Abstract
Introduction:
Menthol cigarettes were banned in Ontario, Canada on January 1st, 2017. We used concept mapping, a mixed-method approach, to describe how menthol cigarette smokers quit smoking after the Ontario menthol ban.
Methods:
Pre-ban daily and non-daily menthol cigarette smokers who reported smoking abstinence 24 months after the ban (n=62; 53.2% women; mean age=43.6, SD=12.5) generated statements describing reasons and strategies for smoking cessation/reduction after the menthol ban. Participants sorted a final list of 57 statements into groups of similar content and rated statements on how true each statement was for them and multidimensional scaling analysis identified thematic clusters.
Results:
Six clusters were identified: Mental and Environment Changes, Direct Ban Impacts, Health Reasons, Cues to Action, Family and Friends, and Cessation Strategies. The highest rated statements (i.e., most true) suggested many participants were motivated to quit smoking before or after the ban and 30.7% of participants believed the menthol ban helped with smoking cessation. Some of the lowest rated statements included using nicotine replacement therapy products, medication (i.e., Champix), or other tobacco products suggesting these strategies were less common. Statement ratings suggested many smokers quit without using replacement products or medication, but modifying cognitions and avoiding smoking cues were common.
Conclusions:
The menthol ban aided some menthol smokers to quit, while others reported the ban did not play a role in smoking cessation. These data suggest the menthol ban had direct and indirect effects on smoking reduction behavior. Campaigns supporting similar bans should target both types of effects.
Keywords: Menthol cigarette ban, smoking cessation, tobacco regulatory science, policy
Introduction
Tobacco use continues to be the leading cause of preventable death worldwide, with an estimated 8 million deaths attributed to tobacco use annually (World Health Organization, 2020). The vast majority of tobacco-related deaths are caused by cigarette smoking, thus, great effort has been placed on cigarette smoking prevention either through preventing cigarette smoking initiation or promoting smoking cessation. One factor that has been shown through multiple evaluations (Food and Drug Administration, 2013; TPSAC, 2011; Villanti, Collins, et al., 2017) to increase the negative impact of cigarette smoking on public health is menthol. Menthol, a flavoring that can decrease irritation (Ferris Wayne & Connolly, 2004) and produce cooling and soothing sensory effects (Ferris Wayne & Connolly, 2004; Garten & Falkner, 2004), has been shown to increase the likelihood of cigarette smoking initiation (Klausner, 2011; Lee & Glantz, 2011; Villanti et al., 2019; Villanti, Johnson, et al., 2017; Yerger, 2011) and is associated with decreased likelihood of smoking cessation among adult smokers (Levy et al., 2011; Transdisciplinary Tobacco Use Research Center (TTURC) Tobacco Dependence et al., 2007), particularly among African-American/Black smokers (P. H. Smith et al., 2020; S. S. Smith et al., 2014). This has led the World Health Organization (WHO) and U.S. Food and Drug Administration (FDA) to conclude that banning menthol from the market would have a public health benefit (Food and Drug Administration, 2013; WHO Study Group on Tobacco Product Regulation (TobReg), n.d.).
Though data supporting recommendations to ban menthol in cigarettes have existed for many years, menthol cigarettes are still widely available around the world. However, some countries have begun implementing policies banning menthol flavoring in cigarettes including Brazil, Ethiopia, Turkey, and European Union (Erinoso et al., 2020), and some states within the United States have also implemented menthol bans such as Massachusetts, New Jersey, New York, Rhode Island, as well as over 270 localities (Campaign for Tobacco-Free Kids, 2020). While the sale of menthol cigarettes is now banned across all of Canada, the province of Ontario was one of the first provinces to prohibit the sale of cigarettes that contain menthol flavoring as an additive. This ban went into effect on January 1st, 2017 (Cork & Tobacco Control Legal Consortium, 2017).
To inform menthol ban policies in other jurisdictions, there is need to evaluate the effects of these bans on smoking and related behaviors. Previous evaluations of hypothetical menthol bans have shown that approximately one to two thirds of menthol smokers would plan to quit smoking in response to a menthol ban (D’Silva et al., 2015; O’Connor et al., 2012; Wackowski et al., 2014). This is consistent with sales data that demonstrate decrease in total cigarette sales and menthol cigarette sales after menthol bans (M. Chaiton et al., 2019). One concern of menthol ban policies is the potential for unintended consequences that may result from limiting the availability of menthol cigarettes, such as switching to other harmful tobacco products or the increase in a “black market” for menthol cigarettes. However, evaluations of the menthol ban in Ontario found that menthol smokers had increased quit attempts and quit success compared to non-menthol cigarette smokers (Chaiton et al., 2018; Chaiton et al., 2020) and limited purchasing of “black market” menthol cigarettes or switching to other products (Soule et al., 2019).
However, not all pre-ban menthol cigarette smokers in Ontario quit smoking after the menthol ban was implemented. Indeed, while 24% of pre-menthol ban daily menthol smokers and 20% of pre-menthol ban non-daily smokers reported having quit one year after implementation of the Ontario menthol ban (Chaiton et al., 2020), some smokers switched to non-menthol cigarettes, sought out locations to purchase menthol cigarettes, and some switched to other menthol tobacco products such as cigars (Soule et al., 2019). Regulators and policy makers interested in optimizing the effectiveness of menthol ban policies would likely benefit from understanding how and why some menthol cigarette smokers were able to quit smoking after a menthol ban. Therefore, the purpose of this study was to characterize and describe cigarette smoking cessation strategies and experiences among menthol cigarette smokers who reported quitting smoking successfully after the implementation of the menthol cigarette ban.
Methods
This study was approved by the [IRB identifiers removed for review] and the [IRB identifiers removed for review]. Participants were recruited from an ongoing longitudinal study of menthol cigarette smokers in Ontario, Canada (Chaiton et al., 2016, 2018; Chaiton et al., 2020). This study used concept mapping (Trochim, 1989), a validated mixed-methods approach that incorporates participant tasks (brainstorming, sorting, and rating) and quantitative analysis to generate a model describing themes related to a research topic. Participants over the age of 18 who reported menthol cigarette smoking (daily or non-daily) before the menthol ban implementation and reported smoking abstinence in 2019 during a follow-up survey were invited to complete the current study. One hundred twenty eligible individuals were invited to complete the current study. At a study website, participants (n=62) first completed a brief questionnaire examining tobacco use behaviors before and after the implementation of the menthol ban and demographic characteristics. Of these participants, 54.8% were identified in the longitudinal study regular menthol smokers (i.e. daily) and 45.2% were occasional menthol (i.e., non-daily) smokers before the menthol ban was implemented.
Participants then completed the brainstorming task by providing statements that completed the prompt: “After the menthol cigarette ban, a specific way I quit/reduced my cigarette smoking, a specific reason I was able to quit/reduce my cigarette smoking, or something that helped me quit/reduce my cigarette smoking was…” Participants completed the task individually, but could see all statements displayed in a list that other participants provided previously. This approach prevents participants from having to wait one’s turn to submit a response as participants can complete the task simultaneously rather than having to allow others to speak, as in a focus group setting. This approach can also generate more (Dennis & Valacich, 1993; Dennis & Williams, 2003; DeRosa et al., 2007) unique (Dugosh et al., 2000; Karen Leggett Dugosh & Paulus, 2005) ideas because after reviewing others’ statements, participants are asked to avoid providing duplicate content. Conversely, when multiple interviews or focus groups are conducted independently, participants do have the opportunity to hear responses from other participants and thus are more likely to duplicate content. After researchers determined statement content reached saturation (i.e., statements provided by additional participants no longer yielded unique content), the brainstorming task was closed. Three researchers reviewed the statements to identify redundant content (e.g., “Exercising” and “Getting more exercise”) and content unrelated to the prompt (e.g., “Ez pz lemon squeezy”). Statements that two or more reviewers identified as being unrelated to the prompt or redundant were removed. For redundant statements, the statement that best captured the idea with the fewest words was retained and all other redundant statements were removed. After review, statements were edited for spelling, grammar, and clarity if necessary and 57 final statements remained after review.
Participants were invited back to the study website to complete the sorting task. For the sorting task, participants grouped statements into “piles” with the following instructions: all piles must be related to a single idea based on content similarity, there could not be an “other/miscellaneous” pile, and there could not be a single pile with all statements. After completing sorting, participants also rated each statement based on the prompt, “This is a specific reason or way I was able to quit or reduce my cigarette smoking after the menthol cigarette ban,” using the scale “1 – Definitely NOT true for me” to “7 – Definitely true for me.” Thirty-eight participants completed sorting and 45 completed rating activities. Previous research shows that this number of sorts is sufficient for generating good model fit (Rosas & Kane, 2012).
Non-metric multidimensional scaling (MDS) was used to generate a “point map” from participant data. Using an algorithm (Kruskal & Wish, 1978), concept mapping software assigned each statement a coordinate in two-dimensional space (x,y) based on aggregated sorting data such that points on the map that were closer together represented statements that were sorted together in piles by more participants and thus represented similar content. The stress value of the MDS analysis was 0.20, consistent with previous concept mapping studies indicating good model fit (Rosas & Kane, 2012).
“Clusters” of statements were identified empirically using an algorithm (Ward, 1963) that identified groups of statements on the point map that limited the distance between all points in the group and the centroid of the identified group of points on the point map. Using a hierarchical cluster analysis, a two-cluster model was examined first. Subsequent models were built from this two-cluster model by separating one cluster into two separate clusters (e.g., “cluster 1” remaining and “cluster 2” divided into two separate clusters). This process was repeated until a final model was achieved. Good model fit for the hierarchical cluster analysis was determined qualitatively through group discussion using parsimony (i.e. fewer clusters preferred) and interpretability (i.e., clusters only described a single theme) as indicators of good model fit. Mean cluster ratings were calculated by taking the average of ratings from all participants for all statements within a single cluster. Mean cluster ratings were compared between sample subgroups including demographic characteristics and frequency of menthol smoking pre-ban, however, all tests were non-significant and thus are not presented.
Results
Participant characteristics
Participant characteristics are displayed in Table 1. Briefly, participants (n=62) were on average 43.6 years old (SD=12.5), close to half were women (53.2%), around two-thirds (66.1%) were White, 14.5% identified as more than race/multiple cultural backgrounds, and 6.5% were Asian. Over one-third (38.7%) had completed a four-year college degree or higher. Participants reported an average of 7.5 years (SD=9.9) smoking menthol cigarettes. In the time since the follow-up screening survey to completing the current study, some participants had likely slipped or relapsed to smoking as 72.6% reported not currently smoking cigarettes, but 17.4% reported smoking cigarettes rarely (16.1%), some days (1.6%), or every day (9.7%). Current other tobacco use was reported by some participants, with the greatest number of participants reporting current electronic cigarette (ECIG) use either rarely, some days, or every day (24.2%). Approximately one-third (30.7%) perceived that the menthol ban helped them with smoking cessation.
Table 1.
Sample demographics and tobacco use characteristics (n=62).
| Characteristic | N | % |
|---|---|---|
| Age (M, SD) | 43.6 (12.5) | |
| Gender | ||
| Women | 33 | 53.2 |
| Men | 29 | 46.8 |
| Transgender or other | 0 | 0 |
| Ethnicity | ||
| Hispanic/Latino(a) | 0 | 0 |
| Race | ||
| Aboriginal | 1 | 1.6 |
| Asian | 4 | 6.5 |
| Arab | 1 | 1.6 |
| Black/African American | 1 | 1.6 |
| White/European American | 41 | 66.1 |
| More than one race | 9 | 14.5 |
| Other | 5 | 8.1 |
| Education | ||
| Some elementary or some high school | 4 | 6.5 |
| Completed high school | 5 | 8.1 |
| Some community or technical college | 5 | 8.1 |
| Completed community or technical college | 17 | 27.4 |
| Some university | 7 | 11.3 |
| Completed university or higher | 24 | 38.7 |
| Currently smoke cigarettes | ||
| Every day | 6 | 9.7 |
| Some days | 1 | 1.6 |
| Rarely | 10 | 16.1 |
| Not at all | 45 | 72.6 |
| Did not respond | 0 | 0 |
| Currently smoke menthol cigarettes | ||
| Every day | 0 | 0 |
| Some days | 1 | 1.6 |
| Rarely | 5 | 8.1 |
| Not at all | 56 | 90.3 |
| Current other tobacco products (every day or somedays) | ||
| Electronic cigarettes | 11 | 17.7 |
| Cigar | 0 | 0 |
| Cigarillo or little cigar | 0 | 0 |
| Smokeless | 1 | 1.6 |
| Waterpipe | l | 1.6 |
| Number of times stopped smoking in past year | ||
| 0 times | 19 | 30.7 |
| 1 time | 15 | 24.2 |
| 2–3 times | 15 | 24.2 |
| 4 or more times | 12 | 19.4 |
| Felt menthol ban helped with smoking cessation | ||
| Yes | 19 | 30.7 |
| No | 42 | 67.7 |
| Electronic cigarette use before menthol ban | ||
| Every Day | 4 | 6.5 |
| Some Days | 5 | 8.1 |
| Rarely | 14 | 22.6 |
| Not at all | 39 | 63.0 |
| Cigar use before menthol ban | ||
| Every Day | 0 | 0 |
| Some Days | 1 | 1.6 |
| Rarely | 14 | 22.6 |
| Not at all | 47 | 75.8 |
| Little cigar/cigarillo use before menthol ban | ||
| Every Day | 0 | 0 |
| Some Days | 6 | 9.7 |
| Rarely | 12 | 19.4 |
| Not at all | 44 | 75.8 |
| Smokeless tobacco use before menthol ban | ||
| Every Day | 0 | 0 |
| Some Days | 1 | 1.6 |
| Rarely | 2 | 3.2 |
| Not at all | 59 | 95.2 |
| Hookah or waterpipe use before menthol ban | ||
| Every Day | 0 | 0 |
| Some Days | 2 | 3.2 |
| Rarely | 9 | 14.5 |
| Not at all | 51 | 82.3 |
Concept mapping results
MDS and hierarchical cluster analysis resulted in cluster map with six thematic clusters (see Table 2 and Figure 1) and are presented from highest to lowest mean cluster rating. The first cluster was the Changes to Support Cessation cluster. This cluster had 10 statements and a mean cluster rating of 4.33 (SD=1.15). The statements in this cluster described changes in cognitions, commitments, or attitudes that promoted smoking cessation. These included the second highest rated statement overall (“I decided I did not want to be a smoker any longer”, M=6.07) as well as statements relating to having “a lot of will power,” or quitting “cold turkey.” Other statements described purposeful actions by participants to avoid being around cigarettes or cigarette smoke such as “I stopped buying cigarettes” (M=5.66) or “I avoided or reduced any association I had with cigarette smoking” (M=4.89).
Table 2.
Clusters and statements describing how and why participant quit or reduced smoking after implementation the menthol ban in Ontario, Canada.
| Cluster Statement | Average Rating |
|---|---|
| Changes to Support Cessation | 4.32 |
| 30. I decided I did not want to be a smoker any longer. | 6.07 |
| 26. I stopped buying cigarettes. | 5.66 |
| 31. I avoided or reduced any association I had with cigarette smoking. | 4.89 |
| 21. I had a lot of will power. | 4.24 |
| 5. Not smoking inside. | 4.16 |
| 2. I quit cold turkey. | 3.89 |
| 16. I avoided smokers and all exposure to any smoking. | 3.52 |
| 38. I convinced myself non-menthol cigarettes were worse, so I should stop smoking. | 2.18 |
| Direct Ban Impacts | 3.98 |
| 1. I had already wanted to, the menthol ban had no effect on me quitting or not. | 5.36 |
| 36. Menthol cigarette ban did not affect my quitting smoking. | 4.51 |
| 33. The menthol ban did not affect me in my effort to quit because I rarely smoked menthol. | 4.09 |
| 25. I had already wanted to quit, but the menthol ban helped. | 3.56 |
| 34. I decided it was a good time now that the cigarettes I enjoyed were unavailable. | 3.22 |
| 50. The taste of smoking was worse after menthol was gone. | 3.13 |
| Health Impacts | 3.96 |
| 43. I wanted to be healthier. | 6.18 |
| 12. Smoking was ruining my health. | 5.49 |
| 47. I was worried about my health. | 5.44 |
| 29. I decided to eat healthier. | 4.27 |
| 6. I was constantly coughing. | 4.14 |
| 39. I was sick of not being able to breathe. | 3.95 |
| 49. I had sore lungs in the morning. | 3.42 |
| 17. Smoking made my skin sag and wrinkle. | 2.78 |
| 8. I got sick with a cold/the flu so I didn’t smoke at all. When I recovered from the cold/flu, my desire to smoke was gone. | 2.09 |
| 24. I had a major health event/scare, such as a heart attack or stroke. | 1.84 |
| Cues to Action | 3.83 |
| 13. I didn’t want to waste more money on cigarettes. | 5.69 |
| 20. I was sick of smelling bad. | 4.96 |
| 45. Smoking no longer appealed to me. | 4.67 |
| 18. I saw the long term effects of smoking on someone close to me and I didn’t want that for myself. | 3.98 |
| 51. I was advised to quit by my doctor. | 3.2 |
| 44. Someone close to me had major health event/scare from cigarette smoking. | 3.09 |
| 10. Pregnancy (my own pregnancy or my partner was pregnant). | 1.27 |
| Family and Friends | 3.57 |
| 19. I wanted to enjoy my family and friends. | 4.76 |
| 11. I had pressure from friends and family to quit. | 3.71 |
| 15. I saw how much it hurt my partner/significant other. | 3.71 |
| 22. I made an agreement to with my significant other/partner to quit smoking. | 3.31 |
| 9. My significant other/partner also quit. | 2.36 |
| Cessation Strategies | 2.82 |
| 27. I tried to distract myself and keep myself busy if I got a craving to smoke. | 4.91 |
| 42. Every time I had a craving I would try and get my mind on something else till the craving went away. | 4.76 |
| 3. I drank lots of water. | 4.47 |
| 53. I started exercising more. | 4.22 |
| 55. I changed habits of when and where I would “light up” such as doing something else instead of going out for a cigarette break. | 4.11 |
| 35. I used the nicotine patch. | 3.07 |
| 37. For a short period of time, vaping helped me gradually reduce my smoking. | 2.76 |
| 41. I ate food or candy when I had a craving to smoke. | 2.71 |
| 4. I started vaping. | 2.71 |
| 28. Using a vape helped with the physical act of smoking that I enjoyed while reducing the dependence on nicotine. | 2.6 |
| 32. I got quit aids from my family doctor’s office. | 2.6 |
| 56. I participated in a community quit smoking program. | 2.4 |
| 7. I read/listened to “The Easy Way” by Allen Carr. | 2.31 |
| 40. I played video games. | 2.23 |
| 23. I stopped drinking heavily on the weekends. | 2.19 |
| 54. I started smoking weed. | 2.11 |
| 52. I limited coffee intake as I would want to smoke with coffee. | 2.09 |
| 57. Wellbutrin helped with smoking cessation. | 2.09 |
| 14. I used Champix to help me quit. | 1.98 |
| 48. I used menthol flavoured nicotine spray. | 1.53 |
| 46. I used the nicotine lozenge. | 1.4 |
Note. Mean ratings are based on responses to the prompt “After menthol cigarettes were no longer sold in Ontario, this is something that I did, something that influenced me, or a reason I chose the cigarettes I smoke now” using a 7-point scale from 1 (Definitely NOT true for me) to 7 (Definitely true for me).
Figure 1.
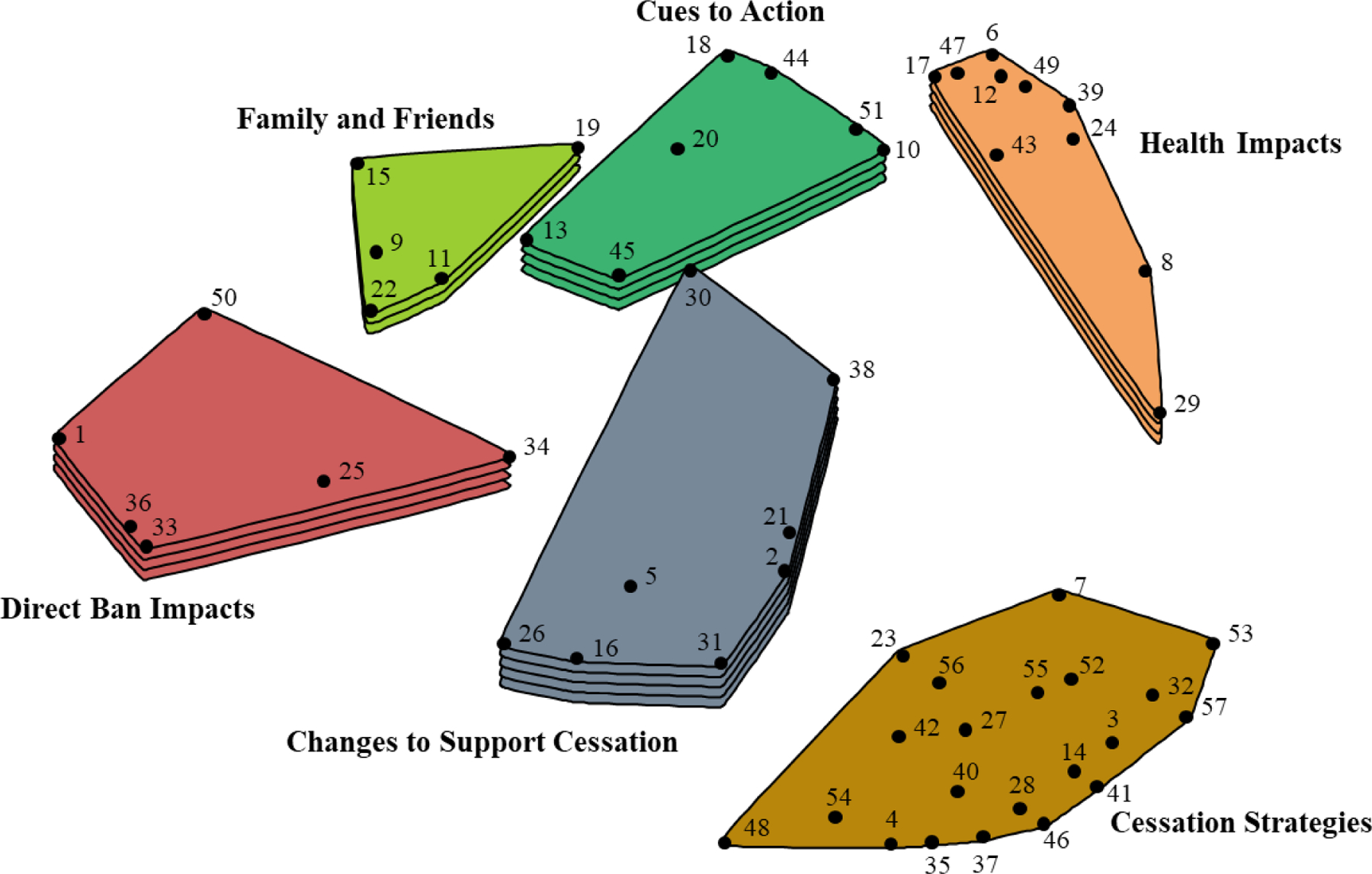
Concept map displaying 6 clusters of user identified statements describing how and why some pre-menthol ban smokers perceived they were able to quit smoking cigarettes after the implementation of a menthol ban in Ontario, Canada. Numbered points on the map that are closer to one another represent statements of more similar content whereas points on the map that are further apart represent statements of less similar content. Greater number of layers in clusters indicate higher mean ratings of statements within each cluster based on the rating task. Mean ratings for clusters with 1 layer range from 2.82 to 3.12, 2 layers from 3.13 to 3.42, 3 layers from 3.43 to 3.72, 4 layers from 3.73 to 4.02, and 5 layers from 4.03 to 4.32.
The next cluster was the Direct Ban Impacts cluster (n=6, M=3.98, SD=0.78). The statements in this cluster referenced the ban specifically regarding smoking cessation outcomes. Some statements suggested that the ban did not play a role in smoking cessation, such as “I had already wanted to [quit], the menthol ban had no effect on me quitting or not” (M=5.36) and “Menthol cigarette ban did not affect my quitting smoking” (M=4.51). Other statements suggested the ban played a supported role in smoking cessation efforts: “I had already wanted to quit, but the menthol ban helped” (M=3.56) and “I decided it was a good time now that the cigarettes I enjoyed were unavailable” (M=3.22).
The Health Impacts cluster (n=10, M=3.98, SD=1.39) included statements describing how health concerns were the driving factor for smoking cessation success. Some statements centered around concerns of how smoking was affecting participants’ health including “Smoking was ruining my health” (M=5.49), with some statements identifying specific symptoms associated with smoking including coughing, sore lungs, wrinkled skin, and getting sick. Other statements focused on the desire to be healthier, including the highest rated of all statements, “I wanted to be healthier” (M=6.18).
The Cues to Action cluster (n=7, M=3.83, SD=1.36) included statements describing triggers that stimulated interest in smoking cessation. This cluster appeared unique from the statements in the Direct Impacts of the Ban cluster as statements did not reference the menthol ban specifically. Rather, statements described perceived negative outcomes and lifestyle related triggers associated with smoking that caused interest in smoking cessation. For example, the highest rated statement in the cluster described not wanting “…to waste more money on cigarettes” (M=5.69). Other statements described triggers to quit related to not wanting to smell bad, being advised to quit from a doctor, having a family member/friend get sick or have a health scare, or pregnancy.
The Family and Friends cluster (n=5, M=3.57, SD=0.77) had the fewest statements of all clusters. The statements described various ways in which family and friends played a role in smoking cessation. The highest rated statement related to participants personal desires for closeness (“I wanted to enjoy family and friends,” M=4.76). Other statements described ways in which family/friends were supportive in cessation efforts (e.g., “I made an agreement with my significant other/partner to quit smoking,” M=3.31). Other content indicated encouragement from family and friends that was not perceived as positively (“I had pressure from friends and family to quit,” M=3.71).
The final cluster with the most statements was the Cessation Strategies cluster (n=21, M=2.82, SD=1.02). The statements within this cluster described a wide variety of behavioral approaches participants used in support of smoking cessation. Some strategies focused on distraction from cravings, such as the highest rated statement in the cluster: “I tried to distract myself and keep myself busy if I got a craving to smoke” (M=4.91). Some statements identified alternative activities to engage in place of smoking including drinking water, exercising more, “changing habits,” or playing video games. Product replacement was described in several statements including engaging in electronic cigarette use (i.e., vaping), eating food or candy, and “smoking weed.” Other statements described using nicotine replacement therapy such as the patch, gum, lozenge, or nicotine spray, as well as using pharmaceuticals for smoking cessation, with many of these statements being some of the lowest rated of all statements.
Discussion
This study identified statements organized into six content themes describing how and why some pre-menthol ban menthol cigarette smokers perceived they were able to quit smoking after the implementation of a menthol ban in Ontario, Canada. Many of the statements described reasons and strategies for smoking cessation that might be expected among individuals who quit smoking without the influence of a menthol ban including health concerns, influence from family and friends, experiencing a cue to action, changing one’s environment or exposure to smoking and smoking cues, as well as using pharmaceutical or behavioral strategies to quit smoking.
Previous evaluations demonstrate that the Ontario menthol ban was associated with increased quit attempts and quit success among pre-ban menthol cigarette smokers relative to non-pre-ban menthol cigarette smokers (Chaiton et al., 2020). However, only 30.7% of the participants in the current study self-reported that the menthol ban helped them quit smoking. That is, while 88.7% reported smoking “not at all” or “rarely”, many did not perceive that the menthol ban played a role in their smoking cessation. Indeed, previous research on the Ontario menthol ban (Soule et al., 2019) as well as other research (Pearson et al., 2012; Rath et al., 2018; Wackowski et al., 2017) indicate menthol bans are often viewed unfavorably by menthol cigarette smokers. Therefore, some menthol smokers who do quit may be hesitant to indicate that the menthol ban had a positive influence on their smoking cessation. Importantly, some statements described that smokers were motivated to quit before the ban, and that the ban either was perceived to help with cessation or not. Indeed, some quitting behaviors that were unrelated to the menthol ban among menthol smokers were to be expected given the lack of education and advertising campaigns that were paired with the menthol ban. Therefore, policy makers and public health professionals may increase the impact of menthol bans by framing the bans positively and trying to increase smokers’ motivation to quit prior to implementation of the ban. While future research is needed to examine the impact of this type of framing and promotion, results from the current study suggest cigarette smokers who are already motivated to quit smoking may view a ban as an opportunity to quit, and others do quit even though they do not attribute their quit attempt to the ban.
Public health campaigns running concurrently when a menthol ban is implemented that support the use of evidence-based practices for smoking cessation may further increase the effectiveness of menthol ban in other jurisdictions. Indeed, tobacco control programs that include mass media campaigns may be more effective in reducing smoking behavior (Bala et al., 2017). Public Health Service reports (“A Clinical Practice Guideline for Treating Tobacco Use and Dependence,” 2008) and the Surgeon General Report on Smoking Cessation (U.S. Department of Health and Human Services, 2020) concluded behavioral counseling and cessation medications, such as varenicline or nicotine replacement therapy (i.e., nicotine patch, gum, or lozenge), increase smoking cessation compared to no treatment or self-help approaches, especially when used in combination. However, statements describing using nicotine replacement therapy or medications were rated low by participants suggesting few participants were using these methods to quit smoking. Without additional social marketing or health promotion campaigns to support smoking cessation efforts, a menthol ban may promote some menthol smokers to seek alternative sources for purchasing menthol cigarettes as was seen after a flavored tobacco ban in San Francisco (Yang et al., 2020), or switch to non-menthol cigarettes (Cadham et al., 2020). This may have been the case for some participants in the current study, given that some participants reported multiple quit attempts after the menthol ban had been implemented. Additionally, while one statement described participating in a “community quit smoking program,” no other statements described any type of behavioral counseling. Instead, numerous statements described various self-help approaches, such as electronic cigarette use (U.S. Department of Health and Human Services, 2020). A concern of the increase in popularity of electronic cigarettes as a self-help smoking cessation aid (Gravely et al., 2021) despite limited evidence of effectiveness (The National Academies of Sciences Engineering Medicine, 2018) is that smokers may be at greater risk for dual use of cigarette and electronic cigarettes. Similarly, those who try electronic cigarettes for smoking cessation may be less likely to try evidence-based smoking cessation approaches.
This study had several limitations. The menthol smoker population in Canada differs from other jurisdictions, such as the United States (Bird et al., 2017; Villanti, Collins, et al., 2017). Therefore, any generalizations of findings from the current study will need to consider differences in population demographics, health care system, and cultural norms that may affect menthol smoker behaviors after implementation of a menthol ban. While participants in the study were identified as having quit smoking at the screening survey, survey responses indicate that some individuals may have relapsed. Future studies should examine which types of reasons or strategies for smoking cessation are most associated with long term smoking abstinence. The focus prompts used in the current study referenced the ban on menthol cigarettes specifically. Because data were collected approximately two and a half years after menthol ban implementation, participants may have had difficulty recalling quit attempts directly related to the menthol ban. However, the data still represent responses from individuals who lived in a location where menthol cigarette were sold previously but are no longer available. Therefore, they can still be informative to other menthol, flavor, or other products bans implemented in the future.
This study found that menthol bans likely have direct and indirect positive effects on smoking cessation among menthol smokers. Menthol bans may have the greatest impact on smoking cessation among those who are already motivated to quit before the implementation of a menthol ban. However, while previous research shows that menthol bans may increase quit attempts among menthol smokers, many may use strategies that are not considered best practices based on available evidence. Future research should examine if incorporating public health campaigns encouraging smokers to quit using evidence-based practices for smoking cessation impact the effectiveness of menthol bans implemented in other jurisdictions.
The Ontario menthol ban likely influence smoking cessation directly and indirectly.
Being motivated to quit prior to menthol ban likely influenced cessation outcomes.
The use of recommended smoking cessation aids (NRT, counseling) was low.
Education and promotional campaigns supporting menthol bans may increase effectiveness.
Role of Funding
This research was supported by grant number R21DA04735801 from the National Institute on Drug Abuse of the National Institutes of Health under Award Number and the Center for Tobacco Products of the U.S. Food and Drug Administration. E.S. and J. C.’s effort is also supported by grant number U54DA036105 from the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the U.S. Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
E.S. is named on a patent application for a smartphone app that determines electronic cigarette device and liquid characteristics.
References
- A Clinical Practice Guideline for Treating Tobacco Use and Dependence: 2008 Update: A U.S. Public Health Service Report. (2008). American Journal of Preventive Medicine, 35(2), 158–176. 10.1016/j.amepre.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala MM, Strzeszynski L, & Topor-Madry R (2017). Mass media interventions for smoking cessation in adults. The Cochrane Database of Systematic Reviews, 2017(11). 10.1002/14651858.CD004704.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird Y, May J, Nwankwo C, Mahmood R, & Moraros J (2017). Prevalence and characteristics of flavoured tobacco use among students in grades 10 through 12: A national cross-sectional study in Canada, 2012–2013. Tobacco Induced Diseases, 15, 20. 10.1186/s12971-017-0124-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadham CJ, Sanchez-Romero LM, Fleischer NL, Mistry R, Hirschtick JL, Meza R, & Levy DT (2020). The actual and anticipated effects of a menthol cigarette ban: A scoping review. BMC Public Health, 20(1), 1055. 10.1186/s12889-020-09055-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campaign for Tobacco-Free Kids. (2020). STATES & LOCALITIES THAT HAVE RESTRICTED THE SALE OF FLAVORED TOBACCO PRODUCTS. https://www.tobaccofreekids.org/assets/factsheets/0398.pdf
- Chaiton M, Diemert L, Cohen JE, Bondy SJ, Selby P, Philipneri A, & Schwartz R (2016). Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open, 6(6), e011045. 10.1136/bmjopen-2016-011045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiton MO, Nicolau I, Schwartz R, Cohen JE, Soule E, Zhang B, & Eissenberg T (2020). Ban on menthol-flavoured tobacco products predicts cigarette cessation at 1 year: A population cohort study. Tobacco Control, 29(3), 341–347. 10.1136/tobaccocontrol-2018-054841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiton M, Schwartz R, Cohen JE, Soule E, & Eissenberg T (2018). Association of Ontario’s Ban on Menthol Cigarettes With Smoking Behavior 1 Month After Implementation. JAMA Internal Medicine. 10.1001/jamainternmed.2017.8650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiton M, Schwartz R, Shuldiner J, Tremblay G, & Nugent R (2019). Evaluating a Real World Ban on Menthol Cigarettes: An Interrupted Time-Series Analysis of Sales. Nicotine & Tobacco Research, 22(4), 576–579. 10.1093/ntr/ntz041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork K, & Tobacco Control Legal Consortium. (2017). Leading from up north: How Canada is solving the menthol tobacco problem.
- Dennis AR, & Valacich JS (1993). Computer brainstorms: More heads are better than one. Journal of Applied Psychology, 78(4), 531–537. 10.1037/0021-9010.78.4.531 [DOI] [Google Scholar]
- Dennis AR, & Williams ML (2003). Electronic brainstorming: Theory, research, and future directions. In Group creativity: Innovation through collaboration (pp. 160–178). Oxford University Press. 10.1093/acprof:oso/9780195147308.003.0008 [DOI] [Google Scholar]
- DeRosa DM, Smith CL, & Hantula DA (2007). The medium matters: Mining the long-promised merit of group interaction in creative idea generation tasks in a meta-analysis of the electronic group brainstorming literature. Computers in Human Behavior, 23(3), 1549–1581. 10.1016/j.chb.2005.07.003 [DOI] [Google Scholar]
- D’Silva J, Amato MS, & Boyle RG (2015). Quitting and Switching: Menthol Smokers’ Responses to a Menthol Ban. Tobacco Regulatory Science, 1(1), 54–60. 10.18001/TRS.1.1.6 [DOI] [Google Scholar]
- Dugosh KL, & Paulus PB (2005). Cognitive and social comparison processes in brainstorming. Journal of Experimental Social Psychology, 41(3), 313–320. 10.1016/j.jesp.2004.05.009 [DOI] [Google Scholar]
- Dugosh KL, Paulus PB, Roland EJ, & Yang HC (2000). Cognitive stimulation in brainstorming. Journal of Personality and Social Psychology, 79(5), 722–735. 10.1037//0022-3514.79.5.722 [DOI] [PubMed] [Google Scholar]
- Erinoso O, Clegg Smith K, Iacobelli M, Saraf S, Welding K, & Cohen JE (2020). Global review of tobacco product flavour policies. Tobacco Control, tobaccocontrol-2019–055454. 10.1136/tobaccocontrol-2019-055454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris Wayne G, & Connolly GN (2004). Application, function, and effects of menthol in cigarettes: A survey of tobacco industry documents. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 6 Suppl 1, S43–54. 10.1080/14622203310001649513 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. (2013). Preliminary Scientific Evaluation of The Possible Public Health Effects of Menthol Versus Nonmenthol Cigarettes. Center for Tobacco Products, Food and Drug Administration. [Google Scholar]
- Garten S, & Falkner RV (2004). Role of mentholated cigarettes in increased nicotine dependence and greater risk of tobacco-attributable disease. Preventive Medicine, 38(6), 793–798. 10.1016/j.ypmed.2004.01.019 [DOI] [PubMed] [Google Scholar]
- Gravely S, Cummings KM, Hammond D, Borland R, McNeill A, East KA, Loewen R, Martin N, Yong H-H, Li L, Liber A, Levy DT, Quah ACK, Ouimet J, Hitchman SC, Thompson ME, Boudreau C, & Fong GT (2021). Self-reported quit aids and assistance used by smokers at their most recent quit attempt: Findings from the 2020 International Tobacco Control Four Country Smoking and Vaping Survey. Nicotine & Tobacco Research, ntab068. 10.1093/ntr/ntab068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner K (2011). Menthol cigarettes and smoking initiation: A tobacco industry perspective. Tobacco Control, 20(Suppl 2), ii12–ii19. 10.1136/tc.2010.041954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal JB, & Wish M (1978). Multidimensional scaling. Sage Publications. [Google Scholar]
- Lee YO, & Glantz SA (2011). Menthol: Putting the pieces together. Tobacco Control, 20(Suppl 2), ii1–ii7. 10.1136/tc.2011.043604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DT, Blackman K, Tauras J, Chaloupka FJ, Villanti AC, Niaura RS, Vallone DM, & Abrams DB (2011). Quit attempts and quit rates among menthol and nonmenthol smokers in the United States. American Journal of Public Health, 101(7), 1241–1247. 10.2105/AJPH.2011.300178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RJ, Bansal-Travers M, Carter LP, & Cummings KM (2012). What would menthol smokers do if menthol in cigarettes were banned? Behavioral intentions and simulated demand. Addiction (Abingdon, England), 107(7), 1330–1338. 10.1111/j.1360-0443.2012.03822.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JL, Abrams DB, Niaura RS, Richardson A, & Vallone DM (2012). A ban on menthol cigarettes: Impact on public opinion and smokers’ intention to quit. American Journal of Public Health, 102(11), e107–114. 10.2105/AJPH.2012.300804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath JM, Greenberg M, Pitzer L, Emelle B, Green M, Liu SM, Willett J, Rose SW, Hair EC, & Vallone D (2018). The Association Between Menthol Perceptions and Support for a Policy Ban Among US Smokers. Ethnicity & Disease, 28(3), 177–186. 10.18865/ed.28.3.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas SR, & Kane M (2012). Quality and rigor of the concept mapping methodology: A pooled study analysis. Evaluation and Program Planning, 35(2), 236–245. 10.1016/j.evalprogplan.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Smith PH, Assefa B, Kainth S, Salas-Ramirez KY, McKee SA, & Giovino GA (2020). Use of Mentholated Cigarettes and Likelihood of Smoking Cessation in the United States: A Meta-Analysis. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 22(3), 307–316. 10.1093/ntr/ntz067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Fiore MC, & Baker TB (2014). Smoking cessation in smokers who smoke menthol and non-menthol cigarettes. Addiction (Abingdon, England), 109(12), 2107–2117. 10.1111/add.12661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule EK, Chaiton M, Zhang B, Hiler MM, Schwartz R, Cohen JE, & Eissenberg T (2019). Menthol Cigarette Smoker Reactions to an Implemented Menthol Cigarette Ban. Tobacco Regulatory Science, 5(1), 50–64. 10.18001/TRS.5.1.5 [DOI] [Google Scholar]
- The National Academies of Sciences Engineering Medicine. (2018). Public Health Consequences of E-Cigarettes. The National Academies Press. [PubMed] [Google Scholar]
- TPSAC. (2011). Menthol cigarettes and public health: Review of the scientific evidence and recommendations.
- Transdisciplinary Tobacco Use Research Center (TTURC) Tobacco Dependence, Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim S-Y, Colby S, Conti D, Giovino GA, Hatsukami D, Hyland A, Krishnan-Sarin S, Niaura R, Perkins KA, & Toll BA (2007). Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 9 Suppl 4, S555–570. 10.1080/14622200701673480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trochim W (1989). An introduction to concept mapping for planning and evaluation. Evaluation and Program Planning, 12, 1–16. [Google Scholar]
- U.S. Department of Health and Human Services. (2020). Smoking Cessation: A Report of the Surgeon General—Executive Summary. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. https://www.hhs.gov/sites/default/files/2020-cessation-sgr-executive-summary.pdf [Google Scholar]
- Villanti AC, Collins LK, Niaura RS, Gagosian SY, & Abrams DB (2017). Menthol cigarettes and the public health standard: A systematic review. BMC Public Health, 17. 10.1186/s12889-017-4987-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, Feirman SP, Tworek C, Glasser AM, Pearson JL, Cohn AM, Conway KP, Niaura RS, Bansal-Travers M, & Hyland A (2017). Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013–2014). American Journal of Preventive Medicine, 53(2), 139–151. 10.1016/j.amepre.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Johnson AL, Glasser AM, Rose SW, Ambrose BK, Conway KP, Cummings KM, Stanton CA, Edwards KC, Delnevo CD, Wackowski OA, Feirman SP, Bansal-Travers M, Bernat JK, Holder-Hayes E, Green VR, Silveira ML, & Hyland A (2019). Association of Flavored Tobacco Use With Tobacco Initiation and Subsequent Use Among US Youth and Adults, 2013–2015. JAMA Network Open, 2(10), e1913804. 10.1001/jamanetworkopen.2019.13804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackowski OA, Evans KR, Harrell MB, Loukas A, Lewis MJ, Delnevo CD, & Perry CL (2017). In Their Own Words: Young Adults’ Menthol Cigarette Initiation, Perceptions, Experiences and Regulation Perspectives. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 10.1093/ntr/ntx048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackowski OA, Manderski MTB, & Delnevo CD (2014). Young adults’ behavioral intentions surrounding a potential menthol cigarette ban. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 16(6), 876–880. 10.1093/ntr/ntu003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JH (1963). Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association, 58, 236–244. [Google Scholar]
- WHO Study Group on Tobacco Product Regulation (TobReg). (n.d.). Banning menthol in tobacco products, 2016. http://www.who.int
- World Health Organization. (n.d.). Tobacco. RetrievedAugust 17, 2020, from https://www.who.int/news-room/fact-sheets/detail/tobacco
- Yang Y, Lindblom EN, Salloum RG, & Ward KD (2020). The impact of a comprehensive tobacco product flavor ban in San Francisco among young adults. Addictive Behaviors Reports, 11, 100273. 10.1016/j.abrep.2020.100273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerger VB (2011). Menthol’s potential effects on nicotine dependence: A tobacco industry perspective. Tobacco Control, 20(Supplement 2), ii29–ii36. 10.1136/tc.2010.041970 [DOI] [PMC free article] [PubMed] [Google Scholar]


