Abstract
Alternative splicing (AS) contributes to the diversity of the proteome by producing multiple isoforms from a single gene. Although short‐read RNA‐sequencing methods have been the gold standard for determining AS patterns of genes, they have a difficulty in defining full‐length mRNA isoforms assembled using different exon combinations. Tropomyosin 1 (TPM1) is an actin‐binding protein required for cytoskeletal functions in non‐muscle cells and for contraction in muscle cells. Tpm1 undergoes AS regulation to generate muscle versus non‐muscle TPM1 protein isoforms with distinct physiological functions. It is unclear which full‐length Tpm1 isoforms are produced via AS and how they are regulated during heart development. To address these, we utilized nanopore long‐read cDNA sequencing without gene‐specific PCR amplification. In rat hearts, we identified full‐length Tpm1 isoforms composed of distinct exons with specific exon linkages. We showed that Tpm1 undergoes AS transitions during embryonic heart development such that muscle‐specific exons are connected generating predominantly muscle‐specific Tpm1 isoforms in adult hearts. We found that the RNA‐binding protein RBFOX2 controls AS of rat Tpm1 exon 6a, which is important for cooperative actin binding. Furthermore, RBFOX2 regulates Tpm1 AS of exon 6a antagonistically to the RNA‐binding protein PTBP1. In sum, we defined full‐length Tpm1 isoforms with different exon combinations that are tightly regulated during cardiac development and provided insights into the regulation of Tpm1 AS by RNA‐binding proteins. Our results demonstrate that nanopore sequencing is an excellent tool to determine full‐length AS variants of muscle‐enriched genes.
Keywords: alternative splicing, heart development, long‐read DNA sequencing, RNA‐binding proteins, tropomyosin
1. INTRODUCTION
Gene regulation by alternative splicing (AS) is an important contributor to development and tissue identity.1 AS not only controls gene expression but also generates different isoforms of genes. Genome‐wide analyses indicate that the majority of human genes undergo AS.2 Currently, many computational approaches are available to investigate AS patterns based on short‐read RNA sequencing. However, with these techniques it can be difficult to determine the connectivity of multiple exons in a given transcript. This becomes more challenging if a given gene has many potential isoforms. Recent advances in nanopore sequencing technology allow sequencing of ultra‐long DNA sequences.3, 4 The Oxford MinION sequencer is a portable device that provides real‐time, high‐throughput and long‐read sequencing with <10% error rate.5, 6, 7 This technology, therefore, is very attractive to study complex AS patterns in the context of full‐length transcripts.
TPM1 is a coiled‐coil protein that wraps around the actin molecules and provides stability to actin filaments. TPM1 is the predominant tropomyosin gene expressed in cardiac muscle and plays a significant role in muscle contraction.8 TPM1 is required for myofibril organization,9 myocardial contraction10 and cardiac development.11 Mutations or aberrant expression of TPM1 is associated with familial hypertrophic cardiomyopathy,12, 13, 14, 15 dilated cardiomyopathy16, 17 and heart failure.18 TPM1 has 15 exons, several of which are alternatively spliced, generating many gene isoforms generated via AS that are tissue specific and developmentally regulated.19 These multiple isoforms render distinct functions including cytoskeleton support and muscle contraction in the heart.20, 21, 22, 23 However, it is still unclear what mechanisms dictate highly coordinated AS of Tpm1 that impacts its expression and function in a cell‐ and development‐specific manner. In this study, we used nanopore sequencing to identify full‐length transcripts of Tpm1 gene with complex AS patterns in the heart.
RBFOX2 is an RNA‐binding protein, which regulates AS by binding to a highly conserved motif ((U)GCAUG) in pre‐mRNAs.24, 25, 26, 27 RBFOX2 is important for muscle differentiation,28, 29 maintaining muscle mass30 and sustaining muscle function.31 We and other groups have shown that RBFOX2 is involved in cardiovascular diseases including hypoplastic left heart syndrome,32, 33, 34 heart failure35 and diabetic cardiomyopathy.36 RBFOX2‐binding sites are enriched near alternative exons that are developmentally regulated postnatally in the heart,37 suggesting a role for RBFOX2 in the regulation of AS during postnatal heart development.
Using nanopore cDNA sequencing, we identified full‐length Tpm1 isoforms with unique exon combinations that are regulated during rat heart development. We found that muscle and non‐striated muscle‐specific Tpm1 isoforms were generated via AS of specific exons during rat heart development. We uncovered that RBFOX2 regulates AS of rat Tpm1 exon 6a. Furthermore, we found that RBFOX2 and PTBP1 antagonistically control AS of Tpm1 exon6a. Overall, our results reveal that Tpm1‐spliced isoforms are tightly regulated during rat cardiac development and that RNA‐binding proteins RBFOX2 and PTBP1 have opposing roles in controlling developmentally regulated Tpm1 AS. Our findings have broad implications in defining complex AS patterns of abundant cardiac muscle‐enriched genes using nanopore cDNA sequencing.
2. MATERIALS AND METHODS
2.1. Cell culture
H9c2 cells (ATCC CRL‐1446) were cultured and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (ATCC 30‐2002), supplemented with 10% foetal bovine serum (ATCC 30–2020) and 100 units/ml penicillin and streptomycin (Thermo Fisher Scientific 15140122).
2.2. Transfections
For siRNA‐KD experiments, H9c2 cells were seeded at 106 cells per 100 mm dish and transfected with 25 nM scrambled siRNA (Invitrogen AM4611), Rbfox2 siRNA (Invitrogen siRNA ID# s96620) or PTBP siRNAs (Qiagen cat# SI02649206 and SI04255146) using Lipofectamine RNAiMAX (Thermo Fisher Scientific). Cells were harvested 72 h post‐transfection for RNA or protein extraction. For rescue experiments, 3 × 106 H9c2 cells were transfected with eGFP (Sigma‐Aldrich), human GFP‐RBFOX2 (transcript variant 3) (Addgene, plasmid #63086) or empty vector (pcDNA 5) together with scrambled or Rbfox2 specific siRNAs using Neon Nucleofection System (Thermo Fisher Scientific) as described previously.38 RNA was harvested 48 h post‐transfection.
2.3. Nanopore sequencing with MinION
RNA was extracted from cells using TRIzol (Invitrogen 15596‐018) by following the manufacturer’s protocol. For nanopore sequencing, three sets of E20 and three sets of 6 M rat heart RNA (Zyagen) were used. Total cellular RNA was first poly(A) enriched (New England Biolabs S1550S) and then amplified using oligo‐dT primers and template switching oligos using Oxford Nanopore Technologies (ONT) cDNA‐PCR sequencing kit (PCS108) as described by the manufacturer. Samples were multiplexed using ONT barcodes. Pooled samples were sequenced on R9.4 flowcells for 36. Reads were demultiplexed and base‐called using Albacore and mapped to the rat genome (rn6) using the splice function of minimap2 as described previously.39 Analysis pipeline is shown in Figure S1.
2.4. RT‐qPCR
Total RNA from cells and rat hearts (purchased from Zyagen) at E13 (pooled), E16 (pooled), E18 (pooled), E20 (pooled), postnatal day1 (D1) (pooled) and 6 M (pooled) stages were extracted using TRIzol. 2μg of total RNA was used for cDNA synthesis using AMV reverse transcriptase (15 units/μg, Life Biosciences). For RT‐qPCR, master mix was set up by mixing 5 μl of cDNA, 3 μl of H2O, 2 μl of PCR gene‐specific primer (10X conc) (Table S1) and 10 μl of master mix (Roche 04707516001) in 20 μl reaction. The RT‐qPCR was conducted using LightCycler 480 Instrument (Roche) using the following conditions: 95°C 10 s; 62°C 15 s; and 72°C 10 s for 40 cycles. Melting curve was obtained to ensure a single product. ΔC t method was adopted for quantification. Semi‐quantitative RT‐PCR instead of RT‐qPCR was used for determining Tpm1 short and Tpm1 long transcript levels due to the AS of exon 9a that generates two different sized DNA bands after amplification. 2 μg of total RNA was used for cDNA synthesis using AMV reverse transcriptase (15 units/μg, Life Biosciences). PCR was performed using 5 μl of cDNA, 25 μM dNTPs, 100 ng of each gene‐specific forward and reverse primer and 0.2 μl of Biolase Taq polymerase (Bioline) in a 20 μl reaction. The amplified products were analysed on 5% acrylamide gel. Hprt was used as an internal control for RT‐PCR quantification.
2.5. Western blot
The membrane was blocked with 5% dry fat‐free milk solution in PBS containing 0.1% Tween (PBST) at RT for 1 h and then incubated with indicated primary antibodies overnight at 4°C. The membrane was washed with PBST for 15 min three times and incubated with HRP‐conjugated secondary antibody for 1 h at RT followed by three washes using PBST. Immobilon Western chemiluminescent (Millipore WBKLS0500) kit was used to detect HRP activity of the secondary antibody. The membrane was then imaged using ChemiDoc Touch imaging system (Bio‐Rad). ImageJ software was used for band intensity quantification. Primary antibodies used for this study are as follows TPM1 (1:1000, Cell Signaling, D12H4), RBFOX2 (1:1000, Abcam, ab57154), PTBP1 (1:5000, a gift from Dr. Mariano Garcia‐Blanco) and α‐tubulin (1:20,000, Sigma‐Aldrich, T6074).
3. RESULTS
3.1. Nanopore sequencing identifies full‐length Tpm1 isoforms that undergo alternative splicing transitions during rat heart development
Tpm1 has 15 exons, several of which undergo AS regulation, generating different Tpm1 isoforms. To determine the exact combination of exons in full‐length Tpm1 isoforms in the heart at different developmental stages, we used total RNA from embryonic day 20 (E20, n = 3) and 6‐month (6 M, n = 3) rat hearts and generated cDNA for nanopore sequencing on the Oxford Nanopore Technologies’s MinION. We picked late embryonic and adult stages because between these stages the heart undergoes structural and functional changes important for cardiac output and contractility relevant to TPM1 function.
We obtained ~310,000–740,000 reads spanning 3000–4300 unique mRNAs, 190–413 of which had a coverage of greater than 100 reads that were mapped to the rn6 genome using Minimap2.40 We obtained average of 200,000 reads mapped to Tpm1. Reads that were mapped to Tpm1 are illustrated in Figure 1A. In E20 rat hearts, we identified Tpm1 isoforms generated via AS of exons 1a/1b, 2a/2b, 6a/6b, 9a, 9b and 9d (Figure 1A, top panel), consistent with previous findings that these mutually exclusive exons are alternatively spliced.41, 42 Strikingly, at this developmental stage Tpm1 transcripts displayed two distinct 3` ends defined by exon usage of either exon 9b or 9d that contain both 3´UTR and coding region (Figure 1A, top panel). There were also transcripts that ended with exon 9a (Figure 1A–C). The differences in the 3` length of Tpm1 were generated via AS of terminal exons 9a‐9b and 9d (Figure 1A). Inclusion of exon 9b resulted in short Tpm1 isoforms whereas inclusion of exon 9d generated long Tpm1 isoforms (Figure 1A, top panel). It has been previously shown that Tpm1 isoforms that contain exon 9b are primarily expressed in striated muscle (muscle‐specific isoform) and that contain exon 9d are expressed in smooth muscle and other cell types (non‐striated muscle isoform, in short non‐muscle).41, 42 Cardiac output increases and contractions become more coordinated at adult stages in comparison with embryonic stages. Consistent with this, muscle‐specific Tpm1 isoforms that end with exon 9b were predominantly expressed at adult stages. On the contrary, non‐muscle Tpm1 isoforms that end with exon 9d (Figure 1A, bottom panel) were present in embryonic hearts but were dramatically decreased in adult hearts. Exon 9c was not detectable in rat hearts as expected because this isoform is mainly brain specific.43
FIGURE 1.
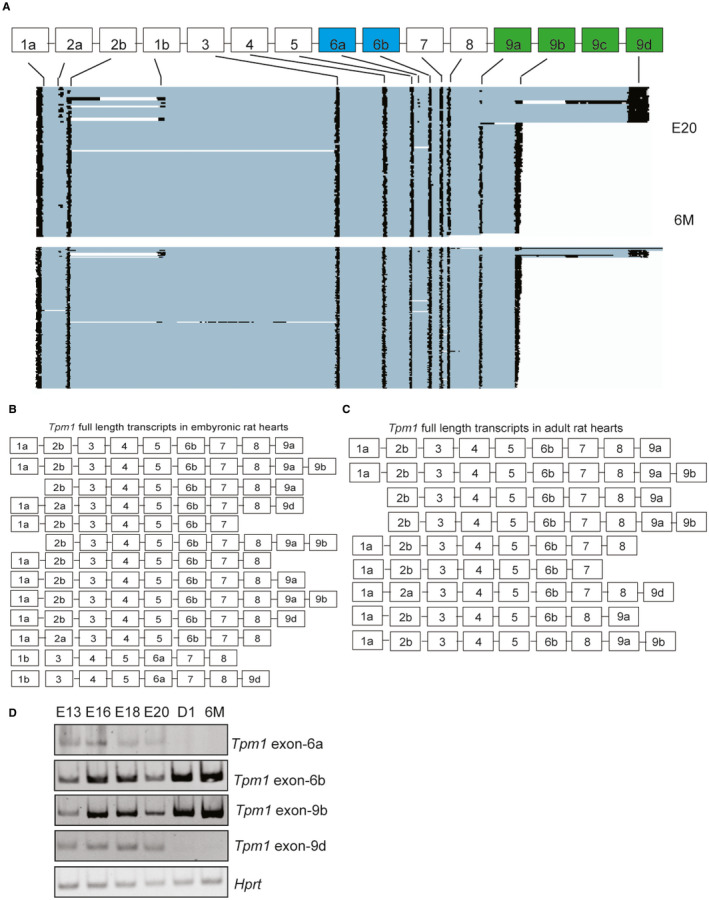
Identification of full‐length Tpm1 isoforms generated via alternative splicing during rat heart development using nanopore sequencing. (A) Representative images of nanopore sequencing reads mapped to Tpm1 gene in rat hearts at different developmental stages: embryonic day 20 (E20) and 6‐months (6 M) old (n = 3). (B) Abundant full‐length Tpm1 isoforms in embryonic day 20 rat hearts determined by nanopore reads. (C) Abundant full‐length Tpm1 isoforms identified in 6‐month‐old rat hearts by nanopore sequencing. (D) Relative ratio of Tpm1 isoforms that include exon 6b vs. 6a or exon 9b vs. 9d in rat hearts at E13, E16, E18, E20, D1 (1‐day‐old) and 6 M
We mapped all the exons and obtained full‐length Tpm1 transcripts and calculated the percentage of each isoform using the nanopore reads (Supplemental Excel File S1). In rat hearts, Tpm1 exons 1a, 2b, 6b, 9a and 9b were more frequently used (Figure 1A–C). Several Tpm1 transcripts started with exon 2b instead of exon 1a or exon 1b (Figure 1B,C). Interestingly, several Tpm1 transcripts ended with exon 7 or 8 instead of exon 9a, 9d or 9d (Figure 1B,C).
In addition, we found unique linkages between internal and terminal exons in a given Tpm1 transcript. For example, exon 9b was present in all muscle‐specific Tpm1 transcripts that included exon 9a in rat hearts (Figure 1A–C). This agrees with predominant expression of Tpm1 isoforms that contain both exon 9a and 9b at adult stages when the heart contracts more coordinately in comparison with the embryonic heart. Like exon9a‐9b linkage, we found that exon 2a was almost always included in non‐muscle Tpm1 isoforms that ended with exon 9d (Figure 1A, top panel and 1B). Exon 2a‐9d containing isoforms were barely detectable in adult rat hearts (Figure 1A, bottom panel and 1C). Also, Tpm1 isoforms that contain exon 6a were present scarcely at E20 rat hearts (Figure 1A,B), but they were diminished in adult rat hearts (Figure 1A,C). When we examined the most abundant Tpm1 full‐length transcripts in E20 hearts, we noticed that alternative exons 1b, 2a, 6a and 9d were more frequently used in Tpm1 full transcripts (Figure 1B, Supplementary Excel File S1) than that adult rat hearts (Figure 1C, Supplementary Excel File S1). These exons are associated with non‐striated muscle isoforms of Tpm1. There was a general increase in striated muscle‐specific exon 6b and 9b inclusions between embryonic and adult stages; however, the inclusion of these isoforms was lower at E20 compared to earlier embryonic stages and postnatal stage. It might be because E20 is a transition time between gestation and postnatal stages when cardiomyocytes start to undergo hypertrophy. Tpm1 muscle‐specific isoforms may be regulated differentially at this stage to accommodate this transition at postnatal stages.
To validate the nanopore sequencing data and assess expression of Tpm1 isoforms in rat hearts during different developmental stages (E13, E16, E18, E20, 1‐day old (D1) and 6‐month old), we designed primers to determine the inclusion of exons 6a, 6b, 9b and 9d via RT‐qPCR (Table S1). Consistent with the nanopore sequencing data, the expression of Tpm1 transcripts that include muscle‐enriched exons 6b and 9b gradually increased after birth (Figure 1D). Exons 9a and 9b are important determinants for actin‐binding affinity of TPM1 and interactions with troponin complex in the presence or absence of Ca2+.41, 44 The exon 6a is required for actin binding and replacing exon 6b with exon 6a increases TPM1 actin‐binding affinity.45 The differences in inclusion of internal exon 6a, exon 2a and terminal exons 9a‐9b and 9d in Tpm1 isoforms expressed in rat hearts correlate with the actin‐binding activity of Tpm1 during muscle contraction at different developmental stages.
3.2. The RNA‐binding protein RBFOX2 controls AS of developmentally regulated exons of Tpm1
RBFOX2 is an AS regulator abundantly expressed in skeletal and heart muscle.30, 36, 46 RBFOX2‐binding sites are enriched in introns flanking alternative exons that are regulated during postnatal mouse hearts.47 To determine the role of RBFOX2 in developmentally regulated AS of Tpm1, we depleted RBFOX2 in embryonic rat heart‐derived H9c2 cells (Figure 2A). We performed RT‐qPCR to validate AS of mutually exclusive exons 6b vs. 6a. Tpm1 isoforms including exon 6a were dramatically increased upon RBFOX2 depletion (Figure 2B). These results show that RBFOX2 KD altered AS and of Tpm1 exon 6a, which is present only in non‐muscle Tpm1 isoforms (Figure 1A).41, 42
FIGURE 2.
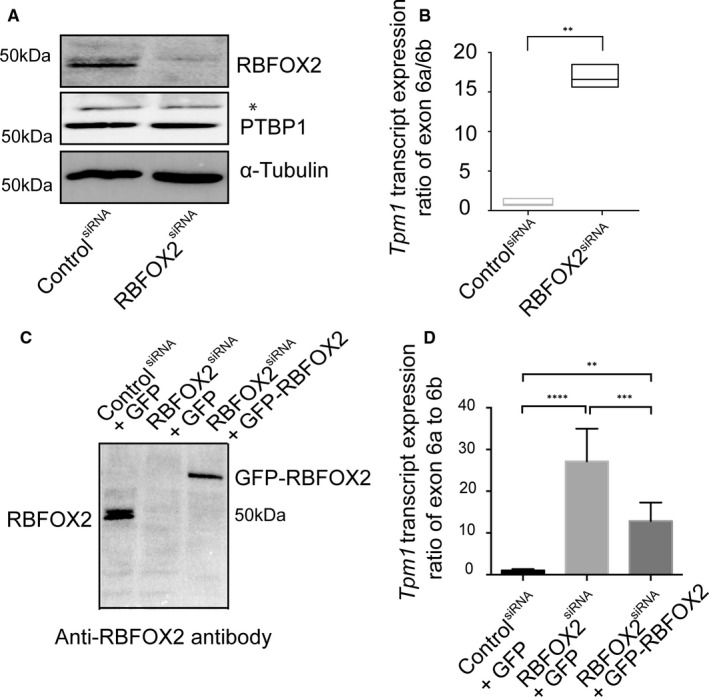
RBFOX2 regulates AS of rat Tropomyosin 1 (Tpm1) exon 6a. (A) Representative Western blot images of RBFOX2 and PTBP1 in control and RBFOX2‐depleted H9c2 cells. α‐tubulin was used as a loading control. (B) The ratio of expression levels of Tpm1 exons 6a vs. 6b in control and RBFOX2‐depleted H9c2 cells determined by RT‐qPCR. Expression levels of Tpm1 exon 6a to 6b in control cells were normalized to 1. Data represent means ± SD. Statistical significance was calculated using t test to compare two different groups in three independent experiments (n = 3). **p = .013. GFP‐REBFOX2 rescue experiments: (C) Western blot analysis of endogenous or GFP‐tagged RBFOX2 protein in (lane1) scrambled siRNA‐treated, (lane 2) RBFOX2 siRNA‐treated, (lane 3) RBFOX2 siRNA‐treated H9c2 cells ectopically expressing of GFP or GFP‐RBFOX2 using anti‐RBFOX2 antibody. (D) The expression level ratios of Tpm1 transcripts containing exons 6a vs. 6b in control, RBFOX2‐depleted or RBFOX2‐depleted GFP or GFP‐RBFOX2 expressing cells. Expression ratios in control (1) cells were normalized to 1. Data represent means ± SD. Statistical significance was calculated using one‐way ANOVA to compare three different groups in three independent experiments (n = 3). p‐Value for ControlsiRNA+GFP vs. RBFOX2siRNA+GFP is p < .000001; for RBFOX2siRNA+ GFP vs. RBFOX2siRNA+ GFP‐RBFOX2 is p = .000683; for ControlsiRNA+GFP vs. RBFOX2siRNA+ GFP‐RBFOX2 is p = .003409
The RNA‐binding protein PTBP1 has been shown to regulate Tpm1 AS.48, 49, 50, 51, 52 RBFOX2 has been shown to regulate AS and mRNA levels of PTBP family member.53 To rule out the possibility that the effect of RBFOX2 on AS of Tpm1 was mediated via changes in PTBP1, we examined PTBP1 protein levels in control and RBFOX2‐depleted H9c2 cells. RBFOX2 KD did not affect PTBP1 protein levels (Figure 2A).
3.3. Ectopic expression of RBFOX2 partially rescues developmentally regulated AS of Tpm1 in RBFOX2‐depleted cells
We tested whether ectopic expression of RBFOX2 can rescue Tpm1 AS changes in RBFOX2‐depleted cells. We expressed GFP tagged RBFOX2 in RBFOX2‐depleted H9c2 cells and found that GFP‐RBFOX2 protein was expressed at low levels like the endogenous RBFOX2 levels in RBFOX2 KD cells (Figure 2C, lane 1 vs. 3). We tested AS of Tpm1 exons 6a/6b in RBFOX2 KD cells expressing either GFP or GFP‐RBFOX2. Expression of GFP‐RBFOX2 partially rescued AS changes of Tpm1 exon 6a/6b (Figure 2D), suggesting that RBFOX2 is a regulator of AS of developmentally regulated Tpm1 alternative exons 6a.
3.4. RBFOX2 and PTBP1 antagonistically control developmentally regulated AS of Tpm1
The RNA‐binding protein PTBP1 is a known regulator of Tpm1 AS of mutually exclusive exons 2a/2b19, 49 and exons 6a/6b19, 50 and terminal exons.51, 52 AS of these Tpm1 exons are also developmentally regulated. We found that RBFOX2 also regulates AS of mutually exclusive exons 6a/6b. Therefore, we examined the ENCODE enhanced cross‐linking immunoprecipitation RNA‐seq (eCLIP) data54, 55 to determine where these RNA‐binding proteins bind with respect to the developmentally regulated alternative exons of Tpm1. We found binding clusters for both PTBP1 and RBFOX2 mapped in or near Tpm1 exons 6a in both eCLIP experiments, suggesting that these RNA‐binding proteins may regulate Tpm1 AS antagonistically (Figure 3A).
FIGURE 3.
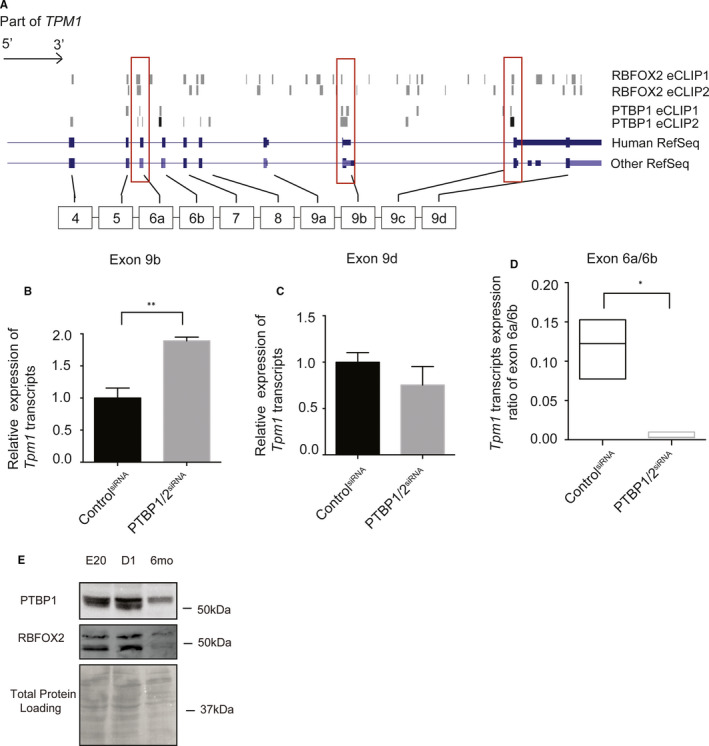
PTBP controls developmentally regulated AS of Tpm1 antagonistically to RBFOX2. (A) RBFOX2 eCLIP and PTBP1 eCLIP‐seq reads mapped to the human Tpm1 gene. (B) Expression levels of Tpm1 transcripts containing exon 9b (muscle) in H9c2 cells treated with control or PTBP1/2 siRNA. mRNA levels in control cells were normalized to 1. Statistical significance was calculated using t test to compare two different groups in three independent experiments (n = 3). p‐value is represented as **p = .0050. (C) Expression levels of Tpm1 transcripts containing exon 9d (non‐muscle) in H9c2 cells treated with control or PTBP1/2 siRNA. mRNA levels in control cells were normalized to 1. (D) Expression of Tpm1 transcripts containing exon 6a vs. 6b in H9c2 cells treated with control or PTBP1/2 siRNA. Data represent means ± SD. Expression ratio of Tpm1 exon 6a to 6b in control cells was normalized to 1. Statistical significance was calculated using t test to compare two different groups in three independent experiments (n = 3). p‐value is represented as *p = .0347. (E) Western blot analysis of PTBP1 and RBFOX2 in rat hearts at different embryonic and postnatal stages. Even protein loading was monitored by Ponceau S stain of the membrane
PTBP1‐binding sites were present in both CLIP experiments near Tpm1 exons 6a, 6b and 9b (Figure 3A). To validate the regulation of these exons by PTBP, we knocked down PTBP proteins using PTBP1/2 siRNAs in embryonic rat heart‐derived H9c2 cells. PTBP depletion increased muscle‐specific Tpm1 transcripts containing exon 9b (Figure 3B). There was no significant change in non‐muscle Tpm1 isoforms that are generated using exon 9d in PTBP1 KD cells (Figure 3C), consistent with the lack of PTBP1‐binding sites within or near this exon in Tpm1 pre‐mRNA (Figure 3A).
Inclusion of Tpm1 exon 6a was inhibited in PTBP‐depleted H9c2 cells (Figure 3D). This was contrary to what was observed in RBFOX2‐depleted H9c2 cells (Figure 2B,D). These findings indicate that RBFOX2 and PTBP1 antagonistically control developmentally regulated AS of Tpm1 exon 6a, contributing to the generation of Tpm1 isoforms with different actin‐binding capabilities.
To better understand how Tpm1 muscle‐specific isoforms are regulated by RBFOX2 and PTBP1 during rat heart development, we checked RBFOX2 and PTBP1 protein levels during rat heart development. Both PTBP1 and RBFOX2 protein levels were abundant at embryonic stages (Figure 3E) but levels went down in adult stages. Because PTBP1 is a repressor of muscle‐specific Tpm1 isoforms, low levels of PTBP1 in adult rat hearts (Figure 3E) correlated well with predominant expression of Tpm1 muscle‐specific isoforms in adult hearts (Figure 1A, bottom panel and 1C).
3.5. Developmentally regulated AS patterns of TPM2 and TPM3
There are four family members of TPM in vertebrates, namely TPM1, TPM2, TPM3 and TPM4. TPM1 and TPM2 are predominantly expressed in muscle and are involved in contraction,56, 57, 58, 59 whereas TPM3 and TPM4 are enriched in non‐muscle cells supporting actin cytoskeleton.20, 21, 42, 56, 57, 60, 61, 62, 63, 64 To determine whether AS of other TPM genes was also regulated during rat heart development, we examined our nanopore sequencing data. Tpm2 and Tpm3 displayed AS transitions between embryonic and adult stages in rat hearts as well as changes in their expression levels (Figure 4A,B top vs. bottom panels). Tpm2 isoforms that end with exon 9d were predominant in adult rat hearts (Figure 4A). Similar to Tpm1, Tpm2 exons 6a/6b and 9b/9d differentially spliced during rat heart development (Figure 4A vs. Figure 1A–C, Figure S2). Tpm3 exons 1a/1b, exons 6a/6b and exons 9a/9b were also developmentally regulated. Interestingly, AS of Tpm4, which is enriched in non‐muscle cells, was not regulated via AS during rat heart development (Figure 4C, Figure S2).
FIGURE 4.
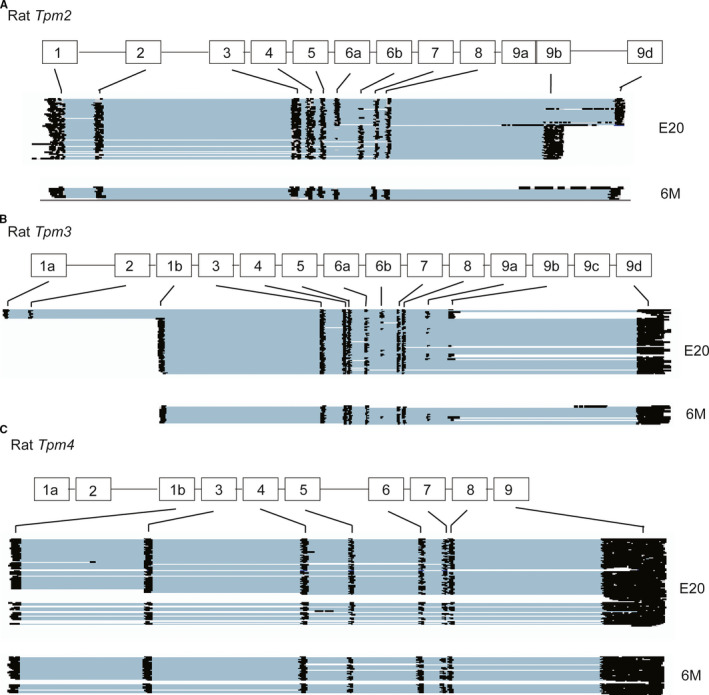
Full‐length isoforms of Tpm2 (A), Tpm3 (B) and Tpm4 (C) identified by nanopore sequencing in embryonic day 20 (E20) and 6‐month‐old (6 M) rat hearts
4. DISCUSSION
Tpm1 is an essential gene required for the organization of the myofibril,9 myocardial contraction10 and heart development.11 Tpm1 has 15 exons, in which internal exons 1a/1b, 2a/2b and 6a/6b and terminal exons 9a, 9b, 9c and 9d are alternatively spliced.19, 20, 49, 52, 65, 66, 67 AS of Tpm1 isoforms is tissue‐ and development‐specific and exhibits distinct physiological functions20, 21, 22, 23 including cytoskeleton support for almost every eukaryotic cells and muscle contraction for striated muscle cells.56, 57, 58, 59 Full‐length Tpm1 isoforms that are generated via extensive AS regulation in different cell types and at different developmental stages have not been obvious using short‐read RNA‐sequencing methods. It is because short‐read RNA sequencing is unable to provide direct information about how different exons are connected and incorporated into full‐length transcripts to generate different Tpm1 isoforms. In addition, bias is generated from fragmentation during library preparation. Using nanopore long‐read sequencing by MinION, we were able to detect full‐length isoforms of abundant cardiac genes including tropomyosin family members without extensive RNA manipulation and gene‐specific PCR amplification.
Nanopore sequencing identified full‐length Tpm isoforms with different internal and terminal exon combinations in rat hearts that are regulated during development. We defined striated muscle vs. non‐striated muscle Tpm1 isoforms based on the specific exon combinations and internal‐terminal exon linkages in embryonic vs. adult hearts. We also observed that striated muscle‐specific Tpm1 isoforms were abundantly expressed at both embryonic and adult stages, but they became the predominant isoform in adult hearts. Embryonic rat hearts displayed more non‐striated muscle Tpm1 isoforms in comparison with adult rat hearts. Loss of non‐muscle‐specific Tpm1 isoforms in adult rats is in agreement with the increased muscle contraction capability of adult hearts in comparison to embryonic hearts.
We identified RBFOX2 as a regulator of developmentally regulated AS of Tpm1, consistent with a recent study that identified a splicing change in TPM1 in mouse embryos in which RBFOX2 was conditionally ablated in neural crest cells.68 Our work provides evidence that changes in Tpm1 AS may contribute to heart and muscle defects observed in RBFOX2 loss of function in human heart diseases and experimental animal models.28, 30, 31, 32, 33, 34, 35, 36, 46
Using nanopore sequencing, we validated previous findings that AS of exons 6a/6b of Tpm1 is developmentally regulated in rat hearts and identified full‐length transcripts of Tpm1 during rat heart development. Here, we showed that AS of exons 6a vs. 6b was controlled antagonistically by RBFOX2 and PTBP. The exon 6a/6b is required for cooperative actin binding.45 Therefore, the dynamic and synergic regulation of specific internal exon 6a/6b via RBFOX2 and PTBP during cardiac development is critical to ensure selective Tpm1 isoforms expressed in equilibrium to exert specific actin‐binding activity during different states of muscle contraction.
Our results support the idea that RBFOX2 is a repressor of Tpm1 exon 6a inclusion. Conversely, PTBP1 is an activator of exon 6a, consistent with the previous reports.51, 52 The expression levels of both PTBP1 and RBFOX2 were high at embryonic stages but both were decreased at adult stages in rat hearts. While the predominant expression of muscle‐specific Tpm1 isoforms at adult stages was consistent with downregulation of PTBP1, the abundance of muscle‐specific Tpm1 isoforms at embryonic stages is consistent with high levels of RBFOX2. The interplay between these RNA‐binding proteins in regulating Tpm1 AS correlates well with their different roles as repressors or activators of exon inclusion.
It is quite common that cardiac structural genes undergo cooperative AS and alternative polyadenylation patterns that determine their specific functions and expression profiles during cardiac development. Mutations in these structural genes, including TPM1, are linked to human heart diseases. Our results using nanopore sequencing provide an efficient way to reveal full‐length isoforms of cardiac structural genes and their regulation during heart development. In addition, our work may pave the way for future studies to determine the functional consequences of non‐coding mutations on post‐transcriptional regulation of cardiac structural genes using nanopore sequencing.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Jun Cao: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Validation (equal); Writing‐original draft (supporting). Andrew L. Routh: Conceptualization (equal); Funding acquisition (supporting); Methodology (lead); Resources (supporting); Software (lead); Writing‐review & editing (equal). Muge N. Kuyumcu‐Martinez: Conceptualization (lead); Data curation (equal); Formal analysis (lead); Funding acquisition (lead); Investigation (equal); Project administration (lead); Resources (lead); Supervision (lead); Writing‐original draft (equal); Writing‐review & editing (lead).
Supporting information
Supplementary Material
Fig S1
Fig S2
Table S1
ACKNOWLEDGEMENTS
The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NHLBI of NIH.
Cao J, Routh AL, Kuyumcu‐Martinez MN. Nanopore sequencing reveals full‐length Tropomyosin 1 isoforms and their regulation by RNA‐binding proteins during rat heart development. J Cell Mol Med. 2021;25:8352–8362. 10.1111/jcmm.16795
Funding information
This work was supported, in part, UTMB Department of Biochemistry and Molecular Biology Bridging funds, a grant from the National Institutes of Health/National Heart Lung Blood Institute [1R01HL135031], a grant from CPRIT [RP190556] and a grant from American Heart Association [20TPA35490206] to M.N.K‐M. J.C. is funded by a post‐doctoral fellowship from American Heart Association [18POST3399018]. A.R. is supported by start‐up funds from UTMB. We thank Dr. Garcia‐Blanco for providing us PTBP1 antibody.
Contributor Information
Andrew L. Routh, Email: alrouth@utmb.edu.
Muge N. Kuyumcu‐Martinez, Email: nmmartin@utmb.edu.
DATA AVAILABILITY STATEMENT
Nanopore sequencing data were deposited to NCBI SRA database with project number PRJNA517125.
REFERENCES
- 1.Baralle FE, Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol. 2017;18(7):437‐451. 10.1038/nrm.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang ET, Sandberg R, Luo S, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470‐476. 10.1038/nature07509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H, Giordano F, Ning Z. Oxford Nanopore MinION sequencing and genome assembly. Genomics Proteomics Bioinformatics. 2016;14(5):265‐279. 10.1016/j.gpb.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain M, Koren S, Miga KH, et al. Nanopore sequencing and assembly of a human genome with ultra‐long reads. Nat Biotechnol. 2018;36(4):338‐345. 10.1038/nbt.4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oikonomopoulos S, Wang YC, Djambazian H, Badescu D, Ragoussis J. Benchmarking of the Oxford Nanopore MinION sequencing for quantitative and qualitative assessment of cDNA populations. Sci Rep. 2016;6:31602. 10.1038/srep31602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong LC, Cree S, Lattimore V, et al. Nanopore sequencing of full‐length BRCA1 mRNA transcripts reveals co‐occurrence of known exon skipping events. Breast Cancer Res. 2017;19:Artn 127. 10.1186/S13058-017-0919-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Qu L, Yang L, Wang Y, Zhu H. NanoReviser: an error‐correction tool for nanopore sequencing based on a deep learning algorithm. Front Genet. 2020;11:900. 10.3389/fgene.2020.00900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai F, Wang L, Kawai M. A study of tropomyosin’s role in cardiac function and disease using thin‐filament reconstituted myocardium. J Muscle Res Cell Motil. 2013;34(3–4):295‐310. 10.1007/s10974-013-9343-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas A, Rajan S, Thurston HL, et al. Expression of a novel tropomyosin isoform in axolotl heart and skeletal muscle. J Cell Biochem. 2010;110(4):875‐881. 10.1002/jcb.22599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolska BM, Wieczorek DM. The role of tropomyosin in the regulation of myocardial contraction and relaxation. Pflugers Arch. 2003;446(1):1‐8. 10.1007/s00424-002-0900-3 [DOI] [PubMed] [Google Scholar]
- 11.England J, Granados‐Riveron J, Polo‐Parada L, et al. Tropomyosin 1: multiple roles in the developing heart and in the formation of congenital heart defects. J Mol Cell Cardiol. 2017;106:1‐13. 10.1016/j.yjmcc.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thierfelder L, Watkins H, MacRae C, et al. Alpha‐tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77(5):701‐712. [DOI] [PubMed] [Google Scholar]
- 13.Jongbloed RJ, Marcelis CL, Doevendans PA, et al. Variable clinical manifestation of a novel missense mutation in the alpha‐tropomyosin (TPM1) gene in familial hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41(6):981‐986. [DOI] [PubMed] [Google Scholar]
- 14.Marques MA, de Oliveira GA. Cardiac troponin and tropomyosin: structural and cellular perspectives to unveil the hypertrophic cardiomyopathy phenotype. Front Physiol. 2016;7:429. 10.3389/fphys.2016.00429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muthuchamy M, Pieples K, Rethinasamy P, et al. Mouse model of a familial hypertrophic cardiomyopathy mutation in alpha‐tropomyosin manifests cardiac dysfunction. Circ Res. 1999;85(1):47‐56. [DOI] [PubMed] [Google Scholar]
- 16.Karam CN, Warren CM, Rajan S, de Tombe PP, Wieczorek DF, Solaro RJ. Expression of tropomyosin‐kappa induces dilated cardiomyopathy and depresses cardiac myofilament tension by mechanisms involving cross‐bridge dependent activation and altered tropomyosin phosphorylation. J Muscle Res Cell Motil. 2011;31(5–6):315‐322. 10.1007/s10974-010-9237-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redwood C, Robinson P. Alpha‐tropomyosin mutations in inherited cardiomyopathies. J Muscle Res Cell Motil. 2013;34(3–4):285‐294. 10.1007/s10974-013-9358-5 [DOI] [PubMed] [Google Scholar]
- 18.Rajan S, Jagatheesan G, Karam CN, et al. Molecular and functional characterization of a novel cardiac‐specific human tropomyosin isoform. Circulation. 2010;121(3):410‐418. 10.1161/CIRCULATIONAHA.109.889725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gooding C, Smith CW. Tropomyosin exons as models for alternative splicing. Adv Exp Med Biol. 2008;644:27‐42. [DOI] [PubMed] [Google Scholar]
- 20.Lin JJ, Eppinga RD, Warren KS, McCrae KR. Human tropomyosin isoforms in the regulation of cytoskeleton functions. Adv Exp Med Biol. 2008;644:201‐222. [DOI] [PubMed] [Google Scholar]
- 21.Perry SV. Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil. 2001;22(1):5‐49. [DOI] [PubMed] [Google Scholar]
- 22.Gunning PW, Hardeman EC, Lappalainen P, Mulvihill DP. Tropomyosin – master regulator of actin filament function in the cytoskeleton. J Cell Sci. 2015;128(16):2965‐2974. 10.1242/jcs.172502 [DOI] [PubMed] [Google Scholar]
- 23.Schevzov G, Whittaker SP, Fath T, Lin JJ, Gunning PW. Tropomyosin isoforms and reagents. Bioarchitecture. 2011;1(4):135‐164. 10.4161/bioa.1.4.17897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeo GW, Coufal NG, Liang TY, Peng GE, Fu XD, Gage FH. An RNA code for the FOX2 splicing regulator revealed by mapping RNA‐protein interactions in stem cells. Nat Struct Mol Biol. 2009;16(2):130‐137. 10.1038/nsmb.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang SC, Ou AC, Park J, et al. RBFOX2 Promotes Protein 4.1R exon 16 selection via U1 snRNP recruitment. Mol Cell Biol. 2012;32(2):513‐526. 10.1128/MCB.06423-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun S, Zhang Z, Fregoso O, Krainer AR. Mechanisms of activation and repression by the alternative splicing factors RBFOX1/2. RNA. 2012;18(2):274‐283. 10.1261/rna.030486.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovci MT, Ghanem D, Marr H, et al. Rbfox proteins regulate alternative mRNA splicing through evolutionarily conserved RNA bridges. Nat Struct Mol Biol. 2013;20(12):1434‐1442. 10.1038/nsmb.2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebastian S, Faralli H, Yao ZZ, et al. Tissue‐specific splicing of a ubiquitously expressed transcription factor is essential for muscle differentiation. Gene Dev. 2013;27(11):1247‐1259. 10.1101/gad.215400.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh RK, Xia Z, Bland CS, et al. Rbfox2‐coordinated alternative splicing of Mef2d and Rock2 controls myoblast fusion during myogenesis. Mol Cell. 2014;55(4):592‐603. 10.1016/j.molcel.2014.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh RK, Kolonin AM, Fiorotto ML, Cooper TA. Rbfox‐splicing factors maintain skeletal muscle mass by regulating calpain3 and proteostasis. Cell Rep. 2018;24(1):197‐208. 10.1016/j.celrep.2018.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallagher TL, Arribere JA, Geurts PA, et al. Rbfox‐regulated alternative splicing is critical for zebrafish cardiac and skeletal muscle functions. Dev Biol. 2011;359(2):251‐261. 10.1016/j.ydbio.2011.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Homsy J, Zaidi S, Shen Y, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. 2015;350(6265):1262‐1266. 10.1126/science.aac9396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma SK, Deshmukh V, Nutter CA, et al. Rbfox2 function in RNA metabolism is impaired in hypoplastic left heart syndrome patient hearts. Sci Rep. 2016;6:Artn 30896. 10.1038/Srep30896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKean DM, Homsy J, Wakimoto H, et al. Loss of RNA expression and allele‐specific expression associated with congenital heart disease. Nat Commun. 2016;7:12824. 10.1038/ncomms12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei CL, Qiu JS, Zhou Y, et al. Repression of the central splicing regulator RBFox2 Is functionally linked to pressure overload‐induced heart failure. Cell Rep. 2015;10(9):1521‐1533. 10.1016/j.celrep.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nutter CA, Jaworski EA, Verma SK, et al. Dysregulation of RBFOX2 is an early event in cardiac pathogenesis of diabetes. Cell Rep. 2016;15(10):2200‐2213. 10.1016/j.celrep.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misra C, Bangru S, Lin F, et al. Aberrant expression of a non‐muscle RBFOX2 isoform triggers cardiac conduction defects in myotonic dystrophy. Dev Cell. 2020;52(6):748‐763.e6. 10.1016/j.devcel.2020.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma SK, Deshmukh V, Liu P, et al. Reactivation of fetal splicing programs in diabetic hearts is mediated by protein kinase C signaling. J Biol Chem. 2013;288(49):35372‐35386. 10.1074/jbc.M113.507426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H. Minimap and miniasm: fast mapping and de novo assembly for noisy long sequences. Bioinformatics. 2016;32(14):2103‐2110. 10.1093/bioinformatics/btw152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34(18):3094‐3100. 10.1093/bioinformatics/bty191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moraczewska J, Nicholson‐Flynn K, Hitchcock‐DeGregori SE. The ends of tropomyosin are major determinants of actin affinity and myosin subfragment 1‐induced binding to F‐actin in the open state. Biochemistry. 1999;38(48):15885‐15892. 10.1021/bi991816j [DOI] [PubMed] [Google Scholar]
- 42.Gunning PW, Schevzov G, Kee AJ, Hardeman EC. Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends Cell Biol. 2005;15(6):333‐341. 10.1016/j.tcb.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 43.Marchenko M, Nefedova V, Artemova N, Kleymenov S, Levitsky D, Matyushenko A. Structural and functional peculiarities of cytoplasmic tropomyosin isoforms, the products of TPM1 and TPM4 genes. Int J Mol Sci. 2021;22(10):5141. 10.3390/ijms22105141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammell RL, HitchcockDeGregori SE. Mapping the functional domains within the carboxyl terminus of alpha‐tropomyosin encoded by the alternatively spliced ninth exon. J Biol Chem. 1996;271(8):4236‐4242. [DOI] [PubMed] [Google Scholar]
- 45.Hammell RL, HitchcockDeGregori SE. The sequence of the alternatively spliced sixth exon of alpha‐tropomyosin is critical for cooperative actin binding but not for interaction with troponin. J Biol Chem. 1997;272(36):22409‐22416. 10.1074/jbc.272.36.22409 [DOI] [PubMed] [Google Scholar]
- 46.Nutter CA, Jaworski E, Verma SK, Perez‐Carrasco Y, Kuyumcu‐Martinez MN. Developmentally regulated alternative splicing is perturbed in type 1 diabetic skeletal muscle. Muscle Nerve. 2017;56(4):744‐749. 10.1002/mus.25599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalsotra A, Xiao X, Ward AJ, et al. A postnatal switch of CELF and MBNL proteins reprograms alternative splicing in the developing heart. Proc Natl Acad Sci USA. 2008;105(51):20333‐20338. 10.1073/pnas.0809045105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gooding C, Edge C, Lorenz M, et al. MBNL1 and PTB cooperate to repress splicing of Tpm1 exon 3. Nucleic Acids Res. 2013;41(9):4765‐4782. 10.1093/nar/gkt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mullen MP, Smith CW, Patton JG, Nadal‐Ginard B. Alpha‐tropomyosin mutually exclusive exon selection: competition between branchpoint/polypyrimidine tracts determines default exon choice. Genes Dev. 1991;5(4):642‐655. 10.1101/gad.5.4.642 [DOI] [PubMed] [Google Scholar]
- 50.Xue Y, Zhou Y, Wu T, et al. Genome‐wide analysis of PTB‐RNA interactions reveals a strategy used by the general splicing repressor to modulate exon inclusion or skipping. Mol Cell. 2009;36(6):996‐1006. 10.1016/j.molcel.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Llorian M, Schwartz S, Clark TA, et al. Position‐dependent alternative splicing activity revealed by global profiling of alternative splicing events regulated by PTB. Nat Struct Mol Biol. 2010;17(9):1114‐1123. 10.1038/nsmb.1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin JC, Tarn WY. Exon selection in alpha‐tropomyosin mRNA is regulated by the antagonistic action of RBM4 and PTB. Mol Cell Biol. 2005;25(22):10111‐10121. 10.1128/Mcb.25.22.10111-10121.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jangi M, Boutz PL, Paul P, Sharp PA. Rbfox2 controls autoregulation in RNA‐binding protein networks. Genes Dev. 2014;28(6):637‐651. 10.1101/gad.235770.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Consortium EP . An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57‐74. 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis CA, Hitz BC, Sloan CA, et al. The encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res. 2018;46(D1):D794‐D801. 10.1093/nar/gkx1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dube DK, McLean MD, Dube S, Poiesz BJ. Translational control of tropomyosin expression in vertebrate hearts. Anat Rec. 2014;297(9):1585‐1595. 10.1002/ar.22978 [DOI] [PubMed] [Google Scholar]
- 57.Yin Z, Ren J, Guo W. Sarcomeric protein isoform transitions in cardiac muscle: a journey to heart failure. Biochem Biophys Acta. 2015;1852(1):47‐52. 10.1016/j.bbadis.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wieczorek DF, Jagatheesan G, Rajan S. The role of tropomyosin in heart disease. Adv Exp Med Biol. 2008;644:132‐142. [DOI] [PubMed] [Google Scholar]
- 59.Jagatheesan G, Rajan S, Ahmed RP, et al. Striated muscle tropomyosin isoforms differentially regulate cardiac performance and myofilament calcium sensitivity. J Muscle Res Cell Motil. 2010;31(3):227‐239. 10.1007/s10974-010-9228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helfman DM, Cheley S, Kuismanen E, Finn LA, Yamawaki‐Kataoka Y. Nonmuscle and muscle tropomyosin isoforms are expressed from a single gene by alternative RNA splicing and polyadenylation. Mol Cell Biol. 1986;6(11):3582‐3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marston SB, Redwood CS. The essential role of tropomyosin in cooperative regulation of smooth muscle thin filament activity by caldesmon. J Biol Chem. 1993;268(17):12317‐12320. [PubMed] [Google Scholar]
- 63.Rao JN, Rivera‐Santiago R, Li XE, Lehman W, Dominguez R. Structural analysis of smooth muscle tropomyosin alpha and beta isoforms. J Biol Chem. 2012;287(5):3165‐3174. 10.1074/jbc.M111.307330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunning P, O'Neill G, Hardeman E. Tropomyosin‐based regulation of the actin cytoskeleton in time and space. Physiol Rev. 2008;88(1):1‐35. 10.1152/physrev.00001.2007 [DOI] [PubMed] [Google Scholar]
- 65.Crawford JB, Patton JG. Activation of alpha‐tropomyosin exon 2 is regulated by the SR protein 9G8 and heterogeneous nuclear ribonucleoproteins H and F. Mol Cell Biol. 2006;26(23):8791‐8802. 10.1128/MCB.01677-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dye DT, Buvoli M, Mayer SA, Lin CH, Patton JG. Enhancer elements activate the weak 3 ‘ splice site of alpha‐tropomyosin exon 2. RNA. 1998;4(12):1523‐1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geeves MA, Hitchcock‐DeGregori SE, Gunning PW. A systematic nomenclature for mammalian tropomyosin isoforms. J Muscle Res Cell Motil. 2015;36(2):147‐153. 10.1007/s10974-014-9389-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cibi DM, Mia MM, Guna Shekeran S, et al. Neural crest‐specific deletion of Rbfox2 in mice leads to craniofacial abnormalities including cleft palate. eLife. 2019;8:e45418. 10.7554/eLife.45418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Fig S1
Fig S2
Table S1
Data Availability Statement
Nanopore sequencing data were deposited to NCBI SRA database with project number PRJNA517125.


