanovski S, … Hutmacher DW (2014). In vitro pre-vascularisation of tissue-engineered constructs A co-culture perspective. Vasc Cell, 6, 13. doi: 10.1186/2045-824x-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt S, Burkhardt V, Kämmerer PW, Pabst AM, Sagheb K, Heller M, … Schiegnitz E (2020). Biofunctionalization of porcine-derived collagen matrices with platelet rich fibrin: influence on angiogenesis in vitro and in vivo. Clin Oral Investig. doi: 10.1007/s00784-020-03213-8 [DOI] [PubMed] [Google Scholar]
- Boccardo S, Gaudiello E, Melly L, Cerino G, Ricci D, Martin I, … Marsano A (2016). Engineered mesenchymal cell-based patches as controlled VEGF delivery systems to induce extrinsic angiogenesis. Acta Biomater, 42, 127–135. doi: 10.1016/j.actbio.2016.07.041 [DOI] [PubMed] [Google Scholar]
- Chan EC, Kuo S-M, Kong AM, Morrison WA, Dusting GJ, Mitchell GM, … Liu G-S (2016). Three Dimensional Collagen Scaffold Promotes Intrinsic Vascularisation for Tissue Engineering Applications. PLoS One, 11(2), e0149799. doi: 10.1371/journal.pone.0149799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P, & Atala A (2019). Engineering blood vessels and vascularized tissues: technology trends and potential clinical applications. Clin Sci (Lond), 133(9), 1115–1135. doi: 10.1042/cs20180155 [DOI] [PubMed] [Google Scholar]
- Chen L, Xing Q, Zhai Q, Tahtinen M, Zhou F, Chen L, … Zhao F (2017). Pre-vascularization Enhances Therapeutic Effects of Human Mesenchymal Stem Cell Sheets in Full Thickness Skin Wound Repair. Theranostics, 7(1), 117–131. doi: 10.7150/thno.17031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JW, Jain D, McCulloch CA, & Santerre JP (2015). Pro-angiogenic character of endothelial cells and gingival fibroblasts cocultures in perfused degradable polyurethane scaffolds. Tissue Eng Part A, 21(9–10), 1587–1599. doi: 10.1089/ten.TEA.2014.0548 [DOI] [PubMed] [Google Scholar]
- Costa-Almeida R, Gomez-Lazaro M, Ramalho C, Granja PL, Soares R, & Guerreiro SG (2015). Fibroblast-endothelial partners for vascularization strategies in tissue engineering. Tissue Eng Part A, 21(5–6), 1055–1065. doi: 10.1089/ten.TEA.2014.0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Almeida R, Granja PL, Soares R, & Guerreiro SG (2014). Cellular strategies to promote vascularisation in tissue engineering applications. Eur Cell Mater, 28, 51–66; discussion 66–57. doi: 10.22203/ecm.v028a05 [DOI] [PubMed] [Google Scholar]
- Costa-Almeida R, Soares R, & Granja PL (2017). Fibroblasts as maestros orchestrating tissue regeneration. Journal of Tissue Engineering and Regenerative Medicine, 12(1), 240–251. doi: 10.1002/term.2405 [DOI] [PubMed] [Google Scholar]
- Datta P, Ayan B, & Ozbolat IT (2017). Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater, 51, 1–20. doi: 10.1016/j.actbio.2017.01.035 [DOI] [PubMed] [Google Scholar]
- Deb S, Mandegaran R, & Di Silvio L (2010). A porous scaffold for bone tissue engineering/45S5 Bioglass derived porous scaffolds for co-culturing osteoblasts and endothelial cells. J Mater Sci Mater Med, 21(3), 893–905. doi: 10.1007/s10856-009-3936-5 [DOI] [PubMed] [Google Scholar]
- DeCicco-Skinner KL, Henry GH, Cataisson C, Tabib T, Gwilliam JC, Watson NJ, … Wiest JS (2014). Endothelial cell tube formation assay for the in vitro study of angiogenesis. J Vis Exp(91), e51312. doi: 10.3791/51312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Paz NG, & D’Amore PA (2009). Arterial versus venous endothelial cells. Cell and Tissue Research, 335(1), 5–16. doi: 10.1007/s00441-008-0706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos BP, Garbay B, Fenelon M, Rosselin M, Garanger E, Lecommandoux S, … Amédée J (2019). Development of a cell-free and growth factor-free hydrogel capable of inducing angiogenesis and innervation after subcutaneous implantation. Acta Biomater, 99, 154–167. doi: 10.1016/j.actbio.2019.08.028 [DOI] [PubMed] [Google Scholar]
- Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, … Motaung K (2018). Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int, 2018, 2495848. doi: 10.1155/2018/2495848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DS, Jones RE, Ransom RC, Longaker MT, & Norton JA (2018). The evolving relationship of wound healing and tumor stroma. JCI Insight, 3(18). doi: 10.1172/jci.insight.99911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudiello E, Melly L, Cerino G, Boccardo S, Jalili-Firoozinezhad S, Xu L, … Marsano A (2017). Scaffold Composition Determines the Angiogenic Outcome of Cell-Based Vascular Endothelial Growth Factor Expression by Modulating Its Microenvironmental Distribution. Advanced Healthcare Materials, 6(24), 1700600. doi: 10.1002/adhm.201700600 [DOI] [PubMed] [Google Scholar]
- Gonzalez AC, Costa TF, Andrade ZA, & Medrado AR (2016). Wound healing - A literature review. An Bras Dermatol, 91(5), 614–620. doi: 10.1590/abd1806-4841.20164741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J-P, & Kiecolt-Glaser JK (2011). The impact of psychological stress on wound healing: methods and mechanisms. Immunology and allergy clinics of North America, 31(1), 81–93. doi: 10.1016/j.iac.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro SG, Brochhausen C, Negrão R, Barbosa MA, Unger RE, Kirkpatrick CJ, … Granja PL (2012). Implanted neonatal human dermal fibroblasts influence the recruitment of endothelial cells in mice. Biomatter, 2(1), 43–52. doi: 10.4161/biom.20063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro SG, Oliveira MJ, Barbosa MA, Soares R, & Granja PL (2014). Neonatal human dermal fibroblasts immobilized in RGD-alginate induce angiogenesis. Cell Transplant, 23(8), 945–957. doi: [DOI] [PubMed] [Google Scholar]
- Guerreiro SG, Unger RE, Cerqueira NMFSA, Sartoris A, Martins MJ, Barbosa MA, … Kirkpatrick CJ (2020). Alkaline phosphatase dual-binding sites for collagen dictate cell migration and microvessel assembly in vitro. Journal of cellular biochemistry, 122(1), 116–129. doi: 10.1002/jcb.29835 [DOI] [PubMed] [Google Scholar]
- Handral HK (2017). IN VITRO ESTABLISHMENT OF VASCULARISED SKIN AND ORAL MUCOSA FROM HUMAN EMBRYONIC STEM CELLS FOR PRE-CLINICAL STUDIES AND INDUSTRIAL APPLICATIONS. [Google Scholar]
- Haro Durand LA, Vargas GE, Vera-Mesones R, Baldi A, Zago MP, Fanovich MA, … Gorustovich A (2017). In Vitro Human Umbilical Vein Endothelial Cells Response to Ionic Dissolution Products from Lithium-Containing 45S5 Bioactive Glass. Materials (Basel), 10(7). doi: 10.3390/ma10070740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M, Bauer HK, Schwab R, Blatt S, Peters K, Nezi-Cahn S, … Brenner W (2020). The impact of intercellular communication for the generation of complex multicellular prevascularized tissue equivalents. J Biomed Mater Res A, 108(3), 734–748. doi: 10.1002/jbm.a.36853 [DOI] [PubMed] [Google Scholar]
- Heller M, Frerick-Ochs EV, Bauer HK, Schiegnitz E, Flesch D, Brieger J, … Brenner W (2016). Tissue engineered pre-vascularized buccal mucosa equivalents utilizing a primary triculture of epithelial cells, endothelial cells and fibroblasts. Biomaterials, 77, 207–215. doi: 10.1016/j.biomaterials.2015.10.073 [DOI] [PubMed] [Google Scholar]
- Herrmann M, Binder A, Menzel U, Zeiter S, Alini M, & Verrier S (2014). CD34/CD133 enriched bone marrow progenitor cells promote neovascularization of tissue engineered constructs in vivo. Stem Cell Research, 13(3, Part A), 465–477. doi: 10.1016/j.scr.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Irvin MW, Zijlstra A, Wikswo JP, & Pozzi A (2014). Techniques and assays for the study of angiogenesis. Exp Biol Med (Maywood), 239(11), 1476–1488. doi: 10.1177/1535370214529386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karppinen S, Heljasvaara R, Gullberg D, Tasanen K, & Pihlajaniemi T (2019). Toward understanding scarless skin wound healing and pathological scarring [version 1; peer review: 2 approved]. F1000Research, 8(787). doi: 10.12688/f1000research.18293.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmaladze A, Ganguly A, Kuo S, Raghavan M, Kainkaryam R, Cole JH, … Morris MD (2013). Tissue-engineered constructs of human oral mucosa examined by Raman spectroscopy. Tissue Eng Part C Methods, 19(4), 299–306. doi: 10.1089/ten.TEC.2012.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick CJ, Fuchs S, & Unger RE (2011). Co-culture systems for vascularization--learning from nature. Adv Drug Deliv Rev, 63(4–5), 291–299. doi: 10.1016/j.addr.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Kniebs C, Kreimendahl F, Köpf M, Fischer H, Jockenhoevel S, & Thiebes AL (2020). Influence of Different Cell Types and Sources on Pre-Vascularisation in Fibrin and Agarose-Collagen Gels. Organogenesis, 16(1), 14–26. doi: 10.1080/15476278.2019.1697597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesky DB, Homan KA, Skylar-Scott MA, & Lewis JA (2016). Three-dimensional bioprinting of thick vascularized tissues. Proceedings of the National Academy of Sciences, 113(12), 3179–3184. doi: 10.1073/pnas.1521342113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimendahl F, Köpf M, Thiebes AL, Duarte Campos DF, Blaeser A, Schmitz-Rode T, … Fischer H (2017). Three-Dimensional Printing and Angiogenesis: Tailored Agarose-Type I Collagen Blends Comprise Three-Dimensional Printability and Angiogenesis Potential for Tissue-Engineered Substitutes. Tissue Eng Part C Methods, 23(10), 604–615. doi: 10.1089/ten.TEC.2017.0234 [DOI] [PubMed] [Google Scholar]
- Lang I, Pabst MA, Hiden U, Blaschitz A, Dohr G, Hahn T, & Desoye G (2003). Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. European Journal of Cell Biology, 82(4), 163–173. doi: 10.1078/0171-9335-00306 [DOI] [PubMed] [Google Scholar]
- Larsson L, Decker AM, Nibali L, Pilipchuk SP, Berglundh T, & Giannobile WV (2016). Regenerative Medicine for Periodontal and Peri-implant Diseases. J Dent Res, 95(3), 255–266. doi: 10.1177/0022034515618887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kim EH, Shin D, & Roh JL (2017). Accelerated oral wound healing using a pre-vascularized mucosal cell sheet. Sci Rep, 7(1), 10667. doi: 10.1038/s41598-017-10991-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee J, Shin D, & Roh JL (2018). Use of a pre-vascularised oral mucosal cell sheet for promoting cutaneous burn wound healing. Theranostics, 8(20), 5703–5712. doi: 10.7150/thno.28754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew AWL, Zhang, & Yilei. (2017). In vitro pre-vascularization strategies for tissue engineered constructs-Bioprinting and others. International Journal of Bioprinting, 3(1), 008–008. doi: 10.18063/IIB.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WC, Chen S, Zheng L, & Qin L (2017). Angiogenesis Assays for the Evaluation of Angiogenic Properties of Orthopaedic Biomaterials - A General Review. Adv Healthc Mater, 6(5). doi: 10.1002/adhm.201600434 [DOI] [PubMed] [Google Scholar]
- Liu X, Chen W, Zhang C, Thein-Han W, Hu K, Reynolds MA, … Xu HHK (2017). Co-Seeding Human Endothelial Cells with Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells on Calcium Phosphate Scaffold Enhances Osteogenesis and Vascularization in Rats. Tissue Eng Part A, 23(11–12), 546–555. doi: 10.1089/ten.tea.2016.0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chan JK, & Teoh SH (2012). Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J Tissue Eng Regen Med, 9(2), 85–105. doi: 10.1002/term.1617 [DOI] [PubMed] [Google Scholar]
- Lu J, Guan F, Cui F, Sun X, Zhao L, Wang Y, & Wang X (2019). Enhanced angiogenesis by the hyaluronic acid hydrogels immobilized with a VEGF mimetic peptide in a traumatic brain injury model in rats. Regenerative Biomaterials, 6(6), 325–334. doi: 10.1093/rb/rbz027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson-Meyers DS, Andrade TAM, Caetano GF, Guimaraes FR, Leite MN, Leite SN, & Frade MAC (2020). Experimental models and methods for cutaneous wound healing assessment. International Journal of Experimental Pathology, 101(1–2), 21–37. doi: 10.1111/iep.12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrullo V, Cathery W, Velliou E, Madeddu P, & Campagnolo P (2020). Angiogenesis in Tissue Engineering: As Nature Intended? Frontiers in Bioengineering and Biotechnology, 8(188). doi: 10.3389/fbioe.2020.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S, Ko IK, & Yoo JJ (2019). State-of-the-Art Strategies for the Vascularization of Three-Dimensional Engineered Organs. Vasc Specialist Int, 35(2), 77–89. doi: 10.5758/vsi.2019.35.2.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor AJ, & Coulombe KLK (2020). Engineering a collagen matrix for cell-instructive regenerative angiogenesis. J Biomed Mater Res B Appl Biomater, 108(6), 2407–2416. doi: 10.1002/jbm.b.34573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin KT, & Tranquillo RT (2013). In vitro models of angiogenesis and vasculogenesis in fibrin gel. Exp Cell Res, 319(16), 2409–2417. doi: 10.1016/j.yexcr.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Annabi N, Nikkhah M, Bae H, Binan L, Park S, … Khademhosseini A (2012). Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng Part B Rev, 18(5), 363–382. doi: 10.1089/ten.TEB.2012.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K, Akagi T, Iwai S, & Akashi M (2019). Construction of Vascularized Oral Mucosa Equivalents Using a Layer-by-Layer Cell Coating Technology. Tissue Eng Part C Methods, 25(5), 262–275. doi: 10.1089/ten.TEC.2018.0337 [DOI] [PubMed] [Google Scholar]
- Novosel EC, Kleinhans C, & Kluger PJ (2011). Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev, 63(4–5), 300–311. doi: 10.1016/j.addr.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Omidi M, Almeida L, & Tayebi L (2020). Microfluidic-assisted fabrication of reverse micelle/PLGA hybrid microspheres for sustained vascular endothelial growth factor delivery. Biotechnol Appl Biochem. doi: 10.1002/bab.1971 [DOI] [PubMed] [Google Scholar]
- Oryan A, & Sahvieh S (2017). Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int J Biol Macromol, 104(Pt A), 1003–1011. doi: 10.1016/j.ijbiomac.2017.06.124 [DOI] [PubMed] [Google Scholar]
- Park KM, & Gerecht S (2014). Hypoxia-inducible hydrogels. Nat Commun, 5, 4075. doi: 10.1038/ncomms5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Amodio S, Tra WM, Rakhorst HA, Hovius SE, & van Neck JW (2011). Hypoxia preconditioning of tissue-engineered mucosa enhances its angiogenic capacity in vitro. Tissue Eng Part A, 17(11–12), 1583–1593. doi: 10.1089/ten.TEA.2010.0429 [DOI] [PubMed] [Google Scholar]
- Politis C, Schoenaers J, Jacobs R, & Agbaje JO (2016). Wound Healing Problems in the Mouth. Frontiers in Physiology, 7(507). doi: 10.3389/fphys.2016.00507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers T, Horvath JM, van Blitterswijk CA, & LaPointe VLS (2019). Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization. Journal of Tissue Engineering and Regenerative Medicine, 13(10), 1815–1829. doi: 10.1002/term.2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L, Ma D, Liu B, Li J, Chen J, Yang D, & Gao P (2014). Preparation of three-dimensional vascularized MSC cell sheet constructs for tissue regeneration. Biomed Res Int, 2014, 301279. doi: 10.1155/2014/301279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger S, Zhao H, Martin P, Abe K, & Lisse TS (2015). The role of nuclear hormone receptors in cutaneous wound repair. Cell Biochem Funct, 33(1), 1–13. doi: 10.1002/cbf.3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizwan M, Yahya R, Hassan A, Yar M, Abd Halim AA, Rageh Al-Maleki A, … Zubairi W (2019). Novel chitosan derivative based composite scaffolds with enhanced angiogenesis; potential candidates for healing chronic non-healing wounds. J Mater Sci Mater Med, 30(6), 72. doi: 10.1007/s10856-019-6273-3 [DOI] [PubMed] [Google Scholar]
- Rouwkema J, & Khademhosseini A (2016). Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol, 34(9), 733–745. doi: 10.1016/j.tibtech.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Saberianpour S, Heidarzadeh M, Geranmayeh MH, Hosseinkhani H, Rahbarghazi R, & Nouri M (2018). Tissue engineering strategies for the induction of angiogenesis using biomaterials. J Biol Eng, 12, 36. doi: 10.1186/s13036-018-0133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi V, Mittermayr R, Hartinger J, Martino MM, Lorentz KM, Wolbank S, … Banfi A (2014). Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proceedings of the National Academy of Sciences, 111(19), 6952–6957. doi: 10.1073/pnas.1404605111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samal J, Weinandy S, Weinandy A, Helmedag M, Rongen L, Hermanns-Sachweh B, … Jockenhoevel S (2015). Co-Culture of Human Endothelial Cells and Foreskin Fibroblasts on 3D Silk-Fibrin Scaffolds Supports Vascularization. Macromol Biosci, 15(10), 1433–1446. doi: 10.1002/mabi.201500054 [DOI] [PubMed] [Google Scholar]
- Sarker M, Chen XB, & Schreyer DJ (2015). Experimental approaches to vascularisation within tissue engineering constructs. J Biomater Sci Polym Ed, 26(12), 683–734. doi: 10.1080/09205063.2015.1059018 [DOI] [PubMed] [Google Scholar]
- Sculean A, Gruber R, & Bosshardt DD (2014). Soft tissue wound healing around teeth and dental implants. J Clin Periodontol, 41 Suppl 15, S6–22. doi: 10.1111/jcpe.12206 [DOI] [PubMed] [Google Scholar]
- Shah R, Domah F, Shah N, & Domah J (2020). Surgical Wound Healing in the Oral Cavity: a Review. Dental Update, 47(2), 135–143. doi: 10.12968/denu.2020.47.2.135 [DOI] [Google Scholar]
- Sharma D, Ross D, Wang G, Jia W, Kirkpatrick SJ, & Zhao F (2019). Upgrading prevascularization in tissue engineering: A review of strategies for promoting highly organized microvascular network formation. Acta Biomater, 95, 112–130. doi: 10.1016/j.actbio.2019.03.016 [DOI] [PubMed] [Google Scholar]
- Shen C, Li Y, Wang H, & Meng Q (2017). Mechanically strong interpenetrating network hydrogels for differential cellular adhesion. RSC Advances, 7(29), 18046–18053. doi: 10.1039/C7RA01271C [DOI] [Google Scholar]
- Shi B, Andrukhov O, Berner S, Schedle A, & Rausch-Fan X (2014). The angiogenic behaviors of human umbilical vein endothelial cells (HUVEC) in co-culture with osteoblast-like cells (MG-63) on different titanium surfaces. Dent Mater, 30(8), 839–847. doi: 10.1016/j.dental.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Simons M, Alitalo K, Annex BH, Augustin HG, Beam C, Berk BC, … Dimmeler S (2015). State-of-the-Art Methods for Evaluation of Angiogenesis and Tissue Vascularization: A Scientific Statement From the American Heart Association. Circ Res, 116(11), e99–132. doi: 10.1161/res.0000000000000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Kato H, Kawakami T, Kodama Y, Shiozawa M, Kuwae H, … Izumi K (2020). Development of microstructured fish scale collagen scaffolds to manufacture a tissue-engineered oral mucosa equivalent. J Biomater Sci Polym Ed, 31(5), 578–600. doi: 10.1080/09205063.2019.1706147 [DOI] [PubMed] [Google Scholar]
- Takei T, Sakai S, & Yoshida M (2016). In vitro formation of vascular-like networks using hydrogels. J Biosci Bioeng, 122(5), 519–527. doi: 10.1016/j.jbiosc.2016.03.023 [DOI] [PubMed] [Google Scholar]
- Tien J (2019). Tissue Engineering of the Microvasculature. Compr Physiol, 9(3), 1155–1212. doi: 10.1002/cphy.c180037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasina C, Bodet T, Mota C, Moroni L, & Camarero-Espinosa S (2019). Bioprinting Vasculature: Materials, Cells and Emergent Techniques. Materials (Basel), 12(17). doi: 10.3390/ma12172701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres AL, Bidarra SJ, Pinto MT, Aguiar PC, Silva EA, & Barrias CC (2018). Guiding morphogenesis in cell-instructive microgels for therapeutic angiogenesis. Biomaterials, 154, 34–47. doi: 10.1016/j.biomaterials.2017.10.051 [DOI] [PubMed] [Google Scholar]
- Torres AL, Bidarra SJ, Vasconcelos DP, Barbosa JN, Silva EA, Nascimento DS, & Barrias CC (2020). Microvascular engineering: Dynamic changes in microgel-entrapped vascular cells correlates with higher vasculogenic/angiogenic potential. Biomaterials, 228, 119554. doi: 10.1016/j.biomaterials.2019.119554 [DOI] [PubMed] [Google Scholar]
- Um Min Allah N, Berahim Z, Ahmad A, & Kannan TP (2017). Biological Interaction Between Human Gingival Fibroblasts and Vascular Endothelial Cells for Angiogenesis: A Co-culture Perspective. Tissue Eng Regen Med, 14(5), 495–505. doi: 10.1007/s13770-017-0065-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterleuthner D, Kramer N, Pudelko K, Burian A, Hengstschläger M, & Dolznig H (2017). An Optimized 3D Coculture Assay for Preclinical Testing of Pro- and Antiangiogenic Drugs. SLAS Discov, 22(5), 602–613. doi: 10.1177/2472555216686529 [DOI] [PubMed] [Google Scholar]
- Wan X, Bovornchutichai P, Cui Z, O’Neill E, & Ye H (2017). Morphological analysis of human umbilical vein endothelial cells co-cultured with ovarian cancer cells in 3D: An oncogenic angiogenesis assay. PLoS One, 12(7), e0180296. doi: 10.1371/journal.pone.0180296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, & Xu J (2020). Effects of Topical Insulin on Wound Healing: A Review of Animal and Human Evidences. Diabetes Metab Syndr Obes, 13, 719–727. doi: 10.2147/dmso.S237294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand A, Beier JP, Arkudas A, Al-Abboodi M, Polykandriotis E, Horch RE, & Boos AM (2016). The Arteriovenous (AV) Loop in a Small Animal Model to Study Angiogenesis and Vascularized Tissue Engineering. J Vis Exp(117). doi: 10.3791/54676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinandy S, Laffar S, Unger RE, Flanagan TC, Loesel R, Kirkpatrick CJ, … Jockenhoevel S (2014). Biofunctionalized microfiber-assisted formation of intrinsic three-dimensional capillary-like structures. Tissue Eng Part A, 20(13–14), 1858–1869. doi: 10.1089/ten.TEA.2013.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Ivan Lam CR, Wen F, Mark Chong SK, Tan NS, Jerry C, … Tan LP (2016). Novel method to improve vascularization of tissue engineered constructs with biodegradable fibers. Biofabrication, 8(1), 015004. doi: 10.1088/1758-5090/8/1/015004 [DOI] [PubMed] [Google Scholar]
- Xiao X, Wang W, Liu D, Zhang H, Gao P, Geng L, … Wang Z (2015). The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci Rep, 5, 9409. doi: 10.1038/srep09409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Zheng W, Guan L, Ai Y, & Liang Q (2020). Engineering of Hydrogel Materials with Perfusable Microchannels for Building Vascularized Tissues. Small, 16(15), e1902838. doi: 10.1002/smll.201902838 [DOI] [PubMed] [Google Scholar]
- Yang G, Mahadik B, Choi JY, & Fisher JP (2020). Vascularization in tissue engineering: fundamentals and state-of-art. Progress in Biomedical Engineering, 2(1), 012002. doi: 10.1088/2516-1091/ab5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yang S, Zhang Y, Sun Z, Xu W, & Ye S (2012). Co-culture of mesenchymal stem cells with umbilical vein endothelial cells under hypoxic condition. J Huazhong Univ Sci Technolog Med Sci, 32(2), 173–180. doi: 10.1007/s11596-012-0031-9 [DOI] [PubMed] [Google Scholar]
- Zucchelli E, Majid QA, & Foldes G (2019). New artery of knowledge: 3D models of angiogenesis. Vasc Biol, 1(1), H135–h143. doi: 10.1530/vb-19-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]