Abstract
Fatty acid nitroalkenes are reversibly-reactive electrophiles that are endogenously detectable at nM concentrations and display anti-inflammatory, pro-survival actions. These actions are elicited through the alteration of signal transduction proteins via a Michael addition on nucleophilic cysteine thiols. Nitrated fatty acids (NO2-FAs), like 9- or 10-nitro-octadec-9-enolic acid, will act on signal transduction proteins directly or on key regulatory proteins to cause an up-regulation or down-regulation of the protein’s expression, yielding an anti-inflammatory response. These responses have been characterized in many organ systems, such as the cardiovascular system, with the pulmonary system less well defined. Macrophages are one of the most abundant immune cells in the lung and are essential in maintaining lung homeostasis. Despite this, macrophages can play a role in both acute and chronic lung injury due to up-regulation of anti-inflammatory signal transduction pathways and down-regulation of pro-inflammatory pathways. Through their propensity to alter signal transduction pathways, NO2-FAs may be able to reduce macrophage activation during pulmonary injury. This review will focus on the implications of NO2-FAs on macrophage activation in the lung and the signal transduction pathways that may be altered, leading to reduced pulmonary injury.
Keywords: Inflammation, macrophage activation, nitrated fatty acid, nitroalkene, signal transduction
Introduction
Nitrated fatty acids (NO2-FAs) are endogenously-formed compounds that play a major role in regulating cellular processes, especially those involved in the inflammatory response.1–4 They are formed via non-enzymatic reactions between unsaturated fatty acids and nitrogen dioxide (NO2) equivalents.5–7 Cell and tissue levels of NO2-FAs can be modulated by diet and oxidative stress.7,8 Similar to other electrophilic fatty acids, NO2-FAs act as potent signaling molecules. Under inflammatory conditions, they are formed at higher concentrations in the body as their formation requires oxidative stress.2,4 Fatty acids with conjugated double bonds are the main targets of this reaction, which has been affirmed via mass spectrometry analysis of human urine.9 NO2-FAs are electrophilic in nature, which allows them to interact readily with nucleophiles, such as cysteine thiols, on susceptible proteins.10–12 Reaction with cysteine residues results in a covalent post-translational modification via Michael addition.13 Through these modifications, NO2-FAs are able to invoke pro-survival, anti-inflammatory responses in many different tissues and organ systems throughout the body.14–17
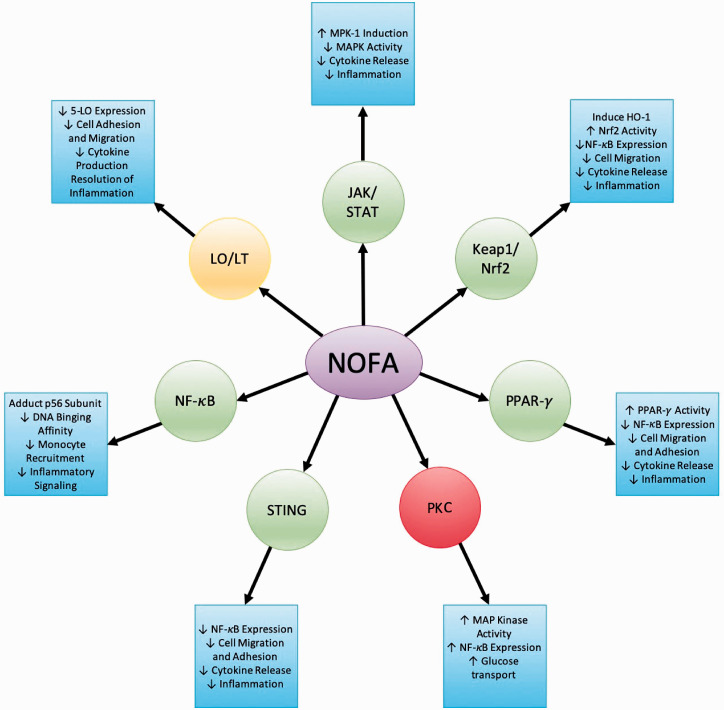
NO2-FAs act on many different signal transduction pathways in which cysteine-containing proteins are involved (Figure 1).
Figure 1.
Signal transduction pathways altered by NOFAs and their subsequent anti-inflammatory responses.
They have been observed altering pathways involved in the initiation of inflammatory responses or migration of inflammatory cells.4,10,18–20 NO2-FAs are pluripotent in nature, meaning that they are capable of altering a number of signal transduction pathways.21 This makes them ideal anti-inflammatory agents in the lung, as the pulmonary immune system is highly complex and involves many different pathways.
Macrophages are one of the most abundant immune cells in the lung and are essential in maintaining lung homeostasis.22 Alveolar macrophages are typically resident in the lung and are the first line of defense against pathogens and toxic or allergic particulates.23 Upon insult, alveolar macrophages will release a wide variety of cytokines and chemokines in response, allowing for recruitment of other immune cells including interstitial macrophages and neutrophils.24 These macrophages are thought to be classically activated (M1). Airway macrophages also play an important role in the resolution of inflammation and injury.25 This population is considered to be alternatively activated (M2). Although both populations of macrophages are essential for immune defense and repair in the lung, they can also contribute to injury should their balance be altered.26 Macrophages play a role in the pathogenesis of both acute lung injury and interstitial lung diseases through an overactivation of the M1 phenotype initially followed by overabundant M2 activation.26 This will lead to acute inflammatory injury in the lung followed by a progression to fibrosis, should resolution falter.
Macrophage activation and phenotypic switching often occurs due to changes in signal transduction, based on cytokines and inflammatory modulators present in the lung. Activation may occur via up-regulation of pro-inflammatory pathways, such as the JAK/signal transducer and activator of transcription proteins (STAT) signaling cascade,27–29 NF-κB,30–32 lipoxygenase (LO)-dependent leukotriene (LT) synthesis,33 protein kinase C,34,35 and stimulator of interferon genes (STING).36 Macrophage activation can also be inhibited through activation of prominent anti-inflammatory pathways such as the kelch-like ECH-associated protein 1(Keap1)/nuclear factor E2-related factor 2 (Nrf2) pathway37 and peroxisome proliferator-activated receptor-gamma (PPAR-γ).38 Alterations to these pathways impact macrophage phenotype greatly and may contribute to their inflammatory and fibrotic potential during lung injury. These pathways are also relevant to NO2-FAs, in that they all have cysteine residues susceptible to Michael addition.
Through their propensity to alter several signal transduction pathways, NO2-FAs have shown potential as a therapeutic in a wide variety of inflammatory diseases. They have demonstrated the ability to reduce inflammation in many different organ system pathologies, which may be beneficial in the mitigation or reversal of symptoms.39 Several studies have been conducted discussing the potential of NO2-FAs as therapeutics in a variety of organs, including the renal, digestive, nervous, respiratory, cardiovascular, and pancreatic systems.3,14,40–43 The pulmonary system has not been studied to the same extent, however, they have great potential to mitigate lung injury by balancing macrophage phenotype and activation.
This review will focus on the implications of NO2-FAs on macrophage activation in the lung. Several of the signal transduction pathways that NO2-FAs alter will be discussed, along with the mechanism by which these alterations are suspected to occur. These alterations will then be discussed in context of pulmonary macrophage activation and their potential use in reducing lung injury.
Signal transduction pathway alterations and macrophage activation
Several studies have been conducted to determine the mechanism by which NO2-FAs elicit anti-inflammatory responses. There are three levels at which signal transduction pathways can be modified: the transcriptional level, the extracellular/intracellular signaling level, and the level of signal being transmitted into the cell itself through receptors. The effects of NO2-FAs on different signal transduction pathways occur through the activation of some pathways and the inhibition of others and many of these pathways are significant in airway macrophage activation. Post-translational modifications to proteins change their signaling abilities and can lead to anti-inflammatory, pro-survival responses through the alterations of macrophage function.
JAK/STAT signaling cascade
The JAK/STAT signaling cascade plays a crucial role in the regulation of bodily defenses against foreign bodies and pathogens via inflammatory response.4 This cascade is located upstream of the NF-B, Keap1/Nrf2, and PPAR-γ pathways and is essential in the IL-6 mediated infiltration of T cells during acute inflammation.44 This pathway is also critical in mediating macrophage responses to cytokines like IL-4, IL-13, and INF-γ and plays an essential role in macrophage phenotypic switching between M1 and M2.45–47 Ichikawa and colleagues demonstrated that NO2-FAs are able to inhibit the inflammatory actions of the JAK/STAT signaling pathway in macrophages.4 NO2-FAs enhance the induction of mitogen-activated protein kinase phosphatase 1 (MKP-1), which acts as a negative regulator of pro-inflammatory cytokine release.4 MKP-1 inactivates MAPK such as c-Jun N-terminal kinase (JNK), which are pro-inflammatory in nature.4 The inactivation of these pro-inflammatory mediators by NO2-FAs will lead to the inhibition of the STAT portion of the JAK/STAT signaling cascade (Figure 1).4 Phosphorylation of STATs via JAKs leads to an up-regulation of pro-inflammatory downstream targets such as inducible NO synthase (iNOS) and monocyte chemoattractant protein-1(MCP-1).27 As NO2-FAs can inhibit STAT phosphorylation in macrophages, they can inhibit activation of these target genes.4
Keap1/Nrf2 pathway
The Keap1/Nrf2 pathway is primarily involved in regulating cellular responses to electrophilic and oxidative stressors.18,48 Keap1 and Nrf2 both have several cysteine residues that are critical to their function and are susceptible to Michael addition.49–51 Nrf2 itself plays a crucial role in the activity and coordinated induction of several different antioxidant genes, including phase 2-detoxifying enzymes and other related proteins and antioxidants like GSH, NADPH, dehydrogenase quinone 1 (NQO1), epoxide hydrolase, thioredoxin, catalase, superoxide dismutase, glutamate cysteine ligase, uridine diphosphate glucuronosyltransferase, and heme oxygensae-1 (HO-1).52,53
Nrf2 is inactivated when it is bound to Keap1.54 The nucleophilic cysteine residues on Keap1 provide a suitable target for electrophiles, like NO2-FAs, and reactions perpetuate conformational changes in Keap1, allowing for the release of Nrf2 from the complex.54 Nrf2 release into the cytoplasm allows for increased Nrf2 synthesis.55 Newly synthesized Nrf2 is able to translocate into the nucleus through the aid of chaperone proteins.14,55 Once in the nucleus, Nrf2 binds to the antioxidant response element (ARE), allowing for the transcription of genes involved in the regulation of several oxidative stress pathways (Figure 1).49
As stated, NO2-FAs are able to induce Nrf2 dependent, cytoprotective gene expression through its interference with Keap1 binding.48 NO2-FAs can covalently adduct Cys237 and Cys288, which are functionally significant in the action of Keap1.17 Unbound Nrf2 is then stabilized in the cytoplasm and translation is increased,55 leading to increased production of Nrf2 targets such as superoxide dismutases (SODs), glutathione peroxidase (GPx), glutathione reductase, glutathione-S-transferase, NQO1, HO-1, glutamate cystine ligases, and multi-drug resistance protein 1.48,56–59 Conditions of inflammation or oxidative stress may lead to the activation of certain pro-inflammatory pathways, such as NF-κB, but their activity is disrupted through the activation of Nrf2 by preventing the up-regulation of pro-inflammatory cytokines.37,60,61 Nrf2 activation by NO2-FAs will also regulate the migration and infiltration of inflammatory cells through cellular adhesion molecule (CAM) inhibition.37,60,61
Nrf2 activation is critical in regulating cellular antioxidants and cytoprotective genes in macrophages.62 Pro-inflammatory cytokine transcription can be blocked via Nrf2 activation, suppressing inflammatory signaling by macrophages.37 These anti-inflammatory effects cannot be elicited when Nrf2 is bound by Keap1 as it is marked for degradation. NO2-FAs have been shown to alter Keap1 binding to Nrf2 in vivo, suggesting it may be able to induce Nrf2 signaling.48,63 This was observed in mouse alveolar macrophages that were exposed to LPS and administered NO2-FAs, leading to increased expression of several genes downstream of Nrf2-activated ARE, such as HO-1, NQO1, and GCLM.17 Therefore, OA-NO2 may elicit anti-inflammatory effects through binding Keap1, leading to decreased cellular migration, cytokine release, and general inflammation via Nrf2-mediated suppression.
NF- B pathway
NF-κB is a nuclear factor that regulates the expression of genes that encode for pro-inflammatory cytokines during inflammatory responses.19 Under normal conditions, NF-κB is sequestered in the cytoplasm through binding with NF-κB inhibitory proteins, IKBs.64,65 During inflammatory conditions, NF-κB is activated via phosphorylation of IKB by the IKB kinase (IKK) complex that is composed of IKK-α, IKK-β, and IKK-γ.58 These phosphorylation events result in the release of NF-κB and the proteasomal degradation of IKB.66–69 NF-κB is key in macrophage activation, especially toward the M1 phenotype, and will induce the expression of inflammatory genes such as IL-6 and IL-1β.70,71
Because NO2-FAs are electrophilic, they can adduct the p65 (Cys38) and p50 (Cys 62) subunits of NF-κB, inhibiting its binding to DNA.17,19 This binding inhibition results in down-regulation of NF-κB target genes, leading to limited downstream pro-inflammatory signaling.19 Monocyte recruitment is also limited by NF-κB inhibition of NO2-FAs due to a down-regulation of intracellular adhesion molecule-1 and vascular cell adhesion molecule-1 (Figure 1).19 Inhibition of the p65 subunit results in the suppression of IL-6, TNF-α, and monocyte chemoattractant protein 1 (MCP-1) secretion, all of which are involved in the inflammatory process.19 NO2-FAs can also inhibit phosphorylation of IKB and IKK, resulting in further NF-κB suppression.69
NO2-FAs can also inhibit NF-κB by impairing upstream signaling events such as TLR4 recruitment into lipid rafts in response to injury.69 Lipid raft microdomains coordinate the initial signaling events for TLR4-mediated inflammatory responses in the membrane.69,72 During inflammatory events, TLR4 and TRAF6 are recruited to flotillin-1-rich fractions of the membrane.69 Recruitment to lipid rafts allows TLR4 signaling complexes to activate NF-κB signaling, ultimately leading to downstream signaling of pro-inflammatory effects.69,73 NO2-FAs are able to inhibit the recruitment of TLR4 and TRAF6 into lipid rafts, preventing their coordination and signaling, thereby inhibiting NF-κB activity downstream.69 This has also been demonstrated with other oxidized lipid molecules, like Eritoran, which has been shown to be protective against pulmonary inflammation in influenza.74
PPAR-γ pathway
PPAR-γ is involved in the regulation of inflammatory responses, cell proliferation, apoptosis, and metabolic function.40 PPARs have very large ligand-binding domains compared with other nuclear receptors, giving them the capacity to accommodate and bind many different molecules, including large fatty acids.75,76 Natural ligands of PPAR-γ include unsaturated fatty acids, 15-hydroxy-eicosatetraenoic acid, 9- and 13- hydroxy-octadecadienoic acid, and prostaglandin PGJ2.75 This activation by large lipid ligands results in PPAR-γ heterodimerizing with retinoid X receptor (RXR) and binding to peroxisome proliferator response elements (PPRE) in the regulatory regions of target genes, allowing for their transcription.40,77 This results in anti-inflammatory effects. PPAR-γ can also directly react with NF-κB through its p50 and p65 subunits, leading to inhibition of pro-inflammatory signals.78 PPAR-γ activation in macrophages attenuates expression of several pro-inflammatory genes.79,80
NO2-FAs are potent agonists of PPAR-γ and exhibit anti-inflammatory actions through this reaction (Figure 1).18,20,81,82 NO2-FAs are capable of binding all three of the PPAR isomers with high affinity but bind with the highest affinity to PPAR-γ.20 NO2-FAs modify PPAR-γ at Cys285 via Michael addition, resulting in partial agonism.43 This modification may serve to protect Cys285 from inflammatory-derived reactive species as Cys285 is highly susceptible to oxidation.43 PPAR-γ association with NO2-FAs additionally promotes interactions between PPAR-γ and NF-κB, leading to further anti-inflammatory effects.5 In classically active macrophages, PPAR-γ inhibits pro-inflammatory responses, including NF-κB activity, but their expression is typically suppressed in environments promoting M1 activation.83–85 The ability of NO2-FAs to activate PPAR-γ and promote its interactions with NF-κB will allow for the pro-survival actions of PPAR-γ to persist and prevent inflammatory activation of macrophages.
LO-LT synthesis
Lipoxygenase-leukotriene (LO-LT) synthesis is a pro-inflammatory process involving phospholipase A2-dependent hydrolysis of arachidonic acid from membrane phospholipids.10,86 These phospholipids are oxidized by 5-LO to form the lipid mediators, LT-A4 and 5-hydroperoxy eicosatetraenoic acid (5-H(P)ETE).86 This leads to the formation of hydroxyeicosatetraenoic acid (HETE), LT-B4, and LT-C4.86 Formation of these eicosanoids promotes regulation of leukocyte recruitment and activation as they are potent modulators of inflammation.10,87
5-LO is the only LO with an appropriate nucleophilic amino acid residue, Cys148, sensitive to undergoing a Michael addition.86 Cys148 is not within the active site of 5-LO, and modification of this residue by NO2-FAs results in a non-competitive inhibition of function.10 5-LO contributes to inflammatory activation in pulmonary macrophages, promoting cellular migration, cytokine production, and a pro-inflammatory phenotype.88 NO2-FAs inhibition of 5-LO function has been seen in vivo within the lung following induction of sepsis, resulting in a suppression of the inflammatory response.10 The overall product formation of 5-LO is inhibited in a concentration-dependent manner, indicating a natural accumulation of NO2-FAs during sepsis.10 This leads to the conclusion that perhaps NO2-FAs act as a feedback mechanism, reducing further inflammatory cell migration and recruitment.10 It is reasonable to suppose that via 5-LO inhibition, NO2-FAs can reduce eicosanoid formation, which may be of benefit in lung injury or asthma.10,87
PKC pathway
PKCs are a large family of proteins that regulate the function of a variety of other proteins through phosphorylation and are one of the major mediators of signal transduction.35,89 Their activation leads to the up-regulation of signaling pathways involved in cell adhesion, motility, and inflammation gene regulation.90,91 There are several ways in which PKCs can be activated, depending on the subfamily to which they belong. Conventional PKCs require calcium and diacylglycerol for their activation, novel PKCs require diacylglycerol alone, and atypical PKCs do not require either substrate for their activation.35 Once activated, PKCs act as effectors for several tyrosine kinases, cytokine receptors, GPCRs, and adhesion receptors, while activating several signaling pathways like NF-κB, JAK/STAT, MEK/ERK, p38, and JNK.91–94 Processes will vary depending on the subfamily that is present and the cell type they are located in.35 All subfamilies of PKC have cysteine residues that may be susceptible to Michael additions.35
NO2-FAs have varied effects on different PKC subtypes and their downstream signaling (Figure 1). The addition of NO2-FAs to pulmonary epithelial cells has been observed to increase the membrane association of the atypical PKCζ, increasing its overall function.35 This will induce the MAP kinase cascade, resulting in an up-regulation of NF-κB. Macrophage activation can occur via PKC-ζ signaling.95 Inflammatory stimuli, such as LPS, will allow PKC-ζ to associate with RhoA, ultimately leading to NF-κB activation.95 Because NO2-FAs have the ability to activate PKC-ζ, they may elicit pro-inflammatory activation in pulmonary macrophages. Another PKC that has been observed to be modified by NO2-FAs is PKC-α. PKC-α is located diffusely throughout the cytosol and will migrate to the membrane in response to secondary messengers such as Ca2+.96 NO2-FAs have been observed to prevent this translocation of PKC-α to the membrane, thereby preventing their inflammatory signaling.96 In macrophages, PKC-α stimulation leads to the release of pro-inflammatory cytokine and NO, contributing to an M1 macrophage phenotype.97 The inhibition of PKC-α translocation and signaling by NO2-FAs in macrophages could prevent inflammation and reduce lung injury.
STING pathway
STING is a transmembrane adaptor protein that is activated via binding of cGMP.98,99 It is located in the endoplasmic reticulum (ER) and the mitochondria-associated ER membrane.36,100 Binding of cGMP leads to the recruitment and phosphorylation of TANK-binding kinase 1 (TBK-1), which plays a role in innate immune system activation via reactions with PRR.99,101,102 TBK-1 activates interferon regulatory factor 3 (IRF3), which will homodimerize and relocate to the nucleus.99,102 The complex will initiate the transcription of several pro-inflammatory genes, leading to increased cytokine production.99,102 This response induces many type 1 IRF genes to be expressed, which are most common in monocytes and fibroblasts.102 TBK-1 activation will also lead to NF-κB induction.103 This will further strengthen the inflammatory response.
A study by Hansen and colleagues demonstrates the potential of NO2-FAs to inhibit STING signaling via adduction to cysteine 88 and 91, as well as the N-terminal histidine (His18).99 This adduction will lead to inhibition of STING and deregulation of STING palmitoylation.99,104 This inhibition will prevent the expression of pro-inflammatory cytokines such as IL-6 and type 1 IRF.99 Inhibition of STING may also lead to downstream inhibition of NF-κB, as TBK-1 will no longer be activated.103 This results in anti-inflammatory, pro-survival responses (Figure 1).
Hematopoietic cells, like macrophages, have high levels of STING expression and its signaling is up-regulated in infiltrating immune cells.100,105 STING is highly up-regulated in macrophages under inflammatory conditions and inflammation was shown to be reduced in STING knockout mice.106 STING activation will lead to downstream activation of inflammatory cytokines, which will further exacerbate inflammation.106 Because STING is up-regulated during injury in the lung, NO2-FAs could be utilized to reduce their response via its adduction.99,107 This will reduce cytokine levels and NF-κB activation via a failure to activate TBK-1.
Acute and chronic inflammatory diseases in the lung
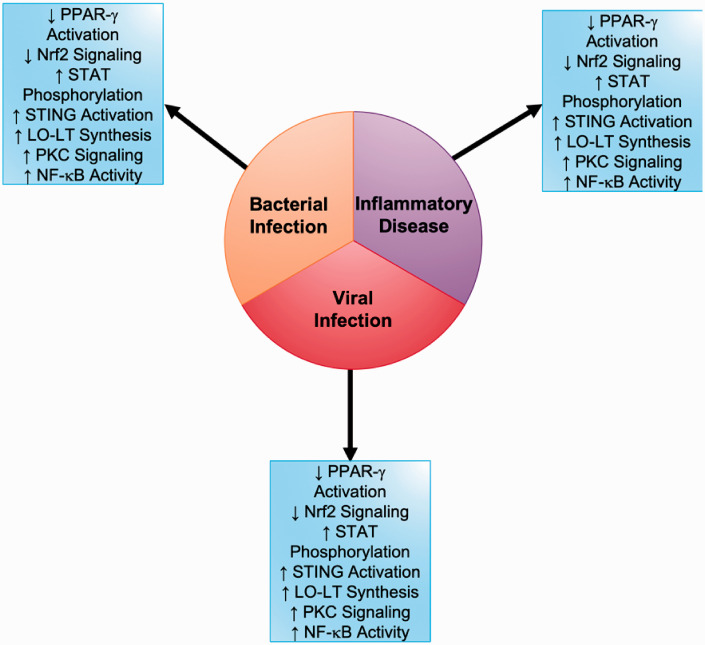
Macrophages play a key role in several acute and chronic inflammatory diseases and infection in the lung (Figure 2).
Figure 2.
Signal transduction pathway alterations in pulmonary macrophages during inflammatory injury and viral and bacterial infections
The lung is highly dependent on innate immune function to defend against inhaled pathogens and particulates.23 This first line of defense is highly dependent on resident alveolar macrophages, which account for the vast majority of cells in the lung lining.22 Macrophages will transition between classically and alternatively activated phenotypes when dealing with these insults and the balance between the two is essential in dealing with the insult and preventing injury.26 When this balance is altered injury prevails and, because of this, the lung is the perfect system to study the therapeutic effects of NO2-FAs. Macrophages are abundant in the lung and their response to insult or particulates is heavily reliant on signal transduction. NO2-FAs have the capacity to alter signaling in several of these key pathways, which may lead to reduced activation and decreased lung injury.
Toxicant exposure, viral and bacterial infections, and idiopathic disease can all elicit injury and disease in the lung. There are few effective treatments once the lung is injured and lasting effects may arise or persist after the initial treatment. Few studies have been conducted describing the potential of NO2-FAs as therapeutics for these disease states but those that have been conducted have shown promising results.
Acute lung injury
During acute lung injury, pulmonary inflammation is mediated by the innate immune system.108,109 Acute lung injury can be caused by a variety of exposures to inhaled toxicants and infectious agents leading to decreased lung function and substantial morbidities and mortalities in severe cases.108,110,111 One model of acute lung injury is through the administration of bleomycin intratracheally.112 The inflammatory response is macrophage dominant, allowing for the examination of macrophage phenotype in response to injury.112 It has been demonstrated that NO2-FAs can reduce acute lung injury in this model, potentially by altering alveolar and interstitial macrophage phenotypes.112 NO2-FAs preserve resident alveolar macrophage populations, which are typically lost in acute lung injury,112 and are thought to maintain the non-inflammatory state of the lung.113 Within the interstitial macrophage population, NO2-FAs inhibit pro-inflammatory activation, as evidenced by reduced expression of Ly6C and CD206.112 This data indicates that NO2-FAs can suppress alveolar and interstitial macrophage activation, which may be due to alterations in inflammatory signaling.112,114
Chronic inflammatory disease
Chronic inflammatory diseases, such as asthma, are also influenced by macrophage activation and may be attenuated by NO2-FA administration. Asthma is characterized by bronchial hyperresponsiveness, airway remodeling, and recruitment of inflammatory cells.115,116 Due to their role in regulating pro- and anti-inflammatory responses in the lung, alveolar macrophages play a critical role in asthma pathology.116 Because of this, NO2-FAs may be able to alter this response. It has been observed that NO2-FAs have the capacity to diminish disease severity in a model of allergic airway disease.117 Infiltration of inflammatory cells into the lungs is suppressed and phagocytosis of neutrophils by alveolar macrophages is induced.117 NO2-FAs also reduce expression of pro-inflammatory cytokines and chemokines, which have the potential to activate macrophages.117 This data indicates that NO2-FAs may be beneficial in treating chronic inflammatory conditions via altering macrophage phenotypes.
Viral and bacterial infection
Macrophages also pay a large role in detecting viral and bacterial infections and respond via modulating their protein expression, post-translational modifications, and subcellular locations.118 Activation of STING in macrophages contributes to inflammation during viral infections.99 NO2-FAs have been observed to be up-regulated during viral infections in response to STING activation.99 INF-1 induction is inhibited with NO2-FA administration leading to decreased STING activity and reduced inflammation. NO2-FAs may be relevant in treating certain effects of COVID-19, such as cytokine storm, which is associated with more severe forms of the syndrome.119 The cytokine storm is characterized by an up-regulation of NF-κB and STAT signaling.119 These pathways both have the potential to be inhibited by NO2-FAs, which may lead to reduced disease severity. It should be noted that this is a double-edged sword, as the inflammatory response may be necessary to rid the body of the virus. Because of this, their role as therapeutics in infection should be further investigated.
Discussion
NO2-FAs are a novel class of compounds that have already shown great potential to modify inflammatory disease states in a variety of organ systems.5,40,41,120,121 Despite this, their effects on the pulmonary system and macrophage activation is less well known. Based on their mechanism of action, NO2-FAs may have great potential for reducing injury in pulmonary inflammatory disease states such as acute lung injury and interstitial lung disease. Macrophages are a major contributor to these disease states and also work through many of the pathways altered by NO2-FAs. NO2-FAs have been observed to elicit anti-inflammatory responses in pathways like STING, PPAR-γ, NF-κ-B, Nrf2, and STAT (Figure 1), all of which are prominent in macrophage activation. They are PPAR-γ agonists, leading to the activation and the transcription of a variety of anti-inflammatory genes.20,82,122 Normally, PPAR-γ is suppressed in classically-activated macrophages but NO2-FAs may be able to overcome this suppression and allow for more robust activation.83,84,123 PPAR-γ activation will also decrease cellular adhesion and migration, which may lead to decreased macrophage migration and recruitment during lung injury.123 This increase in PPAR-γ activation will also lead to downstream inhibition of NF-κB, leading to further inhibition of inflammatory cytokine expression.5 In addition, this response can be further amplified via NO2-FA adduction directly to NF-κB13. NF-κB is essential in lung macrophage activation, especially toward the M1 phenotype.70,71 NO2-FAs readily inhibit NF-κB function, inhibiting the transcription of downstream inflammatory genes and cytokine secretion, resulting in an anti-inflammatory, pro-survival response.19 NF-κB signaling in lung macrophages may be further truncated via NO2-FA inhibition of the STING pathway.99 STING inhibition will prevent TBK-1 induction of NF-κB and will reduce expression of inflammatory type 1 INF genes.99,103 STING is highly expressed on pulmonary macrophages, especially those that are infiltrating the lung, so inhibition of this pathway may reduce cellular infiltration and expression of inflammatory genes during lung injury.
Nrf2 activation will induce cytoprotective and antioxidant signaling in pulmonary macrophages.62 NO2-FAs can enhance Nrf2 signaling through binding to Keap1, resulting in higher concentrations of free Nrf2.37 Nrf2 is then free to translocate to the nucleus, where it transcribes genes to mediate oxidative stress pathways, reducing the inflammatory response.49 NO2-FAs are also able to inhibit JAK/STAT signaling, which will promote pulmonary macrophage activation via iNOS and MCP-1 expression.27 Inhibition of this pathway by NO2-FAs will result in down-regulation of pro-inflammatory genes and increased production of anti-inflammatory MPK-1.4 The LO-LT pathway also plays a big role in macrophage migration and cytokine production.124 NO2-FAs will bind to 5-LO and prevent eicosanoid formation, reducing these pro-inflammatory responses under injury conditions.10 Through all of these different mechanisms, NO2-FAs may be able to reduce excess inflammation in a variety of different pulmonary disease states by altering macrophage signaling.
NO2-FAs can also induce PKC-ζ, which should be considered with caution when discussing this compound as a therapeutic for lung injury.35 PKC is a major extracellular signaling molecule in the body and its overexpression could lead to excess inflammation itself through the activation of NF-κB.35 This may lead to an exacerbated injury response if the signal is magnified to a large extent. Caution is also warranted when considering the effects of NO2-FAs on other pathways that have not yet been studied. There are many other signaling pathways in the body that contain proteins with cysteine thiols and the administration of NO2-FAs may cause unfavorable effects in some of these pathways. For example, channel proteins, such as the ryanodine channel, may be negatively impacted by NO2-FA treatment. The ryanodine channel in the sarcoplasmic reticulum has many calcium channels that contain various cysteine residues that may have the potential to undergo a Michael addition, as they have already shown the potential to be S-nitrosylated.125 These modifications could activate the ryanodine channel, which may lead to unfavorable disruptions in striated muscle function.125 Although they could be highly beneficial in reducing several inflammatory disease states, including those in the lung, we must proceed with caution to ensure the safety of these new compounds.
NO2-FAs are exciting and new compounds that have demonstrated potential as therapeutics in many inflammatory disease states, but there is still so much that is unknown. Based on their potential to alter numerous signal transduction pathways, NO2-FAs could be a highly effective therapeutic in preventing macrophage activation during lung injury. More research is needed to further elicit their effects on macrophage signaling in the lung and to determine if there are any harmful effects that pertain to their use in the lung. That being said, NO2-FAs have successfully cleared five phase I clinical trials in the cardiac system without any toxicity or interference with Qt intervals, suggesting they do not alter cardiac ion channels.17 This absence of toxicity furthers the therapeutic promise of NO2-FAs.17 With further research, NO2-FAs could prove to be novel therapeutics in a number of inflammatory lung injuries.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The study received support from National Institute of Environmental Health Sciences (NIEHSP30ES005022, T32-ES007148) and National Heart and Lung Institute (NIH-HL086621, R01-HL64937).
ORCID iD: Melissa L Wilkinson https://orcid.org/0000-0002-3482-3975
References
- 1.Freeman B, O’Donnell VB, Schopfer FJ.The discovery of nitro-fatty acids as products of metabolic and inflammatory reactions and mediators of adaptive cell signaling. Nitric Oxide 2018; 77: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melo T, Marques SS, Ferreria I, et al. New insights into the anti-inflammatory and antioxidant properties of nitrated phospholipids. Lipids 2018; 53: 117–131. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph V, Schopfer FJ, Khoo NK, et al. Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction. J Biol Chem 2009; 284: 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichikawa T, Zhang J, Chen K, et al. Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: a critical role of mitogen-activated protein kinase phosphatase 1. Endocrinology 2008; 149: 4086–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy AT, Lakshmi SP, Zhang Y, et al. Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages. FASEB J 2014; 28: 5299–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pryor W, Lightsey JW, Church DF.Reaction of nitrogen dioxide with alkenes and polyunsaturated fatty acids: addition and hydrogen abstraction mechanisms. J Am Chem Soc 1982; 104: 6685–6692. [Google Scholar]
- 7.Delmastro-Greenwood M, Hughan KS, Vitturi DA, et al. Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free Radic Biol Med 2015; 89: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudolph V, Rudolph TK, Schopfer FJ, et al. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res 2010; 85: 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvatore SR, Rowart P, Schopfer FJ. Mass spectrometry-based study defines the human urine nitrolipidome. Free Radic Biol Med 2021; 162: 327–337. [DOI] [PMC free article] [PubMed]
- 10.Awwad K, Steinbrink SD, Fromel T, et al. Electrophilic fatty acid species inhibit 5-lipoxygenase and attenuate sepsis-induced pulmonary inflammation. Antioxid Redox Signal 2014; 20: 2667–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceaser EK, Moellering DR, Shiva S, et al. Mechanisms of signal transduction mediated by oxidized lipids: the role of the electrophile-responsive proteome. Biochem Soc Trans 2004; 32: 151–155. [DOI] [PubMed] [Google Scholar]
- 12.Isom AL, Barnes S, Wilson L, et al. Modification of cytochrome c by 4-hydroxy- 2-nonenal: evidence for histidine, lysine, and arginine-aldehyde adducts. J Am Soc Mass Spectrom 2004; 15: 1136–1147. [DOI] [PubMed] [Google Scholar]
- 13.Batthyany C, Schopfer FJ, Baker PR, et al. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem 2006; 281: 20450–20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Li C, Yang T.Protection of nitro-fatty acid against kidney diseases. Am J Physiol Renal Physiol 2016; 310: F697–F704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villacorta L, Gao Z, Schopfer FJ, et al. Nitro-fatty acids in cardiovascular regulation and diseases: characteristics and molecular mechanisms. Front Biosci (Landmark Ed )2016; 21: 873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panati K, Thimmana LV, Narala VR.Electrophilic nitrated fatty acids are potential therapeutic candidates for inflammatory and fibrotic lung diseases. Nitric Oxide 2020; 102: 28–38. [DOI] [PubMed] [Google Scholar]
- 17.Khoo NKH, Li L, Salvatore SR, et al. Electrophilic fatty acid nitroalkenes regulate Nrf2 and NF-kappaB signaling: a medicinal chemistry investigation of structure-function relationships. Sci Rep 2018; 8: 2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kansanen E, Jyrkkanen HK, Volger OL, et al. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J Biol Chem 2009; 284: 33233–33241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui T, Schopfer FJ, Zhang J, et al. Nitrated fatty acids: endogenous anti-inflammatory signaling mediators. J Biol Chem 2006; 281: 35686–35698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schopfer FJ, Baker PR, Giles G, et al. Fatty acid transduction of nitric oxide signaling. Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J Biol Chem 2005; 280: 19289–19297. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed SM, Luo L, Namani A, et al. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 585–597. [DOI] [PubMed] [Google Scholar]
- 22.Byers DE, Holtzman MJ.Alternatively activated macrophages and airway disease. Chest 2011; 140: 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sibille Y, Reynolds HY.Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis 1990; 141: 471–501. [DOI] [PubMed] [Google Scholar]
- 24.Rubins JB.Alveolar macrophages: wielding the double-edged sword of inflammation. Am J Respir Crit Care Med 2003; 167: 103–104. [DOI] [PubMed] [Google Scholar]
- 25.Haslett C.Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med 1999; 160: S5–11. [DOI] [PubMed] [Google Scholar]
- 26.Laskin DL, Malaviya R, Laskin JD.Role of macrophages in acute lung injury and chronic fibrosis induced by pulmonary toxicants. Toxicol Sci 2019; 168: 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irey EA, Lassiter CM, Brady NJ, et al. JAK/STAT inhibition in macrophages promotes therapeutic resistance by inducing expression of protumorigenic factors. Proc Natl Acad Sci U S A 2019; 116: 12442–12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin H, Holdbrooks AT, Liu Y, et al. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol 2012; 189: 3439–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milara J, Hernandez G, Ballester B, et al. The JAK2 pathway is activated in idiopathic pulmonary fibrosis. Respir Res 2018; 19: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moine P, McIntyre R, Schwartz MD, et al. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock 2000; 13: 85–91. [DOI] [PubMed] [Google Scholar]
- 31.Zaynagetdinov R, Sherrill TP, Gleaves LA, et al. Chronic NF-kappaB activation links COPD and lung cancer through generation of an immunosuppressive microenvironment in the lungs. Oncotarget 2016; 7: 5470–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence T.The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009; 1: a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abrial C, Grassin-Delyle S, Salvator H, et al. 15-Lipoxygenases regulate the production of chemokines in human lung macrophages. Br J Pharmacol 2015; 172: 4319–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuschieri J, Umanskiy K, Solomkin J.PKC-zeta is essential for endotoxin-induced macrophage activation. J Surg Res 2004; 121: 76–83. [DOI] [PubMed] [Google Scholar]
- 35.Guo CJ, Schopfer FJ, Gonzales L, et al. Atypical PKCzeta transduces electrophilic fatty acid signaling in pulmonary epithelial cells. Nitric Oxide 2011; 25: 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohkuri T, Kosaka A, Nagato T, et al. Effects of STING stimulation on macrophages: STING agonists polarize into “classically” or “alternatively” activated macrophages? Hum Vaccin Immunother 2018; 14: 285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi EH, Suzuki T, Funayama R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 2016; 7: 11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy RC.Immunomodulatory role of PPAR-gamma in alveolar macrophages. J Investig Med 2008; 56: 522–527. [DOI] [PubMed] [Google Scholar]
- 39.Schopfer FJ, Vitturi DA, Jorkasky DK, et al. Nitro-fatty acids: new drug candidates for chronic inflammatory and fibrotic diseases. Nitric Oxide 2018; 79: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borniquel S, Jansson EA, Cole MP, et al. Nitrated oleic acid up-regulates PPARgamma and attenuates experimental inflammatory bowel disease. Free Radic Biol Med 2010; 48: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diaz-Amarilla P ME, Trostchansky A, Trias E, et al. Electrophilic nitro-fatty acids prevent astrocyte-mediated toxicity to motor neurons in a cell model of familial amyotrophic lateral sclerosis via nuclear factor erythroid 2-related factor activation. Free Radic Biol Med 2016; 95: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy AT, Lakshmi SP, Muchumarri RR, et al. Nitrated fatty acids reverse cigarette smoke-induced alveolar macrophage activation and inhibit protease activity via electrophilic S-alkylation. PLoS One 2016; 11: e0153336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schopfer FJ, Cole MP, Groeger AL, et al. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem 2010; 285: 12321–12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLoughlin RM, Jenkins BJ, Grail D, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A 2005; 102: 9589–9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeh CH, Shih HC, Hong HM, et al. Protective effect of wogonin on proinflammatory cytokine generation via Jak1/3-STAT1/3 pathway in lipopolysaccharide stimulated BV2 microglial cells. Toxicol Ind Health 2015; 31: 960–966. [DOI] [PubMed] [Google Scholar]
- 46.Malyshev I, Malyshev Y.Current concept and update of the macrophage plasticity concept: intracellular mechanisms of reprogramming and M3 macrophage “switch” phenotype. Biomed Res Int 2015; 2015: 341308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharjee A, Shukla M, Yakubenko VP, et al. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med 2013; 54: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kansanen E, Kuosmanen SM, Ruotsalainen AK, et al. Nitro-oleic acid regulates endothelin signaling in human endothelial cells. Mol Pharmacol 2017; 92: 481–490. [DOI] [PubMed] [Google Scholar]
- 49.Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 1999; 13: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito R, Suzuki T, Hiramoto K, et al. Characterizations of three major cysteine sensors of Keap1 in stress response. Mol Cell Biol 2016; 36: 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang DD, Hannink M.Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 2003; 23: 8137–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khoo NK, Freeman BA.Electrophilic nitro-fatty acids: anti-inflammatory mediators in the vascular compartment. Curr Opin Pharmacol 2010; 10: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Liu H, Jia Z, et al. Effects of endogenous PPAR agonist nitro-oleic acid on metabolic syndrome in obese zucker rats. PPAR Res 2010; 2010: 601562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi A KM, Watai Y, Tong KI, et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol 2006; 26: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He F, Ru X, Wen T.NRF2, a transcription factor for stress response and beyond. Int J Mol Sci 2020; 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malhotra D, Portales-Casamar E, Singh A, et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 2010; 38: 5718–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thimmulappa RK, Mai KH, Srisuma S, et al. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 2002; 62: 5196–5203. [PubMed] [Google Scholar]
- 58.Hu R, Xu C, Shen G, et al. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci 2006; 79: 1944–1955. [DOI] [PubMed] [Google Scholar]
- 59.Deng H.Nrf2 and the Nrf2-interacting network in respiratory inflammation and diseases. Nature Public Health Emergency Collection 2020; 85. [Google Scholar]
- 60.Chen XL, Varner SE, Rao AS, et al. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem 2003; 278: 703–711. [DOI] [PubMed] [Google Scholar]
- 61.Soares MP, Seldon MP, Gregoire IP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol 2004; 172: 3553–3563. [DOI] [PubMed] [Google Scholar]
- 62.Zhu H, Jia Z, Zhang L, et al. Antioxidants and phase 2 enzymes in macrophages: regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Exp Biol Med (Maywood) 2008; 233: 463–474. [DOI] [PubMed] [Google Scholar]
- 63.Tsujita T, Li L, Nakajima H, et al. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: a study using green fluorescent protein transgenic zebrafish. Genes Cells 2011; 16: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh G, van Duyne G, Ghosh S, et al. Structure of NF-kappa B p50 homodimer bound to a kappa B site. Nature 1995; 373: 303–310. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh S, May MJ, Kopp EB.NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998; 16: 225–260. [DOI] [PubMed] [Google Scholar]
- 66.Davis M, Hatzubai A, Andersen JS, et al. Pseudosubstrate regulation of the SCF(beta-TrCP) ubiquitin ligase by hnRNP-U. Genes Dev 2002; 16: 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madrid LV, Mayo MW, Reuther JY, et al. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem 2001; 276: 18934–18940. [DOI] [PubMed] [Google Scholar]
- 68.Mercurio F, Zhu H, Murray BW, et al. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science 1997; 278: 860–866. [DOI] [PubMed] [Google Scholar]
- 69.Villacorta L, Chang L, Salvatore SR, et al. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc Res 2013; 98: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang N, Liang H, Zen K.Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol 2014; 5: 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu T, Zhang L, Joo D, et al. NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Legler DF, Micheau O, Doucey MA, et al. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity 2003; 18: 655–664. [DOI] [PubMed] [Google Scholar]
- 73.Nakahira K, Kim HP, Geng XH, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med 2006; 203: 2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shirey KA, Lai W, Scott AJ, et al. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 2013; 497: 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grygiel-Gorniak B.Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutr J 2014; 13: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varga T, Czimmerer Z, Nagy L.PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 2011; 1812: 1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gampe RT, Jr., Montana VG, Lambert MH, et al. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell 2000; 5: 545–555. [DOI] [PubMed] [Google Scholar]
- 78.Ogawa S LJ, Benner C, Pascual G, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 2005; 122: 707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang C, Ting AT, Seed B.PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998; 391: 82–86. [DOI] [PubMed] [Google Scholar]
- 80.Ricote M, Li AC, Willson TM, et al. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998; 391: 79–82. [DOI] [PubMed] [Google Scholar]
- 81.Ferreira AM, Ferrari MI, Trostchansky A, et al. Macrophage activation induces formation of the anti-inflammatory lipid cholesteryl-nitrolinoleate. Biochem J 2009; 417: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forman BM, Tontonoz P, Chen J, et al. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995; 83: 803–812. [DOI] [PubMed] [Google Scholar]
- 83.Pascual G, Fong AL, Ogawa S, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 2005; 437: 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chawla A.Control of macrophage activation and function by PPARs. Circ Res 2010; 106: 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barish GD, Downes M, Alaynick WA, et al. A nuclear receptor atlas: macrophage activation. Mol Endocrinol 2005; 19: 2466–2477. [DOI] [PubMed] [Google Scholar]
- 86.Egan RW, Gale PH.Inhibition of mammalian 5-lipoxygenase by aromatic disulfides. J Biol Chem 1985; 260: 11554–11559. [PubMed] [Google Scholar]
- 87.Montuschi P, Peters-Golden ML.Leukotriene modifiers for asthma treatment. Clin Exp Allergy 2010; 40: 1732–1741. [DOI] [PubMed] [Google Scholar]
- 88.Sorgi CA, Zarini S, Martin SA, et al. Dormant 5-lipoxygenase in inflammatory macrophages is triggered by exogenous arachidonic acid. Sci Rep 2017; 7: 10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lipp P, ReitherG. Protein kinase C: the “masters” of calcium and lipid. Cold Spring Harb Perspect Biol 2011; 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leppanen T, Tuominen RK, Moilanen E.Protein kinase C and its inhibitors in the regulation of inflammation: inducible nitric oxide synthase as an example. Basic Clin Pharmacol Toxicol 2014; 114: 37–43. [DOI] [PubMed] [Google Scholar]
- 91.Garg R, Caino MC, Kazanietz MG.Regulation of transcriptional networks by PKC isozymes: identification of c-Rel as a key transcription factor for PKC-regulated genes. PLoS One 2013; 8: e67319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garg R, Blando J, Perez CJ, et al. Activation of nuclear factor kappaB (NF-kappaB) in prostate cancer is mediated by protein kinase C epsilon (PKCepsilon). J Biol Chem 2012; 287: 37570–37582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boyle WJ, Smeal T, Defize LH, et al. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell 1991; 64: 573–584. [DOI] [PubMed] [Google Scholar]
- 94.Aziz MH, Manoharan HT, Church DR, et al. Protein kinase Cepsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res 2007; 67: 8828–8838. [DOI] [PubMed] [Google Scholar]
- 95.Huang X, Chen LY, Doerner AM, et al. An atypical protein kinase C (PKC zeta) plays a critical role in lipopolysaccharide-activated NF-kappa B in human peripheral blood monocytes and macrophages. J Immunol 2009; 182: 5810–5815. [DOI] [PubMed] [Google Scholar]
- 96.Bonilla L, O'Donnell VB, Clark SR, et al. Regulation of protein kinase C by nitroarachidonic acid: impact on human platelet activation. Arch Biochem Biophys 2013; 533: 55–61. [DOI] [PubMed] [Google Scholar]
- 97.St-Denis A, Chano F, Tremblay P, et al. Protein kinase C-alpha modulates lipopolysaccharide-induced functions in a murine macrophage cell line. J Biol Chem 1998; 273: 32787–32792. [DOI] [PubMed] [Google Scholar]
- 98.Diner EJ, Burdette DL, Wilson SC, et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep 2013; 3: 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hansen AL, Buchan GJ, Ruhl M, et al. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc Natl Acad Sci U S A 2018; 115: E7768–E7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ishikawa H, Barber GN.STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008; 455: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Louis C, Burns C, Wicks I.TANK-binding kinase 1-dependent responses in health and autoimmunity. Front Immunol 2018; 9: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tanaka Y, Chen ZJ.STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal 2012; 5: ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abe T, Barber GN.Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-kappaB activation through TBK1. J Virol 2014; 88: 5328–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mukai K, Konno H, Akiba T, et al. Activation of STING requires palmitoylation at the Golgi. Nat Commun 2016; 7: 11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ishikawa H, Ma Z, Barber GN.STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009; 461: 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao Q, Wei Y, Pandol SJ, et al. STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology 2018; 154: 1822–1835 e1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benmerzoug S, Rose S, Bounab B, et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat Commun 2018; 9: 5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson ER, Matthay MA.Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 2010; 23: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matthay MA, Zimmerman GA.Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 2005; 33: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dowdy DW, Eid MP, Dennison CR, et al. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med 2006; 32: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 111.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 112.Wilkinson ML, Abramova E, Guo C, et al. Fatty acid nitroalkenes inhibit the inflammatory response to bleomycin-mediated lung injury. Toxicol Appl Pharmacol 2020; 407: 115236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Evren E, Ringqvist E, Willinger T.Origin and ontogeny of lung macrophages: from mice to humans. Immunology 2020; 160: 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arora S, Dev K, Agarwal B, et al. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology 2018; 223: 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Robinson DS.The role of the T cell in asthma. J Allergy Clin Immunol 2010; 126: 1081–1091; quiz 1092-1083. [DOI] [PubMed] [Google Scholar]
- 116.Balhara J, Gounni AS.The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol 2012; 5: 605–609. [DOI] [PubMed] [Google Scholar]
- 117.Reddy AT, Lakshmi SP, Dornadula S, et al. The nitrated fatty acid 10-nitro-oleate attenuates allergic airway disease. J Immunol 2013; 191: 2053–2063. [DOI] [PubMed] [Google Scholar]
- 118.Nyman TA, Matikainen S.Proteomics to study macrophage response to viral infection. J Proteomics 2018; 180: 99–107. [DOI] [PubMed] [Google Scholar]
- 119.Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen 2020; 40: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mollenhauer M, Mehrkens D, Rudolph V. Nitrated fatty acids in cardiovascular diseases. Nitric Oxide 2018; 146–153. [DOI] [PubMed]
- 121.Agarwal R.Anti-inflammatory effects of short-term pioglitazone therapy in men with advanced diabetic nephropathy. Am J Physiol Renal Physiol 2006; 290: F600–605. [DOI] [PubMed] [Google Scholar]
- 122.Ferreira A, Minarrieta L, Bervejillo ML, et al. Nitro-fatty acids as novel electrophilic ligands for peroxisome proliferator-activated receptors. Free Radic Biol Med 2012; 53: 1654–1663. [DOI] [PubMed] [Google Scholar]
- 123.Reddy AT, Lakshmi SP, Kleinhenz JM, et al. Endothelial cell peroxisome proliferator-activated receptor gamma reduces endotoxemic pulmonary inflammation and injury. J Immunol 2012; 189: 5411–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brock TG, McNish RW, Peters-Golden M.Capacity for repeatable leukotriene generation after transient stimulation of mast cells and macrophages. Biochem J 1998; 329 (Pt 3): 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stoyanovsky D, Murphy T, Anno PR, et al. Nitric oxide activates skeletal and cardiac ryanodine receptors. Cell Calcium 1997; 21: 19–29. [DOI] [PubMed] [Google Scholar]