Abstract
Introduction
The prevalence and significance of acute liver injury in patients with COVID-19 are poorly characterized.
Methods
Patients with confirmed COVID-19 who were hospitalized in geographically diverse medical centers in North America were included. Demographics, symptoms, laboratory data results, and outcomes were recorded. Linear and logistic regression identified factors associated with liver injury, in-hospital mortality, and length of stay (LOS).
Results
Among 1555 patients in the cohort, most (74%) had an elevated alanine aminotransferase (ALT) during hospitalization, which was very severe (> 20 × upper limit of normal [ULN]) in 3%. Severe acute liver injury (ALI) was uncommon, occurring in 0.1% on admission and 2% during hospitalization. No patient developed acute liver failure (ALF). Higher ALT was associated with leukocytosis (per mL3) (β 10.0, 95% confidence interval (CI) 6.7–12.6, p < 0.001) and vasopressors use (β 80.2, 95%CI 21.5–138.8, p = 0.007). In-hospital mortality was associated with ALT > 20 × ULN (unadjusted OR 6.0, 95%CI 3.1–11.5, p < 0.001), ALP > 3 × ULN (unadjusted OR 4.4, 95%CI 2.5–7.7, p < 0.001), and severe ALI (unadjusted OR 6.8, 95%CI 3.0–15.3, p < 0.001) but lost significance after adjusting for covariates related to severe COVID-19 and hemodynamic instability. Elevated ALP and ALT were associated with longer LOS, admission to intensive care, mechanical ventilation, vasopressor use, and extracorporeal membrane oxygenation use (p < 0.001).
Conclusions
Transaminase elevation is common in hospitalized patients with COVID-19. Severe ALI is rare, and ALF may not be a complication of COVID-19. Extreme elevations in liver enzymes appear to be associated with mortality and longer LOS due to more severe systemic disease rather than SARS-CoV-2-related hepatitis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-021-07230-9.
Keywords: Transaminase, Alkaline phosphatase, Bilirubin, Acute liver failure, Outcomes
Introduction
In November 2019, a cluster of patients with a severe, atypical pneumonia were identified in mainland China. Investigation of these cases resulted in the identification of a novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus that is responsible for coronavirus disease 2019 (COVID-19) infection [1–3]. The clinical presentation of COVID-19 ranges from asymptomatic to a severe infection characterized by multisystem organ failure with need for mechanical ventilation, vasopressor support, or extracorporeal membrane oxygenation (ECMO) that can result in death [4–7].
It has been reported that a large proportion of hospitalized patients with COVID-19 develop abnormal liver enzymes [4, 5, 8–11]. Over 50% of patients hospitalized for COVID-19 appear to have at least one abnormal liver enzyme on admission, and over 75% will develop abnormal liver enzymes over the course of hospitalization. These abnormalities are typically an elevation in aminotransferases between 1 and 3 times (x) the upper limit of normal (ULN), although a small number of patients have been reported to experience transaminases greater than 10 × the ULN [5, 8, 12, 13]. Several case reports have promulgated the concept that COVID-19 is associated with severe acute livery injury (ALI) and acute liver failure (ALF) [13–15]. Currently, it remains unclear whether SARS-CoV-2 is truly a hepatotropic virus or whether abnormal liver enzymes are a reflection of a systemic inflammatory syndrome and/or severe sepsis [9].
The clinical significance of liver enzyme abnormalities, specifically their correlation to COVID-19 disease severity (i.e., the need for intensive care and mortality), has been controversial [8, 12]. While it is suspected that severe elevations in transaminases correlate with increasing COVID-19 severity, this remains uncertain based on the current literature. In this study, we aimed to determine the prevalence and severity of liver enzyme abnormalities in a large population of patients hospitalized with COVID-19 at an alliance of geographically diverse medical centers across North America and also to determine whether SARS-CoV-2 caused severe ALI and/or ALF.
Methods
Data Source and Study Design
This was an observational cohort study that utilized information from patients hospitalized at 36 geographically diverse medical centers throughout North America during the earliest phase of the pandemic [5]. Each center obtained institutional review board approval prior to patient identification and data collection.
Study Sample
Adult patients were eligible for review if they were hospitalized and were documented to have COVID-19 through either rapid viral testing or polymerase chain reaction (PCR). Eligible patients were identified by site investigators utilizing multiple methods including data warehouse queries, electronic research identification tools, and lists provided by infectious diseases services. Depending on the number of hospitalized patients with COVID-19 at each institution, the first 50 to 100 consecutive hospitalized patients with documented COVID-19 were included to ensure a systemic and unbiased sample. Patients were excluded from this analysis if liver tests were not recorded on admission or during hospitalization (Supplementary Fig. 1).
Data Collection and Quality Assurance
De-identified data were directly entered into an electronic data collection form by study personnel under direct supervision of a clinician investigator at each institution. Multiple interventions were instituted to ensure data quality and veracity. Once data were received by the data coordinating center (Medical University of South Carolina), they were manually reviewed by a dedicated data manager. Missing or duplicate data, inconsistencies, outlier data, and conflicting results were directly queried to the site until resolution.
Variables and Definitions
Demographics, laboratory test results on admission and peak laboratory abnormalities during hospitalization, radiographic data, presenting symptoms, medical comorbidities, treatments received for COVID-19, and patient outcomes were collected for each patient as previously described. Patients with severe acute livery injury also had assessment of their mental status using the West Haven criteria [16].
Elevation in serum transaminases was defined as normal, mild (1–3 × ULN), moderate (3–10 × ULN), severe (10–20 × ULN), or very severe (> 20 × ULN). The ULN of alanine aminotransferase (ALT) was defined as 29 U/L for men and 22 U/L for women [17]. Elevation of alkaline phosphatase (ALP) was defined as normal, mild (1–2 × ULN), moderate (2–3 × ULN), or severe (> 3 × ULN). ULN of ALP was defined as 120 IU/L [17–19].
In patients with at least moderate elevation of ALT (> 3 × ULN) or at least moderate elevation of ALP (> 2 × ULN), pattern of liver injury was further characterized as hepatocellular (R factor ≥ 5), cholestatic (R factor ≤ 2), or mixed (R factor 2–5) [20]. Severe ALI was defined as ALT greater than 10 × ULN with total bilirubin greater than 2 mg/dL. Acute liver failure (ALF) was defined as the presence of severe ALI with altered mental status in the absence of preexisting liver disease as previously defined [21]. Only 60% of patients had reported admission and peak international normalized ratio (INR), and thus, INR was not included in our definition of severe ALI and ALF to avoid excluding a significant portion of the sample. Sites that contributed data on patients who meet criteria for severe ALI were queried whether, based on their clinical judgment, severe ALI was due to SARS-CoV-2 and whether the patient met clinical criteria for ALF.
Study Outcomes
The primary study outcome was the prevalence of abnormal liver enzymes and liver injury in patients hospitalized with COVID-19. Secondary outcomes included severe ALI or ALF, in-hospital mortality, discharge to skilled nursing facility (SNF), hospital length of stay (LOS), and intensive care requirements (mechanical ventilation, vasopressor use, and/or ECMO).
Statistical Analysis
Continuous and categorical variables were summarized as means, median, and proportions. Patient groups were compared using analysis of variance and Chi-square for continuous and categorical variables, respectively. Independent predictors of peak ALT, peak ALP, and severe ALI were analyzed through stepwise backward regression that used a cutoff p-value of 0.1 for inclusion in the final model. Predictor variables included demographic data (e.g., age, sex, race, smoking, and BMI), comorbidities, laboratory test values (e.g., peak white blood cell count, nadir hemoglobin, and nadir platelet count), markers of hemodynamic instability (e.g., need for vasopressors, mechanical ventilation, and ECMO), and markers of organ failure (e.g., AKI). The association of peak ALT, peak ALP, and severe ALI to in-hospital mortality and hospital LOS was examined using hierarchical multiple logistic and linear regression where separate covariable blocks composed of demographic features, medical comorbidities, and markers of critical illness were sequentially added to the regression model. Model 1 is composed of demographic covariables, model 2 is composed of model 1 plus comorbidity variables, and model 3 (fully adjusted model) is composed of model 2 plus hemodynamic instability and organ failure variables. A subgroup analysis was performed using discharge to SNF as outcome utilizing the same regression models, but which excluded patients who died or were still hospitalized at the end of the observation period (n = 1224). Kaplan–Meier curves were fit to evaluate inpatient mortality based on admission ALT, ALP, or pattern of liver injury. Statistical analyses were performed using Stata v14.2 (StataCorp, College Station, TX).
Results
Data were collected on 1992 patients hospitalized with COVID-19. A total of 437 patients were excluded due to missing admission and peak ALT or ALP, leaving 1555 patients (78%) for analysis (Table 1, Supplementary Table 1, Supplementary Fig. 1).
Table 1.
Characteristics of the study cohort (n = 1555)
| Mean age (SD) | 60 (16) |
| Male sex, n (%) | 879 (57) |
| Race, n (%) (unspecified 17%) | |
| Black | 684 (44) |
| White | 571 (37) |
| Other | 44 (3) |
| Hispanic, n (%) | 251 (16) |
| Healthcare worker, n (%) (missing 11%) | 93 (6) |
| Smoking, n (%) (unknown 5%) | |
| Current | 107 (7) |
| Former | 418 (27) |
| Never | 946 (61) |
| Alcohol use, n (%) (unknown 10%) | |
| Current | 147 (10) |
| Former | 83 (5) |
| Never | 1163 (75) |
| Mean BMI (SD) (missing 6%) | 32 (9) |
| Comorbidities, n (%) | |
| Hypertension | 994 (64) |
| Diabetes mellitus | 587 (38) |
| Cardiovascular disease* | 453 (29) |
| Chronic lung disease** | 426 (27) |
| End-stage renal disease | 155 (10) |
| Cancer | 95 (6) |
| Dementia | 86 (6) |
| Connective tissue disease | 83 (5) |
| HIV | 25 (2) |
| Chronic liver disease, n (%) | 55 (4) |
| Viral hepatitis | 20 (36) |
| Nonalcoholic fatty liver disease | 15 (27) |
| Alcohol | 4 (7) |
| Other*** | 3 6) |
| Unspecified | 13 (4) |
| Cirrhosis, n (%) | 21 (1) |
| Decompensated cirrhosis, n (%) | 7 (1) |
| Lab tests | |
| Peak WBC (SD) (missing 0.2%) | 13 (9) |
| Nadir WBC (SD) (missing 0.3%) | 5 (3) |
| Nadir hemoglobin (SD) (missing 0.2%) | 10 (3) |
| Nadir platelet (SD) (missing 0.3%) | 17 (82) |
| Peak creatinine (SD) (missing 1%) | 2.4 (3) |
| Liver tests | |
| Admission AST, U/L (SD) | 51 (6) |
| Peak AST, U/L (SD) | 191 (1099) |
| Admission ALT, U/L (SD) | 37 (36) |
| Peak ALT, U/L (SD) | 119 (452) |
| Admission ALP, U/L (SD) | 82 (53) |
| Peak ALP, U/L (SD) | 123 (119) |
| Admission total bilirubin, mg/dL (SD) | 0.6 (0.7) |
| Peak total bilirubin, mg/dL (SD) | 1.7 (10) |
| Admission INR (SD) (missing 50%) | 1.3 (1) |
| Peak INR (SD) (missing 35%) | 1.5 (1) |
| Acute kidney injury, n (%) | 228 (15) |
| (Peak Cr > 2 × admission Cr + no history of ESRD) | |
| Abdominal imaging, n (%) | 244 (16) |
| Computed tomography | 199 (13) |
| Ultrasound | 65 (4) |
| Magnetic resonance imaging | 5 (0.3) |
| Received SARS-CoV-2 treatment, n (%) | 1197 (77) |
| Hydroxychloroquine | 848 (55) |
| Azithromycin | 823 (53) |
| Corticosteroids | 186 (12) |
| Tocilizumab | 101 (7) |
| Remdesivir | 97 (6) |
| Convalescent plasma | 32 (2) |
Abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, BMI = body mass index, Cr =creatinine, ESRD = end-stage renal disease, INR = international normalized ratio, SD—standard deviation, WBC = white blood cell
*Cardiovascular disease is a composite of the following
Coronary artery disease 231 (15)
Heart failure 192 (12)
Stroke 141 (9)
Peripheral vascular disease 77 (5)
**Chronic lung disease is a composite of the following
Asthma 188 (12)
COPD 132 (9)
OSA 171 (11)
Interstitial lung disease 15 (1)
Pulmonary hypertension 10 (1)
***Other liver disease includes autoimmune hepatitis (1), hemochromatosis (1), and cardiac cirrhosis (1)
Prevalence of Abnormal Liver Tests
A majority of patients (53%) had an elevated ALT on admission, which was mild in most cases (45%). No patient had a very severe elevation in ALT on admission. Overall, 74% of patients developed an elevation in ALT during hospitalization, most of which were mild elevation (45%); however, 43 (3%) developed pronounced peak elevations in ALT (Fig. 1). Virtually all patients had normal or, at most, mildly elevated ALP (normal 88%, mild elevation 11%) on admission, and most (70%) continued to have normal ALP during hospitalization. Of those who had elevated peak ALP levels, most were mild elevations; however, 56 (4%) developed a severe elevation in ALP (Fig. 1B). Bilirubin levels were typically normal on presentation, and only a mild elevation was noted on peak laboratory analysis (Table 1). Only 270 (17%) patients had a liver injury defined as at least moderate elevation in ALT or any elevation in ALP on admission which increased to 677 (44%) during the course of hospitalization. Liver injury on admission was cholestatic in 10%, hepatocellular in 5%, and mixed in 3%. Peak liver injury was hepatocellular in 17%, cholestatic in 16%, and mixed in 11% (Fig. 1C).
Fig. 1.
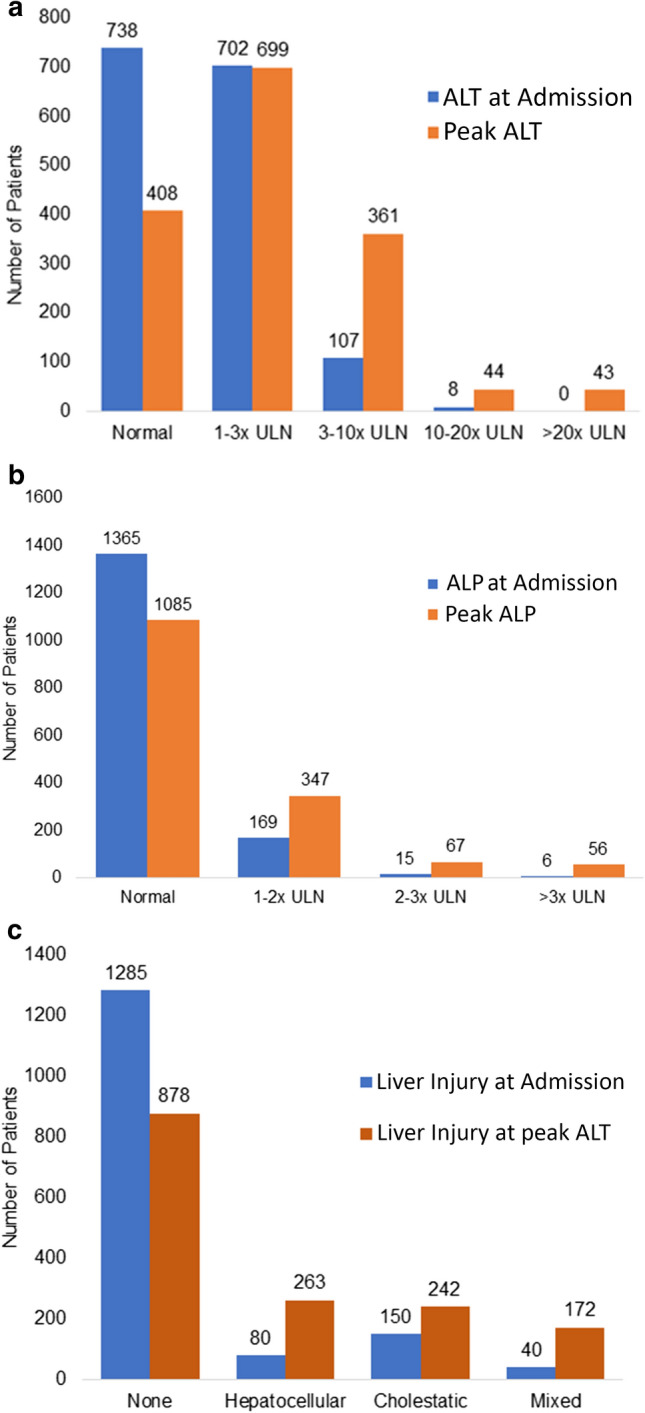
Frequency of liver test abnormalities and liver injury patterns. In a and b are shown the number of patients with abnormal alanine aminotransferase (ALT) or alkaline phosphatase (ALP) (of the total 1,555) at admission or at peak. In c liver injury (defined as hepatocellular, cholestatic or mixed) based on R factor at the time of admission and at peak are shown
Predictors of Abnormal ALT and ALP
On multivariable analysis, peak ALT was independently associated with leukocytosis, need for vasopressor support, and presence of end-stage renal disease, while peak ALP was independently associated with leukocytosis, anemia, thrombocytopenia, acute kidney injury (AKI), low BMI, Hispanic ethnicity, and need for vasopressors (Table 2).
Table 2.
Predictors of elevated ALT and ALP and liver injury in hospitalized patients with COVID-19
| Variables | Fully adjusted regression model | |||||
|---|---|---|---|---|---|---|
| Peak ALT beta (95% CI) | p-value | Peak ALP beta (95% CI) | p-value | Severe ALI OR (95% CI) | p-value | |
| WBC count (per mL3) | 9.7 (6.7–12.6) | < 0.001 | 2.8 (2.0–3.5) | < 0.001 | 1.0 (1.01–1.1) | 0.015 |
| Hemoglobin (g/dL) | NS | − 6.5 (− 9.1 to − 3.9) | < 0.001 | NS | ||
| Platelet count (per 109/L) | NS | − 0.1 (− 0.2 to − 0.02) | 0.013 | 1.0 (0.98–1.0) | 0.001 | |
| African-American race | NS | NS | 2.5 (1.01–6.1) | 0.047 | ||
| Hispanic ethnicity | NS | 26.5 (10.8–42.2) | 0.001 | NS | ||
| BMI (per unit) | NS | − 1.3 (− 2.0 to − 0.6) | < 0.001 | NS | ||
| AKI | NS | 19.9 (2.3–37.4) | 0.027 | NS | ||
| ESRD | 106.3 (27.6–185.1) | 0.008 | NS | NS | ||
| Vasopressor requirement | 80.1 (21.5–138.8) | 0.007 | 18.8 (3.7–33.9) | 0.015 | 4.2 (1.4–12.5) | 0.009 |
Abbreviations: AKI = acute kidney injury, ALI = acute liver injury, ALP = alkaline phosphatase, ALT = alanine aminotransferase, BMI = body mass index, CI = confidence interval, ESRD = end-stage renal disease, OR = odds ratio, NS = not significant, WBC = white blood cell
Patient Outcomes
Out of 1,555 patients, 19% died in the hospital, while 65% were discharged to home and 15% were discharged to a care facility. Median hospital LOS was 13 ± 12 days. Forty-seven percent were admitted in intensive care for a mean duration of 13 ± 11 days. Mechanical ventilation, vasopressors, and ECMO were needed in 35%, 30%, and 1%, respectively (Table 3).
Table 3.
Outcomes in hospitalized patients with COVID-19 and relationship to liver tests and injury
| ALT (times ULN) | ALP (times ULN) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | 1-3x | 3-10x | 10-20x | > 20x | p-value | Normal | 1-2x | 2-3x | > 3x | p-value | |
| A. Admission ALT and ALP values | |||||||||||
| Mean LOS, days (SD) | 14 (12) | 13 (12) | 13 (14) | 16 (13) | – | 0.634 | 13 (12) | 15 (15) | 16 (12) | 7.3 (5) | 0.203 |
| Critical care needs | |||||||||||
| ICU admission, % | 44 | 50 | 43 | 75 | – | 0.069 | 46 | 52 | 60 | 17 | 0.180 |
| ICU LOS, days (SD) | 12 (11) | 13 (10) | 14 (11) | 16 (4) | – | 0.776 | 13 (10) | 12 (12) | 14 (13) | 7 (0) | 0.903 |
| Mech. ventilation, % | 34 | 36 | 31 | 75 | – | 0.069 | 34 | 37 | 47 | 17 | 0.505 |
| Vasopressors, % | 29 | 32 | 22 | 75 | – | 0.004 | 30 | 31 | 4 | 3 | 0.829 |
| ECMO, % | 1 | 1 | 4 | 13 | – | 0.001 | 1 | 1 | – | – | 0.969 |
| Outcomes | |||||||||||
| Death, % | 20 | 18 | 11 | 38 | – | 0.196 | 18 | 22 | 33 | 17 | 0.090 |
| Discharged to home, % | 62 | 66 | 77 | 50 | – | 66 | 54 | 47 | 67 | ||
| Discharged to SNF, % | 17 | 14 | 11 | 13 | – | 14 | 22 | 20 | 17 | ||
| Still hospitalized, % | 1 | 1 | 1 | - | – | 1 | 2 | – | – | ||
| B. Peak ALT and ALP values | |||||||||||
| Mean LOS, days (SD) | 10 (10) | 12 (12) | 18 (13) | 21 (14) | 20 (13) | < 0.001 | 11 (10) | 18 (14) | 25 (17) | 23 (16) | < 0.001 |
| Critical care needs | |||||||||||
| ICU admission, % | 31 | 42 | 65 | 84 | 88 | < 0.001 | 38 | 63 | 82 | 77 | < 0.001 |
| ICU LOS, days (SD) | 9 (9) | 19 (11) | 15 (10) | 15 (10) | 16 (12) | < 0.001 | 10 (9) | 15 (12) | 18 (10) | 20 (13) | < 0.001 |
| Mech. ventilation, % | 2 | 29 | 54 | 73 | 79 | < 0.001 | 25 | 53 | 79 | 70 | < 0.001 |
| Vasopressors, % | 17 | 24 | 46 | 64 | 70 | < 0.001 | 20 | 48 | 69 | 66 | < 0.001 |
| ECMO, % | 0.5 | 0.1 | 2 | 7 | 9 | < 0.001 | 1 | 2 | 5 | 4 | < 0.001 |
| Outcomes | < 0.001 | ||||||||||
| Death, % | 19 | 17 | 19 | 18 | 58 | < 0.001 | 14 | 28 | 37 | 41 | |
| Discharged to home, % | 66 | 69 | 60 | 55 | 28 | 73 | 50 | 30 | 30 | ||
| Discharged to SNF, % | 14 | 13 | 20 | 27 | 14 | 12 | 19 | 31 | 29 | ||
| Still hospitalized, % | 1 | 2 | 1 | - | - | 1 | 3 | 2 | 2 | ||
Abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, ECMO = extracorporeal membrane oxygenation, LOS = length of stay, SNF = skilled nursing facility, ULN = upper limit of normal
Association Between In-Hospital Mortality and Peak Abnormal ALT and ALP
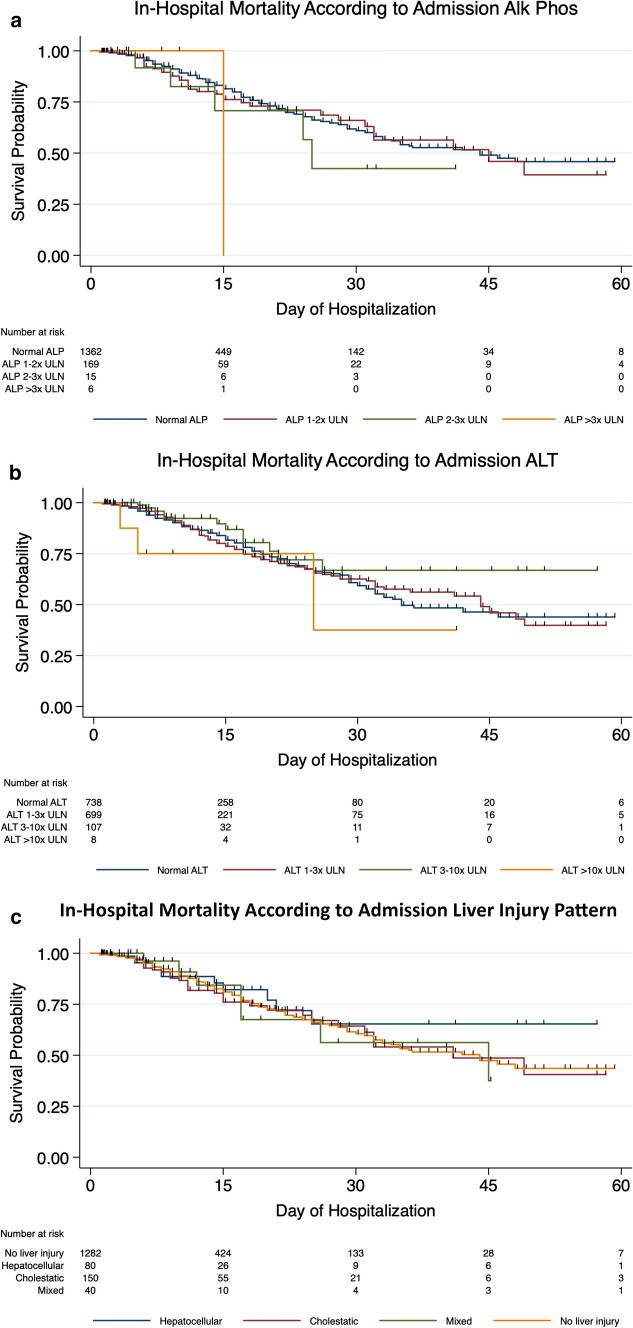
In-hospital mortality was not significantly different based on ALT or ALP elevations on presentation (p 0.196 and p 0.090, respectively) (Table 3A, Fig. 2A, B). A significantly higher proportion of patients with very severe elevation in peak ALT or ALP died in the hospital (58% vs. < 20% for ALT vs. those with less severe ALT elevation, p < 0.001 and 41% vs. 14% for ALP vs. those with less severe ALP elevation, p < 0.001, respectively) (Table 3B). Patients with moderate-to-severe elevations in peak ALT or elevated ALP had a greater frequency of ICU admission, mechanical ventilation, vasopressor use, and ECMO use. The severity of elevation in ALT or ALP on admission did not appear to be associated with in-hospital mortality (Table 3).
Fig. 2.
Kaplan–Meier curves evaluating in-hospital mortality based on admission liver tests and pattern of liver injury
On univariable regression, patients with very severe elevation in peak ALT had sixfold higher risk and patients with severe elevation in peak ALP had fourfold higher risk of in-hospital mortality compared to patients with normal liver enzymes. The association between peak ALT and ALP elevation and in-hospital mortality remained statistically significant after adjusting for demographic variables and comorbidities. Statistical significance was lost after adjusting for covariables of hemodynamic instability and organ failure (e.g., AKI and need for vasopressors) (Table 4A). In the fully adjusted models, age, non-white and non-black race, underlying cancer or dementia, development of AKI, and need for vasopressors or mechanical ventilation predicted in-hospital mortality (Supplementary Tables 3 and 4).
Table 4.
Association Between Liver Test Abnormalities and Outcomes in Hospitalized Patients with COVID-19
| Unadjusted | Model 1 (demographic variables) * | Model 2 (model 1 + comorbidity Variables) ** | Model 3 (models 1 and 2 + severe illness variables) *** | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| A. Association with in-hospital mortality | ||||||||
| Peak ALT | ||||||||
| Normal | Reference | Reference | Reference | Reference | ||||
| 1–3 × ULN | 0.9 (0.6–1.2) | 0.305 | 0.9 (0.7–1.3) | 0.730 | 1.1 (0.7–1.5) | 0.736 | 0.8 (0.5–1.2) | 0.213 |
| 3–10 × ULN | 1.0 (0.7–1.4) | 0.912 | 1.3 (0.9–1.9) | 0.222 | 1.7 (1.1–2.5) | 0.014 | 0.6 (0.4–1.01) | 0.055 |
| 10–20 × ULN | 1.0 (0.4–2.1) | 0.911 | 1.3 (0.6–3.1) | 0.537 | 1.5 (0.6–4.0) | 0.432 | 0.4 (0.2–1.3) | 0.132 |
| > 20 × ULN | 6.0 (3.1–11.5) | < 0.001 | 7.1 (3.1–14.2) | < 0.001 | 7.8 (3.7–16.3) | < 0.001 | 2.2 (0.9–5.4) | 0.075 |
| Peak ALP | ||||||||
| Normal | Reference | Reference | Reference | Reference | ||||
| 1–2 × ULN | 2.4 (1.8–3.2) | < 0.001 | 2.7 (2.0–3.7) | < 0.001 | 2.8 (2.0–3.8) | < 0.001 | 1.4 (0.9–2.1) | 0.064 |
| 2–3 × ULN | 3.8 (2.2–6.4) | < 0.001 | 4.2 (2.4–7.4) | < 0.001 | 4.7 (2.6–8.6) | < 0.001 | 1.6 (0.8–3.1) | 0.149 |
| > 3 × ULN | 4.4 (2.5–7.7) | < 0.001 | 5.1 (2.8–9.3) | < 0.001 | 4.8 (2.5–9.4) | < 0.001 | 2.0 (0.9–4.2) | 0.067 |
| Severe ALI | 6.8 (3.0–15.3) | < 0.001 | 7.4 (3.1–17.7) | < 0.001 | 7.3 (3.0–17.8) | < 0.001 | 2.8 (1.01–7.9) | 0.047 |
| B. Association with hospital LOS | ||||||||
| Peak ALT | ||||||||
| Normal | Reference | Reference | Reference | Reference | ||||
| 1–3 × ULN | 2.4 (0.9–3.8) | 0.001 | 2.7 (1.3–4.2) | < 0.001 | 2.6 (1.2–4.1) | < 0.001 | 1.6 (0.4–2.9) | 0.012 |
| 3–10 × ULN | 7.7 (6.0–9.4) | < 0.001 | 8.3 (6.6–10.0) | < 0.001 | 8.6 (7.0–10.3) | < 0.001 | 4.3 (2.8–5.9) | < 0.001 |
| 10–20 × ULN | 11.1 (7.4–14.8) | < 0.001 | 11.7 (8.0–15.4) | < 0.001 | 11.3 (7.5–15.1) | < 0.001 | 5.0 (1.6–8.4) | 0.004 |
| > 20 × ULN | 10.2 (6.4–14.0) | < 0.001 | 10.1 (6.4–13.9) | < 0.001 | 9.9 (6.2–13.5) | < 0.001 | 2.8 (-0.6–6.1) | 0.107 |
| Peak ALP | ||||||||
| Normal | Reference | Reference | Reference | Reference | ||||
| 1–2 × ULN | 7.5 (6.2–8.8) | < 0.001 | 7.7 (6.3–9.1) | < 0.001 | 7.2 (5.8–8.6) | < 0.001 | 3.8 (2.4–5.1) | < 0.001 |
| 2–3 × ULN | 13.9 (11.2–16.6) | < 0.001 | 13.7 (10.9–16.6) | < 0.001 | 11.7 (8.8–14.6) | < 0.001 | 5.3 (2.6–7.9) | < 0.001 |
| > 3 × ULN | 11.8 (8.8–14.8) | < 0.001 | 11.9 (8.8–15.0) | < 0.001 | 11.7 (8.5–14.9) | < 0.001 | 6.4 (3.5–9.3) | < 0.001 |
| Severe ALI | 3.3 (-1.6–8.2) | 0.185 | 2.8 (-2.1–7.7) | 0.259 | 3.3 (-1.5–8.1) | 0.180 | -3.8 (-8.0–0.4) | 0.073 |
Abbreviations:ALI =acute liver injury, ALP = alkaline phosphatase, ALT = alanine aminotransferase, CI = confidence interval, OR = odds ratio, ULN = upper limit of normal
*Model 1 adjusted for age (per 10 years), sex, race, Hispanic ethnicity, smoking, and alcohol use
**Model 2 adjusted for model 1 variables plus body mass index (per 5 units), hypertension, diabetes mellitus, cardiovascular disease, chronic lung disease, end-stage liver disease, cancer, dementia, connective tissue disease, HIV infection, and chronic liver disease
***Model 3 adjusted for models 1 and 2 variables plus acute kidney injury, need for vasopressors, need for mechanical ventilation, and need for ECMO
Association Between Hospital Length of Stay and Peak Abnormal Liver Enzymes
Hospital LOS was twice longer in patients with severe and very severe elevation in ALT. Patients with any elevation in peak ALP also had longer hospital LOS. Severity of elevations in ALT or ALP on admission was not associated with hospital LOS (Table 3). Adjusting for demographic and comorbidity variables did not diminish the magnitude and statistical significance of the association between elevation in peak ALT and ALP and hospital LOS. After adjusting for variables related to hemodynamic instability in the fully adjusted model, very severe elevation in peak ALT was no longer associated with hospital LOS; associations in patients with less severe elevations in peak ALT remained significant. Adjusting for variables related to hemodynamic instability also decreased the magnitude of associations between peak ALP and hospital LOS, but these remained significant and showed a trend toward longer LOS with more severe elevation in peak ALP (Table 4B).
Association Between Discharge to SNF and Peak Abnormal ALT and ALP
In a subgroup analysis that only included patients who were discharged, moderate-to-severe elevations in ALT were not associated with discharge to SNF in fully adjusted regression models. However, patients who had elevations in ALP were more likely to be discharged to SNF and the magnitude of the association increased with severity of ALP elevation (1–2 × ULN: OR 1.6, p = 0.001; 2–3 × ULN: OR 2.6, p = 0.025; > 3 × ULN: OR 5.3, p < 0.001).
Severe ALI in COVID-19
Only 1 patient (0.1%) had severe ALI on hospital admission, while 25 patients (2%) had severe ALI based on peak laboratory values. None of the patients met criteria for ALF defined by the absence of underlying liver disease, new ALI, and hepatic encephalopathy. [21] On average, patients with severe ALI were aged 61 ± 14 years old, 68% male, and 68% African-American. Twenty-three (92%) had chronic medical conditions. Average peak AST, ALT, ALP, and total bilirubin levels were 3,625 ± 4,166 U/L, 1,561 ± 1,498 U/L, 276 ± 203 U/L, and 5.2 ± 5.6 mg/dL, respectively. The suspected etiology of severe ALI was shock liver in 44%, drug-induced liver injury in 4%, and reported as “unknown” in the remainder. Based on the individual clinical picture of the patients with severe ALI, none were suspected by site investigators to have had liver injury as a result of direct SARS-CoV-2 injury (Supplementary Table 7). Notably, the mortality in this group was 60% (15/25). Only 24% of patients were discharged home.
Development of severe ALI was independently associated on multivariate regression with need for vasopressors, leukocytosis, thrombocytopenia, and African-American race (Table 2). Patients who developed severe ALI were more likely to have had severe COVID-19 given frequent admissions to intensive care (84% vs 47%, p < 0.001), mechanical ventilation (80% vs 35%, p < 0.001), vasopressor use (80% vs 30%, p < 0.001), and ECMO use (12% vs 1%, p < 0.001). The in-hospital mortality rate in patients with severe ALI was over 3 times higher than in those without severe ALI (60% vs. 19%, p < 0.001). On multivariable regression, severe ALI was associated with increased in-hospital mortality (OR 2.8, 95%CI 1.01–7.9, p = 0.047), but the magnitude of the association was reduced after adjusting for covariables of hemodynamic instability and organ failure. Severe ALI was not associated with hospital LOS or discharge to a skilled care facility.
Discussion
In this large and geographically diverse cohort of hospitalized patients with confirmed COVID-19, we found that many patients presented with mild elevation in ALT on admission and 10% presented with mild elevation in ALP. The overall likelihood of developing very high elevations in ALT or ALP was extremely low (3% and 4%, respectively), and no patient met criteria for ALF.
The prevalence of liver injury in our study is similar to other studies that have examined abnormal liver enzymes in patients with COVID-19 [8, 22, 23]. It is crucial to highlight that none of the patients had severe elevations in ALT and ALP on admission, and other studies have noted similar findings [8, 12]. This suggests that severe elevations in liver enzymes are likely due to severe inflammatory response and hemodynamic instability that are characteristic of COVID-19 rather than SARS-CoV-2 hepatotropism and related viral hepatitis or cholestatic disease [24–27]. This is further supported by our findings that factors such as leukocytosis, thrombocytopenia, vasopressor requirement, and renal dysfunction, which correlate with severe sepsis and hemodynamic instability, are associated with greater hepatocellular injury and cholestasis and the occurrence of severe ALI [28, 29].
A critical finding in our study was that severe ALI was particularly uncommon, and no patients in our cohort met formal criteria for ALF. We speculate that the majority of patients who developed severe ALI most likely had shock liver and/or multisystem organ failure. Shock liver was specifically identified as the etiology of severe ALI in 11 patients (44%), and moreover, among the 14 patients in whom the etiology of severe ALI was not specified, nine (64%) required vasopressors. In aggregate, the data lead us to speculate that markedly abnormal ALT levels in patients with COVID-19 are most likely simply a marker of severe underlying systemic disease and that COVID-19 is not a cause of severe primary acute viral hepatitis as has been hypothesized in previous case reports. [13–15]
Severe elevation in ALT and ALP and severe ALI were associated with increased in-hospital mortality. That the strength of these associations was attenuated or statistical significance was lost after adjusting for variables related to hemodynamic instability and critical illness demonstrate that elevated liver enzymes and liver injury per se do not predict death or indicate viral hepatitis or cholestatic liver disease due to SARS-CoV-2, but are best regarded as markers of severe COVID-19. In keeping with this proposition that liver injury is a marker of severe COVID-19, we also observed longer hospital LOS in patients with elevated ALT and ALP and increased likelihood of discharge to SNF in patients with elevated ALP in the fully adjusted regression models. Patients with very severe elevations in ALT had a shorter hospital LOS than patients with mild-to-severe elevations in ALT in the fully adjusted model. This finding is probably due to high in-hospital mortality and poor prognosis in this patient group, which translates to fewer hospitalization days.
We recognize the limitations of this study. First, data collection was retrospective. Additionally, contributing centers were not required to obtain abdominal imaging or comprehensive serologic testing in order to evaluate the etiology of abnormal liver enzymes. The primary liver enzyme measurements recorded were those on admission and peak values during hospitalization. Therefore, temporal trends in liver enzymes in relation to medications, clinical status, or other critical events could not be determined. However, we utilized multiple approaches to overcome these limitations including rigorous data collection and quality assurance processes employed by participating centers and monitored by the data coordinating center. Further, for all cases of severe ALI, contributing centers were also contacted individually to obtain their expert opinion on the etiology of liver injury. The study is also subject to selection bias since all study patients were hospitalized and had more severe COVID-19. The findings may not be generalizable to patients with less severe COVID-19. For example, demonstration of new liver test elevations in a patient with milder COVID-19 raises the possibility of SARS-CoV-2 hepatotropism.
In summary, abnormal liver tests are frequently identified in patients hospitalized with COVID-19. The majority of these abnormalities are mild elevations in ALT or ALP. More severe elevations in ALT levels occur in a subset of patients with severe COVID-19, typically those with hemodynamic instability, systemic inflammation, severe sepsis, and multiorgan failure. Severe ALI is rare and typically due to shock liver rather than SARS-CoV-2 viral hepatitis, and ALF does not appear to be a complication of COVID-19.
Financial Support
DCR supported by the NIDDK, Grant P30 DK 123704.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix
First author
Lindsay A. Sobotka DO
Senior author
Don C. Rockey MD
Primary authors
Lindsay A. Sobotka DO, James Esteban MD, Michael L. Volk MD, MSc, B.Joseph Elmunzer MD, Don C. Rockey MD.
Statisticians
James Esteban MD
Data Coordinating Center
Teldon B. Alford BA, Haley Nitchie MHA, Collins O. Ordiah MBBS, Rebecca L. Spitzer MPH, Lauren Wakefield MHA.
Steering Committee
Olga C. Aroniadis MD, Darwin L. Conwell MD, Amar R. Deshpande MD, Christopher J. DiMaio MD, Rebekah E. Dixon BS, B. Joseph Elmunzer MD (Chair), Jennifer M. Kolb MD, Robin B. Mendelsohn MD, Raman Muthusamy MD, Collins O. Ordiah MBBS, MS, Swati Pawa MD, MSc, MAS, Don C. Rockey MD, Rebecca L. Spitzer MPH, William M. Tierney MD, Sachin Wani MD, Dhiraj Yadav MD, MPH.
Clinical sites in order of number of patients contributed
Emory University
Ambreen A. Merchant MBBS, Vaishali A. Patel MD, Field F. Willingham MD, MPH.
Vanderbilt University
Eric F. Howard RN, BSN, Mary K. West RN, BSN, Casey L Koza BS, Patrick S. Yachimksi MD.
Grady Memorial Hospital
Emad Qayed MD, MPH, Rosemary Nustas MD.
Ascension Providence Hospital/Michigan State University
Ali Zakaria MD, Marc S. Piper MD, MSc.
Saint Louis University
Jason R. Taylor MD, Lujain Jaza MD.
University of Calgary
Nauzer Forbes MD, MSc, Millie Chau BSc.
The Ohio State University Wexner Medical Center
Luis F. Lara MD, Georgios I. Papachristou MD, PhD, Uchechi Okafor BSc, Darwin L. Conwell MD, MSc.
Loma Linda University
Michael L. Volk MD, MSc, Evan Mosier MD, Mohamed Azab MD, Anish Patel MD.
University of Southern California
Liam G. Hilson MD, Selena Zhou MD, James Buxbaum MD.
Washington University School of Medicine
Vladimir M. Kushnir MD, Alexandria M. Lenyo BS, Ian P. Sloan BS, Thomas Hollander RN, BSN.
Medical University of South Carolina
Caroline G. McLeod BS, Rebecca L. Spitzer MPH, Lauren Wakefield MHA (1), Haley Nitchie MHA, Collins O. Ordiah MBBS, MPH, Don C. Rockey MD, B. Joseph Elmunzer MD.
University of Miami Miller School of Medicine
Sunil Amin MD, MPH, Gabriela N. Kuftinec MD, MPH, Amar R. Deshpande MD.
University of Pittsburgh
Dhiraj Yadav MD, MPH, Melissa Saul MS (14), Melanie Mays BS (14), Gulsum Anderson PhD (14), Kelley Wood BS (14), Laura Mathews BS (14).
University of Colorado
Charlie Fox MD (15) Jennifer M. Kolb MD, MS, Sachin Wani MD.
Wake Forest University School of Medicine
Swati Pawa MD, Rishi Pawa MD.
Boston University
Andrew Canakis DO, Christopher Huang MD.
Beaumont Health
Laith H. Jamil MD, Andrew M. Aneese MD, V. Mihajlo Gjeorgjievski, MD, Zaid Imam MD, Fadi Odish MD, Ahmed I. Edhi MD, Molly Orosey DO, Abhinav Tiwari MD, Soumil Patwardhan MBBS.
University Hospitals of Cleveland Medical Center
Benita K. Glamour BA, Zachary L Smith DO, Amy E. Hosmer MD, Nancy Furey RN, BSN, MBA, Amitabh Chak MD.
Northwestern University Feinberg School of Medicine
Katherine A. Hanley MMS, PAC, Jordan Wood BS, Rajesh N. Keswani MD, MS.
Ochsner Health
Harsh K. Patel MBBS, Janak N. Shah MD.
Columbia University Medical Center
Emil Agarunov BS, Nicholas G. Brown MD, Anish A. Patel MD, Amrita Sethi MD.
Indiana University School of Medicine
Evan L. Fogel MD, MSc, Gail McNulty RN, BSN.
Loyola University Medical Center
Abdul Haseeb MD, Judy A. Trieu MD.
Icahn School of Medicine at Mount Sinai
Rebekah E. Dixon BS, Jeong Yun Yang MD, Christopher J. DiMaio MD.
Memorial Sloan Kettering Cancer Center
Robin B. Mendelsohn MD, Delia Calo MD.
Renaissance School of Medicine at Stony Brook University
Olga C. Aroniadis MD, MSc, Joseph F. LaComb BS, Lilian Cruz BS, Olga Reykhart BS.
University of Virginia Medical School
James M. Scheiman MD, Bryan G. Sauer MD, Galina Diakova MS.
Henry Ford Health System
Duyen T. Dang MD, Cyrus R. Piraka MD.
Dartmouth-Hitchcock Health and VA White River Junction
Eric D. Shah MD, MBA, Molly Caisse BS, Natalia H. Zbib MD (31), John A. Damianos MD, Heiko Pohl MD.
University of Oklahoma Health Sciences Center
William M. Tierney MD, Stephanie Mitchell RN, Michael S. Bronze MD.
David Geffen School of Medicine at UCLA
Ashwinee Condon MD (34), Adrienne Lenhart MD, Raman Muthusamy MD, MAS.
Medical College of Wisconsin
Kulwinder S. Dua MD, Vikram S. Kanagala MD, James Esteban MD.
Johns Hopkins Medical Institutions
Ayesha Kamal MD, Marcia I. Canto MD, Vikesh K. Singh MD, MS.
McMaster University Hamilton Health Sciences
Maria Ines Pinto-Sanchez MD, MSc (37), Joy M. Hutchinson RD, MSc.
Michigan Medicine
Richard S. Kwon MD (38), Sheryl J. Korsnes MA.
University of Manitoba
Harminder Singh MD, MPH, Zahra Solati MSc, Nick Hajidiacos MD.
Declarations
Conflict of interest
None of the authors have potential competing interests.
Footnotes
*Full list of authors in Appendix 1
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. The New England Journal of Medicine. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munster VJ, Koopmans M, van Doremalen N, et al. A Novel Coronavirus Emerging in China–Key Questions for Impact Assessment. The New England Journal of Medicine. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult patients with COVID-19 in Wuhan, Chine: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan Wj, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England Journal of Medicine. 2020; 382:1708-1720. [DOI] [PMC free article] [PubMed]
- 5.Elmunzer BJ, Spitzer RL, Foster LD, et al. Digestive Manifestations in Patients Hospitalized with COVID-19. Clin Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed]
- 6.Herold T, Jurinovic V, Arnreich C, et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. Journal of Allergy and Clinical Immunology. 2020;14(1):128–136. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian J, Yuan XY, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan China: a multicentre, retrospective, cohort study. The Lancet Oncology. 2020;21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Q, Huang D, Yu H, et al. COVID-19: Abnormal liver function tests. Journal of Hepatology. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv. 2020.
- 10.Chen N, Zhou M, Dong X, et al. Epidemiologic and clinic characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronovirus-infected pneumonia in Wuhan China 2020;323:1061-1069. [DOI] [PMC free article] [PubMed]
- 12.Ali N. Relationship Between COVID-19 Infection and Liver Injury: A Review of Recent Data. Front Med. 2020;7:458. doi: 10.3389/fmed.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurala D, Moussawi HA, Philipose J, et al. Acute Liver Failure in a COVID-19 Patients Without any Preexisting Liver Disease. Cureus 2020;12:e10045 [DOI] [PMC free article] [PubMed]
- 14.Weber S, Mayerle J, Irlbeck M, et al. Severe liver failure during SAR-CoV-2 infection. Gut. 2020;69:1365–1367. doi: 10.1136/gutjnl-2020-321350. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar S, Rapista N, Jean LG. Coronavirus Disease-19-Induced Acute Liver Failure Leading to Severe Metabolic Acidosis. Chest 2020;158:a1002
- 16.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guidelines by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 17.Clark JM, Brancati JM, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2020;122(6):1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 18.American Gastroenterologic Association Medical position statement: evaluation of liver chemistry tests. Gastroenterology. 2002;123:1364–1366. doi: 10.1053/gast.2002.36060. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez HC, Imam Z, Wong R, et al. Normal alkaline phosphatase levels are dependent on race/ethnicity: National Health and Nutrition Examination Survey Data. BMJ Open Gastroenterology 2020;7:e000502 [DOI] [PMC free article] [PubMed]
- 20.Robles-Diaz M, Lucena MI, Kaplowitz N, et al. Use of Hy’s Law and a New Composite Algorithm to Predict Acute Liver Failure in Patients with Drug-Induced Liver Injury. Gastroenterology. 2014;147:109–118. doi: 10.1053/j.gastro.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 21.Lee WM, Squires RH, Nyberg SL, et al. Acute liver failure: summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phipps MM, Barraza LH, LaSota ED, et al. Acute Liver Injury in COVID-19:Prevalence and Association with Clinical Outcomes in a Large U.S.Cohort. Hepatology 2020;72:807–817. [DOI] [PMC free article] [PubMed]
- 23.Da BL, Kushner T, El Halabi M, et al. Liver Injury in Patients Hospitalized with Coronovirus Disease 2019 Correlates with Hyperinflammatory Response and Elevated Interleukin-6. Hepatology Communications. 2020;5:177–188. doi: 10.1002/hep4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertolini A, van de Peppel IP, Bodewes FAJA, et al. Abnormal Liver Function Tests in Patients with COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiersinga WJ, Rhodes A, Cheng A, et al. Pathophysiology, Transmission, Diagnossi, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review 2020;324:782–793. [DOI] [PubMed]
- 26.Chand N, Sanyal AJ. Sepsis-Induced Cholestasis. Hepatology. 2007;45:230–241. doi: 10.1002/hep.21480. [DOI] [PubMed] [Google Scholar]
- 27.Guglielmi FW, Regano N, Mazzuoli S, et al. Cholestasis Induced by Total Parental Nutrition. Clinical Liver Diseases. 2008;12:97–110. doi: 10.1016/j.cld.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Darkas JD. The Complete Blood Count to Diagnose Septic Shock. Journal of Thoracic Disease. 2020;12:S16–S21. doi: 10.21037/jtd.2019.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auchet T, Regnier MA, Girerd N, et al. Outcome of Patients with Septic Shock and High-Dose Vasopressor Therapy. 2017;7:43. doi: 10.1186/s13613-017-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.