Abstract
Brain functional network (BFN) has become an increasingly important tool to explore individual differences and identify neurological/mental diseases. For estimating a “good” BFN (with more discriminative information for example), researchers have developed various methods, in which the most popular and simplest is Pearson's correlation (PC). Despite its empirical effectiveness, PC only encodes the low-order (second-order) statistics between brain regions. To model high-order statistics, researchers recently proposed to estimate BFN by conducting two sequential PCs (denoted as PC2 in this paper), and found that PC2-based BFN can provide additional information for group difference analysis. This inspires us to think about (1) what will happen if continuing the correlation operation to construct much higher-order BFN by PCn (n>2), and (2) whether the higher-order correlation will result in stronger discriminative ability. To answer these questions, we use PCn-based BFNs to predict individual differences (Female vs. Male) as well as identify subjects with mild cognitive impairment (MCI) from healthy controls (HCs). Through experiments, we have the following findings: (1) with the increase of n, the discriminative ability of PCn-based BFNs tends to decrease; (2) fusing the PCn-based BFNs (n>1) with the PC1-based BFN can generally improve the sensitivity for MCI identification, but fail to help the classification accuracy. In addition, we empirically find that the sequence of BFN adjacency matrices estimated by PCn (n = 1,2,3,⋯ ) will converge to a binary matrix with elements of ± 1.
Keywords: brain functional network, Pearson's correlation, mild cognitive impairment, gender prediction, higher-order correlation
1. Introduction
Brain functional network (BFN), learning from the resting-state functional magnetic resonance imaging (rs-fMRI), has become an increasingly important tool to understand the brain working mechanism (Liu et al., 2015; Jiang et al., 2020; Xue et al., 2020; Chen et al., 2021), reveal the biomarkers of neurological/mental disorders (Bijsterbosch et al., 2017; Li et al., 2017; Liu et al., 2017; Sun et al., 2020), and predict the individual differences (Dubois and Adolphs, 2016). However, establishing a “good” BFN (with more discriminative information for example) is currently a challenging problem, due to the low-quality of fMRI data and the high complexity of our brain.
In the past decades, numerous BFN construction methods (McLntosh and Gonzalez-Lima, 1994; Marrelec et al., 2006; Ramsey et al., 2010; Qiao et al., 2016; Li et al., 2017; Jiang et al., 2019) have been proposed. The main difference among these methods lies in the calculation of the relationship between different brain regions of interest (ROIs). Pearson's correlation (PC) is the commonly used method for constructing BFN due to its relative simplicity and reliability (Smith et al., 2013).
However, traditional PC cannot capture the complex relationship (e.g., non-linearity and non-Gaussianity) among ROIs because it can only encode the simple linear correlation (Wan et al., 2006; Xie et al., 2008; Zhang et al., 2016). In contrast, the two sequential PCs (denoted as PC2 in this paper) provide a high-order statistic that, in theory, can encode more complex relationship (Swami et al., 1997; Zhou et al., 2018). In fact, PC2 has been used to estimate BFN and some findings have been obtained in recent years (Chen et al., 2016; Zhang et al., 2016; Zhou et al., 2018). For instance, Chen et al. (2016) revealed that PC2 can encode the complex interactions between different ROIs, and Zhang et al. (2016) found that PC2 is more sensitive to AD identification.
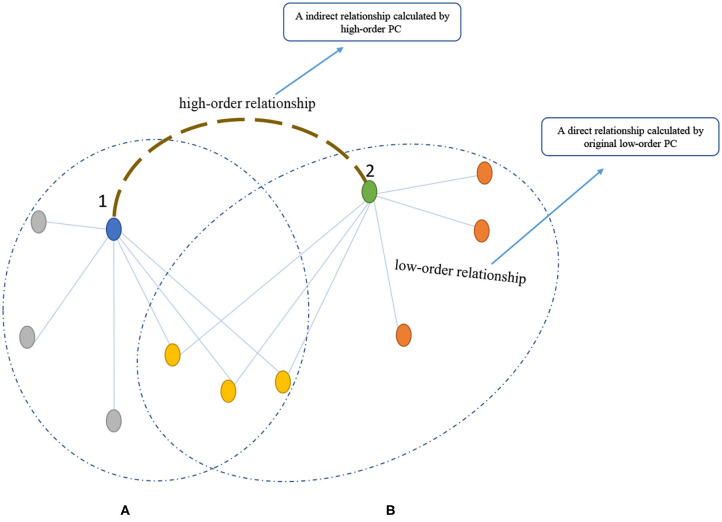
In order to have an intuitive explanation of the original low-order PC and high-order PC (i.e., PC2), we provide a simple analogy via a friendship network in Figure 1, where each node corresponds to a person and each edge means existence of friendship between two nodes. As shown in Figure 1, we note that there is no direct edge between Node 1 and Node 2, meaning that these two persons have no relationship in a low-order form. However, from a high-order view, Nodes 1 and 2 may have some kinds of relationship since they share three common friends. Such a friend-sharing relationship (with similar friend circles) can be easily captured by the high-order PC, according to its definition (i.e., two sequential correlations). This indicates that the high-order PC can find more complex relationship that cannot be directly modeled in a low-order way.
Figure 1.
An analogy of PC2 to a friendship network. The (A,B) are the friendship networks of nodes 1 and 2, respectively.
Inspired by the potential powerfulness of PC2, a natural question is (1) what will happen if continuing the correlation operation to construct much higher-order BFN by PCn (n>2), and (2) whether the higher-order correlation will result in stronger discriminative ability or other useful information.
For investigating the above problems, we construct PCn-based BFNs on two public datasets, and then apply the constructed BFNs to conduct the classification task. Through the experiments, we get two interesting findings: (1) for the PCn-based BFNs, with the increase of n, their discriminative ability tends to decrease; (2) by fusing the PCn-based BFNs (n>1) with the original PC-based BFN, the sensitivity for MCI identification is generally increased, but the classification accuracy has no significant improvement. In addition, we have empirically found that the BFN adjacency matrix sequence constructed by PCn (n = 1,2,3 ⋯ ) is convergent, where PC1corresponds to the original PC.
The rest of this paper is organized as follows. In section 2, we introduce the data acquisition, data preprocessing and the PCn-based BFN construction scheme. In section 3, we design two classification tasks for evaluating the proposed methods. In section 4, we discuss our findings and analyze the convergence of the PCn-matrix sequence, and also introduce several limitations of this work with possible research plans in the future. Finally, we briefly summarize this paper in section 5.
2. Materials and Methods
2.1. Data Acquisition
Two public datasets, from Alzheimer's Disease Neuroimaging Initiative (ADNI)1 and Human Connectome Project (HCP)2, respectively, are used in our experiments. For ADNI dataset, we first remove the subjects if (1) the maximum translation/rotation of the head motion exceeds 2.0, or (2) the volumes with big frame-to-frame displacement (>0.5) accumulate more than 2.5 min (Power et al., 2012), which leaves us 299 scans (including 154 HCs and 145 MCIs) from 143 participants (with some participants scanned at one or more times). The scanning parameters are given as follows: the image resolution is from 2.29 to 3.31 mm for in-plane and the slice thickness equal to 3.31 mm, TE (echo time) = 30 ms, TR (repetition time) is from 2.2 to 3.1 s, flip angle = 800, field of view (FOV) = 198.75 × 212 mm2, matrix size = 64 × 64, voxel size = 3.3 × 3.3 × 3.3mm3 and the scanning time for each subject is 7 min (resulting in 140 volumes) (Wang et al., 2019). The demographic information of 143 subjects is shown in Table 1.
Table 1.
The main demographic information of 143 subjects.
| Category | Scan # | Age (Years) | Gender (M/F) |
|---|---|---|---|
| MCI | 145 | 71.99 ± 7.67 | 95/50 |
| HC | 154 | 75.36 ± 6.16 | 67/87 |
For HCP dataset, 1,003 subjects, including 469 males and 534 females, are involved in our experiment. Each subject was scanned in four sessions, each of which generates 1,200 volumes, thus resulting in 4,800 volumes. Data acquisition is performed as follows: TR = 720 ms, TE = 33.1 ms with 72 slices, flip angle = 520, FOV = 208 × 180 mm2, matrix size = 104 × 90, and voxel size = 2 × 2 × 2 mm3 (WU-Minn, 2017). The demographic information of 1,003 subjects is shown in Table 2.
Table 2.
The main demographic information of 1, 003 subjects.
| Category | Scan # | Age (Years) |
|---|---|---|
| Female | 534 | 22–36+ |
| Male | 469 | 22–36+ |
2.2. Data Preprocessing
For each subject from ADNI dataset, we discard the first three volumes for magnetization balance, and then use the FSL FEAT software3 for preprocessing the remaining 137 volumes, including slice timing correction, head motion estimation, bandpass filtering, and regression of nuisance covariates (white matter, cerebrospinal fluid, and motion parameters). After that, we align the fMRIs (that are skull-stripped based on T1-weighted MRI) onto the Montreal Institute of Neurology (MNI) space, and perform spatial smoothing by the Gaussian kernel with full-width-at-half-maximum (FWHM) of 6 mm. Finally, we divide the preprocessed volumes into 116 ROIs based on the automatic anatomical labeling (AAL) template (Tzourio-Mazoyer et al., 2002), and extract its mean blood oxygen level dependent (BOLD) signals (fMRI series) (Wang et al., 2019).
For the subjects from HCP dataset, they are pre-processed according to WU-Minn (2017). More specifically, structural noise in the fMRI was removed through the pairsed independent component analysis (ICA) (Beckmann and Smith, 2004) with FMRIB's ICA-based Xnoiseifier (FIX) in FSL toolbox (Griffanti et al., 2014). Then, inter-subject registration of cerebral cortex was carried out using areal-feature-based alignment and Multimodal Surface Matching algorithm (MSMAII) (Smith et al., 2011; Glasser et al., 2016). In order to divide the ROIs of the brain, the data was first temporally normalized and fed into group-PCA (Beckmann and Smith, 2004; Smith et al., 2014), and then the group-ICA based on FSL's MELODIC tool (Griffanti et al., 2014) was applied to the data obtained by group-PCA (Beckmann and Smith, 2004). Note that the data dimension after group ICA determines the number of ROIs, which is simply set to 100 in this paper.
2.3. PC-Based BFN Estimation
As described earlier, PC is the simplest and most popular approach to construct BFNs (Smith et al., 2013). The edge weight between the ith and jth ROIs of PC-based BFN is defined as follows:
| (1) |
where is the fMRI signal from the ith ROI, and is a constant signal obtained by averaging the elements in xi. Without loss of generality, we suppose that xi is centralized by and normalized by . Then, Equation ( 1) can be simplified to , and its matrix form is defined as follows:
| (2) |
where is the data matrix, and R is the edge weight matrix of PC-based BFN. For the convenience of presentation in what follows, we simply call the original PC as PC1, and the corresponding edge weight matrix of PC-based BFN as R1 = R.
2.4. From PC1 to PCn
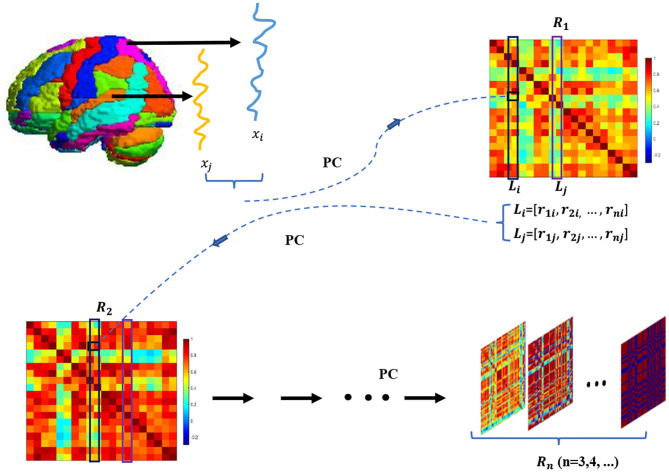
Despite its simplicity and popularity, PC1-BFN can only model the low-order statistics that may be insufficient to capture more complicated relationship between ROIs. As an alternative to PC1, Zhang et al. (2016) recently proposed to construct the high-order BFN by correlation's correlation. Specifically, as shown in Figure 2, the PC1-based BFN (i.e., R1) is first established by Equations ( 1) or ( 2). Then, the R1 is used as a data matrix for constructing the high-order PC2-based BFN (i.e., R2) by considering each row (or column) of R1 as a new “signal” associated with each ROI. Interestingly, the researchers find that R2 can provide additional information in group difference analysis (Zhang et al., 2016).
Figure 2.
The pipeline of building Rn. R1 is first obtained via computing PC coefficient of the BOLD signals associated with different ROIs. Then, R1 is seen as the new data matrix to calculate R2, where the feature vector Li is considered as a new “signal” that indicates the correlation between the ith ROI and other ROIs. By continuously conducting the above operation, we calculate Rn (n = 2,3,⋯ ).
Motived by PC2, in this paper we go further to construct higher-order PCn-based BFN (i.e., Rn, n>2) by continuously conducting the correlation operation. As a result, we obtain a sequence of BFN adjacency matrices, and this sequence from R1 to Rn can be achieved by the following iteration formula (please see Appendix for the details of formula derivation):
| (3) |
where is a constant matrix (with I and E being the identity and all-one matrix, respectively), and diag(A) is a diagonal matrix that shares the same diagonal elements with A.
3. Experiment
In this section, we conduct experiments to answer the questions about the higher-order BFNs: (1) whether the higher-order BFN are more discriminative for achieving better classification performance, and (2) by combinating with PC1-based BFN, whether the PCn-BFNs (n>2) can provide additional information as PC2-based BFNs.
3.1. Experimental Setting
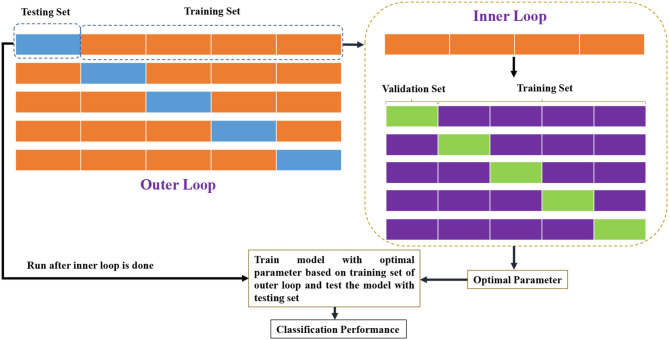
In this experiment, the classification performance is evaluated by five-fold subject-level cross validation (5-F CV), to ensure that fMRI scans of the subject do not appear in both training and testing sets (Wong and Tzu-Tsung, 2015). Specifically, as shown in Figure 3, the 143 (or 1,003) subjects of ADNI (or HCP) are first divided into five-fold, each of which contains almost the same number of subjects. Then, four-fold are used to train the classifier, and the remaining one is used to test the classification performance. Linear support vector machine (SVM) (Chang and Lin, 2011) with default parameter C = 1 is utilized to perform the classification task. The edge weights of the BFN are adopted as features for classification. In consideration of the fact that the number of edges far exceeds the sample size, t-test is used to select edge weight features prior to the classification task. Two binary classification tasks are conducted to evaluate the constructed BFNs with different orders. One is to identify subjects with MCI from HCs based on the ADNI dataset, and the other is to predict gender based on the HCP dataset.
Figure 3.
The pipeline of 5-F CV in this study.
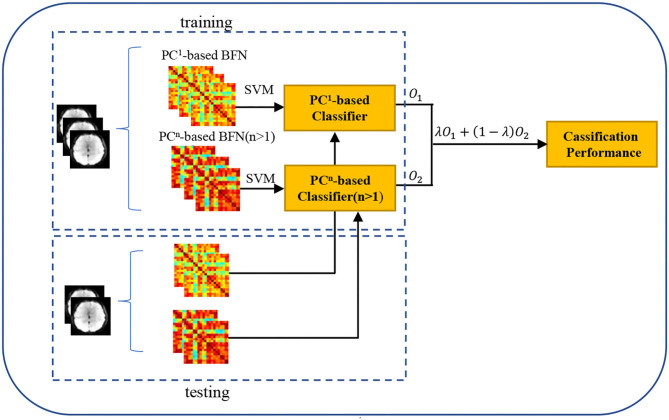
Furthermore, we design experiment to verify whether the PCn-BFNs (n>2) can provide additional information by combining the PCn- and PC1-based BFNs. In particular, we first train two classifiers (SVM with C = 1) based on PC1- and PCn (n>1)-based BFNs, respectively, and then fuse the outputs of these two classifiers. As shown in Figure 4, the outputs, O1 and O2, of the two classifiers measure the probabilities that the current subject belongs to the positive class. With the two probabilistic outputs, we can achieve the fused classification result via λO1+(1−λ)O2, where the optimal parameter λ is selected in the range of [0.1, 0.2, 0.3, 0.4, ⋯ , 0.9] by an inner 5-F CV on the training set. Finally, we retrain the classifier with the optimally selected parameter, and put the BFNs of the testing subject into the trained classifiers for the ultimate performance as shown in Figure 3.
Figure 4.
The classification pipeline based on the PC1- and PCn (n>1)-based BFNs. We first train two classifiers based on the PC1- and PCn (n>1)-based BFNs, respectively, and then fuse the outputs (i.e., O1 and O2) of the two classifiers via λO1+(1−λ)O2, where λ is the optimal parameter obtained by an inner 5-F CV.
3.2. Estimated Functional Brain Networks
In the first group of experiments, we visualize the sequence of BFN constructed by PCn.
In Figure 5, we randomly select a subject from ADNI dataset and visualize the BFNs constructed by different methods. It can be observed that the BFNs estimated by PC16 and PC17 are almost the same, indicating that the adjacency matrix sequence may converge to a binary matrix with entries of 1 and −1. This phenomenon motivates us to explore and prove it in theory. In the discussion section, we will further analyze and discuss this problem.
Figure 5.
The visualization of BFNs with different order n. We empirically note that the adjacency matrix sequence will converge after dozens of iterations.
3.3. Experiment Results
In this group of experiments, two binary classification tasks (i.e., MCI vs. HC, Female vs. Male) based on the constructed BFNs with different orders are first conducted to evaluate whether the higher-order BFN are more discriminative, and then to verify whether the PCn-BFNs (n>2) can provide additional information by combining the PCn- and PC1-based BFNs. We evaluate the classification performance of different methods by a set of quantitative measures, including accuracy (ACC), sensitivity (SEN), and specificity (SPE). Their expressions are defined as follows:
| (4) |
| (5) |
| (6) |
where TP, TN, FP, and FN denote the number of true positive, true negative, false positive, and false negative numbers, respectively.
As described in the experimental setting, we construct BFNs with different orders via multiple sequential PCs and apply them in the classification task. The classification results on two datasets are reported in Figures 6, 7.
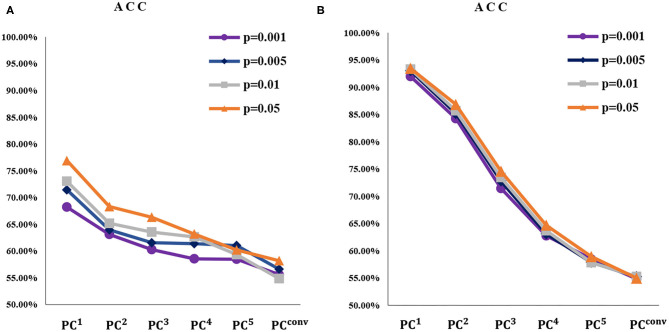
Figure 6.
The ACC of (A) MCI identification and (B) gender prediction based on BFNs with six different orders and four different p-values (for filtering features). The PCconv-based BFN is the empirically convergent adjacency matrix through thirty iterations. Note that, with the increase of n, the ACC of PCn-based BFN tends to decrease in all the cases.
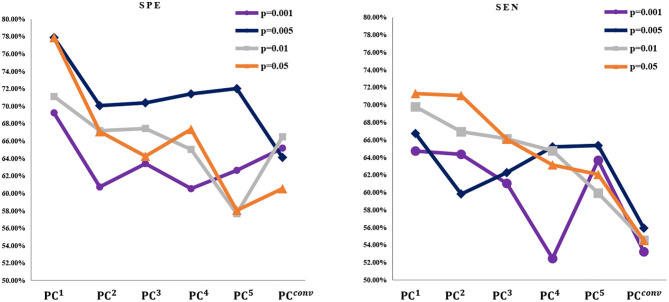
Figure 7.
The SEN and SPE based on six kinds of BFNs for MCI identification, respectively. It can be observed that the SEN and SPE of PC1 is better than other cases. In addition, under different p-values of t-test, the SEN and SPE tend to change greatly.
Furthermore, the classification results based on the fusion of the low-order and higher-order BFNs are reported in Figures 8, 9, respectively.
Figure 8.
The ACC of gender prediction based on the fusion of PCn-based BFN and PC1-based BFN under different p-values. The PCn&1 corresponds to the fused classification accuracy of PCn and PC1, n = 2,3,4, convergence(conv). The dotted line is the ACC of PC1 itself (without fusion with PCn).
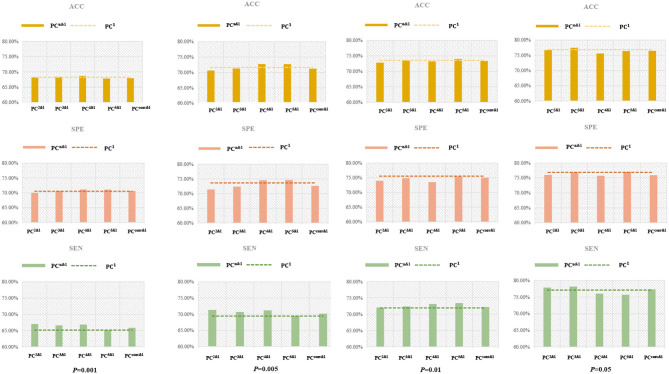
Figure 9.
The performance (ACC, SPE, and SEN) for MCI identification based on fusion of PCn-based BFN and PC1-based BFN under different p-values. The PCn&1 corresponds to the fused performance of PCn and PC1, n = 2,3,4, conv. The dotted line is the performance of PC1 itself (without fusion with PCn). We can observe that the fusion of low- and higher-order BFNs fails to improve the ACC and SPE in most cases, but generally improves SEN.
4. Discussion
In this section, we first study the discrimination of features and the additional information provided by higher-order BFN. Then, we further analyze the convergence of BFNs with different orders and show some related findings about it. Finally, we illustrate the limitations of the current work and the direction of future work.
4.1. Discrimination of Higher-Order BFN
Based on the classification results, we analyze the discrimination of higher-order BFN. Based on Figures 6, 7.
(1) As n increases, the ACC of PCn generally decreases for both MCI identification and gender prediction, even with various p-values in t-test for selecting different groups of feature. This illustrates that the higher-order does not result in stronger discriminative ability. Especially when the BFN converges with a big n, the ACC approaches 50%, meaning that its discrimination almost disappears since our task is a binary classification.
(2) Different from the ACC that decreases monotonically, the SPE and SEN generally fluctuate for the MCI identification task under most p-values. However, the overall performance shows a downtrend for both SPE and SEN. This further illustrates that the higher-order BFNs in the sense of multiple correlations do not necessarily help the discrimination.
(3) As a byproduct of our experiments, we find that the p-value generally has a great impact on the final performance. For example, as shown in Figure 6, the PCn-based BFNs have better performance when the p-value is 0.05. In terms of the fused classification results, it can also be observed that p = 0.05 will result in the best ACC, SEN, and SPE, as shown in Figure 9. This suggests that we should carefully select features (edge weights) prior to the classification.
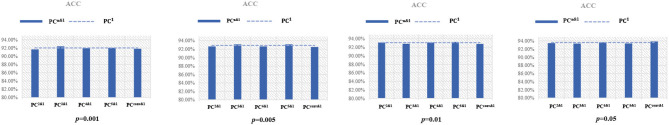
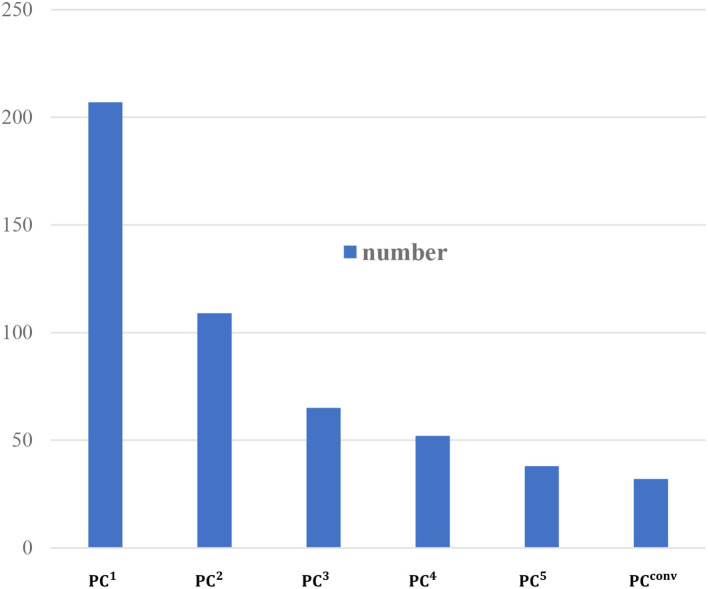
In order to better explain our classification results, we further investigate the number of features involved in the PCn-based MCI classification, and report the results with p-value of 0.001 in Figure 10.
Figure 10.
The number of selected features with p-values = 0.001.
It can be seen, from Figure 10, that PC1 includes the largest number of features (207 features) and the number of features selected by PCn decrease with the increase of n. This means that the higher-order BFN instead contains less discriminative information than the low-order BFN.
4.2. Supplementary Information of Higher-Order BFNs
The higher-order BFNs can provide some additional information for the classification task. As shown in Figure 9, although it cannot benefit the ACC and SPE, the combination of PCn-based BFN and PC1-based BFN can generally improve the SEN. The improvement of SEN is generally important, because it may help to detect the brain disorders in their early stage. In addition, this also give a reason to believe that PCn(n>1) would provide potentially additional information for encoding complex relationship between different ROIs.
4.3. Convergence Analysis
In the process of constructing the BFN based on the sequential PCs, we have an interesting finding that the sequence of BFN adjacency matrices estimated by PCn (n = 1,2,3⋯ ) will converge to a binary matrix with entries of 1 and −1 as shown in Figure 5. Although we cannot provide a direct explanation of this phenomenon from a biological mechanism, it will inspire us to think whether the interaction between different ROIs promotes or inhibits each other at a certain working stage of the brain.
Currently, the convergence of the BFN sequence cannot be rigorously proved in theory. We will explore this problem in the future work. However, in the process of searching the rigorous proof of convergence, we achieve several interesting theoretical findings. In particular, let be the sequence of correlation coefficient matrices generated by the iterative formula shown in Equation ( 3), and then we have the following results or conjecture:
After iterating the matrix for a certain number of times, the elements in the matrix will be monotonic with the number of iterations, and the sign of a small number of elements will change once.
Supposing that the rank of Rn is , we can achieve a sequence of rank that is monotonically decreasing, since the rank of the matrix product is not greater than the rank of any one of them (Lancaster and Tismenetsky, 1985).
According to Schauder's fixed-point theorem (Kellogg, 1976), we find that the iterative formula given in Equation ( 3) has a fixed-point4.
4.4. Limitations and Future Work
First, in this paper we only study the HOBFN constructed by multiple sequential PCs. In fact, however, many traditional BFN estimation methods such as sparse representation (Lee et al., 2011) can be extended to higher-order versions through the similar way (i.e., multiple sequential calculations). In the future, we plan to discuss more kinds of HOBFNs and systematically compare their discrimination. Second, we cannot rigorously prove the convergence of matrix sequence in theory, and the biological mechanism of HOBFN is unclear at present. We will try to prove its convergence and explore its biological mechanism in the future.
5. Conclusion
Recent researches have proposed many methods to construct high-order BFNs (HOBFNs) that can generally provide some additional information for poring cerebral mechanisms. For instance, Guo et al. (2017) constructed HOBFN using minimum spanning tree and Zhang et al. (2016) recently proposed to construct BFNs by conducting two sequential PCs. In this paper, we mainly focus on constructing the PCn-based BFN by multiple sequential PCs, and exploring its performance in the classification tasks. Through experiments, we find that the higher-order does not necessarily result in stronger discriminative ability, but the additional information provided by PCn (n>1) is helpful for PC1 to improve the sensitivity of the classifier. In addition, we empirically find that the matrix sequence constructed by PCn (n=1,2,3 ⋯ ) converges to a matrix with elements of −1 and 1.
Data Availability Statement
The original contributions generated for the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by public datasets HCP and ADNI. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LQ and TG contributed to the conception and design of this research. LQ, YZ, and TG derived and designed the theoretical and experimental parts in the article. TG, LQ, YX, and DS wrote the first draft of the manuscript. All authors participated in the revision, reading, and approval of the manuscript.
Conflict of Interest
DS was employed by the company Shanghai United Imaging Intelligence Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
4Schauder fixed point: suppose X is a banach space, and M∈X is a bounded closed convex set. If F: M→M is a compact-operator, there is x∈M such that F(x)=x.
Funding. This work was partly supported by National Natural Science Foundation of China (Nos. 61976110 and 11931008).
Appendix
Construction Details of the Iterative Formula
Suppose that is an initial Pearson's correlation coefficient matrix estimated from observed data of an n-dimensional random vector X = (X1, X2, ⋯ , Xn). If considering R1 as new observed data, each row (or equivalently each column, due to the symmetry) of which corresponds to a random variable in X, we can estimate another correlation coefficient matrix R2 based on R1. Further, by continuously conducting the operation of “correlation's correlation,” we can have a sequence of correlation coefficient matrices , and we empirically find that the matrix sequence will converge, if all the involved calculations are legal (for example, the denominator is not zero).
In order to prove the conjecture of convergence, we first express the sequence of correlation's correlation as an iterative formula. Specifically, let ri and rj be the ith and jth rows of current correlation matrix , respectively. Then, the Pearson's correlation coefficient, denoted by , between the corresponding variables is given as follows,
| (7) |
where , (i, j = 1, 2, ⋯ , n), and 1∈R(1 × n) is an all-one vector. As a result, the new correlation matrix can be expressed as follows:
| (8) |
Note that
| (9) |
| (10) |
where diag(A) is a diagonal that shares the same diagonal elements with A, and , with I and E being the identity and all-one matrix, respectively. Therefore, we have , and accordingly have the iterative formula is given as follows below:
| (11) |
References
- Beckmann C. F., Smith S. M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imag. 23, 137–152. 10.1109/TMI.2003.822821 [DOI] [PubMed] [Google Scholar]
- Bijsterbosch J., Smith S., Beckmann C. (2017). An Introduction to Resting State FMRI Functional Connectivity. Oxford University Press. [Google Scholar]
- Chang C., Lin C. (2011). Libsvm: a library for support vector machines. ACM Trans. Intell. Syst. Technol. 2, 1–27. 10.1145/1961189.1961199 [DOI] [Google Scholar]
- Chen H., Zhang Y., Zhang L., Qiao L., Shen D. (2021). Estimating brain functional networks based on adaptively-weighted fMRI signals for MCI identification. Front. Aging Neurosci. 12:595322. 10.3389/fnagi.2020.595322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhang H., Gao Y., Wee C.-Y., Li G., Shen D. (2016). High-order resting-state functional connectivity network for MCI classification. Hum. Brain Mapp. 37, 3282–3296. 10.1002/hbm.23240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Adolphs R. (2016). Building a science of individual differences from fMRI. Trends Cogn. Sci. 20, 425–443. 10.1016/j.tics.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M. F., Coalson T. S., Robinson E. C., Hacker C. D., Harwell J., Yacoub E., et al. (2016). A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L., Salimi-Khorshidi G., Beckmann C. F., Auerbach E. J. Douaud, G., Sexton C. E., et al. (2014). ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage 95, 232–247. 10.1016/j.neuroimage.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Lei L., Chen J., Xu Y., Xiang J. (2017). Alzheimer classification using a minimum spanning tree of high-order functional network on fMRI dataset. Front. Neurosci. 11:639. 10.3389/fnins.2017.00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Zhang H., Zeng L., Shen H., Shen D. (2020). Dynamic neural circuit disruptions associated with antisocial behaviors. Hum. Brain Mapp. 42, 329–344. 10.1002/hbm.25225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Zhang L., Qiao L., Shen D. (2019). Estimating functional connectivity networks via low-rank tensor approximation with applications to MCI identification. IEEE Trans. Biomed. Eng. 67, 1912–1920. 10.1109/TBME.2019.2950712 [DOI] [PubMed] [Google Scholar]
- Kellogg R. (1976). Uniqueness in the schauder fixed point theorem. Proc. Am. Math. Soc. 60, 207–210. 10.1090/S0002-9939-1976-0423137-6 [DOI] [Google Scholar]
- Lancaster P., Tismenetsky M. (1985). The Theory of Matrices: With Applications. New York, NY: Academic Press. [Google Scholar]
- Lee H., Lee D, S., Kang H., Kim B, N., Chung M. (2011). Sparse brain network recovery under compressed sensing. IEEE Trans. Med. Imag. 30, 1154–1165. 10.1109/TMI.2011.2140380 [DOI] [PubMed] [Google Scholar]
- Li W., Wang Z., Zhang L., Qiao L., Shen D. (2017). Remodeling Pearson's correlation for functional brain network estimation and autism spectrum disorder identification. Front. Neuroinformatics 11:55. 10.3389/fninf.2017.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Guo W., Fouche J.-P., Wang Y., Wang W., Ding J., et al. (2015). Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Struct. Funct. 220, 101–115. 10.1007/s00429-013-0641-4 [DOI] [PubMed] [Google Scholar]
- Liu F., Wang Y., Li M., Wang W., Li R., Zhang Z., et al. (2017). Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum. Brain Mapp. 38, 957–973. 10.1002/hbm.23430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelec G., Krainik A., Duffau H., Plgrini-Issac M., Lehricy S., Doyon J., et al. (2006). Partial correlation for functional brain interactivity investigation in functional MRI. NeuroImage 32, 228–237. 10.1016/j.neuroimage.2005.12.057 [DOI] [PubMed] [Google Scholar]
- McLntosh A. R., Gonzalez-Lima F. (1994). Structural equation modeling and its application to network analysis in functional brain imaging. Hum. Brain Mapp. 2, 2–22. 10.1002/hbm.460020104 [DOI] [Google Scholar]
- Power J. D., Barnes K. A., Snyder A. Z., Schlaggar B. L., Petersen S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L., Zhang H., Kim M., Teng S., Zhang L., Shen D. (2016). Estimating functional brain networks by incorporating a modularity prior. NeuroImage 141, 399–407. 10.1016/j.neuroimage.2016.07.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J. D., Hanson S. J., Hanson C., Halchenko Y. O., Poldrack R. A., Glymour C. (2010). Six problems for causal inference from FMRI. NeuroImage 49, 1545–1558. 10.1016/j.neuroimage.2009.08.065 [DOI] [PubMed] [Google Scholar]
- Smith S. M., Hyvrinen A., Varoquaux G., Miller K. L., Beckmann C. F. (2014). Group-PCA for very large fMRI datasets. NeuroImage 101, 738–749. 10.1016/j.neuroimage.2014.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Miller K. L., Salimi-Khorshidi G., Webster M., Beckmann C. F., Nichols T. E., et al. (2011). Network modelling methods for FMRI. NeuroImage 54, 875–891. 10.1016/j.neuroimage.2010.08.063 [DOI] [PubMed] [Google Scholar]
- Smith S. M., Vidaurre D., Beckmann C. F., Glasser M. F., Jenkinson M., Miller K. L., et al. (2013). Functional connectomics from resting-state fMRI. Trends Cogn. Sci. 17, 666–682. 10.1016/j.tics.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Xue Y., Zhang Y., Qiao L., Liu M. (2020). Estimating sparse functional connectivity networks via hyperparameter-free learning model. Artif. Intell. Med. 111:102004. 10.1016/j.artmed.2020.102004 [DOI] [PubMed] [Google Scholar]
- Swami A., Giannakis G. B, Zhou G. (1997). Bibliography on higher-order statistics. Signal Process. 60, 65–126. 10.1016/S0165-1684(97)00065-0 [DOI] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15, 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Wan X., Riera J., Iwata K., Takahashi M., Wakabayashi T., Kawashima R. (2006). The neural basis of the hemodynamic response nonlinearity in human primary visual cortex: implications for neurovascular coupling mechanism. NeuroImage 32, 616–625. 10.1016/j.neuroimage.2006.03.040 [DOI] [PubMed] [Google Scholar]
- Wang M., Lian C., Yao D., Zhang D., Shen D. (2019). Spatial-temporal dependency modeling and network hub detection for functional MRI analysis via convolutional-recurrent network. IEEE Trans. Bio-Med. Eng. 67, 2241–2252. 10.1109/TBME.2019.2957921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.-T. (2015). Performance evaluation of classification algorithms by K-fold and leave-one-out cross validation. Pattern Recogn. 48, 2839–2846. 10.1016/j.patcog.2015.03.009 [DOI] [Google Scholar]
- Wu-Minn H. (2017). 1200 Subjects Data Release Reference Manual. Available online at: https://www.humanconnectome.org.
- Xie X., Cao Z., Weng X. (2008). Spatiotemporal nonlinearity in resting-state fMRI of the human brain. NeuroImage 40, 1672–1685. 10.1016/j.neuroimage.2008.01.007 [DOI] [PubMed] [Google Scholar]
- Xue Y., Zhang L., Qiao L., Shen D. (2020). Estimating sparse functional brain networks with spatial constraints for MCI identification. PLoS ONE 15:e0235039. 10.1371/journal.pone.0235039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chen X., Shi F., Li G., Kim M., Giannakopoulos P., et al. (2016). Topographical information-based high-order functional connectivity and its application in abnormality detection for mild cognitive impairment. J. Alzheimer's Dis. 54, 1095–1112. 10.3233/JAD-160092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Qiao L., Li W., Zhang L., Shen D. (2018). Simultaneous estimation of low- and high-order functional connectivity for identifying mild cognitive impairment. Front. Neuroinform. 12:3. 10.3389/fninf.2018.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions generated for the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.