Abstract
Objective: This study aimed to determine whether the psoas muscle volume (PMV) and its muscle attenuation (MA) are associated with hospital readmission after transcatheter aortic valve implantation (TAVI). Method: We included 113 older patients with aortic stenosis who underwent TAVI at Sakakibara Heart Institute (mean age 85 ± 5 years, 69% women). We measured PMV and psoas muscle area (PMA) as well as total muscle area (TMA) at the third lumbar vertebra using preoperative computed tomography (CT) images. The crude values of the PMV, PMA, and TMA were normalized by dividing by height squared. Results: The median follow-up period was 724 days (interquartile range: 528-730 days), and there were 25 all-cause readmissions during the follow-up period (22% of all patients). In the multivariate Cox regression analysis adjusted for age, sex, and EuroSCORE II, the PMV and its MA and crude PMA were significantly associated with all-cause readmission [HR: 0.957 (0.930-0.985), p = 0.003, HR: 0.927 (0.862-0.997), p = 0.040], whereas the PMA and TMA and each MA were not (all p > 0.05). The groups with low PMV and MA had significantly higher incidences of all-cause readmission than that with high PMV and MA (log-rank test: p = 0.011). Conclusion: PMV and its MA measured from preoperative CT images were independent predictors of all-cause readmission in TAVI patients.
Keywords: Muscle mass, Sarcopenia, Transcatheter aortic valve implantation, Aortic stenosis, Readmission
The incidence of aortic stenosis increases with age, and the number of surgical operations for aortic stenosis has increased with the aging of the population1,2). Patients with severe aortic stenosis who are at high risk for surgery, including older patients and those with comorbidities, are chosen to undergo transcatheter aortic valve implantation (TAVI). Readmissions after TAVI are relatively frequent, and readmissions within one year after TAVI occur at a frequency of approximately 20-50%3). Preventing readmission is an important issue for maintaining quality of life and reducing health care costs.
Low muscle mass or sarcopenia are a prognostic factor for all-cause mortality in patients with cardiac disease, including patients undergoing TAVI4,5). In these patients, the percentage of those with low muscle mass, as determined by the cutoffs of previous studies, was reported to be around 60%5-7). In elderly patients admitted to the acute care ward, the diagnosis of sarcopenia, including low muscle mass, has been reported to increase readmission rates after discharge8). Nevertheless, it is unclear whether low muscle mass predicts readmission in patients who undergo TAVI.
Reports of muscle mass measured using computed tomography (CT) have increased in recent years. The measurement site for the evaluation of muscle mass is usually the cross-sectional area of the total skeletal muscle or the psoas muscle at the third lumbar vertebra (L3)9,10). In patients with major vascular disease, the risk of all-cause mortality was greater in groups with smaller cross-sectional area in the total skeletal muscle and psoas muscle at L3 than in those with larger areas11,12).
The European Working Group on Sarcopenia in Older People reported that the total skeletal muscle and the psoas muscle at the L3 measured using CT require further verification to be used for sarcopenia evaluation13). Because patients under undergo TAVI are elderly and often have spinal deformations, there may be limits to assessing muscle mass in one cross section. In elderly patients with substantial spinal deformations, the volume of the psoas muscle on CT may be evaluated more accurately than measurements of cross-sectional area. There are reports of measurements of muscle mass using volume in other fields such as oncology, and the relationship with prognosis has also been reported14,15). Nevertheless, there are few reports of patients with cardiac disease, including patients undergoing TAVI. Reports on the assessment of muscle quality using CT values are increasing, and the attenuation of muscle quality is reportedly associated with a decline in physical function and prognosis16,17). Assessing muscle mass and quality deterioration using standard pre-TAVI protocol may lead to early identification of the risk of worse outcomes, and early referral to physical therapy. Therefore, this study aimed to determine whether psoas muscle volume (PMV) and its muscle attenuation (MA) are associated with all-cause hospital readmission after TAVI.
Methods
Study population
This study was conducted at a single center, and the study design was retrospective. The study included 116 consecutive patients with aortic stenosis who underwent TAVI at the Sakakibara Heart Institute between 2016 January and 2017 March. We excluded three patients who had undergone spinal fusion surgery in the past because implanted orthopedic hardware caused metal artifacts. This study was approved by the ethics board of the Sakakibara Heart Institute (ID: 18-011), and written informed consent was obtained from all patients. Study procedures were carried out in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects (Japanese Ministry of Education, Culture, Sports, Science and Technology and Ministry of Health, Labour and Welfare).
Assessment of physical function
Grip strength was used to evaluate the maximum muscle strength and was measured using a handgrip dynamometer. Patients were measured in a sitting position with elbow flexion of 90 degrees, 3 times on each side. Grip strength was calculated by averaging the maximum values of the left and right sides. Gait speed was calculated by dividing 4 m by the time required to walk the distance. Patients were instructed to walk at a comfortable speed and were allowed to use walking aids such as canes and walkers. Patients unable to walk the distance were recorded as having a gait speed of 0 m/sec.
Assessment of skeletal muscle in CT images
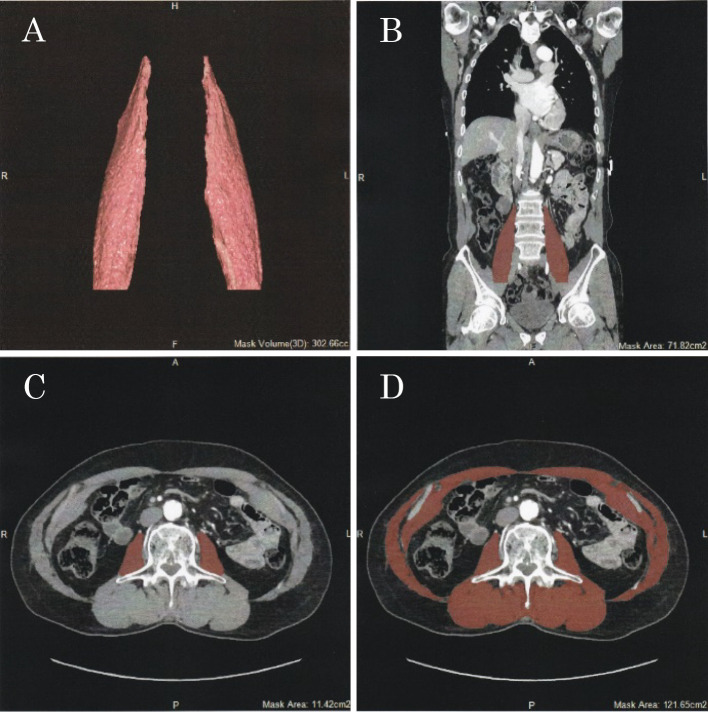
PMV, psoas muscle area (PMA), and total muscle area (TMA) were obtained from preprocedural CT images. The PMV, PMA, and TMA were measured using semi-automatic methods on Ziostation2 (Ziosoft, Tokyo, Japan), as shown in Figure 1. The PMV was measured from the level of the lumbar vertebra to the plane connecting the bilateral anterior superior iliac spine in the sagittal axis (Fig. 1A, 1B). The PMA and TMA were analyzed in the cross-sectional area in the axial plane at the middle of L3 (Fig. 1C, 1D). In the selected PMV, PMA, and TMA, thresholds of CT values were -30 to +150 Hounsfield Units (HU) because of the exclusion of fat or other tissues except for the muscle. The PMV and PMA were calculated as the sum of the left and right psoas muscles. The crude values of the PMV, PMA, and TMA were normalized by dividing by height squared. To evaluate the MA, average CT values were evaluated in the measured PMV, PMA, and TMA. The measurements of skeletal muscle were made by an independent observer. To evaluate interobserver agreements for the measurements, the intraclass correlation coefficient (ICC) was used in the analysis of randomly selected 20 patients by two independent observers. The ICCs for the PMV, PMA, and TMA were 0.984, 0.989, and 0.989, respectively, and the ICCs for the MA of the PMV, PMA, and TMA were 0.997, 0.975, and 0.972, respectively.
Fig. 1.
Assessment of skeletal muscle in CT images.
Psoas muscle volume in the three dimensions (A) and in the coronal plane (B). Psoas muscle area (C) and total muscle (D) at the third lumbar vertebra in the axial plane.
Endpoint and follow-up
The endpoint of this study was unplanned all-cause readmission following TAVI. We obtained follow-up data from medical records or by telephone interviews. The follow-up period was a maximum of two years.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation for normal distribution or median (interquartile range: 25th to 75th percentiles) for non-normal distribution, and categorical variables are expressed as numbers or percentages. The distribution of each continuous variable was assessed for normality using the Shapiro-Wilk test. Comparisons between gender in muscle mass and attenuation were performed using Student's t-test. Cox regression analysis was used to examine the association between muscle mass and attenuation and all-cause readmissions. In multivariate Cox regression analysis, the covariates included age, sex, and EuroSCORE II. The Kaplan-Meier curve and log-rank test were performed to examine the impact of muscle quantity and quality on all-cause readmissions using stratified median PMV and MA. A p-value of less than 0.05 was considered significant. Statistical analyses were performed using SPSS 21.0 (IBM Corp., New York, USA).
Results
Baseline characteristics
Clinical characteristics at baseline are shown in Table 1. The mean age was 85 ± 5 years, and women accounted for 69% of all patients. In terms of New York Heart Association (NYHA) class, 15 patients were in class I, 63 patients were in class II, 32 patients were in class III, and three patients were in class IV. The median left ventricular ejection fraction was 61.7% (interquartile range: 56.4-65.0%) in all patients. EuroSCORE II, a risk score for cardiac surgery, was a median 3.4% (interquartile range: 2.1-5.2%) in all patients. Mean grip strength was 16.2 ± 6.7 kg, and mean gait speed was 0.75 ± 0.26 m/sec in all patients.
Table 1.
Baseline characteristics
| Overall (n = 113) | |
|---|---|
| Values are mean ± standard deviation, percentages, or median (interquartile range). NYHA, New York Heart Association; eGFR, estimated glomerular filtration rate. | |
| Age (years) | 85 ± 5 |
| Women (%) | 69 |
| Height (cm) | 150.2 ± 8.5 |
| Body mass index (kg/m2) | 22.5 ± 3.3 |
| NYHA class (I/II/III/IV) (%) | 13/56/28/3 |
| Comorbidity | |
| Hypertension (%) | 72 |
| Dyslipidemia (%) | 48 |
| Diabetes mellitus (%) | 22 |
| Previous myocardial infarction (%) | 5 |
| Previous angina pectoris (%) | 19 |
| Chronic obstructive pulmonary disease (%) | 4 |
| Previous stroke (%) | 12 |
| Previous motor disorder (%) | 25 |
| Transthoracic echocardiography | |
| Left ventricular ejection fraction (%) | 61.7 (56.4-65.0) |
| Aortic valve area (cm2) | 0.7 ± 0.1 |
| Mean pressure gradient (mmHg) | 51.6 (42.1-62.3) |
| Laboratory data | |
| Albumin (g/dl) | 3.9 ± 0.4 |
| eGFR (ml/min/1.73 m2) | 52.8 (38.4-61.5) |
| Creatinine (mg/dl) | 0.92 (0.73-1.11) |
| EuroSCORE II (%) | 3.4 (2.1-5.2) |
| Physical function | |
| Grip strength (kg) | 16.2 ± 6.7 |
| Gait speed (m/sec) | 0.75 ± 0.26 |
Muscle mass and attenuation of skeletal muscle
PMV, PMA, TMA, and their MA are shown in Table 2. The crude or normalized for height squared PMV, PMA, and TMA were significantly larger in men than in women (all p < 0.001). The MA of the PMV and TMA were significantly higher in men than in women (p < 0.001 and p = 0.006, respectively).
Table 2.
Muscle mass and attenuation of Skeletal muscle in CT images
| Overall (n = 113) | Women (n = 78) | Men (n = 35) | p-value | |
|---|---|---|---|---|
| Values are presented as mean ± standard deviation. PMV, psoas muscle volume; PMA, psoas muscle area; TMA, total muscle area; HU, Hounsfield unit. | ||||
| Crude | ||||
| PMV (cm3) | 170.7 ± 59.9 | 141.1 ± 33.5 | 236.6 ± 52.8 | < 0.001 |
| PMA (cm2) | 9.4 ± 3.2 | 8.1 ± 2.3 | 12.3 ± 2.9 | < 0.001 |
| TMA (cm2) | 80.4 ± 24.0 | 71.0 ± 15.6 | 101.3 ± 26.4 | < 0.001 |
| Normalized for height squared | ||||
| PMV (cm3/m2) | 74.4 ± 20.4 | 66.1 ± 15.1 | 93.0 ± 18.7 | < 0.001 |
| PMA (cm2/m2) | 4.1 ± 1.2 | 3.8 ± 1.1 | 4.9 ± 1.2 | < 0.001 |
| TMA (cm2/m2) | 36.1 ± 7.8 | 33.9 ± 6.8 | 41.2 ± 7.5 | < 0.001 |
| Muscle attenuation (HU) | ||||
| PMV | 37.3 ± 5.6 | 36.0 ± 4.8 | 40.2 ± 6.0 | < 0.001 |
| PMA | 35.9 ± 6.4 | 35.3 ± 6.0 | 37.3 ± 7.0 | 0.133 |
| TMA | 28.8 ± 8.5 | 27.4 ± 7.5 | 32.1 ± 9.8 | 0.006 |
Endpoint and follow-up
The median follow-up period in all patients was 724 days (interquartile range: 528-730 days). There were 25 all-cause readmissions during the follow-up period (22% of all patients). There were 13 readmissions due to cardiac causes (52% of the all-cause total), and eight among cardiac causes were because of heart failure (32% of the all-cause total). There were 12 readmissions due to non-cardiac causes (48% of the all-cause); of these, fractures were most common (six events, 24% of the all-cause total).
Association of muscle mass and attenuation with all-cause readmission
The results of the Cox regression analysis for all-cause readmission are shown in Table 3. In the univariate analysis, PMV and MA were significant predictors of all-cause readmission, whereas the PMA and TMA and each of the MA were not. In the multivariate analysis adjusted for age, sex, and EuroSCORE II, the PMV and its MA and crude PMA were significantly associated with all-cause readmission. Our findings demonstrated that PMV, MA, and crude PMA were inversely related to risk of all-cause readmission.
Table 3.
Association of muscle mass and attenuation with all-cause readmission
| Unadjusted | Adjusted for age/sex | Adjusted for age/sex/ EuroSCORE II | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| HR, hazard ratio; CI, confidence interval; PMV, psoas muscle volume; PMA, psoas muscle area; TMA, total muscle area. | ||||||
| Crude | ||||||
| PMV | 0.988 (0.980-0.997) | 0.008 | 0.980 (0.968-0.992) | 0.001 | 0.978 (0.965-0.991) | 0.001 |
| PMA | 0.879 (0.764-1.012) | 0.073 | 0.858 (0.719-1.024) | 0.090 | 0.812 (0.667-0.988) | 0.037 |
| TMA | 0.996 (0.980-1.012) | 0.585 | 0.999 (0.979-1.021) | 0.947 | 0.997 (0.976-1.020) | 0.816 |
| Normalized for height squared | ||||||
| PMV | 0.970 (0.949-0.992) | 0.008 | 0.961 (0.936-0.988) | 0.005 | 0.957 (0.930-0.985) | 0.003 |
| PMA | 0.792 (0.556-1.129) | 0.197 | 0.813 (0.549-1.205) | 0.303 | 0.722 (0.472-1.105) | 0.134 |
| TMA | 0.994 (0.942-1.049) | 0.840 | 1.006 (0.947-1.069) | 0.845 | 0.999 (0.937-1.065) | 0.979 |
| Muscle attenuation | ||||||
| PMV | 0.922 (0.864-0.985) | 0.016 | 0.922 (0.859-0.989) | 0.023 | 0.927 (0.862-0.997) | 0.040 |
| PMA | 0.973 (0.916-1.034) | 0.383 | 0.977 (0.918-1.041) | 0.474 | 0.978 (0.916-1.044) | 0.506 |
| TMA | 0.962 (0.924-1.001) | 0.057 | 0.963 (0.923-1.004) | 0.079 | 0.968 (0.925-1.012) | 0.151 |
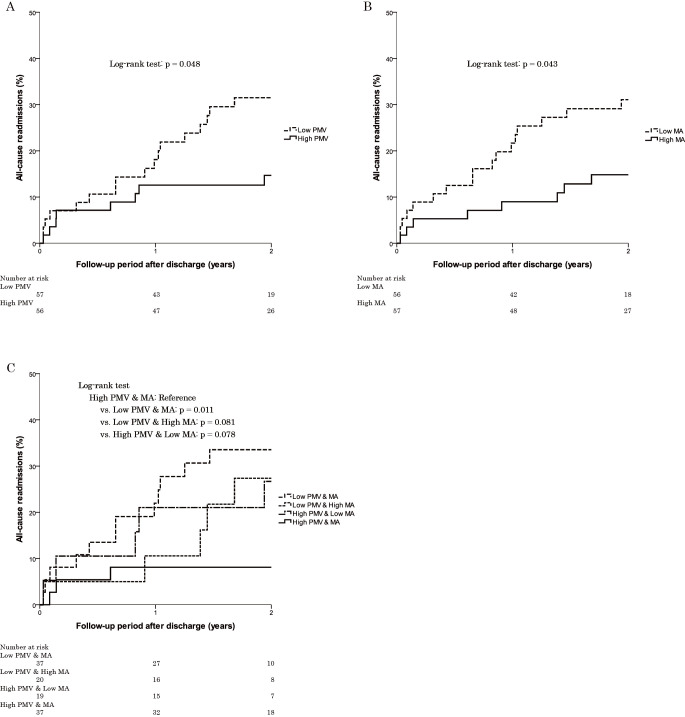
The Kaplan-Meier survival curves for all-cause readmission divided according to the normalized PMV and its MA are shown in Figure 2. The median of the normalized PMV was 68.0 cm3/m2 for women and 96.3 cm3/m2 for men, and the median of the MA of the normalized PMV was 36.4 HU for women and 41.8 HU for men. In the results divided according to normalized PMV, the low PMV group had a significantly higher incidence of all-cause readmission than the high PMV group (log-rank test: p = 0.048) (Fig. 2A). The low MA group had a significantly higher incidence of all-cause readmission than the high MA group (log-rank test: p = 0.043) (Fig. 2B). The group with low PMV and MA had a significantly higher incidence of all-cause readmission than the group with high PMV and MA (log-rank test: p = 0.011) (Fig. 2C).
Fig. 2.
Kaplan-Meier curves for all-cause readmission divided according to the normalized PMV and its MA for each sex.
Divided according to PMV (A), its MA (B), and both PMV and its MA (C). PMV, psoas muscle volume; MA, muscle attenuation.
Discussion
This was the first report to show that loss of the PMV assessed by CT was an independent predictor of post-discharge all-cause readmission in patients who underwent TAVI. The novelty of this study was the evaluation of not only the psoas muscle mass, but also its MA. The risk of readmission after discharge was significantly greater in the group with both low values of the PMV and its MA than in the group with both high values. Assessing muscle quality in addition to muscle mass may more accurately predict all-cause readmission after TAVI.
It has been reported that the loss of muscle mass assessed using CT is associated with prognosis after TAVI. The decrease in the PMA at L3 or the fourth lumbar vertebra (L4) independently predicted 30-day and cumulative mortality rates after TAVI18); although this result showed different prognostic importance than our result, decreased muscle mass was associated with poor outcomes in a similar fashion to what we showed in this study. To the best of our knowledge, there are no studies examining the association between CT-assessed skeletal muscle mass and readmission in patients who underwent TAVI; however, there are several reports regarding other diseases19,20). Although the subjects and the survey period differed from those described above, the results of this study showed similar trends. Loss of PMV was an independent predictor of readmission after discharge in TAVI patients.
There are several reports on the association between PMA and all-cause mortality after discharge in patients who underwent TAVI; however the association remains controversial. In these patients, the PMA at L4 was a predictor of 12-month all-cause mortality in receiver operating characteristic analysis21). In contrast, the PMA at L3 was not associated with an increased risk of all-cause mortality at 1 year in patients who underwent TAVI22). It was reported that the PMA at the L3 or L4 was associated with all-cause mortality only in women23,24) or only in men25) in patients with TAVI.
In recent years, reports on the evaluation of the psoas muscle by CT images included evaluations of not only the cross-sectional area but also the volume. In patients with pancreatic cancer who underwent pancreatic resection, the PMV, and not the PMA, predicted postoperative complications and all-cause mortality14). In the present study, we showed that low PMV was an independent predictor of post-discharge readmission in TAVI patients.
It was speculated that more accurate evaluation of the psoas muscle was possible by using the volume rather than the cross-sectional area. Spinal deformity tends to become more pronounced with age26,27). Patients undergoing TAVI tend to be very old and often have substantial spinal deformities; it was speculated that the PMV was more suitable as a method for evaluating the muscle mass of the psoas muscle than the PMA. Several reports have revealed that frailty is an independent predictor of readmission after TAVI28,29). It was speculated that patients with reduced muscle mass may be more prone to the frailty cycle owing to reduced physical reserve and higher risk of readmission.
In the evaluation of skeletal muscle, the importance of evaluating not only muscle mass but also muscle quality has been drawing attention. In CT images, increasing lipid content in the skeletal muscle was associated with decreased MA30). In a report of volunteers aged 59-85 years, the MA of the TMA at the level of the second lumbar vertebra correlated with grip strength16). Lower MA of the TMA at L3 was an independent predictor of all-cause mortality, and both low MA and muscle mass were associated with increased risk of all-cause mortality in patients who underwent TAVI31). Similar to previous reports, in this study, the MA of the PMV independently predicted readmission after discharge, with both low MA and muscle mass indicating high risks of readmission. Low MA has been shown to be correlated with insulin resistance32), increased inflammatory cytokine levels33), and physical inactivity34) all of which are associated with worse clinical outcome.
CT examinations are routinely performed preoperatively in TAVI patients. Muscle quantity and quality assessment are possible to grasp the risk of readmission and early referral to physical therapy. Early detection of muscle quantity and quality and early referral to physical therapy may reduce the risk of readmission.
This study has several limitations. First, this study was conducted at a single center, and the study design was retrospective. Second, the number of samples was relatively small. Multicenter prospective studies with large sample sizes are needed to test whether the findings of this study are widely applicable to patients undergoing TAVI. Third, skeletal muscle mass was only assessed in individual muscles by CT images and was not assessed in systemic muscle using dual-energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis, both of which are standards for sarcopenia evaluation. It is unclear whether the PMV-decreased cases in this study caused systemic muscle loss; however, a previous study showed a high correlation between systemic muscle mass evaluated by DXA and cross-sectional area of muscle evaluated using CT images9).
Conclusion
We showed that the PMV and its MA measured from preoperative CT images independently predicted post-discharge all-cause readmission in patients who underwent TAVI. In addition, both low PMV and its MA were associated with increased post-discharge readmissions. These findings suggest that accurate evaluations of muscle mass and quality are necessary to assess the risk of post-discharge readmissions in TAVI patients.
Conflict of Interest
The authors have no conflict of interest to disclose.
Funding
This research was supported by a Sakakibara Heart Institute (Tokyo, Japan) research grant.
Acknowledgments
The authors thank Kazuo Awai, RT, Tatsunori Niwa, RT, and all members of the Department of Rehabilitation, Sakakibara Heart Institute.
References
- 1.Nkomo VT, Gardin JM, et al.: Burden of valvular heart diseases: a population-based study. Lancet. 2006; 368: 1005-1011. [DOI] [PubMed] [Google Scholar]
- 2.Committee for Scientific Affairs TJAfTS, Shimizu H, et al.: Thoracic and cardiovascular surgeries in Japan during 2017: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2020; 68: 414-449. [DOI] [PubMed] [Google Scholar]
- 3.Li YM, Mei FY, et al.: Causes and predictors of readmission after transcatheter aortic valve implantation: A meta-analysis and systematic review. Herz. 2019. 10.1007/s00059-019-04870-6 [Online ahead of print]. [Google Scholar]
- 4.Zhang N, Zhu WL, et al.: Prevalence and prognostic implications of sarcopenia in older patients with coronary heart disease. J Geriatr Cardiol. 2019; 16: 756-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidari B, Al-Hijji MA, et al.: Transcatheter aortic valve replacement outcomes in patients with sarcopaenia. EuroIntervention. 2019; 15: 671-677. [DOI] [PubMed] [Google Scholar]
- 6.Mok M, Allende R, et al.: Prognostic Value of Fat Mass and Skeletal Muscle Mass Determined by Computed Tomography in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am J Cardiol. 2016; 117: 828-833. [DOI] [PubMed] [Google Scholar]
- 7.Damluji AA, Rodriguez G, et al.: Sarcopenia and health-related quality of life in older adults after transcatheter aortic valve replacement. Am Heart J. 2020; 224: 171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang M, Hu X, et al.: Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle. 2017; 8: 251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mourtzakis M, Prado CM, et al.: A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008; 33: 997-1006. [DOI] [PubMed] [Google Scholar]
- 10.Baracos V and Kazemi-Bajestani SM: Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int J Biochem Cell Biol. 2013; 45: 2302-2308. [DOI] [PubMed] [Google Scholar]
- 11.Koter S, Cohnert TU, et al.: Increased hospital costs are associated with low skeletal muscle mass in patients undergoing elective open aortic surgery. J Vasc Surg. 2019; 69: 1227-1232. [DOI] [PubMed] [Google Scholar]
- 12.Cheng BT, Soult MC, et al.: Sarcopenia predicts mortality and adverse outcomes after endovascular aneurysm repair and can be used to risk stratify patients. J Vasc Surg. 2019; 70: 1576-1584. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Jentoft AJ, Bahat G, et al.: Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48: 16-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amini N, Spolverato G, et al.: Impact Total Psoas Volume on Short- and Long-Term Outcomes in Patients Undergoing Curative Resection for Pancreatic Adenocarcinoma: a New Tool to Assess Sarcopenia. J Gastrointest Surg. 2015; 19: 1593-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsubara Y, Nakamura K, et al.: Pre-treatment psoas major volume is a predictor of poor prognosis for patients with epithelial ovarian cancer. Mol Clin Oncol. 2019; 11: 376-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Yin L, et al.: Muscle Density, but Not Size, Correlates Well With Muscle Strength and Physical Performance. J Am Med Dir Assoc. 2020. 10.1016/j.jamda.2020.06.052 [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita M, Kamiya K, et al.: Prognostic Value of Psoas Muscle Area and Density in Patients Who Undergo Cardiovascular Surgery. Can J Cardiol. 2017; 33: 1652-1659. [DOI] [PubMed] [Google Scholar]
- 18.Kofler M, Reinstadler SJ, et al.: Prognostic implications of psoas muscle area in patients undergoing transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2019; 55: 210-216. [DOI] [PubMed] [Google Scholar]
- 19.Fintelmann FJ, Troschel FM, et al.: Thoracic Skeletal Muscle Is Associated With Adverse Outcomes After Lobectomy for Lung Cancer. Ann Thorac Surg. 2018; 105: 1507-1515. [DOI] [PubMed] [Google Scholar]
- 20.Looijaard S, Meskers CGM, et al.: Computed Tomography-Based Body Composition Is Not Consistently Associated with Outcome in Older Patients with Colorectal Cancer. Oncologist. 2020; 25: e492-e501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleczynski P, Tokarek T, et al.: Usefulness of Psoas Muscle Area and Volume and Frailty Scoring to Predict Outcomes After Transcatheter Aortic Valve Implantation. Am J Cardiol. 2018; 122: 135-140. [DOI] [PubMed] [Google Scholar]
- 22.Hebeler KR, Baumgarten H, et al.: Albumin Is Predictive of 1-Year Mortality After Transcatheter Aortic Valve Replacement. Ann Thorac Surg. 2018; 106: 1302-1307. [DOI] [PubMed] [Google Scholar]
- 23.Mamane S, Mullie L, et al.: Psoas Muscle Area and All-Cause Mortality After Transcatheter Aortic Valve Replacement: The Montreal-Munich Study. Can J Cardiol. 2016; 32: 177-182. [DOI] [PubMed] [Google Scholar]
- 24.van Mourik MS, Janmaat YC, et al.: CT determined psoas muscle area predicts mortality in women undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2019; 93: E248-E254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foldyna B, Troschel FM, et al.: Computed tomography-based fat and muscle characteristics are associated with mortality after transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2018; 12: 223-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uehara M, Takahashi J, et al.: Sagittal spinal alignment deviation in the general elderly population: a Japanese cohort survey randomly sampled from a basic resident registry. Spine J. 2019; 19: 349-356. [DOI] [PubMed] [Google Scholar]
- 27.Asai Y, Tsutsui S, et al.: Sagittal spino-pelvic alignment in adults: The Wakayama Spine Study. PLoS One. 2017; 12: e0178697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik AH, Yandrapalli S, et al.: Impact of Frailty on Mortality, Readmissions, and Resource Utilization After TAVI. Am J Cardiol. 2020; 127: 120-127. [DOI] [PubMed] [Google Scholar]
- 29.Saji M, Higuchi R, et al.: Impact of Frailty Markers for Unplanned Hospital Readmission Following Transcatheter Aortic Valve Implantation. Circ J. 2018; 82: 2191-2198. [DOI] [PubMed] [Google Scholar]
- 30.Goodpaster BH, Kelley DE, et al.: Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000; 89: 104-110. [DOI] [PubMed] [Google Scholar]
- 31.Tokuda T, Yamamoto M, et al.: Importance of combined assessment of skeletal muscle mass and density by computed tomography in predicting clinical outcomes after transcatheter aortic valve replacement. Int J Cardiovasc Imaging. 2020; 36: 929-938. [DOI] [PubMed] [Google Scholar]
- 32.Kelley DE, Goodpaster BH, et al.: Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002; 22: 325-346. [DOI] [PubMed] [Google Scholar]
- 33.Zoico E, Rossi A, et al.: Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010; 65: 295-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodpaster BH, Chomentowski P, et al.: Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985). 2008; 105: 1498-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]