Abstract
Objectives:
Intensive care unit (ICU) costs comprise a significant proportion of the total inpatient charges for cardiac surgery. No reliable method for predicting intensive care unit length of stay following cardiac surgery exists, making appropriate staffing and resource allocation challenging. We sought to develop a predictive model to anticipate prolonged ICU length of stay (LOS).
Methods:
All patients undergoing coronary artery bypass grafting (CABG) and/or valve surgery with a Society of Thoracic Surgeons (STS) predicted risk score were evaluated from an institutional STS database. Models were developed using 2014–2017 data; validation used 2018–2019 data. Prolonged ICU LOS was defined as requiring ICU care for at least three days postoperatively. Predictive models were created using lasso regression and relative utility compared.
Results:
A total of 3,283 patients were included with 1669 (50.8%) undergoing isolated CABG. Overall, 32% of patients had prolonged ICU LOS. Patients with comorbid conditions including severe COPD (53% vs. 29%, p<0.001), recent pneumonia (46% vs. 31%, p<0.001), dialysis-dependent renal failure (57% vs. 31%, p<0.001) or reoperative status (41% vs. 31%, p<0.001) were more likely to experience prolonged ICU stays. A prediction model utilizing preoperative and intraoperative variables correctly predicted prolonged ICU stay 76% of the time. A preoperative variable-only model exhibited 74% prediction accuracy.
Conclusions:
Excellent prediction of prolonged ICU stay can be achieved using STS data. Moreover, there is limited loss of predictive ability when restricting models to preoperative variables. This novel model can be applied to aid patient counseling, resource allocation, and staff utilization.
Keywords: Critical Care, Length of Stay
Introduction
Intensive care unit (ICU) costs make up a significant proportion of the total inpatient expenses (1, 2). From a clinical perspective it is well reported that patients with prolonged ICU length of stay following cardiac surgery experience higher rates of sepsis, postoperative pneumonia, renal failure and gastrointestinal tract complications as well as worse in-hospital and long-term mortality (3, 4, 5).
While numerous models exist to predict ICU length of stay, they are not without limitations. A recent systematic review identified 11 studies detailing 31 models found that none fully satisfied all of their main requirements including: published variables, lack of organizational characteristic variables, low level of bias and accurate predictions (6). Even in well-established models like the Acute Physiology and Chronic Health Evaluation (APACHE) score, they are better suited for comparing case-mix adjusted ICU metrics instead of single patient utilization because of higher group precision and poorer individual accuracy (7).
In 2018, Meadows and colleagues investigated the use of the preoperative cardiac surgery risk tool EuroSCORE to predict ICU LOS after cardiac surgery. They demonstrated through logistic regression models that both additive and logistic EuroSCORE values yield reliable and accurate predictors of prolonged ICU stay, 79.77% and 79.73% respectively (8). The Society of Thoracic Surgeons (STS) risk calculator provides an estimate of an individual patient’s chance of prolonged total hospital stay, but does not stratify this result into ICU versus other levels of care.
In order to increase efficiency within our cardiac surgery postoperative intensive care unit, we sought to develop a predictive model utilizing commonly acquired patient variables captured within the STS database. Our objective was to translate these results into a transferable and translatable predictive tool.
Patients and Methods
Patient Population and Data Collection
All patients who underwent adult cardiac surgery between 2014 – 2017 with calculated STS risk scores were extracted from an institutional STS database. Patients undergoing CABG, valvular or combined CABG and valvular procedures were included, and patients without risk score calculation were excluded. Total time in the ICU was also extracted from the STS database. Short ICU length of stay was defined as less than 72 hours (three days) while long ICU length of stay was defined as greater than or equal to 72 hours. The Institutional Review Board for Human Subjects Research at the University of Virginia (Protocol #17806) approved the study.
Patients were stratified by the duration of their ICU stay (short vs. long). Other outcomes of interest were defined according to standard STS definitions. Iterative models predicting short vs. long length of stay were created and serially examined. Importantly, models using only preoperative factors and models using preoperative and intraoperative factors were considered separately and performance compared.
The current ICU staffing model at our institution consists of critical care intensivists, surgical and anesthesia resident physicians, and advanced practice providers including nurse practitioners and physician assistants, along with dedicated ICU nursing and respiratory therapy. No major changes to the staffing model occurred during the entirety of the study period.
Our institution employs rapid-ICU discharge protocols for select patients, and in October 2018, we began patient enrollment into an enhanced recovery after cardiac surgery (ERACS) program.
Model Construction and Evaluation
Prediction models for long length of stay were developed using STS data from 2014–2017 and then validated using STS data from 2018–2019. Two categories of prediction models were generated based on sets of variables: (1) using both preoperative and intraoperative factors, and (2) using only preoperative factors. Variables were assessed for collinearity prior to model fitting. Highly correlated variables (>|0.9|) were clinically assessed to determine inclusion in the candidate set based on definition, clinical relevance, and potential for missing information. For each model, the best predictors of ICU stay were derived using penalized logistic regression. Lasso regression was chosen in order to achieve predictive parsimony while minimizing prediction errors, while being tolerant of any multicollinearity undetected in the potential predictors. Lasso regression was performed using the glmnet R package with smoothing parameter selected to minimize the cross-validated area under the curve (AUC) (9, 10). The prediction cut-point selected for each model balanced sensitivity and specificity. The two models were compared using tests for receiver operating characteristic (ROC) curves (11). The performance metrics were used to select the optimal model, which was then compared with the performance obtained using the STS risk scores, and validated against the 2018–2019 cohort.
Missing data was handled individually for each variable. For the majority of variables, any missing or unknown and/or not applicable were included as valid categories. Variables with a large percentage of unexplainable missing data (i.e. the information was applicable but not entered) were excluded from consideration since the validity of the data was questionable and the probability of bias large. Characteristics recorded in multiple variables due to changes in STS coding were individually inspected and collapsed to the finest clinically relevant categorization possible.
Results
Patient and Operative Characteristics
A total of 3,283 patients met inclusion criteria and were included in the study with approximately half of patients, 1669 (51%), undergoing isolated coronary artery bypass grafting (CABG). Long ICU stay affected 1044 (31.8%) of included patients. Overall, the median ICU stay was 46.8 hours (IQR=66.2), with a median stay of 28.8 hours (IQR=24.9) in the short ICU stay group and 115.5 hours (IQR=66.5) in the long ICU stay group. Baseline characteristics and operations performed between groups are shown in Table 1. Patients with long ICU stays had higher prevalence of respiratory comorbidities including moderate to severe lung disease (53% vs. 29%, p<0.001), recent pneumonia (46% vs. 31%, p<0.001), and obstructive sleep apnea (38% vs. 30%, p<0.001). Other major comorbidities or preoperative factors affecting patients with prolonged ICU stay included: advanced age (median 71.0 vs. 67.0, p<0.001), female gender (36% vs. 30%, p=0.001), hypertension (33% vs. 25%, p<0.001), dyslipidemia (33% vs. 27%, p=0.008), diabetes (37% vs. 28%, p<0.001), dialysis-dependent renal failure (57% vs. 31% p<0.001), reoperative status (41% vs. 31%, p<0.001), peripheral arterial disease (43% vs. 30%, p<0.001), and higher STS prediction scores for risk of mortality and predicted morbidity or mortality (both p<0.001). Patients who experienced long ICU stay were more likely to have undergone operations other than isolated CABG (p<0.001).
Table 1:
Baseline Characteristics and demographics comparing patients with short ICU length of stay vs. long ICU length of stay. Subcategory percentages are of total category, i.e. Long ICU Stay.
| Characteristic | Overall n=3283 | Long ICU Stay n=1044 (31.8%) | Short ICU Stay n=2239 (68.2%) | Standardized Differences | p-value - |
|---|---|---|---|---|---|
| Age; median (Q1, Q3) | 68 (60, 75) | 71.0 (62.0, 77.0) | 67.0 (59.0, 74.0) | 0.295 | <0.001 |
| Female | 997 (30.4%) | 357 (34.2%) | 640 (28.6%) | 0.121 | 0.001 |
| Procedure | |||||
| Isolated CABG | 1669 (50.8%) | 476 (45.6%) | 1193 (53.3%) | −0.154 | <0.001 |
| AVR | 1103 (33.6%) | 365 (35.0%) | 738 (33.0%) | 0.042 | <0.001 |
| MV Repair | 309 (9.4%) | 94 (9.0%) | 215 (9.6%) | −0.021 | <0.001 |
| MV Replacement | 202 (6.2%) | 109 (10.4%) | 93 (4.2%) | 0.244 | <0.001 |
| Hypertension | 2675 (81.5%) | 895 (85.7%) | 1780 (79.5%) | 0.165 | <0.001 |
| Dyslipidemia1 | 2747 (83.7%) | 899 (86.2%) | 1848 (82.5%) | 0.101 | 0.008 |
| Diabetes | 1378 (42.0%) | 507 (48.6%) | 871 (38.9%) | 0.196 | <0.001 |
| Severe COPD | 342 (10.4%) | 180 (17.2%) | 162 (7.2%) | 0.309 | <0.001 |
| Obstructive Sleep Apnea2 | 552 (16.9%) | 209 (20.1%) | 343 (15.4%) | 0.125 | <0.001 |
| Recent Pneumonia | 100 (3.0%) | 46 (4.4%) | 54 (2.4%) | 0.110 | 0.002 |
| Dialysis1 | 87 (2.7%) | 50 (4.8%) | 37 (1.7%) | 0.179 | <0.001 |
| Peripheral Arterial Disease | 528 (16.1%) | 228 (21.8%) | 300 (13.4%) | 0.223 | <0.001 |
| Prior Stroke3 | 305 (43.5%) | 133 (45.1%) | 172 (42.4%) | 0.055 | 0.473 |
| Reoperation | 306 (9.3%) | 125 (12.0%) | 181 (8.1%) | 0.130 | <0.001 |
| Intra-Aortic Balloon Pump | 197 (6.0%) | 120 (11.5%) | 77 (3.4%) | 0.310 | <0.001 |
| STS Predicted Risk of Mortality; median (Q1, Q3) | 1.7 (0.9, 3.8) | 3.3 (1.6, 6.2) | 1.3 (0.7, 2.7) | 0.601 | <0.001 |
| STS Predicted Risk of Morbidity or Mortality; median (Q1, Q3) | 15.5 (9.6, 25.2) | 23.5 (14.9, 35.6) | 12.5 (8.6, 19.7) | 0.829 | <0.001 |
Missing data on 1 subject (i.e. n=3282)
Missing data on 11 subjects (n=3272)
Missing data on 2,582 subjects (n=701)
Model Performance and Key Variables
Models were created and derived as described above. The best model based on both pre-and intraoperative factors selected 28 predictors with an AUC of 0.76. The cut-point of 0.31 yielded a sensitivity and specificity of 69.6% and 69.8% for long length of stay, and a positive predictive value (PPV) of 52.0%. The best model based on only the preoperative factors selected 23 predictors with an AUC of 0.74. Both the sensitivity and specificity of this model was 69.1% under its cut-point of 0.31, and PPV was 51.4%. Comparison of the two models found a statistically significant difference (p<0.001) due to the large sample size. However, when the optimal cut point for model prediction was applied, there was no clinically meaningful difference in predictive ability between each of the models and their functionality was essentially unchanged. Hence, the preoperative factors-only model was selected as the final model.
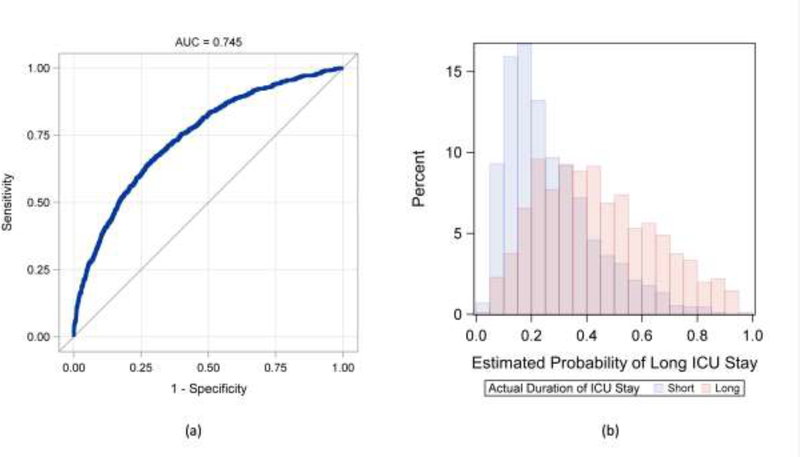
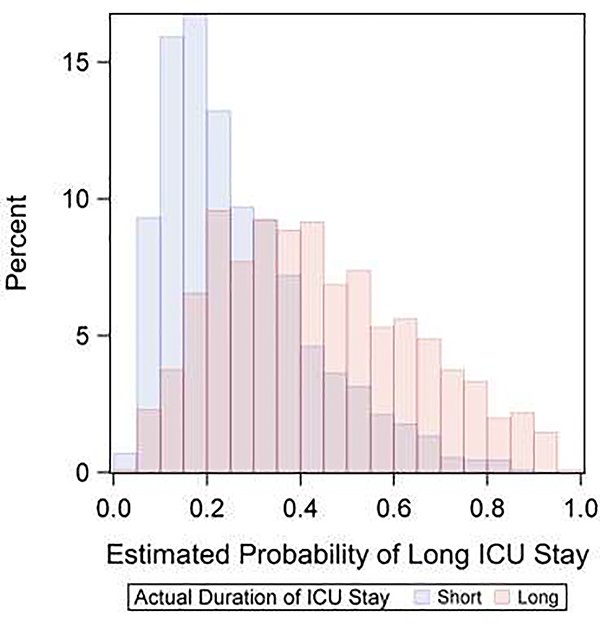
The performance of the preoperative model is shown in Figure 1. Visual analysis of the predicted probabilities demonstrates that long ICU stay can be predicted with a fair amount of confidence. However, there is overlap in the mid-range of predicted risk indicating imperfection and reduced discretionary ability in patients with intermediate risk. Uniform among all models, respiratory comorbidities had a relatively strong effect size. In the final model, other variables that were important included: preoperative intra-aortic balloon pump (IABP) (0.88), isolated CABG (0.77), race (0.56), home oxygen use (0.47) and chronic lung disease (0.44). Analysis of performance analysis in the training data pre- versus post-ERACS implementation demonstrated a slightly better AUC post-ERACS (0.74 vs. 0.71) with an improved PPV of 65.9% vs. 57.7%. A complete list of variables included in the final model and their relative impact can be found in Table 2.
Figure 1:
Performance of the preoperative variable-only, final model for predicting long length of stay. Panels: (a) Receiver operating characteristic (ROC) curve, (b) Distribution of predicted probability of long length of stay by actual length of stay.
Table 2:
Relative importance of each model variable used in final model.
| Parameter Estimate | Standard Error | |
|---|---|---|
| Preoperative IABP | 0.8803 | 0.1958 |
| Isolated CABG | −0.8665 | 0.1345 |
| CABG performed | 0.769 | 0.1386 |
| Race | 0.5636 | 0.1643 |
| Other Cardiac Operation Performed | 0.5173 | 0.1335 |
| Home Oxygen | 0.4678 | 0.2468 |
| Chronic Lung Disease | 0.4404 | 0.1436 |
| Total Albumin | −0.4276 | 0.1094 |
| Diabetes | 0.4189 | 0.1206 |
| Heart Failure | 0.3957 | 0.0961 |
| Reoperation | 0.3917 | 0.1537 |
| Alcohol Use | −0.3695 | 0.0967 |
| Prior MI | 0.3128 | 0.1039 |
| Status | −0.259 | 0.111 |
| Cerebrovascular Disease | 0.252 | 0.1028 |
| Smoking | 0.2464 | 0.0929 |
| Sex | −0.2438 | 0.1005 |
| Sleep Apnea | 0.212 | 0.1155 |
| Operative Approach | −0.1209 | 0.2231 |
| Inhaled or oral Bronchodilator Therapy | 0.0919 | 0.1233 |
| MELD Score | 0.0703 | 0.0124 |
| Patient Age | 0.0254 | 0.00418 |
| Last Hematocrit | 0.000174 | 0.00891 |
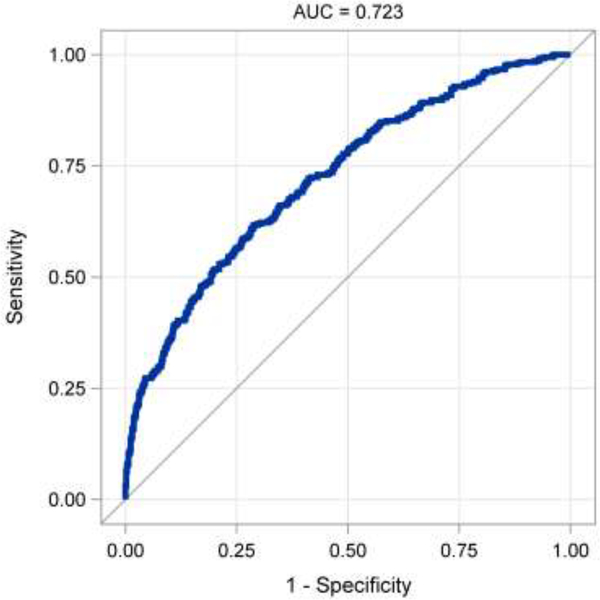
The final model was compared to the STS prediction scores for length of stay. There was no difference in the performance of the two predictors (AUC difference = 0.002, p=0.75). In the validation cohort, the final model produced an AUC of 0.72 (Figure 3), with a sensitivity of 62.5%, specificity of 68%, and PPV of 54.4%.
Figure 3.
Receiver operating characteristic curve for preoperative prediction model in the validation cohort.
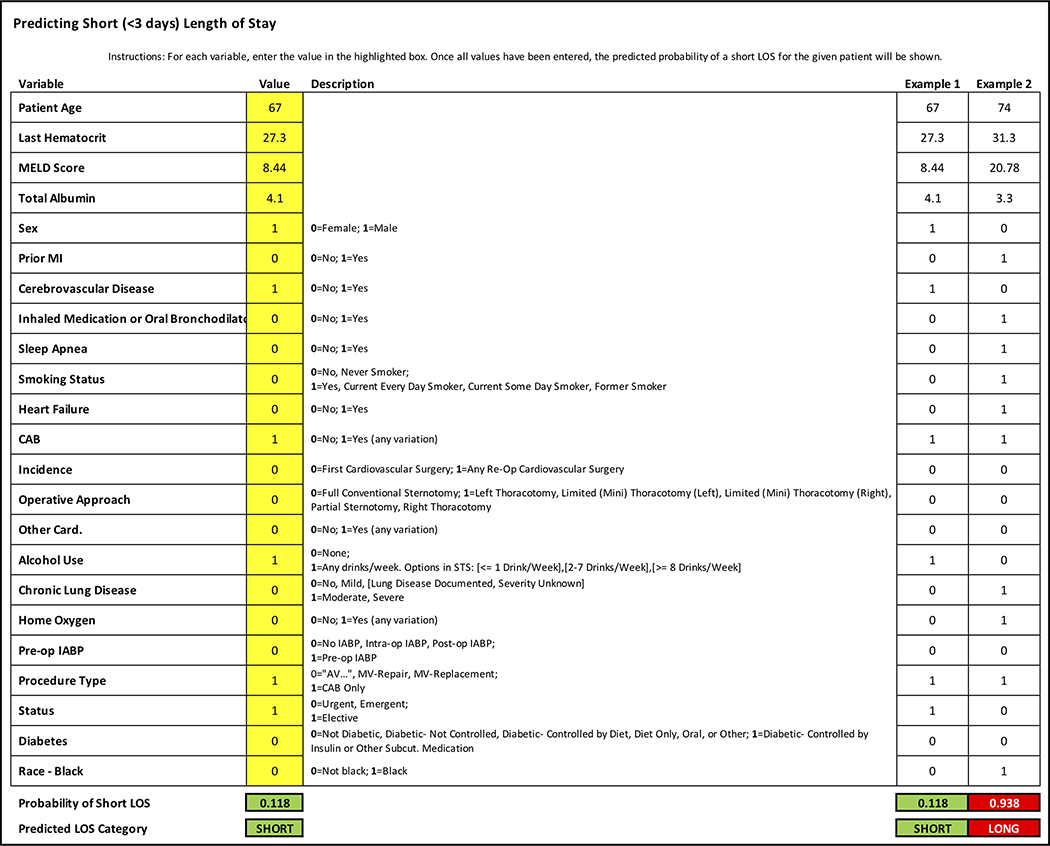
An easy to use, first generation platform was created to be made available to providers. The user interface is demonstrated in Figure 2.
Figure 2:
User interface for Predictive Model calculator.
Discussion
Postoperative cardiac intensive care units are unique in that patients often follow a predetermined pathway. Beyond this general feature of the field, there are two contemporary factors that underscore the importance of continuing to work towards accurate ICU utilization models in the current era. First, there is increasing interest in the development and implementation of enhanced recovery after cardiac surgery pathways which provide a source of consistency in the postoperative care (12). The value of an ICU LOS prediction tool would do well to promote these efforts by adding another identifying factor of who may or may not benefit from these protocols. Second, with the emergence of the novel coronavirus pandemic, short- and medium-term prognostication of post-operative ICU needs has never been more important (13).
In this single institution study of over 3000 patients, we created a functional model to predict individual patient ICU utilization using STS data. Our results demonstrated adequate discriminatory function and found minimal decrease in model performance when only using regularly assessed preoperative variables. This is important since intraoperative variables may not yet be available to unit leadership during strategic planning for staff and resource allocation. Not only does the final model perform at or above the discriminatory ability of other published models, but we have also translated it into a user-friendly format that could be transferred and accessed by other institutions and programs.
In all of our models examined, and in our final model, we found a consistent impact of respiratory comorbidities on ICU length of stay. One of the most consistently identified risk factors for prolonged ICU stay in unselected populations stay has been the need for mechanical ventilation (14, 15, 16). While the vast majority of cardiac surgery patients will require mechanical ventilation for some period of time, perturbations in the respiratory system appear to be a powerful signal to indicate the potential for an extended ICU stay in both mixed ICU and post-cardiac surgery populations.
Pre-existing comorbid conditions provide more meaningful prognostication for patients following cardiac surgery than in unselected populations. While these factors in and of themselves may not drive prolonged ICU LOS, they can be surrogates of higher risk patients. Compared to the varied indications for general ICU admissions, cardiac surgery patients have relatively predictable and conserved insults inherent to their pathology, and outcomes are highly dependent on preoperative function. This difference is exemplified by studies in mixed ICU populations that have compared the utility of preadmission variables to those obtained after admission, demonstrating significantly better predictive ability of post-admission data. In one study of mixed ICU populations, the need for invasive ventilation and the oxygenation ratio following ICU admission accounted for over 50% of the model variance while chronic comorbidities accounted for less than 1% (16). Prediction based solely on pre-incision data has been successfully demonstrated in neural network modeling in cardiac surgery and underscores the importance of applying specific models to appropriate patient populations (17). In the setting of cardiac surgery, preoperative comorbidities appear to be especially important, particularly respiratory comorbidities.
Significant variability exists within the literature defining a long ICU LOS following cardiac surgery. This is highlighted by a 2010 systematic review by Ettema and colleagues where they restricted their analyses to of a range of 24 to 72 hours, which required the elimination of two studies using a cutoff of over 72 hours (18). While 48 hours was ultimately used for their validation study, they found minimal impact on their results when performing their analyses using 24 or 72 hours as their cut off point. Herman and colleagues sought to predict prolonged length of stay for patients undergoing CABG and also utilized 72 hours as the cutpoint (19). We investigated the distribution of ICU days prior to modeling to identify any external factors that might influence the definition. We discovered both 24 and 48 hours were highly influenced by the time of day at which surgery was scheduled. In general, rounding and determinations to transfer patients are made at set times, and definitions based on 24 or 48 hours were affected by this timing. At 72 hours, the time of surgery was no longer driving the ICU LOS distribution. Therefore, the use of 72 hours throughout existing literature, its clinical relevance, and the improved discriminatory ability in our cohort supports our use of this time point. Historically, the lack of consistency of definitions coupled with the increasingly streamlined processes for post-cardiac surgery care in the era of enhanced recovery programs, points to the need to refocus on the development on models fitting contemporary post cardiac surgery care with large, multi-institutional, standardized data (20).
The field of cardiac surgery has already embraced the use of risk scores both for internal clinical risk assessment and for external accountability through the use of the star rating system (21). The STS risk models have been praised as the gold standard of surgical risk assessment tools and their importance cannot be understated, especially as pertains to specific operations. Refinement and implementation of an extended ICU prediction model should be of equally critical importance and may be used in a similar fashion to benchmark outcomes in the future, but more importantly, is now ready to aid in resource utilization. Inefficiencies within our system of care must be eliminated as they pose a threat to patient outcomes and come with significant financial repercussions. A 2018 study conducted at Yale New Haven hospital demonstrated systematic inefficiencies in the form of delayed operating room to ICU patient transfer resulted in significantly longer ICU length of stay (13% increase), 30-day mortality of 34%, and an estimated loss in revenue of $15 million annually (22). Similar financial consequences could be anticipated from inefficiencies in patient movement elsewhere throughout the hospital such as discharge from the ICU without proper estimates of expected lengths of stay. Now more than ever, we must refine our risk assessment as the cost of delivering high quality healthcare continues to rise. These costs in conjunction with staffing shortages reinforce the need to allocate resources to areas of high-need patient care. We propose that by utilization of preoperative prediction tools, we can better prepare for nursing, respiratory therapy, pharmacy and other critical service needs based on the upcoming scheduled operative case load. Importantly, in the era of the ongoing novel coronavirus pandemic, it is the expectation that ICU volume will need to rapidly adjust to accommodate waves of critically ill patients; knowledge of anticipated utilization will dramatically assist in achieving this goal (13). Currently, our institution admits all post-cardiac surgery patients to the intensive care unit. We do employ rapid ICU-discharge protocols to an intermediate care unit (IMU) where qualification mandates patients have stable blood pressure with minimal or no need for vasopressors or inotropes, decreasing oxygen requirements, and no pulmonary artery catheter. As ICU space is further constrained by the ongoing pandemic, efforts to create fast-track protocols for optimal patients utilizing direct transfer to care wards from post-anesthesia care units (PACU) may be of increasing value (23, 24).
Risk prediction is routinely utilized during preoperative planning and evaluation in clinic. Although we have not yet implemented the proposed model into practice, we envision this prediction tool to be part of the standard evaluation implemented throughout ambulatory and inpatient settings alike. Translation of this model into an easy-to-use application is our ultimate goal. ICU managers, charge nurses, or hospital bed control centers would be able to quickly enter patient data to position patients in the most appropriate location given their predicted needs. Figure 2 demonstrates a preliminary user interface for required input variables. Ultimately, validation of these models with regional or national STS data would further strengthen this initiative, which we plan to evaluate in forthcoming analyses.
Limitations
This study is limited in its retrospective nature which exposes the data to some degree of selection bias. However, we included all patients’ operations that are eligible for STS risk prediction which represents a large proportion of adult cardiac operations that are regularly performed. In addition, data was derived from a single institution and determinations about transfer out of the ICU are dependent on provider preferences and institutional culture. Finally, the data captured represent actual time in the ICU rather than readiness for transfer which can be impacted by numerous variables including, but not limited to: time of day of the operation, receiving unit capacity and staffing availability, patient isolation status, timing of patient transfer orders, discharge timing, and other incoming unit transfers. The multitude of competing variables represent a source of unaccounted variability even though these factors should have affected all patients equally or on a random basis. Ultimately, these variables are limitations to any study of ICU LOS. Since our model is a predictor of real-time LOS, these other factors would be present independent of the institution implementing this prediction model. Furthermore, this model was evaluated and validated using a large patient cohort which diminishes the impact of many of these sources of error.
Conclusion
Predicting ICU length of stay has significant impact for patients and hospital systems alike. Tailoring care and establishing appropriate staffing and resource allocation is critical. We have demonstrated that by utilizing well-established and universal STS data, factors predicting prolonged ICU LOS can be readily identified and utilized for predictive modeling (Figure 4). The utility of a model exclusively reliant on preoperative information has the potential for earlier preparation of post-operative resources. We have successfully created a predictive model that can now be used to guide patient flow through the ICU. We plan to continue these efforts utilizing multi-institutional data for further validation of the predictive model, identification of surgery-specific predictive metrics and opportunities for improved resource allocation for those who are predicted to have prolonged ICU length of stay.
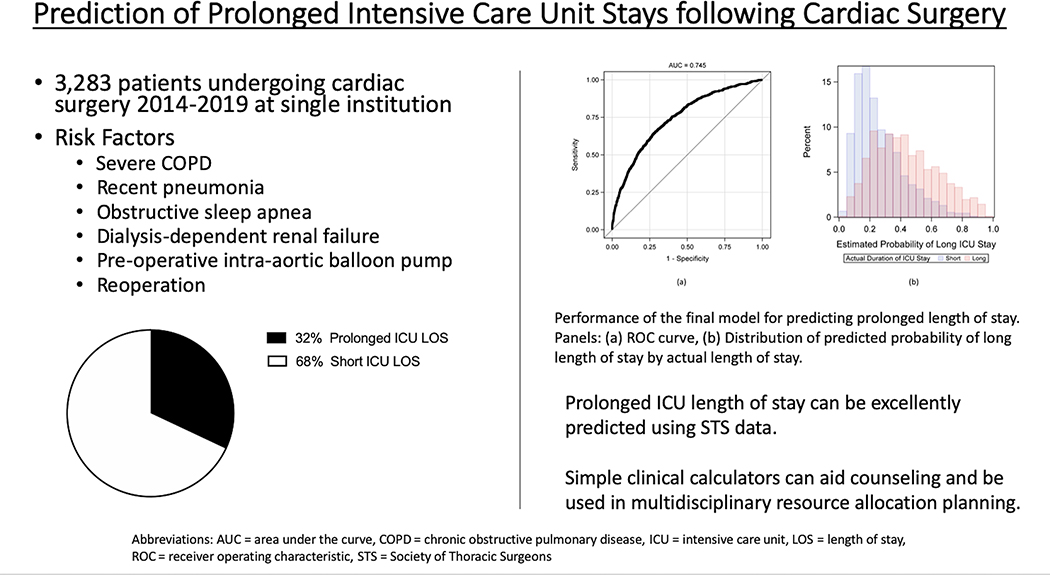
Figure 4.
Graphical Abstract. Prediction of Prolonged Intensive Care Unit Stay following Cardiac Surgery. Total patients undergoing cardiac surgery at a single institution (3,283) enrolled for prediction calculator of prolonged ICU LOS. Significant risk factors and graphical representation of total cohort with prolonged ICU LOS (32%). Final predictive model performance metrics (ROC curve, distribution of predictive ability).
Central Picture.
Distribution of predicted probability of long length of stay by actual length of stay.
Central Message:
Prediction of prolonged intensive care unit length of stay can be successfully achieved using data available prior to entering the operating room.
Perspective Statement:
A prediction tool that identifies patients at high risk for prolonged intensive care length of stay will aid in appropriate staffing decisions and resource allocation to improve efficiency within a cardiac surgery intensive care unit.
Sources of Funding:
This work was supported by the National Heart, Lung, and Blood Institute (grant T32 HL007849)
Glossary of Abbreviations:
- AUC
area under the curve
- CABG
coronary artery bypass grafting
- COPD
chronic obstructive pulmonary disease
- IABP
intra-aortic balloon pump
- ICU
intensive care unit
- IMU
intermediate care unit
- LOS
length of stay
- MI
myocardial infarction
- MELD
Model for End-Stage Liver Disease
- PPV
positive predictive value
- ROC
receiver operating characteristic
- STS
Society of Thoracic Surgeons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Gorav Ailawadi discloses consulting for Abbott, Medtronic, Edwards Lifesciences, Admedus, and Cephea Valve Technologies. All other authors report no disclosures.
References
- 1.Talmor D, Shapiro N, Greenberg D, Stone PW, Neumann PJ. When is critical care medicine cost-effective? A systematic review of the cost-effectiveness literature. Crit Care Med. 2006;34(11):2738–2747. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin AM, Hardt J, Canavan JB, Donnelly MB. Determining the economic cost of ICU treatment: a prospective "micro-costing" study. Intensive Care Med. 2009;35(12):2135–2140. [DOI] [PubMed] [Google Scholar]
- 3.Yu PJ, Cassiere HA, Fishbein J, Esposito RA, Hartman AR. Outcomes of Patients With Prolonged Intensive Care Unit Length of Stay After Cardiac Surgery. J Cardiothorac Vasc Anesth. 2016;30(6):1550–1554. [DOI] [PubMed] [Google Scholar]
- 4.Williams MR, Wellner RB, Hartnett EA, Thornton B, Kavarana MN, Mahapatra R, et al. Long-term survival and quality of life in cardiac surgical patients with prolonged intensive care unit length of stay. Ann Thorac Surg. 2002;73(5):1472–1478. [DOI] [PubMed] [Google Scholar]
- 5.Hassan A, Anderson C, Kypson A, Kindell L, Ferguson TB, Chitwood WR, et al. Clinical Outcomes in Patients with Prolonged Intensive Care Unit Length of Stay After Cardiac Surgical Procedures. Ann Thorac Surg. 2012;93(2):565–569. [DOI] [PubMed] [Google Scholar]
- 6.Verburg IW, Atashi A, Eslami S, Holman R, Abu-Hanna A, de Jonge E, et al. Which Models Can I Use to Predict Adult ICU Length of Stay? A Systematic Review. Crit Care Med. 2017;45(2):e222–e231. [DOI] [PubMed] [Google Scholar]
- 7.Kramer AA. Are ICU Length of Stay Predictions Worthwhile?. Crit Care Med. 2017;45(2):379–380. [DOI] [PubMed] [Google Scholar]
- 8.Meadows K, Gibbens R, Gerrard C, Vuylsteke A. Prediction of Patient Length of Stay on the Intensive Care Unit Following Cardiac Surgery: A Logistic Regression Analysis Based on the Cardiac Operative Mortality Risk Calculator, EuroSCORE. J Cardiothorac Vasc Anesth. 2018;32(6):2676–2682. [DOI] [PubMed] [Google Scholar]
- 9.Tibshirani R Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society. Series B (Methodological). 1996;58(1):267–288. [Google Scholar]
- 10.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. Journal of Statistical Software. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 11.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxter R, Squiers J, Conner W, Kent M, Fann J, Lobdell K, et al. Enhanced Recovery After Surgery: A Narrative Review of its Application in Cardiac Surgery. Ann Thorac Surg. 2020;109(6):1937–1944. [DOI] [PubMed] [Google Scholar]
- 13.Ross SW, Lauer CW, Miles WS, Green JM, Christmas AB, May AK, et al. Maximizing the Calm before the Storm: Tiered Surgical Response Plan for Novel Coronavirus (COVID-19). J Am Coll Surg. 2020;230(6):1080–1091.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin CM, Hill AD, Burns K, Chen LM. Characteristics and outcomes for critically ill patients with prolonged intensive care unit stays. Crit Care Med. 2005;33(9):1922–1936. [DOI] [PubMed] [Google Scholar]
- 15.Higgins TL, McGee WT, Steingrub JS, Rapoport J, Lemeshow S, Teres D. Early indicators of prolonged intensive care unit stay: impact of illness severity, physician staffing, and pre-intensive care unit length of stay. Crit Care Med. 2003;31(1):45–51. [DOI] [PubMed] [Google Scholar]
- 16.Kramer AA, Zimmerman JE. A predictive model for the early identification of patients at risk for a prolonged intensive care unit length of stay. BMC Med Inform Decis Mak. 2010;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaFaro RJ, Pothula S, Kubal KP, Inchiosa ME, Pothula VM, Yuan SC, et al. Neural Network Prediction of ICU Length of Stay Following Cardiac Surgery Based on Pre-Incision Variables. PLoS One. 2015;10(12):e0145395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ettema RG, Peelen LM, Schuurmans MJ, Nierich AP, Kalkman CJ, Moons KG. Prediction models for prolonged intensive care unit stay after cardiac surgery: systematic review and validation study. Circulation. 2010;122(7):682–689. [DOI] [PubMed] [Google Scholar]
- 19.Herman C, Karolak W, Yip AM, Buth KJ, Hassan A, Légaré JF. Predicting prolonged intensive care unit length of stay in patients undergoing coronary artery bypass surgery--development of an entirely preoperative scorecard. Interact Cardiovasc Thorac Surg. 2009. Oct;9(4):654–8. [DOI] [PubMed] [Google Scholar]
- 20.Messaoudi N, De Cocker J, Stockman B, Bossaert LL, Rodrigus IE. Prediction of prolonged length of stay in the intensive care unit after cardiac surgery: the need for a multi-institutional risk scoring system. J Card Surg. 2009. Mar-Apr;24(2):127–33. [DOI] [PubMed] [Google Scholar]
- 21.Shahian DM, He X, Jacobs JP, Kurlansky PA, Badhwar V, Cleveland JC Jr, et al. The Society of Thoracic Surgeons Composite Measure of Individual Surgeon Performance for Adult Cardiac Surgery: A Report of The Society of Thoracic Surgeons Quality Measurement Task Force. Ann Thorac Surg. 2015;100(4):1315–1325. [DOI] [PubMed] [Google Scholar]
- 22.Somlo DRM, Repenning NP, Mangi AA. In-Hospital Delays Result in Worse Patient Outcomes and Higher Cost After Cardiac Surgery. Ann Thorac Surg. 2018;106(4):1143–1149. [DOI] [PubMed] [Google Scholar]
- 23.Haanschoten MC, van Straten AH, ter Woorst JF, et al. Fast-track practice in cardiac surgery: results and predictors of outcome. Interact Cardiovasc Thorac Surg. 2012;15(6):989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waseem Z, Lindner J, Sgouropoulou S, Eibel S, Probst S, Scholz M, Ender J. Independent Risk Factors for Fast-Track Failure Using a Predefined Fast-Track Protocol in Preselected Cardiac Surgery Patients. J Cardiothorac Vasc Anesth. 2015. Dec;29(6):1461–5. [DOI] [PubMed] [Google Scholar]