Abstract
Purpose.
Acute uncomplicated urinary tract infections (UTIs) are among the most common indications for antibiotic prescriptions in otherwise healthy women. We compared the risk of treatment failure of antibiotic regimens for outpatient treatment of UTI in real-world practice.
Methods.
We identified non-pregnant, premenopausal women diagnosed with uncomplicated, lower tract UTI and prescribed an oral antibiotic with activity against common uropathogens. We used propensity score-weighted Kaplan-Meier functions to estimate 30-day risks and risk differences (RD) for pyelonephritis and UTI-related antibiotic prescription switch.
Results.
Of 1,140,602 patients, the distribution of index prescriptions was 44% fluoroquinolones (non-first-line), 28% trimethoprim-sulfamethoxazole (TMP/SMX) (first-line), 24% nitrofurantoin (first-line), 3% narrow-spectrum β-lactams (non-first-line), 1% broad-spectrum β-lactams (non-first-line), and 1% amoxicillin/ampicillin (non-recommended). Compared to the risk of pyelonephritis for nitrofurantoin (0.3%), risks were higher for TMP/SMX (RD, 0.2%; 95% CI, 0.2%–0.2%) and broad-spectrum β-lactams (RD, 0.2%; 95% CI, 0.1%–0.4%). Compared to the risk of prescription switch for nitrofurantoin (12.7%), the risk was higher for TMP/SMX (RD 1.6%; 95% CI 1.3%–1.7%) but similar for broad-spectrum β-lactams (RD −0.7%; 95% CI −1.4%–0.1%) and narrow-spectrum β-lactams (RD −0.3%; 95% CI −0.8%–0.2%). Subgroup analyses suggest TMP/SMX treatment failure may be due in part to increasing uropathogen resistance over time.
Conclusions.
The risk of treatment failure differed by antibiotic agent, with higher risk associated with TMP/SMX versus nitrofurantoin, and lower or similar risk associated with broad- versus narrow-spectrum β-lactams. Given serious safety warnings for fluoroquinolones, these results suggest that nitrofurantoin may be preferable as the first-line agent for outpatient treatment of uncomplicated UTI.
Keywords: Administrative data, anti-bacterial agents, cohort study, comparative effectiveness, propensity score, treatment failure, urinary tract infections
INTRODUCTION
Acute uncomplicated urinary tract infections (UTIs) are one of the most common bacterial infections and indications for antibiotic prescriptions in otherwise healthy women. UTIs account for approximately 10.5 million ambulatory visits annually and are associated with morbidity, reduced quality of life, lost time from work, and costs.1 Uncomplicated UTI symptoms usually resolve in three days, however, infections can progress to pyelonephritis, bloodstream infection (BSI), and other severe complications in the absence of effective therapy. Failure of antibiotic therapy for uncomplicated UTI is detrimental from a public health perspective because subsequent antibiotic prescribing can lead to antibiotic resistance,2,3 drug-related adverse events,4–7 and increased healthcare costs.8
Clinical practice guidelines for the treatment of uncomplicated UTI in women recommend several antibiotic regimens for empirical therapy.9 The guidelines are based on efficacy from randomized trials and prevalence of antibiotic resistance.9 First-line therapies for uncomplicated UTI include nitrofurantoin, trimethoprim-sulfamethoxazole (TMP/SMX), if local resistance rates of uropathogens do not exceed 20% or if the infecting strain is known to be susceptible.9,10 Fluoroquinolones are also highly efficacious, but given high propensity of bacteria to develop resistance and risk of serious adverse effects, fluoroquinolones should only be considered as alternative antibiotics for uncomplicated UTI.9,11 β-Lactam agents generally have inferior efficacy compared with fluoroquinolones, particularly amoxicillin and ampicillin (AMX/AMP), and should be avoided given poor efficacy and high prevalence of antibiotic resistance.9
Despite these recommendations, clinical equipoise exists among providers regarding the selection of antibiotic therapy regimen for the treatment of uncomplicated UTI,9,10,12,13 as demonstrated by wide variation in prescribing practices.14–16 This equipoise is likely due in part to limitations of previous studies including small sample sizes, heterogeneous study populations, short follow-up duration, lack of head-to-head comparisons of antibiotic regimens, lack of subgroup analyses, and study periods that preceded increasing resistance of uropathogens to antibiotic agents.17,18
Given the ubiquitous use of antibiotics to treat uncomplicated UTI and increases in the prevalence of antibiotic resistance, robust epidemiologic evidence based on contemporary real-world data in the US population is needed to guide optimal prescribing of antibiotics. We sought to compare the risk of treatment failure of antibiotic regimens for the outpatient treatment of uncomplicated UTI among premenopausal women. We further explored whether associations varied across subgroups of age, calendar time, geographic region, and initial laboratory testing.
METHODS
Data Source
We used the IBM® MarketScan® Commercial Database (2006–2015), which contains individual-level health insurance enrollment and billing information, including inpatient and outpatient procedures and diagnoses, and outpatient pharmacy-dispensed medications for millions of commercial insurance individuals across the U.S.19
Study Design and Population
We performed an active comparator, new-user cohort study.20 We identified women 18–44 years coded in an outpatient setting for a lower tract UTI (i.e., cystitis, urinary tract infection) (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code 595.0, 595.9, 599.0) and prescribed an oral antibiotic on the day of or after the UTI diagnosis from July 1, 2006 to June 30, 2015. The index date represented the date of a filled prescription for an oral antibiotic with activity against common uropathogens. The 180-day baseline period preceded the index date. Uncomplicated UTI was defined in accordance with guidelines for treatment of uncomplicated UTI;9 thus, patients were excluded if they received diagnoses or prescription medications during baseline for pregnancy, urinary comorbidities or abnormalities, pyelonephritis, diabetes, systemic autoimmune conditions, spinal cord injuries, or hematologic or solid organ malignancies (Table S1–3). We further excluded patients without continuous enrollment and prescription drug coverage during baseline; hospitalized within 90 days prior to index date; prescribed antibiotics or diagnosed with bacterial infection within 30 days prior to index date (Table S4); prescribed multiple antibiotics, days’ supply of >10 days,9 or non-standard agents or doses (Table S5) on index date.
Exposure
We categorized UTI-related antibiotic agents as nitrofurantoin, TMP/SMX, fluoroquinolones, broad-spectrum β-Lactam/β-Lactamase inhibitor combinations (henceforth broad-spectrum β-Lactams), narrow-spectrum β-Lactam/β-Lactamase inhibitor combinations (henceforth narrow-spectrum β-Lactams), and AMX/AMP (Table S5).9 Fosfomycin and trimethoprim monotherapy were not included due to rare use in the U.S (Figure S1). We further categorized agents as first-line; non-first-line; and non-recommended agents.9
Outcomes
Pyelonephritis, the primary outcome, was defined as the presence of any of the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes: 590.10, 590.11, 590.2, 590.3, 590.80, 590.81, or 590.9.21 UTI-related antibiotic switch, the secondary outcome, was defined as a filled pharmacy claim for a different UTI-related antibiotic agent category as the index prescription; we did not require a UTI diagnosis code since subsequent antibiotics are often prescribed over the phone without assignment of a UTI diagnosis code. Pyelonephritis was directly related to treatment failure due to ascending infection from the bladder to the kidney. UTI-related antibiotic switch was a pragmatic measure of treatment failure reflecting a subsequent antibiotic prescription due to absence of symptom resolution, antibiotic resistance, or medication intolerance / adverse drug event. We examined two outcomes in sensitivity analyses: (1) pyelonephritis and/or BSI 22 (Table S6); and (2) UTI-related antibiotic prescription switch/repeat, a broader outcome definition allowing receipt of the same or different UTI-related antibiotic agent category as the index prescription.
Follow-up
Follow-up for pyelonephritis and BSI started 3 days after the index prescription to allow for diagnostic delay because pyelonephritis or BSI diagnosed within 3 days of the UTI diagnosis was almost certainly present initially but not yet identified. Follow-up for pyelonephritis and BSI ended 30 days following the index prescription. For all outcomes, we performed intention-to-treat (first treatment carried forward) analyses where follow-up ended at the earliest of: the outcome-specific follow-up period, health plan disenrollment, or administrative end-of-study (September 30, 2015). For the subsequent antibiotic analysis, patients were additionally censored for non-UTI bacterial infections (Table S4), to account for the possibility of an antibiotic prescribed for an indication other than UTI.
Covariates
We ascertained the following baseline covariates: age, month and year of prescription, geographic region, provider specialty, initial receipt of urine testing, and comorbid conditions defined using the Elixhauser classification.23
Statistical Analyses
We summarized the distribution of baseline covariates within each treatment group. To examine the relationship between antibiotic agents and treatment failure outcomes, we used Kaplan-Meier methods to estimate and plot daily cumulative risk since the start of therapy. We calculated unadjusted and standardized mortality ratio (SMR)-weighted cumulative risk and risk difference estimates. We computed 95% confidence intervals (CIs) using non-parametric bootstrap sampling with replacement (N=500 samples). SMR weights were calculated from five separate propensity scores (p) using multivariable logistic regression, where p represented the probability that a patient initiated nitrofurantoin versus each of the other antibiotic categories of interest, accounting for several baseline covariates. Age was modeled using restricted cubic splines.24 SMR weights allowed us to standardize the covariate distribution in each comparator cohort to that in the reference cohort (i.e., nitrofurantoin).25 Therefore, under the assumption of no unmeasured confounding, our treatment comparisons would be unconfounded with respect to their effect on the outcome. We calculated and plotted the absolute standardized mean differences of baseline covariates in the unweighted and SMR-weighted populations to determine whether weighting the population reduced imbalances of observed covariates and made the treatment groups more exchangeable.26
We conducted subgroup analyses of treatment failure outcomes by age, geographic region, year, and initial laboratory testing. We performed sensitivity analyses excluding UTI patients with high severity of illness, defined by proxy of kidney imaging to rule-out pyelonephritis at UTI diagnosis (Table S7); because the inclusion of patients suspected but not coded for pyelonephritis at UTI diagnosis could potentially bias the results. We performed sensitivity analyses for the pyelonephritis analysis, which additionally censored for BSI to account for the possibility that pyelonephritis was related to the BSI rather than the UTI. We performed as-treated analyses for the pyelonephritis and BSI outcomes, which additionally censored follow-up for a new prescription for a different UTI-related antibiotic than the index prescription.
RESULTS
We identified 1,140,602 eligible women who met study eligibility criteria (Figure S1). The majority of women initiated fluoroquinolones (44%), nitrofurantoin (24%), or TMP/SMX (28%). We observed differences between treatment groups by age, year, geographic region, provider type, and laboratory testing at diagnosis (Table 1, Table S8, Table S9 and Figure S2). Nitrofurantoin initiators were more likely to have OBGYN providers, TMP/SMX and fluoroquinolone initiators were more likely to have family medicine/pediatrics providers, and narrow-spectrum β-lactam initiators were more likely to have emergency medicine providers. Nitrofurantoin and broad-spectrum β-lactam initiators were most likely to receive urine culture at diagnosis. After SMR weighting, measured patient characteristics were well-balanced between treatment groups (standardized mean differences < 0.10) (Figure S3).27
TABLE 1.
Baseline Characteristics of Uncomplicated Lower Tract UTI Patients by Index Antibiotic Agent, IBM® MarketScan® Commercial Database, 2006–2015a
| First-line agents | Non-first-line agents | Non-recommended agents | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | Nitrofurantoin (%) N=271,189 |
TMP/SMX (%) N=322,052 |
Fluoroquinolone (%) N=497,279 |
Broad-spectrum β-lactam (%) N=12,491 |
Narrow-spectrum β-lactam (%) N=30,087 |
AMX/AMP (%) N=7,613 |
| Age, median (IQR), y | 30 (23–37) | 29 (22–37) | 32 (24–39) | 29 (20–36) | 29 (21–36) | 30 (23–37) |
| Age group, y | ||||||
| 18–24 | 30.8 | 35.3 | 27.1 | 39.1 | 36.2 | 30.0 |
| 25–29 | 17.0 | 15.5 | 15.8 | 13.7 | 16.7 | 18.7 |
| 30–34 | 17.2 | 15.8 | 16.8 | 15.9 | 16.7 | 19.0 |
| 35–39 | 17.6 | 16.6 | 19.2 | 16.0 | 15.6 | 17.4 |
| 40–44 | 17.5 | 16.8 | 21.0 | 15.3 | 14.7 | 14.9 |
| Region of residence | ||||||
| Northeast | 13.9 | 13.9 | 13.7 | 14.9 | 8.8 | 19.0 |
| Midwest | 21.0 | 29.0 | 21.8 | 16.3 | 23.3 | 25.1 |
| South | 41.5 | 40.6 | 45.7 | 55.3 | 29.9 | 40.8 |
| West | 23.6 | 16.5 | 18.8 | 13.5 | 38.0 | 15.0 |
| Provider type | ||||||
| Emergency medicine | 6.8 | 7.1 | 7.4 | 8.8 | 18.9 | 3.0 |
| Internal medicine | 11.3 | 13.3 | 16.8 | 13.7 | 10.3 | 18.2 |
| Family medicine / Pediatrics | 32.3 | 40.0 | 38.3 | 41.0 | 30.5 | 41.4 |
| OBGYN | 14.4 | 4.3 | 4.2 | 4.0 | 5.3 | 5.4 |
| MD / DO | 6.1 | 7.6 | 6.3 | 5.1 | 7.0 | 6.8 |
| Non-MD | 5.3 | 6.7 | 4.6 | 3.6 | 2.8 | 3.3 |
| Other | 20.4 | 17.7 | 19.4 | 21.8 | 23.0 | 19.4 |
| Missing | 3.2 | 3.3 | 3.1 | 2.0 | 2.3 | 2.5 |
| Outpatient Encounter | ||||||
| In-person b | 97.1 | 97.1 | 97.3 | 98.3 | 98.0 | 98.1 |
| Laboratory only | 2.9 | 2.9 | 2.7 | 1.7 | 2.0 | 1.9 |
| Initial receipt of urine tests | ||||||
| Urinalysis | 78.6 | 78.7 | 78.9 | 78.2 | 75.3 | 74.0 |
| Culture | 53.3 | 43.7 | 48.0 | 56.9 | 47.1 | 49.3 |
Abbreviations: AMX/AMP, amoxicillin or ampicillin; DO, doctor of osteopathic medicine; IQR, interquartile range; OBGYN, obstetrics and gynecology; TMP/SMX, trimethoprim-sulfamethoxazole.
Baseline covariates were assessed during the 180-day period prior to the index prescription.
Includes emergency department visits, office visits, phone call consultations, and online consultations.
Risk of Pyelonephritis
The 30-day weighted risks for pyelonephritis ranged from 0.3% for nitrofurantoin to 0.5% for TMP/SMX or broad-spectrum β-lactams (Table 2). Compared to nitrofurantoin, the weighted 30-day risks were highest for TMP/SMX and broad-spectrum β-lactams, corresponding to 2 more events per 1,000 patients (Table 3). Figure 1 presents the weighted cumulative risk and risk difference curves for pyelonephritis. Among first-line regimens, we observed higher risk of pyelonephritis for TMP/SMX versus nitrofurantoin throughout the follow-up period, with the largest increase in risk during the first week. Among non-first-line regimens, the daily risk of pyelonephritis was similar except the risk among broad-spectrum β-lactam initiators continued to increase steeply after the first week.
TABLE 2.
30-day Cumulative Risk Estimates of Treatment Failure Outcomes by Index Antibiotic Agent (Intention-to-treat Analyses)
| Antibiotic agent | No. of events | No. of censoring events | Crude Cumulative Risk, % (95% CI) |
Weighted Cumulative Risk, % (95% CI)a |
|---|---|---|---|---|
| Pyelonephritis | ||||
| Nitrofurantoin | 820 | 269,149 | 0.3 (0.3–0.3) | 0.3 (0.3–0.3) |
| TMP/SMX | 1567 | 318,834 | 0.5 (0.5–0.5) | 0.5 (0.5–0.5) |
| Fluoroquinolone | 1849 | 492,595 | 0.4 (0.4–0.4) | 0.4 (0.3–0.4) |
| Broad-spectrum β-lactam | 74 | 12,348 | 0.6 (0.4–0.7) | 0.5 (0.4–0.7) |
| Narrow-spectrum β-lactam | 146 | 29,761 | 0.5 (0.4–0.6) | 0.4 (0.3–0.5) |
| AMP/AMX | 32 | 7,554 | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) |
| Antibiotic prescription switch | ||||
| Nitrofurantoin | 34,311 | 236,878 | 12.7 (12.5–12.8) | 12.7 (12.5–12.8) |
| TMP/SMX | 44,457 | 277,595 | 13.8 (13.7–13.9) | 14.2 (14.1–14.4) |
| Fluoroquinolone | 40,871 | 456,408 | 8.2 (8.1–8.3) | 8.3 (8.2–8.4) |
| Broad-spectrum β-lactam | 1,462 | 11,029 | 11.7 (11.1–12.2) | 12.0 (11.3–12.7) |
| Narrow-spectrum β-lactam | 3,603 | 26,484 | 12.0 (11.6–12.4) | 12.4 (11.9–12.8) |
| AMP/AMX | 1,244 | 6,369 | 16.4 (15.4–17.2) | 16.3 (15.3–17.3) |
Abbreviations: AMX/AMP indicates amoxicillin or ampicillin; CI, confidence interval; TMP/SMX, trimethoprim-sulfamethoxazole; UTI, urinary tract infection.
Propensity score weighting was implemented by standardized mortality ratio weighting where patients were weighted to reflect the covariate distributions in the nitrofurantoin treatment group. The nitrofurantoin cohort was given a weight of 1 and each comparator PS/(1 – PS), in which PS is the propensity score of nitrofurantoin initiators in the study population. The PS models were estimated using the following covariates: age, month, year, geographic region, provider type, drug or alcohol abuse, deficiency anemias, chronic pulmonary disease, depression, psychoses, hypertension, obesity, initial receipt of urinalysis, and initial receipt of urine culture.
TABLE 3.
30-day Risk Difference Estimates of Pyelonephritis versus Nitrofurantoin Initiators by Index Antibiotic Agent, Overall and by Subgroup (Intention-to-treat Analyses)
| Risk Difference, % (95% CI) versus Nitrofurantoina | |||||
|---|---|---|---|---|---|
|
| |||||
| First-line agents | Non-first-line Agents | Non-recommended agents | |||
|
| |||||
| TMP/SMX | Fluoroquinolone | Broad-spectrum β-lactam | Narrow-spectrum β-lactam | AMX/AMP | |
| Overall analyses | |||||
| Crude | 0.2 (0.2–0.2) | 0.1 (0.0–0.1) | 0.3 (0.2–0.4) | 0.2 (0.1–0.3) | 0.1 (0.0–0.3) |
| Weighted | 0.2 (0.2–0.2) | 0.1 (0.0–0.1) | 0.2 (0.1–0.4) | 0.1 (0.0–0.2) | 0.1 (0.0–0.3) |
| Subgroup analyses (weighted) | |||||
| Age group, y | |||||
| 18–24 | 0.2 (0.1–0.2) | 0.0 (−0.1–0.0) | 0.3 (0.0–0.6) | 0.0 (−0.1–0.2) | 0.2 (−0.2–0.6) |
| 25–29 | 0.3 (0.2–0.4) | 0.1 (0.1–0.2) | 0.5 (0.1–0.9) | 0.1 (−0.1–0.3) | 0.3 (−0.1–0.9) |
| 30–34 | 0.3 (0.2–0.4) | 0.1 (0.1–0.2) | 0.5 (0.0–1.0) | 0.0 (−0.1–0.2) | −0.2 (−0.3−−0.1) |
| 35–39 | 0.1 (0.1–0.2) | 0.1 (0.0–0.1) | 0.0 (−0.2–0.1) | 0.1 (−0.1–0.3) | 0.1 (−0.2–0.5) |
| 40–44 | 0.1 (0.0–0.2) | 0.0 (0.0–0.1) | 0.1 (−0.2–0.3) | 0.1 (−0.1–0.4) | 0.1 (−0.2–0.6) |
| Year | |||||
| 2006–2008 | 0.2 (0.1–0.2) | 0.1 (0.0–0.2) | 0.3 (0.0–0.7) | 0.1 (−0.1–0.3) | 0.2 (−0.1–0.6) |
| 2009–2011 | 0.2 (0.1–0.2) | 0.0 (0.0–0.1) | 0.2 (0.0–0.5) | 0.0 (−0.1–0.1) | 0.2 (−0.1–0.6) |
| 2012–2015 | 0.2 (0.2–0.3) | 0.0 (0.0–0.1) | 0.2 (0.0–0.4) | 0.1 (0.0–0.2) | 0.1 (−0.1–0.3) |
| Region of residence | |||||
| Northeast | 0.1 (0.0–0.2) | 0.0 (0.0–0.1) | 0.0 (−0.2–0.4) | 0.2 (−0.1–0.5) | 0.1 (−0.2–0.5) |
| Midwest | 0.2 (0.1–0.3) | 0.1 (0.0–0.1) | 0.3 (0.0–0.6) | 0.1 (0.0–0.3) | 0.4 (0.0–0.8) |
| South | 0.2 (0.1–0.2) | 0.0 (0.0–0.1) | 0.2 (0.0–0.4) | 0.0 (−0.1–0.1) | 0.0 (−0.2–0.3) |
| West | 0.3 (0.2–0.3) | 0.1 (0.0–0.2) | 0.4 (0.1–0.8) | 0.1 (0.0–0.2) | 0.2 (−0.2–0.7) |
| Initial laboratory testingb | |||||
| None | 0.2 (0.1–0.3) | 0.1 (0.0–0.1) | 0.2 (−0.2–0.5) | −0.1 (−0.2–0.0) | −0.1 (−0.4–0.2) |
| Urinalysis only | 0.2 (0.2–0.3) | 0.0 (0.0–0.1) | 0.3 (0.0–0.6) | 0.0 (−0.1–0.1) | 0.3 (0.0–0.7) |
| Culture ± urinalysis | 0.1 (0.1–0.2) | 0.1 (0.0–0.1) | 0.2 (0.0–0.5) | 0.2 (0.0–0.3) | 0.1 (−0.1–0.4) |
| No culture | 0.2 (0.2–0.3) | 0.0 (0.0–0.1) | 0.3 (0.1–0.5) | 0.0 (−0.1–0.1) | 0.2 (0.0–0.5) |
Abbreviations: AMX/AMP indicates amoxicillin or ampicillin; CI, confidence interval; PS, propensity score, TMP/SMX, trimethoprim-sulfamethoxazole.
Propensity score (PS) weighting was implemented by standardized mortality ratio weighting where patients were weighted to reflect the covariate distributions in the nitrofurantoin treatment group. The nitrofurantoin cohort was given a weight of 1 and each comparator PS/(1 – PS), in which PS is the propensity score of nitrofurantoin initiators in the study population. The PS models were estimated using the following covariates: age, month, year, geographic region, provider type, drug or alcohol abuse, deficiency anemias, chronic pulmonary disease, depression, psychoses, hypertension, obesity, initial receipt of urinalysis, and initial receipt of urine culture.
Sample size of the subgroups for the pyelonephritis analysis: none (n=163,184), urinalysis only (n=425,718), culture ± urinalysis (n=545,772), and no culture (n=588,902).
Sample size of the subgroups for the prescription switch analysis: none (n=164,088), urinalysis only (n=427,793), culture ± urinalysis (n=548,830), and no culture (n=591,881).
FIGURE 1.
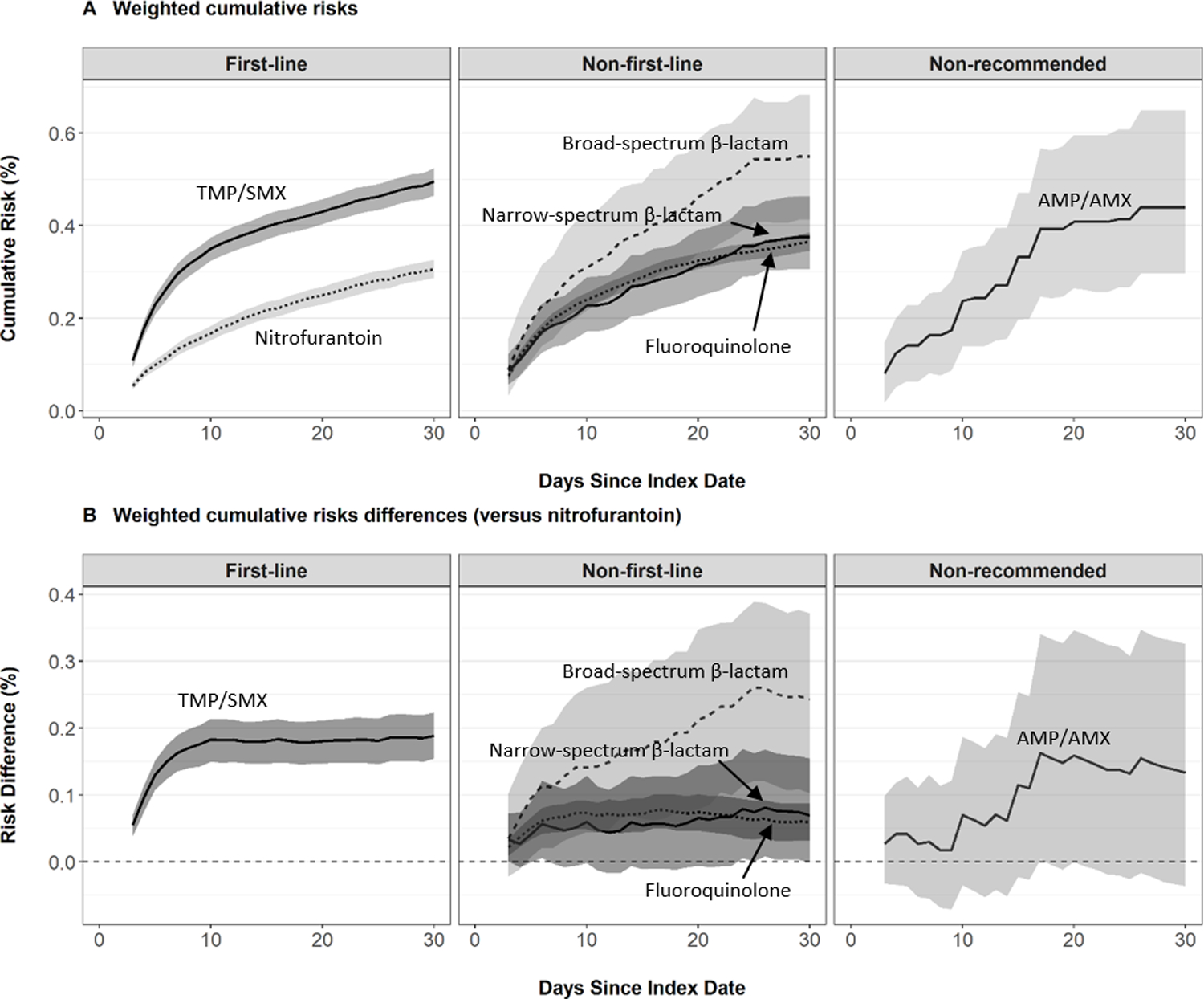
Risk of Pyelonephritis by Index Antibiotic Agent, IBM® MarketScan® Commercial Database, 2006–2015. Cumulative risks and risk differences were estimated using standardized mortality ratio- weighted Kaplan-Meier functions. 95% confidence intervals were computed using a non-parametric bootstrap. Risk difference estimates greater than zero indicate higher risk of pyelonephritis compared to nitrofurantoin (reference). Estimates were adjusted for age, month, year, geographic region, provider type, drug or alcohol abuse, deficiency anemias, chronic pulmonary disease, depression, psychoses, hypertension, obesity, initial receipt of urinalysis, and initial receipt of urine culture.
In subgroup analyses, the associations between antibiotic agent and pyelonephritis varied by age, year, region of residence, and type of initial laboratory testing (Table 3, Table S10). Compared to nitrofurantoin, the risk of pyelonephritis was elevated for all subgroups of TMP/SMX initiators and some subgroups of non-first line fluoroquinolone initiators.
Results of sensitivity analyses were consistent with primary findings. These sensitivity analyses included: broadening the pyelonephritis outcome to a composite outcome of pyelonephritis and/or BSI (Table S11); excluding 24,963 (2.2%) patients who received kidney imaging at UTI diagnosis (Table S12); and censoring the pyelonephritis analysis for BSI (Table S13). As-treated analyses for both pyelonephritis outcomes (i.e., including and excluding BSI) revealed similar results as the primary intention-to-treat analysis, except for broad-spectrum β-lactams compared to nitrofurantoin where the risk difference remained constant (0.2) but the 95% CI limits shifted slightly down and toward the null (95% CI 0.0–0.3) (Table S14).
Risk of Antibiotic Prescription Switch
The 30-day weighted risks for prescription switch ranged from 12.7% (nitrofurantoin) to 14.2% (TMP/SMX) for first-line agents, 8.3% (fluoroquinolone) to 12.4% (narrow-spectrum β-lactam) for non-first-line agents, and 16.3% (AMP/AMX) for non-recommended agents (Table 2). Compared to nitrofurantoin, we observed higher 30-day risks of prescription switch, with risk difference estimates ranging from 1.6% (TMP/SMX) to 3.6% (AMP/AMX), corresponding to 16 to 36 more prescription switches per 1,000 patients (Table 4). Conversely, we observed a 4.4% lower risk of prescription switch for fluoroquinolones vs. nitrofurantoin, corresponding to 44 less prescription switches per 1,000 patients treated over the 30-day follow-up period (Table 4). Figure 2 presents the weighted cumulative risk and risk difference curves for prescription switch. Among first-line regimens, we observed higher risk of prescription switch for TMP/SMX versus nitrofurantoin throughout the follow-up period, due to a steeper risk in the first week. Among non-first-line regimens, we observed similar risk of prescription switch for broad- and narrow-spectrum β-lactams throughout most of the 30-day follow-up period; however, the risk of prescription switch for fluoroquinolones remained substantially lower throughout follow-up.
TABLE 4.
30-day Risk Difference Estimates of Antibiotic Prescription Switch versus Nitrofurantoin Initiators by Index Antibiotic Agent, Overall and by Subgroup (Intention-to-treat Analyses)
| Risk Difference, % (95% CI) versus Nitrofurantoina | |||||
|---|---|---|---|---|---|
|
| |||||
| First-line agents | Non-first-line Agents | Non-recommended agents | |||
|
| |||||
| TMP/SMX | Fluoroquinolone | Broad-spectrum β-lactam | Narrow-spectrum β-lactam | AMX/AMP | |
| Overall analyses | |||||
| Crude | 1.2 (1.0–1.3) | −4.4 (−4.6−−4.3) | −1.0 (−1.5−−0.4) | −0.7 (−1.1−−0.3) | 3.7 (2.8–4.6) |
| Weighted | 1.6 (1.3–1.7) | −4.4 (−4.5−−4.2) | −0.7 (−1.4–0.1) | −0.3 (−0.8–0.2) | 3.6 (2.5–4.6) |
| Subgroup analyses (weighted) | |||||
| Age group, y | |||||
| 18–24 | 1.1 (0.8–1.5) | −4.4 (−4.7−−4.1) | −1.4 (−3.2–0.4) | −1.2 (−1.9−−0.3) | 1.9 (0.0–3.6) |
| 25–29 | 2.6 (2.1–3.1) | −3.5 (−3.9−−3.2) | 0.2 (−1.8–2.0) | 0.3 (−0.9–1.5) | 6.3 (3.8–9.0) |
| 30–34 | 2.1 (1.6–2.6) | −4.0 (−4.4−−3.6) | 0.3 (−1.5–2.2) | 1.0 (−0.3–2.1) | 5.6 (3.1–8.4) |
| 35–39 | 1.3 (0.9–1.8) | −4.6 (−4.9−−4.2) | −0.1 (−2.0–1.8) | −1.1 (−2.3–0.2) | 2.9 (0.6–5.0) |
| 40–44 | 1.0 (0.5–1.5) | −5.4 (−5.8−−5.0) | −0.6 (−2.3–1.4) | 0.6 (−0.7–1.9) | 2.8 (−0.2–5.4) |
| Year | |||||
| 2006–2008 | 1.0 (0.6–1.4) | −5.1 (−5.4−−4.8) | −1.1 (−2.5–0.7) | 0.3 (−0.8–1.4) | 4.5 (2.3–6.5) |
| 2009–2011 | 1.7 (1.4–2.0) | −4.2 (−4.5−−4.0) | 0.5 (−0.9–2.0) | −0.1 (−0.9–0.9) | 2.7 (1.1–4.4) |
| 2012–2015 | 1.6 (1.4–2.0) | −4.2 (−4.5−−3.9) | −1.8 (−2.8−−0.7) | −0.6 (−1.3–0.1) | 3.8 (2.3–5.5) |
| Region of residence | |||||
| Northeast | 1.5 (1.0–2.1) | −4.2 (−4.7−−3.8) | −1.5 (−3.3–0.4) | 0.9 (−0.8–2.7) | 6.1 (3.5–9.4) |
| Midwest | 1.1 (0.8–1.5) | −4.5 (−4.8−−4.1) | 0.4 (−1.3–2.4) | −0.4 (−1.3–0.5) | 4.7 (2.6–6.7) |
| South | 1.4 (1.1–1.7) | −4.4 (−4.6−−4.2) | −1.6 (−2.5−−0.6) | −0.5 (−1.3–0.2) | 2.4 (0.8–3.8) |
| West | 2.2 (1.8–2.6) | −4.3 (−4.7−−4.0) | 0.8 (−1.2–2.8) | −0.4 (−1.1–0.4) | 3.4 (1.1–5.7) |
| Initial laboratory testingc | |||||
| None | 0.9 (0.5–1.4) | −3.9 (−4.4−−3.6) | −2.0 (−3.5−−0.5) | −0.1 (−1.1–1.0) | 1.2 (−1.1–3.6) |
| Urinalysis only | 1.0 (0.7–1.3) | −3.8 (−4.0−−3.5) | −0.5 (−1.7–0.8) | −0.8 (−1.5−−0.2) | 1.2 (−0.2–2.6) |
| Culture ± urinalysis | 2.1 (1.8–2.3) | −4.9 (−5.1−−4.7) | −0.4 (−1.4–0.7) | 0.3 (−0.5–1.0) | 5.5 (4.0–7.3) |
| No culture | 1.0 (0.7–1.2) | −3.8 (−4.0−−3.6) | −1.0 (−2.0–0.1) | −0.6 (−1.2–0.0) | 1.5 (0.2–2.7) |
Abbreviations: AMX/AMP indicates amoxicillin or ampicillin; CI, confidence interval; PS, propensity score, TMP/SMX, trimethoprim-sulfamethoxazole.
Propensity score (PS) weighting was implemented by standardized mortality ratio weighting where patients were weighted to reflect the covariate distributions in the nitrofurantoin treatment group. The nitrofurantoin cohort was given a weight of 1 and each comparator PS/(1 – PS), in which PS is the propensity score of nitrofurantoin initiators in the study population. The PS models were estimated using the following covariates: age, month, year, geographic region, provider type, drug or alcohol abuse, deficiency anemias, chronic pulmonary disease, depression, psychoses, hypertension, obesity, initial receipt of urinalysis, and initial receipt of urine culture.
Sample size of the subgroups for the pyelonephritis analysis: none (n=163,184), urinalysis only (n=425,718), culture ± urinalysis (n=545,772), and no culture (n=588,902).
Sample size of the subgroups for the prescription switch analysis: none (n=164,088), urinalysis only (n=427,793), culture ± urinalysis (n=548,830), and no culture (n=591,881).
FIGURE 2.
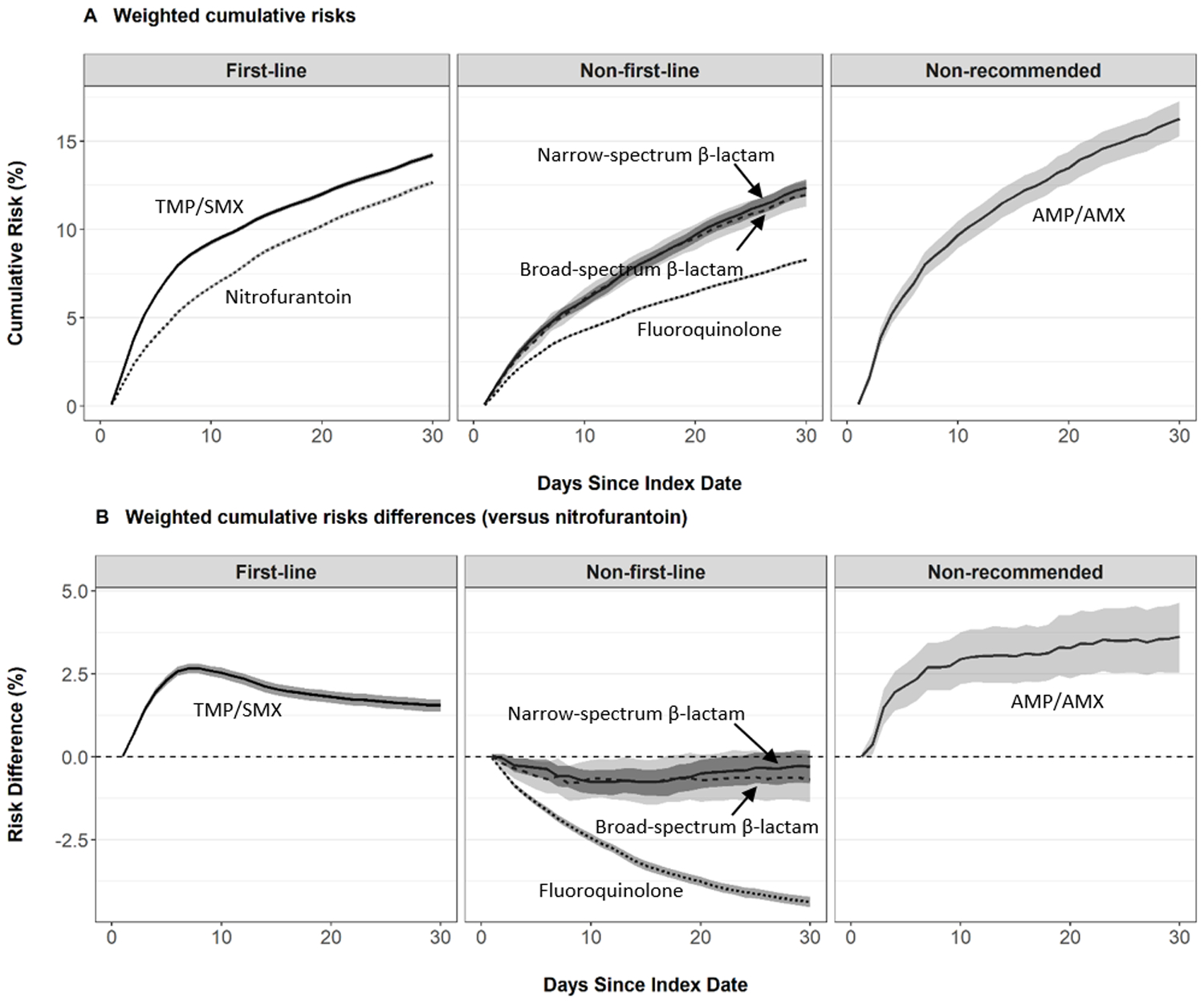
Risk of Antibiotic Prescription Switch by Index Antibiotic Agent, IBM® MarketScan® Commercial Database, 2006–2015. Cumulative risks and risk differences were estimated using standardized mortality ratio- weighted Kaplan-Meier functions. 95% confidence intervals were computed using a non-parametric bootstrap. Risk difference estimates greater than zero indicate higher risk of prescription switch compared to nitrofurantoin (reference). Estimates were adjusted for age, month, year, geographic region, provider type, drug or alcohol abuse, deficiency anemias, chronic pulmonary disease, depression, psychoses, hypertension, obesity, initial receipt of urinalysis, and initial receipt of urine culture.
In subgroup analyses, the associations between antibiotic agent and prescription switch varied by age, year, region of residence, and type of initial laboratory testing (Table 4 and Table S15). Compared to nitrofurantoin, the 30-day risk of prescription switch was higher in all subgroups of TMP/SMX, lower in all subgroups of fluoroquinolones, similar in most subgroups of broad- or narrow-spectrum β-lactam, and higher in most subgroups of AMX/AMP.
Results of sensitivity analyses were consistent with primary findings. These sensitivity analyses included using the broader outcome definition of prescription switch/repeat (Table S11 and Table S16) and excluding patients who received kidney imaging at UTI diagnosis (Table S12). Regardless of index antibiotic, the largest proportion of subsequent antibiotic prescriptions were fluoroquinolones, followed by nitrofurantoin, and TMP/SMX (Figure S4).
DISCUSSION
In this active comparator, new-user study conducted in a commercially-insured U.S. population of non-pregnant, premenopausal women with uncomplicated UTI, we observed 30-day risks of treatment failure outcomes which varied by antibiotic agent. The risk of treatment failure differed between first-line agents, with TMP/SMX associated with higher 30-day risk of pyelonephritis or prescription switch compared to nitrofurantoin. Although the differences in 30-day risk of pyelonephritis were small in magnitude and residual confounding cannot be ruled out, this finding warrants attention due to very high annual prescribing of antibiotic therapy for uncomplicated UTI. As expected from guidelines, the risk of treatment failure was low among fluoroquinolone initiators and high among AMP/AMX initiators. Among non-first-line agents, broad-spectrum β-lactams were not associated with better outcomes than narrow-spectrum β-lactams.
The observed differences in treatment failure by antibiotic agent raise concerns about guideline recommendations for TMP/SMX as a first-line therapy for uncomplicated UTI in outpatient settings.9 Our subgroup analyses demonstrate higher TMP/SMX treatment failure in recent years, possibly due in part to increasing uropathogen resistance over time. Among TMP/SMX initiators, prescription switch was more likely for recipients of urine culture, presumably in response to confirmation of TMP/SMX resistance. Although guidelines recommend avoidance of TMP/SMX when local resistance rates exceed 20%, local data on uropathogen resistance is typically unavailable.9 Separately, TMP/SMX has a relatively poor drug tolerability and safety profile.28 Adverse effects (e.g., gastrointestinal disturbances, allergic skin reactions) are usually managed by drug discontinuation, which may decrease effectiveness and lead to subsequent prescriptions with alternative agents.29
Our results confirm that fluoroquinolones are highly efficacious, but the U.S. Food and Drug Administration reserves these agents for important uses other than uncomplicated UTI, as the risk of serious side effects (e.g., aortic aneurysm, aortic dissection, retinal detachment, tendon rupture, peripheral neuropathy, central nervous system effects) generally outweighs the benefits.11 Yet, in this study, fluoroquinolones remained the most commonly prescribed agent each year, as we have previously reported.14
Our study contributes to a small but emerging body of evidence demonstrating no difference in treatment failure outcomes between broad- and narrow-spectrum β-lactams.30,31 Although broad-spectrum β-lactams cover a wider spectrum of pathogens, they are disadvantageous due to selection for and spread of resistance across multiple bacterial species,32 and patients may disproportionately discontinue because of deleterious effects on the host microbiome leading to adverse health effects (e.g., Clostridioides difficile infection).4,5,33,34
Our study has some limitations. Given the nonrandomized nature of the exposure, the results are subject to residual confounding, and should be interpreted with caution. Our study employed an active comparator, new-user study design, which helped reduce measured and unmeasured confounding by restricting the study population to women with the indication for treatment and comparing commonly prescribed antibiotic therapies.20 We also attempted to reduce confounding by including a wide range of variables in our propensity score model. However, there may be some uncontrolled differences between treatment groups (e.g., unmeasured UTI severity). Despite these concerns, it is reassuring that sensitivity analyses excluding UTI patients with high severity of illness, as defined by proxy of kidney imaging to rule-out pyelonephritis at UTI diagnosis, did not change conclusions. In addition, crude and weighted analyses yielded similar results, indicating relatively little residual confounding by measured covariates.
The interpretation of our results is limited by the validity of the antibiotic exposure definition. A strength of our study was the use of pharmacy dispensing billing claims -- considered the gold standard of prescription drug ascertainment versus self-report or medical records -- because insurance reimbursement is based on complete and accurate claims.35 However, the index antibiotic exposure could be misclassified if patients did not initiate the antibiotic. Moreover, adherence may differ by antibiotic exposure category, with possibly lower completion among therapies with poorer tolerability or longer regimens (e.g., 7-day β-lactam versus 3-day fluoroquinolones). But, the effects of shorter course regimens has recently been shown to be similar to recommended durations for several agents.36 Additionally, our findings were generally robust regardless of censoring for antibiotic switching, indicating that these censoring events were not differentially associated with risk of pyelonephritis.
Our study is also limited by the lack of information on results of urinalysis or urine culture tests in the claims data; we were unable to confirm true bacterial UTI cases or account for uropathogen susceptibility results in the analyses. Given the empiric nature of treatment, the distribution of patients without true UTI cases (e.g., asymptomatic bacteriuria) is likely non-differential with respect to antibiotic exposure; thus, any bias due to inappropriate inclusion of these patients would be towards the null. In addition, our study does not account for local antibiotic resistance. However, these results are useful for healthcare providers who likely do not have access to local uropathogen susceptibility data. Also, our study was restricted to clinician-observed outcomes, and did not capture patient-centered outcomes such as pain or symptom resolution. Lastly, our study was conducted in a population of healthy premenopausal, non-pregnant women identified from a database that oversamples residents of the South and under-samples residents of the West. Thus, results may not generalize to children, men, or women who are pregnant, post-menopausal, medically complex (e.g., diabetes, systemic autoimmune conditions, spinal cord injuries), or insured by Medicaid or uninsured, and may not directly generalize to the U.S. commercially-insured population.
Among premenopausal women treated for uncomplicated UTI in the outpatient setting, our results comparing first-line antibiotic agents suggest that TMP/SMX is associated with higher risk of pyelonephritis and prescription switch compared to nitrofurantoin. Among non-first-line therapies, broad-spectrum β-lactams were associated with a marginally higher comparative risk of pyelonephritis but similar risk of a prescription switch than narrow-spectrum β-lactams. Considering serious safety warnings for fluoroquinolones, these results suggest that nitrofurantoin may be preferable as the first-line agent for outpatient treatment of uncomplicated UTI. Our large national study provides valuable evidence regarding antibiotic treatment failure outcomes in a real-world setting in which therapy is prescribed empirically despite escalating antibiotic resistance. Given the large magnitude and wide variation in outpatient prescribing of antibiotics for uncomplicated UTI,1,14 our results can be used to guide antimicrobial stewardship efforts37 and appropriate selection of antibiotic therapy, which is critical to optimize treatment of infections, decrease occurrence of drug-related adverse events, and limit the spread of antibiotic resistance.
Supplementary Material
KEY POINTS.
The weighted risks of UTI-related treatment failure differed between first-line agents, with higher 30-day risks of pyelonephritis or prescription switch among TMP/SMX versus nitrofurantoin users. Broad-spectrum β-lactams were not associated with better outcomes than narrow-spectrum β-lactams.
Findings were similar in sensitivity analyses using broader outcome definitions or excluding UTI cases with potentially higher severity of illness.
Subgroup analyses suggested TMP/SMX treatment failure may be due to increasing uropathogen resistance over time.
Given serious safety warnings for fluoroquinolones, these results support UTI treatment guidelines regarding nitrofurantoin as a first-line therapy, but warrant reappraisal to consider reclassifying TMP/SMX as a non-first-line therapy.
ACKNOWLEDGMENT:
We thank Caroline A. O’Neil, MA, MPH for editorial assistance.
FUNDING INFORMATION:
National Center for Advancing Translational Sciences at the National Institutes of Health, Grant/Award Number: KL2 TR002346. Data programming for this study was conducted by the Center for Administrative Data Research, which is supported in part by the Washington University Institute of Clinical and Translational Sciences from the National Center for Advancing Translational Sciences at the National Institutes of Health, Grant/Award Number: UL1 TR002345; through the Agency for Healthcare Research and Quality, Grant/Award Number: R24 HS19455.
Footnotes
PRESENTATION: This work was previously presented as an oral presentation at the 2018 International Conference on Pharmacoepidemiology & Therapeutic Risk Management in Prague, Czech Republic; and the 2019 Association for Clinical and Translational Science Conference in Washington, D.C; and as a poster presentation at the 2019 annual meeting of the Infectious Diseases Society of America in San Francisco, CA.
ETHICS STATEMENT: The institutional review board at Washington University School of Medicine deemed this study exempt from human subject review.
CONFLICT OF INTEREST: Dr. Olsen reported receiving investigator-initiated research funds from Sanofi Pasteur, Pfizer, and Merck and serving as a consultant for Pfizer. Dr. Powderly reported receiving investigator-initiated research funds from Merck and serving as an advisor for Merck and Gilead. Dr. Dharnidharka reported receiving research funds from Atara Bio and CareDx and serving as a consultant to Atara Bio. No other disclosures were reported.
Publisher's Disclaimer: DISCLAIMER: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Foxman B Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infectious disease clinics of North America. 2014;28(1):1–13. [DOI] [PubMed] [Google Scholar]
- 2.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ (Clinical research ed). 2010;340:c2096. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC;2019. [Google Scholar]
- 4.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US Emergency Department Visits for Outpatient Adverse Drug Events, 2013–2014. Jama. 2016;316(20):2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;47(6):735–743. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Kotsantis IK, Vouloumanou EK, Rafailidis PI. Antibiotics versus placebo in the treatment of women with uncomplicated cystitis: a meta-analysis of randomized controlled trials. The Journal of infection. 2009;58(2):91–102. [DOI] [PubMed] [Google Scholar]
- 7.Geller AI, Lovegrove MC, Shehab N, Hicks LA, Sapiano MRP, Budnitz DS. National Estimates of Emergency Department Visits for Antibiotic Adverse Events Among Adults-United States, 2011–2015. J Gen Intern Med. 2018;33(7):1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. In.
- 9.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(5):e103–120. [DOI] [PubMed] [Google Scholar]
- 10.Grigoryan L, Trautner BW, Gupta K. Diagnosis and management of urinary tract infections in the outpatient setting: a review. Jama. 2014;312(16):1677–1684. [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. FDA drug safety communication: fluoroquinolone antibiotics. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM513019.pdf. AccessedFebruary 5, 2018.
- 12.Grigoryan L, Nash S, Zoorob R, et al. Qualitative Analysis of Primary Care Provider Prescribing Decisions for Urinary Tract Infections. Antibiotics (Basel, Switzerland). 2019;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinkerton M, Bongu J, James A, Lowder J, Durkin M. A qualitative analysis of diagnostic testing, antibiotic selection, and quality improvement interventions for uncomplicated urinary tract infections. PloS one. 2020;15(9):e0238453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durkin MJ, Keller M, Butler AM, et al. An Assessment of Inappropriate Antibiotic Use and Guideline Adherence for Uncomplicated Urinary Tract Infections. Open forum infectious diseases. 2018;5(9):ofy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez GV, Roberts RM, Albert AP, Johnson DD, Hicks LA. Effects of knowledge, attitudes, and practices of primary care providers on antibiotic selection, United States. Emerg Infect Dis. 2014;20(12):2041–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark AW, Durkin MJ, Olsen MA, et al. Rural-urban differences in antibiotic prescribing for uncomplicated urinary tract infection. Infection Control and Hospital Epidemiology (In Press). 2021. [DOI] [PMC free article] [PubMed]
- 17.Foxman B The epidemiology of urinary tract infection. Nature reviews Urology. 2010;7(12):653–660. [DOI] [PubMed] [Google Scholar]
- 18.Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Annals of internal medicine. 2001;135(1):41–50. [DOI] [PubMed] [Google Scholar]
- 19.Truven Health MarketScan® Research Databases. MarketScan user guide, commercial claims and encounters, medical supplemental and coordination of benefits 2015.
- 20.Lund JL, Richardson DB, Sturmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Current epidemiology reports. 2015;2(4):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiese AD, Griffin MR, Stein CM, et al. Validation of discharge diagnosis codes to identify serious infections among middle age and older adults. BMJ open. 2018;8(6):e020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Mourik MS, van Duijn PJ, Moons KG, Bonten MJ, Lee GM. Accuracy of administrative data for surveillance of healthcare-associated infections: a systematic review. BMJ open. 2015;5(8):e008424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 24.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ Jr. Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circulation Cardiovascular quality and outcomes. 2013;6(5):604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. Paper presented at: SAS Global Forum2012; Orlando, FL. [Google Scholar]
- 27.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. 2012.
- 28.Pfizer Laboratories Div Pfizer Inc. Trimethoprim/Sulfamethoxazole (Septra) Package Insert. https://rsc.niaid.nih.gov/sites/default/files/Trimethoprim-Sulfamethoxazle%20%28Septra%29%20PI%20dated%20August%202018.pdf. AccessedOctober 9, 2020.
- 29.Hooton TM, Gupta K. Acute simple cystitis in women. 2020; https://www.uptodate.com/contents/acute-simple-cystitis-in-women#H899949213.
- 30.Gerber JS, Ross RK, Bryan M, et al. Association of Broad- vs Narrow-Spectrum Antibiotics With Treatment Failure, Adverse Events, and Quality of Life in Children With Acute Respiratory Tract Infections. Jama. 2017;318(23):2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams DJ, Hall M, Shah SS, et al. Narrow vs broad-spectrum antimicrobial therapy for children hospitalized with pneumonia. Pediatrics. 2013;132(5):e1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melander RJ, Zurawski DV, Melander C. Narrow-Spectrum Antibacterial Agents. Medchemcomm. 2018;9:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein RD, Hultgren SJ. Urinary tract infections: microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat Rev Microbiol. 2020;18(4):211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–337. [DOI] [PubMed] [Google Scholar]
- 36.Kim DK, Kim JH, Lee JY, et al. Reappraisal of the treatment duration of antibiotic regimens for acute uncomplicated cystitis in adult women: a systematic review and network meta-analysis of 61 randomised clinical trials. Lancet Infect Dis. 2020. [DOI] [PubMed]
- 37.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. 2016;65(6):1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


