Abstract
Purpose:
To compare the therapeutic impact of combining intravitreal injections of bevacizumab (IVB) with micropulse laser (MPL) in central diffuse diabetic macular edema (DME) versus IVB monotherapy during 12 months follow-up.
Methods:
We conducted a retrospective comparative study of 98 treatment-naive eyes (63 patients) with central diffuse DME. The first group of patients (IVB + MPL group, n = 49) was treated with 3 monthly IVB followed by MPL within 1 week after the third injection. Patients were then followed and treated on a pro re nata (PRN) basis, with MPL retreatment if necessary. The changes in best-corrected visual acuity (BCVA), central macular thickness (CMT), number of IVB injections and MPL sessions were evaluated at 4, 8, and 12 months. A control group of diabetic patients with treatment-naive DME was treated with standard protocol of 3 monthly IVB as monotherapy then followed on a PRN basis (IVB group, n = 49). Statistic comparaison of BCVA, CMT, and IVB number variation was interpreted at 12 months between both groups.
Results:
In IVB + MPL group, baseline BCVA improvement was not significant at 4 and 8 months (p = 0.90, p = 0.08), and was statistically significant (p = 0.01) at 12 months. Mean CMT significantly decreased at 4, 8, and 12 months (p < 0.01) in IVB + MPL group. The difference in BCVA (p = 0.091) and CMT (p = 0.082) variation at 12 months between both groups was not significant but the number of injections was significantly lower in IVB + MPL group (4.1 ± 1.5 injections) compared to IVB group (7.2 ± 1.3 injections) (p < 0.005).
Conclusion:
Combining intravitreal injections of bevacizumab and MPL in the treatment of DME is effective and safe. This protocol may decrease the number of IVB and its frequency. It offers the advantage of lasting therapeutic response with fewer recurrences.
Keywords: anti-VEGF, bevacizumab, diabetic macular edema, micropulse laser, subthreshold laser
Introduction
Diabetic macular edema (DME) is the most common cause of vision loss in diabetic patients.1 For many years, laser photocoagulation was offered as the gold standard treatment for DME according to Early Treatment of Diabetic Retinopathy Study (ETDRS).2 However, complications such as loss of contrast sensitivity, impaired colour vision, accidental foveal damage, choroidal neovascularization, and expansion of macular scars have largely limited macular laser indications.3–6
A protocol combining physical and pharmacological treatments might induce a synergy of action of the two techniques with less side effects.7,8 Recently, a new subthreshold micropulse laser (MPL) has been introduced into the therapeutic arsenal of DME.9 Micropulse technology divides laser power into trains of microsecond “on” pulses with longer “off” times that allow complete relaxation of energy, avoiding thermal build-up and preventing retinal damage.9,10
We present a retrospective comparative study evaluating the visual and anatomical outcomes in eyes with treatment-naive central diffuse DME treated with intravitreal bevacizumab (IVB) associated to MPL photostimulation during 12 months follow-up and compared to 1-year visual and anatomical outcomes of IVB monotherapy.
Patients and methods
We conducted a retrospective comparative study from January 2015 to January 2019. Patients’ information was collected from medical records. We included two groups of type 2 diabetic patients with treatment-naive central diffuse DME.
Forty-nine eyes (32 patients) were treated with 3 monthly IVB doses associated to adjuvant MPL within 1 week after the last injection. Patients were then treated with IVB on a Pro Re Nata (PRN) regimen every 4 weeks,7,11–13 with MPL retreatment if necessary (IVB + MPL group, n = 49).
Criteria of IVB retreatment were the association of:
BCVA ⩽ 20/25
Presence of intraretinal fluid (IRF) and/or subretinal fluid (SRF).
They were compared to 49 eyes (31 patients) treated with 3 monthly doses of IVB and then followed on a PRN regimen every 4 weeks,7,11–13 with bevacizumab monotherapy (IVB group, n = 49). The same criteria of IVB retreatment were applied in this group.
The use of MPL protocol or not was retained after discussing the therapeutic options with all the patients.
Patients enrolled in the study were aged over 18 and presenting treatment-naïve central DME with best-corrected visual acuity (BCVA) ⩾ 20/400, central macular thickness (CMT) measured by spectral domain optical coherence tomography (SD-OCT) ⩽ 500 μm, HbA1C < 9% and with 12 months follow-up data.
Exclusion criteria were proliferative diabetic retinopathy, large central hard exudates, macular ischaemia on fluorescein angiography (FA), epiretinal membrane or tractional maculopathy on structural OCT, media opacity, the presence of other concomitant retinal diseases that could lead to central macular edema and history of any intraocular surgery or intravitreal injection during the prior 6 months. Patients were also excluded if they had a history of severe heart disease, renal failure, uncontrolled high blood pressure, permanent, or transient cerebral ischemic attack. Non-inclusion criteria were the presence of focal or multifocal DME and cases of extra-macular edema.
All patients underwent complete ophthalmic examination. BCVA was measured using ETDRS visual acuity charts at 4 metres. BCVA was converted to logarithm of the minimum angle of resolution (logMAR) units for statistical analysis. Central macular thickness measurement was performed on structural SD-OCT at baseline and each monthly control (3D-OCT 2000 Topcon, Tokyo, Japan). Fundus autofluorescence (FAF) and FA were performed at baseline and at the end of follow-up (Spectralis HRA2, Heidelberg Engineering, Heidelberg, German). During follow-up, eventual treatment adverse effects as inflammation, retinal tears, cataract evolution or laser scars were sought and recorded.
Study protocol
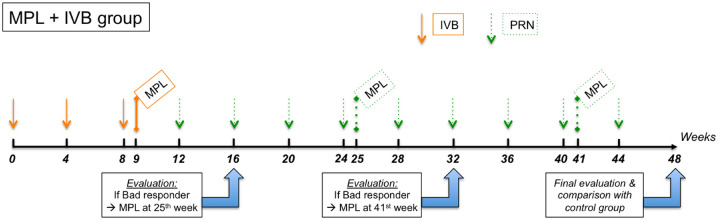
In IVB + MPL group, all patients received initial 3 monthly doses (0, fourth, and eighth week) of IVB (1.25 mg/0.05 ml). MPL 577 nm session was performed within 1 week after the third injection (ninth week). Then patients were followed and injected with IVB on a PRN regimen every 4 weeks.
An evaluation was performed at 16th and 32nd week. We defined depending on their therapeutic responses:
Good responders
Improvement of BCVA by one line or more (corresponding to a gain of ⩾ 5 letters) or stabilization of BCVA, meaning a variation by less than one line (< 5 letters).
Poor responder
Decrease of BCVA by one line or more (⩾ 5 letters).
The good responders at 16th and/or 32nd week were followed and treated with IVB on a PRN regimen until next evaluation. On the other hand, the poor responders at 16th week were treated with a supplementary MPL session at 25th week, and/or the poor responders at 32nd week were treated with a supplementary MPL session at 41st week. Poor responders continued to receive IVB doses on a PRN regimen every 4 weeks, between MPL sessions (Figure 1).
Figure 1.
Therapeutic regimen in MPL + IVB group.
At 48th week (12 months follow-up), a final evaluation was performed and a comparison was done with control group in terms of mean variation in BVCA and CMT and mean number of injections.
In control group (IVB group): All patients received initial 3 monthly doses (0, fourth, and eighth week) of IVB (1.25 mg/0.05 ml). Then patients were followed and injected with IVB on a PRN regimen every 4 weeks. A final evaluation and comparison were done at 48th week.
MPL protocol
We performed micropulse laser using a 577 nm yellow-light laser (Iridex IQ 577, California, USA) with confluent impacts. An Area Centralis contact lens was utilized for macular impacts. Laser parameters were: power = 400 mW, spot size = 200 microns, pulse duration = 0.2 seconds and duty cycle = 5%, after micropulse mode activation. The number of spots was variable and MPL was applied with no spacing application of spots using a 2 x 2 or 4 x 4 treatment grid to cover the entire edematous area based on OCT.
Retreatments were performed if necessary, using the same protocol.
Statistical analysis
The primary outcomes measures were the changes in BCVA and CMT, at 16, 32, and 48 weeks and the number of IVB injections and MPL sessions at 48 weeks (12 months) in IVB + MPL group.
The secondary outcome measures were the comparison between both groups (IVB + MPL group versus IVB group) in terms of variation between baseline and final BCVA and CMT and the comparison in final number of IVB injections at 48 weeks.
Statistical analysis was performed with Statistical Package for Social Sciences software (SPSS, version 20; IBM Corporation, New York, USA). Mean deviation and standard deviation (SD) were calculated for quantitative data. Paired t test was calculated to determine numerical data differences in the same group and student t test was calculated in order to compare numerical data between both groups. Significance level was 0.05.
Results
Baseline characteristics
Our study included 98 eyes of 63 patients with DME. There were 49 eyes of 32 patients in IVB + MPL group and 49 eyes of 31 patients in IVB group. Mean age was, respectively, 67.7 ± 5.23 and 61.3 ± 4.11 (p = 0.366) and the sex ratio male/female was respectively 19/13 and 20/11 (p = 0.215). All patients had type 2 diabetes mellitus in both groups; the mean duration of known diabetes was 13.67 ± 6.63 years in IVB + MPL group and 18.65 ± 3.72 years in IVB group (p = 0.291), and mean haemoglobin A1c level was respectively 7.70 ± 0.81% and 7.60 ± 0.62% (p = 0.419). In IVB + MPL group, 14 eyes were phakic and 35 eyes were pseudophakic; and in IVB group, 17 eyes were phakic and 32 eyes were pseudophakic (p = 0.493).
In IVB + MPL group, 34 eyes had moderate NPDR and 15 eyes had severe NPDR; and in IVB group, 37 eyes had moderate NPDR and 12 eyes had severe NPDR (p = 0.167). DME was diffuse in all eyes of both groups. There was intraretinal fluid on SD-OCT in all eyes as well, while subretinal fluid was present in 28 eyes in IVB + MPL group (57%) and 33 eyes in IVB group (67%) (p = 0.537). There were no anatomical nor visual differences in this SRF sub-group.
Mean time between DME diagnosis and first IVB was 5.22 days [0–13] in IVB + MPL group and 5.18 days in IVB group [0–13]. The difference was not statistically significant (p = 0.18). Demographic data are available in Table 1, with no significant differences between both groups.
Table 1.
Demographics and clinical characteristics of the two groups.
| Parameters | IVB + MPL group N = 49 eyes | IVB group N = 49 eyes | p value |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 67.7 ± 5.23 | 61.3 ± 4.11 | 0.366 |
| Gender | |||
| Male/female | 19/13 | 20/11 | 0.215 |
| Type of diabetes | |||
| Type 1/Type 2 | 0/32 | 0/31 | – |
| Duration of known diabetes | |||
| Mean ± SD | 13.67 ± 6,.63 | 18.65 ± 3.72 | 0.291 |
| Haemoglobin A1c level (%) | 7.70 ± 0.81% | ||
| Mean ± SD | 7.60 ± 0.62% | 0.419 | |
| Lens status | 0.493 | ||
| Phakic | 14 eyes | 17 eyes | |
| Pseudophakic | 35 eyes | 32 eyes | |
| Severity of DR | 0.167 | ||
| • Moderate NPDR | 34 eyes | 37 eyes | |
| • Severe NPDR | 15 eyes | 12 eyes | |
| Type of DME | |||
| • Diffuse | 49 eyes | 49 eyes | – |
| Type of retinal fluid | |||
| • Intraretinal fluid | 49 eyes | 49 eyes | – |
| • Subretinal fluid | 28 eyes | 33 eyes | 0.537 |
| Mean time between DME diagnosis and first IVB | 5.22 days [0–13] |
5.18 days [0–13] |
0.180 |
DME, diabetic macular edema; DR, diabetic retinopathy; IVB, intravitreal injections of bevacizumab; MPL, micropulse laser; NPDR, non-proliferative diabetic retinopathy; SD, standard deviation.
Visual outcomes
In IVB + MPL group, baseline logMAR BCVA was 0.692 ± 0.35 (range from 0.15 to 1.3). At 16 and 32 weeks, we observed non-significant BCVA improvement (p = 0.90; p = 0.08). At 48 weeks, BCVA improvement was statistically significant (p < 0.001) with an average logMAR of 0.501 ± 0.37. During follow-up, we noted 69.4% of “good responders” at 16 weeks, while they reached 93.8% at 32 and 48 weeks. Mean logMAR BCVA values of IVB + MPL group at 16, 32 and 48 weeks are shown in Table 2.
Table 2.
Evolution of mean BCVA, mean CMT, mean IVB injections and mean MPL sessions in IVB + MPL group during the follow-up.
| Parameters | Baseline | 16 weeks | 32 weeks | 48 weeks |
|---|---|---|---|---|
| Mean BVCA (logMAR) Mean ± SD | 0.692 ± 0.35 | 0.689 ± 0.38 (p = 0.90) |
0.639 ± 0.33 (p = 0.08) |
0.501 ± 0.37 (p < 0.001) |
| Mean CMT (μm) Mean ± SD | 479.1 ± 14.3 | 409.8 ± 15.8 (p < 0.01) |
353.2 ± 17.2 (p < 0.01) |
289.6 ± 15 (p < 0.01) |
| Mean IVB injections (n) Mean ± SD | 0 | 3.4 ± 2,68 | 3.9 ± 1.16 | 4.1 ± 1.58 |
| Mean MPL sessions (n) | 0 | 1 | 1.37 | 1.41 ± 0.37 |
BVCA, best-corrected visual acuity; CMT, central macular thickness; IVB, intravitreal injections of bevacizumab; MPL, micropulse laser; SD, standard deviation.
Significance at p ⩽ 0.05.
In IVB group, baseline logMAR BCVA was 0.598 ± 0.42 (range from 0.15 to 1.8). At 48 weeks, BCVA improvement was statistically significant (p < 0.001) with an average logMAR of 0.491 ± 0.32.
In both groups, variation between baseline and final BCVA was statistically significant (p < 0.001). Final BVCA improvement was greater in IVB + MPL group compared to IVB group but the difference was not statistically significant (p = 0.114). Comparison between IVB + MPL group and IVB group is shown in Table 3.
Table 3.
Comparison between both groups: variation in mean BVCA, variation in mean CMT and final mean number of IVB injections.
| Parameters | IVB + MPL group | IVB group | p value | |
|---|---|---|---|---|
| Mean BVCA (logMAR) Mean ± SD |
Baseline | 0.692 ± 0.35 | 0.598 ± 0.42 | 0.145 |
| Final | 0.501 ± 0.37 | 0.491 ± 0.32 | 0.091 | |
| Mean CMT (μm) Mean ± SD |
Baseline | 479.1 ± 14.3 | 359.9 ± 22.9 | 0.113 |
| Final | 289.6 ± 15 | 305.9 ± 0.38 | 0.082 | |
| Mean final IVB injections (n) Mean ± SD |
4.1 ± 1.5 | 7.2 ± 1.3 | <0.005 |
BVCA, best-corrected visual acuity; CMT, central macular thickness; IVB, intravitreal injections of bevacizumab; MPL, micropulse laser; SD, standard deviation.
Significance at p ⩽ 0.05.
Anatomic outcomes
In IVB + MPL group, mean CMT was 479.1 ± 14.3 μm at baseline and decreased significantly to 409.8 ± 15.8 μm at 16 weeks (p < 0.01), 353.2 ± 17.2 μm at 32 weeks (p < 0.01) and reached 289,6 ± 15 μm at 48 weeks (p < 0.01).
Mean CMT values of IVB + MPL group at 16, 32, and 48 weeks are shown in Table 2.
In IVB group, mean CMT was 359.9 ± 22.9 μm at baseline and decreased significantly to 305.5 ± 17 μm at 48 weeks (p < 0.01).
In both groups, variation between baseline and final CMT was statistically significant (p < 0.001). Final CMT reduction was greater in IVB + MPL group compared to IVB group but the difference was not statistically significant (p = 0.09). Comparison between IVB + MPL group and IVB group is shown in Table 3.
Correlations between CMT and visual acuity are summarized below:
In IVB + MPL group: at baseline, p < 0.01; at 16 weeks, p = 0.07; at 32 weeks, p = 0.103 and at 48 weeks, p = 0.09.
In IVB group: at baseline, p < 0.01; at 16 weeks, p = 0.09; at 32 weeks, p = 0.164 and at 48 weeks, p = 0.113.
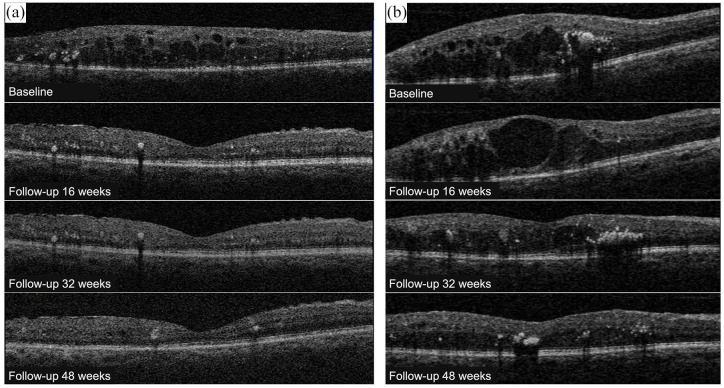
An example of CMT and BCVA evolution in 2 patients (one good responder at 16 weeks receiving a total of 1 MPL session, and one bad responder at 16 weeks receiving a total of 2 MPL sessions) is illustrated in Figure 2.
Figure 2.
SD-OCT follow-up of 2 patients from IVB + MPL group. Patient (a): Baseline examination: CMT = 388 μm and BCVA = 20/80. Follow-up 16 weeks (after 3 IVB + MPL): CMT = 280 μm and BCVA = 20/40 (good responder at 16 weeks). Follow-up 32 weeks (no IVB / no MPL): CMT = 278 μm and BCVA = 20/32 (good responder at 32 weeks). Final follow-up 48 weeks (no IVB / no MPL): CMT = 262 μm and BCVA = 20/25 (good responder at 48 weeks). Patient (b): Baseline examination: CMT = 489 μm and BCVA = 20/320. Follow-up 16 weeks (after 3 IVB + MPL + 1 IVB): CMT = 497 μm and BCVA = 20/400 (bad responder at 16 weeks). Follow-up 32 weeks (after 3 more IVB + second MPL): CMT = 375 μm and BCVA = 20/80 (good responder at 32 weeks). Final follow-up 48 weeks (after 2 more IVB): CMT = 271 μm and BCVA = 20/40 (good responder at 48 weeks).
IVB retreatment and complications
In IVB + MPL group, mean number of IVB injections was 4.1 ± 1.5 at last control (48 weeks). Only 3 eyes (6.2 %) required new injections at 32nd week control. Mean number of IVB injections of IVB + MPL group at 16, 32, and 48 weeks is shown in Table 2.
In IVB group, mean number of IVB injections was 7.2 ± 1.3 (range from 6 to 12) at last control. Twenty-two eyes (44.8%) required new injections at 32nd week control.
Number of injections was significantly lower in IVB + MPL group compared to control group (IVB group) (p < 0.005). Comparison between IVB + MPL group and IVB group is shown in Table 3.
The only ocular complication observed was subconjunctival haemorrhage in the injection site, in 12 eyes (12.2%). We did not record any cases of ocular inflammation.
We did not observe any case of progression from moderate to severe NPDR nor from severe NPDR to proliferative diabetic retinopathy (PDR). Besides, no patient underwent pan-retinal photocoagulation since there was no evidence of neovascularization on FA at baseline and last follow-up.
MPL retreatment and complications
Mean number of MPL sessions at last control was 1.41 ± 0.37 (range 1–3). Retreatment with MPL was performed in 20 eyes (40.8 %): retreatment once in 18 eyes (36.7%) and retreatment twice in 2 eyes (4.1%). Mean number of MPL sessions at 16, 32, and 48 weeks is shown in Table 2.
No patient complained about scotoma. There were nor laser scars on fundus photography, fundus autofluorescence nor SD-OCT.
Discussion
In our study, the combination therapy (IVB followed by MPL photostimulation) was as effective as IVB monotherapy in treating DME at 12 months follow-up. Adjuvant MPL decreased the number of IVB from 7.2 to 4.1 (p < 0.005). Anatomical and visual outcomes were comparable at 12 months control.
Previous large randomized studies using anti-VEGF injections (RESOLVE, RESTORE, READ-2, REVEAL, DRCRnet) showed a significant improvement in BCVA at 1 year.5,14–17 However, recurrences of macular edema and the necessity of reinjection remain the two major problems of this pharmacological treatment. Indeed, 30% of diabetic patients are non-responders to anti-VEGF alone.15–17
Laser photocoagulation has been considered as the gold standard treatment for clinically significant DME according to ETDRS2 but laser therapy has known side effects such as enlarged scars from impacts that can threaten visual function.14
In 2008, the DRCR network published the largest cohort of patients treated with laser since ETDRS14. They showed that laser results were better than we thought, compared to ETDRS. Laser allowed visual acuity (VA) stabilization during the first year with a slowly improvement over 3 years. Average VA gain at 3 years was close to 5 letters and 32% of patients had a gain of 10 letters or more at 2 years. This difference between both studies is explained by the different inclusion criteria and the better management of systemic factors in DRCRnet study.14 REVEAL study showed a significant improvement in VA (p < 0.0001) at 12 months with an average gain of 1.8 letters.15
Nowadays, micropulse laser is an alternative to the conventional continuous-wave laser for the treatment of retinal diseases.10,18 MLP efficiency and safety treating DME have been proven in different studies.10
In our study, we used the Iridex micropulse 577 nm yellow-light laser with confluent application of impacts, on a 5% duty cycle. The 577 nm yellow wavelength presents the peak of absorption of oxy-haemoglobin, which is advantageous in the treatment of diffusing microvascular anomalies such as microaneurysms.19 Moreover, it is not absorbed by the xanthophyll pigment, making it safe for the fovea.20 Subthreshold micropulse impacts are used to minimize retinal damage10,21 with a series of very brief micro-pulses22,23 followed by a longer phase with no laser exposure: “long relaxation phase”.24 The site of action of MPL is supposed to be at the level of retinal pigment epithelium.24,25 MPL therapy restores the oxidant/antioxidant balance within retinal layers and modulates programmed forms of cell death.26 Lately, a research study on humans showed a reduction in levels of VEGF associated to Müller cells function restoration, resulting of MPL therapy.25 Midena and colleagues25 suggested that reduction in inner nuclear layer thickness and changes in biomarker levels, secondary to MPL, would improve Müller cells metabolism and function. They showed that MPL is responsible for localized metabolic modifications, reducing the inflammation processes due to Müller cells activity, hence having a beneficial effect on Müller cells function.25
In our study, MPL was safe as reported in the literature.10,27–29 No patient developed laser scars on fundus photography, fundus autofluorescence nor OCT, even after retreatments. Vujosevic and colleagues27 reported no changes neither in fundus autofluorescence nor in microperimetry signal in the MPL group. Besides, these authors evaluated the effect of MPL on DME using OCT-Angiography. They reported more pronounced changes in the deep capillary plexus than in the superficial capillary plexus. Microvascular changes were observed starting from 3 months after MPL session.30
MPL therapy have some limitations. Treatment power is titrated individually for each patient with a risk of under-treatment since laser surgeon cannot see the MPL impact. In our protocol, MPL was applied with no spacing application of spots using a 2 x 2 or 4 x 4 treatment grid to cover the entire edematous area based on OCT. Nowadays, it is preferred to apply panmacular MPL treatment rather than focal treatment focused on the area involved by DME.31 However, since we included only diffuse types of DME, MPL was panmacular in most cases.
More recent studies evaluated the results of combined anti-VEGF treatment with MPL.32–34 Khattab and colleagues showed that this combined treatment may be effective and safe. It decreased the burden of aflibercept injection frequency with comparable anatomical and visual outcomes.32 Abouhussein and colleagues33 showed that 577 nm micropulse laser adjuvant to Aflibercept was effective for treatment-naive DME and was associated with decreased number of injections. The combined treatment compared to intravitreal injections alone allows reduction of annual injections frequency.32–34 Recently, Gawecki published a systematic review about subthreshold MPL combined with intravitreal injections in macular edema treatment.35 Analysing different studies about MPL and anti-VEGF in DME,32,34,36–39 author concluded that combining MPL would reduce the number of required intravitreal injections in cases of limited macular edema with non-inferior functional and morphological outcomes to those of anti-VEGF monotherapy, as observed in our study. Of course, author suggested that larger randomized trials were need to delineate the exact role of MPL in the treatment of DME.
On the other hand, we did not observe in our study any case of progression from moderate to severe NPDR nor from severe NPDR to PDR during the 1-year-follow-up, most probably because patients were treated with intravitreal anti-VEGF. Indeed, anti-VEGF shown effective reducing and treating retinal neovascularization in protocol S for patients with PDR.40,41
Our current study is limited by the relatively short period of follow-up, the absence of macular function tests and its retrospective nature.
Besides, our study has some other limitations. First, we included eyes with CMT ⩽ 500 μm, while a recent study has established that MPL is more efficient when CMT is lower than 400 μm42 and another study indicated that MPL would provide a statistically significant improvement in BCVA and a reduction in CMT in patients with a CMT of 300 μm or less,43 but the aforementioned results were not available yet during the recruitment of our study participants. However, we did indicate three monthly IVB doses initially, to eventually reduce CMT before the MPL session and enhancing MPL efficacy. Second, we included both eyes of some patients in the study, while statistical analysis would have been stronger if only eye per participant was included.
Generalized estimated acquisition (GEE) should have been implemented since both eyes of same patients were included. However, in this preliminary study we tried to look for correlations between the groups of 49 eyes treated with IVB + MPL and the group of 49 eyes treated with IVB. Nevertheless, we are planning to include more patients for a larger prospective study and we will certainly apply GEE in statistical analysis. Another drawback was related to the structural analysis on SD-OCT. We did not record the presence of microaneurysms (hyperreflective or hyporeflective) and hyperreflective intraretinal foci. Their presence or absence could have an influence on therapeutic responses to MPL. Neither did we record the presence of lesions within outer retinal layers at baseline and at the end of follow-up. Their integrity status could influence the functional outcomes in both groups.
Finally, the two groups were not perfectly matched. Patients in the IVB group were younger, with longer duration of diabetes, however there were more cases of severe NPDR in the MPL + IVB group. Besides, differences between both groups were not significant, and HbA1C was similar in both groups. Our study strength relies in its comparative design, adequate simple size and the choice to include only treatment-naïve patients.
Our results suggest that associating MPL with IVB therapy could be a safe combination with efficient outcomes treating DME for everyday practice. This work endorses larger and more prolonged prospective studies in this subject for the validity of the conclusion.
In conclusion, our therapeutic protocol with micropulse laser adjuvant to Bevacizumab injections in treatment-naive diabetic macular edema resulted in statistically significant functional and anatomical improvement at 12 months. In most cases, efficacy was delayed and observed starting from 32 weeks (8 months) with a possibility of retreatment with MPL.
The major outcome of this therapeutic protocol study is the advantage of having a satisfactory and lasting therapeutic response with a decreased number of injections and fewer recurrences. It could be an alternative treatment in cases not responding sufficiently to anti-VEGF therapy, patients presenting contraindications to anti-VEGF or patients unable to follow a long-term treatment for socio-economic reasons.
Acknowledgments
The authors thank Iridex company for providing micropulse laser 577 nm device used in the study.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics statement: This work was approved by the ethical board of Institut Hédi Rais d’ophtalmologie de Tunis (approval numberEC15/2019). All patients gave written informed consent for taking part in the study and having the study results submitted to a scientific journal for publication.
ORCID iD: Khaled El Matri  https://orcid.org/0000-0002-7939-3251
https://orcid.org/0000-0002-7939-3251
Contributor Information
Leila El Matri, Department B, Institut Hedi Rais d’ophtalmologie de Tunis, Tunis, Tunisia; Oculogenetic Research Laboratory (LR14SP01), Tunis, Tunisia; Faculté de médecine de Tunis, University de Tunis El Manar, Tunis, Tunisia.
Ahmed Chebil, Department B, Institut Hedi Rais d’ophtalmologie de Tunis, Tunis, Tunisia; Oculogenetic Research Laboratory (LR14SP01), Tunis, Tunisia; Faculté de médecine de Tunis, University de Tunis El Manar, Tunis, Tunisia.
Khaled El Matri, Department B, Institut Hedi Rais d’ophtalmologie de Tunis, Boulevard 9 avril 1938, 1006 Tunis, Tunisia; Oculogenetic Research Laboratory (LR14SP01), Tunis, Tunisia; Faculté de médecine de Tunis, University de Tunis El Manar, Tunis, Tunisia.
Yousra Falfoul, Department B, Institut Hedi Rais d’ophtalmologie de Tunis, Tunis, Tunisia; Oculogenetic Research Laboratory (LR14SP01), Tunis, Tunisia; Faculté de médecine de Tunis, University de Tunis El Manar, Tunis, Tunisia.
Zouheir Chebbi, Department B, Institut Hedi Rais d’ophtalmologie de Tunis, Tunis, Tunisia; Faculté de médecine de Tunis, University de Tunis El Manar, Tunis, Tunisia.
References
- 1.Bandello F, Battaglia Parodi M, Lanzetta P, et al. Diabetic macular edema. Dev Ophthalmol 2017; 58: 102–138, http://www.ncbi.nlm.nih.gov/pubmed/28351052 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 2.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. early treatment diabetic retinopathy study report number 1. Arch Ophthalmol 1985; 103: 1796–1806, http://archopht.jamanetwork.com/article.aspx?doi=10.1001/archopht.1985.01050120030015 (accessed 22 July 2020). [PubMed] [Google Scholar]
- 3.Schatz H, Madeira D, McDonald HR, et al. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol 1991; 109: 1549–1551, http://www.ncbi.nlm.nih.gov/pubmed/1755735 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 4.Guyer DR, D’Amico DJ, Smith CW.Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am J Ophthalmol 1992; 113: 652–656, http://www.ncbi.nlm.nih.gov/pubmed/1376019 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 5.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012; 119: 789–801, http://www.ncbi.nlm.nih.gov/pubmed/22330964 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 6.Chhablani J, Alshareef R, Kim DT, et al. Comparison of different settings for yellow subthreshold laser treatment in diabetic macular edema. BMC Ophthalmol 2018; 18: 168, https://bmcophthalmol.biomedcentral.com/articles/10.1186/s12886-018-0841-z (accessed 13 September 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai S, Bressler NM.Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: recent clinically relevant findings from DRCR.net Protocol T. Curr Opin Ophthalmol 2017; 28: 636–643, http://www.ncbi.nlm.nih.gov/pubmed/28837425 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 8.Antonetti DA, Klein R, Gardner TW.Diabetic retinopathy. N Engl J Med 2012; 366: 1227–1239, http://www.nejm.org/doi/10.1056/NEJMra1005073 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 9.Chen G, Tzekov R, Li W, et al. Subthreshold micropulse diode laser versus conventional laser photocoagulation for diabetic macular edema: a meta-analysis of randomized controlled trials. Retina 2016; 36: 2059–2065, http://www.ncbi.nlm.nih.gov/pubmed/27096529 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 10.Brader HS, Young LHY. Subthreshold diode micropulse laser: a review. Semin Ophthalmol 2016; 31: 30–39, http://www.ncbi.nlm.nih.gov/pubmed/26959127 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 11.The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015; 372: 1193–1203, http://www.nejm.org/doi/10.1056/NEJMoa1414264 (accessed 10 July 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017; 237: 185–222, http://www.ncbi.nlm.nih.gov/pubmed/28423385 (accessed 10 July 2021). [DOI] [PubMed] [Google Scholar]
- 13.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016; 123: 1351–1359, http://www.ncbi.nlm.nih.gov/pubmed/26935357 (accessed 10 July 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2008; 115: 1447–1449, 1449.e1–1449.e10, http://www.ncbi.nlm.nih.gov/pubmed/18662829 (accessed 22 July 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishibashi T, Li X, Koh A, et al. The REVEAL study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology 2015; 122: 1402–1415, http://www.ncbi.nlm.nih.gov/pubmed/25983216 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 16.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011; 118: 615–625, http://www.ncbi.nlm.nih.gov/pubmed/21459215 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 17.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010; 33: 2399–2405, http://www.ncbi.nlm.nih.gov/pubmed/20980427 (accessed 22 July 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scholz P, Altay L, Fauser S.A review of subthreshold micropulse laser for treatment of macular disorders. Adv Ther 2017; 34: 1528–1555, http://link.springer.com/10.1007/s12325-017-0559-y (accessed 13 September 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inagaki K, Ohkoshi K, Ohde S, et al. Comparative efficacy of pure yellow (577-nm) and 810-nm subthreshold micropulse laser photocoagulation combined with yellow (561-577-nm) direct photocoagulation for diabetic macular edema. Jpn J Ophthalmol 2015; 59: 21–28, http://www.ncbi.nlm.nih.gov/pubmed/25392274 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 20.Bandello F, Polito A, Del Borrello M, et al. “Light” versus “classic” laser treatment for clinically significant diabetic macular oedema. Br J Ophthalmol 2005; 89: 864–870, http://www.ncbi.nlm.nih.gov/pubmed/15965168 (accessed 22 July 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luttrull JK, Dorin G.Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: a review. Curr Diabetes Rev 2012; 8: 274–284, http://www.ncbi.nlm.nih.gov/pubmed/22587512 (accessed 22 July 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorin G.Evolution of retinal laser therapy: minimum intensity photocoagulation (MIP). Can the laser heal the retina without harming it? Semin Ophthalmol 2004; 19: 62–68, http://www.ncbi.nlm.nih.gov/pubmed/15590536 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 23.Lanzetta P, Dorin G, Pirracchio A, et al. Theoretical bases of non-ophthalmoscopically visible endpoint photocoagulation. Semin Ophthalmol 2001; 16: 8–11, http://www.ncbi.nlm.nih.gov/pubmed/15487692 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 24.Luttrull JK, Sinclair SH.Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina 2014; 34: 2010–2020, https://pubmed.ncbi.nlm.nih.gov/24837050/ (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 25.Midena E, Bini S, Martini F, et al. Changes of aqueous humor Müller cells’ biomarkers in human patients affected by diabetic macular edema after subthreshold micropulse laser treatment. Retina 2020; 40: 126–134, https://pubmed.ncbi.nlm.nih.gov/30300267/ (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 26.De Cillà S, Vezzola D, Farruggio S, et al. The subthreshold micropulse laser treatment of the retina restores the oxidant/antioxidant balance and counteracts programmed forms of cell death in the mice eyes. Acta Ophthalmol 2019; 97: e559–e567, http://www.ncbi.nlm.nih.gov/pubmed/30585429 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 27.Vujosevic S, Bottega E, Casciano M, et al. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010; 30: 908–916, http://www.ncbi.nlm.nih.gov/pubmed/20168272 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 28.Inagaki K, Ohkoshi K, Ohde S.Spectral-domain optical coherence tomography imaging of retinal changes after conventional multicolor laser, subthreshold micropulse diode laser, or pattern scanning laser therapy in Japanese with macular edema. Retina 2012; 32: 1592–1600, http://www.ncbi.nlm.nih.gov/pubmed/22466485 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 29.Nakamura Y, Mitamura Y, Ogata K, et al. Functional and morphological changes of macula after subthreshold micropulse diode laser photocoagulation for diabetic macular oedema. Eye 2010; 24: 784–788, http://www.ncbi.nlm.nih.gov/pubmed/19680274 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 30.Vujosevic S, Gatti V, Muraca A, et al. Optical coherence tomography angiography changes after subthreshold micropulse yellow laser in diabetic macular edema. Retina 2020; 40: 312–321, http://www.ncbi.nlm.nih.gov/pubmed/30418391 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 31.Keunen JEE, Battaglia-Parodi M, Vujosevic S, et al. International Retinal Laser Society guidelines for subthreshold laser treatment. Transl Vis Sci Technol 2020; 9: 15, http://www.ncbi.nlm.nih.gov/pubmed/32879771 (accessed 11 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khattab AM, Hagras SM, AbdElhamid A, et al. Aflibercept with adjuvant micropulsed yellow laser versus aflibercept monotherapy in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 2019; 257: 1373–1380, http://www.ncbi.nlm.nih.gov/pubmed/31127381 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 33.Abouhussein MA, Gomaa AR.Aflibercept plus micropulse laser versus aflibercept monotherapy for diabetic macular edema: 1-year results of a randomized clinical trial. Int Ophthalmol 2020; 40: 1147–1154, http://www.ncbi.nlm.nih.gov/pubmed/31919773 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]
- 34.Moisseiev E, Abbassi S, Thinda S, et al. Subthreshold micropulse laser reduces anti-VEGF injection burden in patients with diabetic macular edema. Eur J Ophthalmol 2018; 28: 68–73, http://www.ncbi.nlm.nih.gov/pubmed/28731494 (accessed 11 April 2021). [DOI] [PubMed] [Google Scholar]
- 35.Gawęcki M.Subthreshold diode micropulse laser combined with intravitreal therapy for macular edema – a systematized review and critical approach. J Clin Med 2021; 10: 1394, https://www.mdpi.com/2077-0383/10/7/1394 (accessed 12 July 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inagaki K, Hamada M, Ohkoshi K.Minimally invasive laser treatment combined with intravitreal injection of anti-vascular endothelial growth factor for diabetic macular oedema. Sci Rep 2019; 9: 7585, http://www.nature.com/articles/s41598-019-44130-5 (accessed 12 July 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thinda S, Patel AP, Hunter AA, et al. Combination therapy with subthreshold diode laser micropulse photocoagulation and intravitreal anti-vascular endothelial growth factor injections for diabetic macular edema. Invest Ophthalmol Vis Sci 2014; 55: 6363, https://iovs.arvojournals.org/article.aspx?articleid=2272060 (accessed 12 July 2021). [Google Scholar]
- 38.Luttrull JK, Sramek C, Palanker D, et al. Long-term safety, high-resolution imaging, and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina 2012; 32: 375–386, http://www.ncbi.nlm.nih.gov/pubmed/21971077 (accessed 12 July 2021). [DOI] [PubMed] [Google Scholar]
- 39.Kanar H, Arsan A, Altun A, et al. Can subthreshold micropulse yellow laser treatment change the anti-vascular endothelial growth factor algorithm in diabetic macular edema? A randomized clinical trial. Indian J Ophthalmol 2020; 68: 145–151, http://www.ijo.in/text.asp?2020/68/1/145/273237 (accessed 12 July 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2015; 314: 2137–2146, http://www.ncbi.nlm.nih.gov/pubmed/26565927 (accessed 11 July 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun JK, Glassman AR, Beaulieu WT, et al. Rationale and application of the Protocol S anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology 2019; 126: 87–95, https://pubmed.ncbi.nlm.nih.gov/30096354/ (accessed 11 July 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lois N, Gardner E, Waugh N, et al. Diabetic macular oedema and diode subthreshold micropulse laser (DIAMONDS): study protocol for a randomised controlled trial. Trials 2019; 20: 122, http://www.ncbi.nlm.nih.gov/pubmed/30755274 (accessed 11 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Citirik M.The impact of central foveal thickness on the efficacy of subthreshold micropulse yellow laser photocoagulation in diabetic macular edema. Lasers Med Sci 2019; 34: 907–912, http://www.ncbi.nlm.nih.gov/pubmed/30368640 (accessed 22 July 2020). [DOI] [PubMed] [Google Scholar]