Abstract
Introduction:
High-flow nasal cannula (HFNC) therapy in patients with hypoxemic respiratory failure due to COVID-19 is poorly understood and remains controversial.
Methods:
We evaluated a large cohort of patients with COVID-19-related hypoxemic respiratory failure at the temporary COVID-19 hospital in Mexico City. The primary outcome was the success rate of HFNC to prevent the progression to invasive mechanical ventilation (IMV). We also evaluated the risk factors associated with HFNC success or failure.
Results:
HFNC use effectively prevented IMV in 71.4% of patients [270 of 378 patients; 95% confidence interval (CI) 66.6–75.8%]. Factors that were significantly different at admission included age, the presence of hypertension, and the Charlson comorbidity index. Predictors of therapy failure (adjusted hazard ratio, 95% CI) included the comorbidity-age-lymphocyte count-lactate dehydrogenase (CALL) score at admission (1.27, 1.09–1.47; p < 0.01), Rox index at 1 hour (0.82, 0.7–0.96; p = 0.02), and no prior steroid treatment (0.34, 95% CI 0.19–0.62; p < 0.0001). Patients with HFNC success rarely required admission to the intensive care unit and had shorter lengths of hospital stay [19/270 (7.0%) and 15.0 (interquartile range, 11–20) days, respectively] than those who required IMV [104/108 (96.3%) and 26.5 (20–36) days, respectively].
Conclusion:
Treating patients with HFNC at admission led to improvement in respiratory parameters in many patients with COVID-19.
Keywords: COVID-19, high-flow nasal cannula, hypoxia, invasive mechanical ventilation, Mexico, SARS-CoV-2 pneumonia
Introduction
In the management of acute respiratory failure in patients with SARS-CoV-2 pneumonia, experts have agreed that following conventional oxygen therapy, the use of high-flow nasal cannula (HFNC) constitutes the next step in the therapeutic pyramid, before endotracheal intubation.1 HFNC has emerged as an alternative, non-invasive respiratory support option that can reduce the mortality rate as well as prevent or delay the need for intubation in patients with severe acute respiratory distress caused by SARS-CoV-2 pneumonia.2 However, despite evidence of the beneficial effect of HFNC use in patients with acute respiratory distress,3 at present, there is limited evidence on the efficacy of HFNC in patients with severe SARS-CoV-2 pneumonia.4 While some concerns have been raised regarding the risk of dispersion of SARS-CoV-2 through bio-aerosols,5 the evidence to date challenges the presumption of aerosol generating procedures.6,7 Some concerns have also been raised regarding the risk of delayed intubation resulting in worse outcomes.8 Based on the known usefulness of HFNC in patients with acute respiratory failure,3 it is crucial to determine the efficacy of this non-invasive respiratory support method to avoid the need for invasive mechanical ventilation (IMV) in patients with SARS-CoV-2.
The present study, which was performed at the temporary COVID-19 hospital located in the Citibanamex Exhibition Center in Mexico City, aimed to evaluate the efficacy of HFNC in patients with hypoxemic respiratory failure due to severe SARS-CoV-2 pneumonia to reduce the risk of requiring IMV. We also aimed to identify the risk factors of disease progression among patients with severe SARS-CoV-2 pneumonia and treated with HFNC.
Methods
Study design
This prospective observational study was conducted between 16 May 2020 and 9 November 2020 in accordance with the Declaration of Helsinki. The study protocol was approved by an independent Ethical Review Board at the National Autonomous University of Mexico (FM/DI/099/2020). All patients provided written informed consent prior to participation.
Patients
Patients aged ⩾18 years who were admitted to the temporary COVID-19 hospital with a confirmed diagnosis of COVID-19 [as verified by a positive polymerase chain reaction (PCR) test] and hypoxemic respiratory failure (PaO2 ⩽60 mmHg) due to severe SARS-CoV-2 pneumonia were included. For HFNC use, a respiratory rate of >24–30 breaths per minute, a PAFI ratio (PaO2/FiO2) of 100–200,9 and requiring a FiO2 > 50% to achieve an oxygen saturation (SpO2) of ⩾92% was necessary.
Procedures
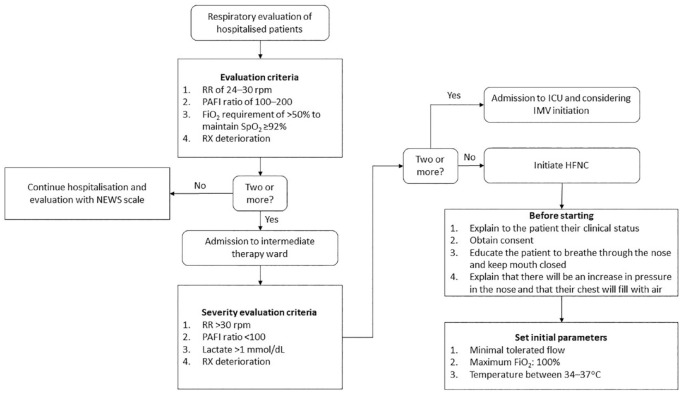
After admission, patients were initially evaluated for treatment with HFNC (Figure 1). In addition, patients were also assessed for HFNC according to the Rox index (see Supplemental Figure 1 online). The Rox index is a measure of hypoxemia severity that predicts the need for IMV.10,11 In this study, cut-off values of <2.85, <3.47, <3.85, and <4.88 after 2 hours, 6 hours, 12 hours, and 16 hours were considered failure of HFNC, as previously described.10 A Rox index of ⩾4.88 after 16 hours was considered HFNC success.
Figure 1.
Clinical protocol for the management of hypoxemic respiratory failure in the temporary COVID-19 Hospital.
FiO2, fractional inspired oxygen; HFNC, high-flow nasal cannula; ICU, intensive care unit; NEWS, National Early Warning Score; PAFI, ratio of PaO2 over FIO2; RR, respiratory rate; RX, radiography; SpO2, oxygen saturation.
HFNC (Precision Flow Plus, Vapotherm, New Hampshire, USA) was started with an oxygen flow rate of 40 l/min and FiO2 of 100%, humidified and heated to 34–37°C. Patients were instructed to keep their mouths closed to avoid loss of gas flow. To conform with local standards of infection control, the use of N95 masks, ocular protection, surgical gowns, and disposable gloves were mandatory for all healthcare personnel.
After the initial respiratory evaluation, patients who were admitted to the intermediate therapy ward and fulfilled two or more of the following criteria were admitted to the intensive care unit (ICU): (1) respiratory rate >30 rpm, (2) PAFI ratio <100, (3) lactate >1 mmol/dl, and (4) radiographic deterioration (Figure 1). Patients who were admitted to the ICU were then considered for intubation according to ICU admission criteria. Among patients who initiated HFNC, admission to the ICU was based on the Rox index evaluation as follows: patients whose Rox index was <3.47 at 6 hours after initiating HFNC after a first HFNC failure, patients whose Rox index was <3.85 at 12 hours after initiating HFNC, and patients whose Rox index was <4.88 at 16 hours after initiating HFNC (see Supplemental Figure 1 online).
Variables
We evaluated demographic and clinical characteristics, duration of symptoms prior to admission, duration of HFNC or IMV, length of hospital stay, Rox index measurements, and respiratory and blood gas parameters at the start of HFNC and 16 hours after HFNC. The success of HFNC was defined as the non-progression from HFNC to IMV, with a CALL score cutoff of >6 points used to stratify the risk of progression in patients with COVID-19.12
Statistical methods
A convenience sampling approach was used to select patients for inclusion in the study. Descriptive statistics were used for baseline demographic and clinical characteristics, with n (%) for categorical variables and median [interquartile range (IQR)] for continuous variables. The Wilcoxon rank-sum test was used to compare continuous data between patients who did or did not require IMV. A Chi-square or Fisher’s exact test was used to compare categorical data between the groups. Statistical significance was set at a p-value < 0.05.
Survival analysis (Cox regression) was fitted to calculate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for IMV among patients treated with HFNC. A multivariate survival analysis was performed, adjusting for the following confounders: age, sex, CALL score, diabetes, hypertension, obesity, Rox index at 1 hour, treatment with steroids, absolute lymphocytes, D-dimer, PAFI, lactate, and PaCO2. Only patients with complete clinical data were included in the survival analysis. Data were analyzed from the start of HFNC until failure (the need of IMV) or censure (discharge date). STATA v.15 (Stata Corp., College Station, TX, USA) and R version 3.6.3 were used for statistical analyses.
Results
Patients
The characteristics of patients included in this study are summarized in Table 1. A total of 378 patients were included in this study and data were collected between 16 May 2020 and 9 November 2020. The median (IQR) age was 54.5 (46–64) years, and 66.7% (n = 252) were male. The proportions of patients with type 2 diabetes, hypertension, and obesity were 35.5% (n = 134), 36.8% (n = 139), and 47.6% (n = 180), respectively.
Table 1.
Patient characteristics.
| Total | HFNC only | HFNC + IMV | p-value | |
|---|---|---|---|---|
| N = 378 | n = 270 | n = 108 | ||
| Age, years | 54.5 (46–64) | 53 (45–61) | 60 (51–70) | <0.0001 |
| Sex | 0.63 | |||
| Female | 126 (33.3) | 92 (34.1) | 34 (31.5) | – |
| Male | 252 (66.7) | 178 (65.9) | 74 (68.5) | – |
| Diabetes | 134 (35.5) | 92 (34.1) | 42 (38.9) | 0.38 |
| Uncontrolled diabetesa | 71 (53.0) | 44 (47.8) | 27 (64.3) | 0.08 |
| Glucose, n = 348 | 128 (106–172) | 124 (105.5–166.5) | 135 (107.5–187) | 0.26 |
| Hypertension | 139 (36.8) | 90 (33.3) | 49 (45.4) | 0.03 |
| Uncontrolled hypertensionb | 30 (7.9) | 17 (6.3) | 13 (12) | 0.30 |
| BMI (kg/m2) | 0.80 | |||
| Normal (18.5–24.9) | 43 (11.4) | 31 (11.5) | 12 (11.1) | – |
| Overweight (25.0–29.9) | 140 (37.0) | 101 (37.4) | 39 (36.1) | – |
| Obesity (⩾30) | 180 (47.6) | 124 (45.9) | 56 (51.9) | – |
| Unknown | 15 (4.0) | 14 (5.2) | 1 (0.9) | – |
| Charlson comorbidity index | <0.0001 | |||
| No comorbidities | 162 (42.9) | 130 (48.2) | 32 (29.6) | – |
| Low-risk category (1–2) | 178 (47.1) | 121 (44.8) | 57 (52.8) | – |
| High-risk category (⩾3) | 38 (10.1) | 19 (7.0) | 19 (17.6) | – |
| Duration of symptoms prior to admission, days | 8 (5–11) | 9 (6–12) | 6 (4–8.5) | <0.0001 |
| ⩽5 days | 109 (28.8) | 59 (21.8) | 50 (46.3) | <0.0001 |
| >5 days | 268 (70.9) | 210 (77.8) | 58 (53.7) | <0.0001 |
| Unknown | 1 (0.3) | 1 (0.4) | 0 (0.0) | <0.0001 |
| Time from admission to HFNC, days | 1.99 (2.4) | 1.8 (2.3) | 2.5 (2.6) | 0.02 |
| Duration of HFNC, (days) | 11 (4–16) | 13 (10–18) | 2 (1–3) | <0.0001 |
| Steroid treatment at hospitalization | 270 (71.4) | 211 (78.2) | 59 (54.6) | <0.0001 |
| ICU admission | 123 (32.5) | 19 (7.0) | 104 (96.3) | <0.0001 |
| ICU duration, days | 10 (5–19) | 3 (2–3) | 13 (7–20) | <0.0001 |
| Hospital duration, days | 18 (12–25) | 15 (11–20) | 26.5 (20–36) | <0.0001 |
Data are presented as n (%) or median (IQR)
Uncontrolled diabetes defined as glucose >180 mg/dl.
Uncontrolled hypertension defined as a blood pressure of >140/100 mmHg.
BMI, body mass index; HFNC, high-flow nasal cannula; ICU, intensive care unit; IMV, invasive mechanical ventilation; IQR, interquartile range.
Outcomes
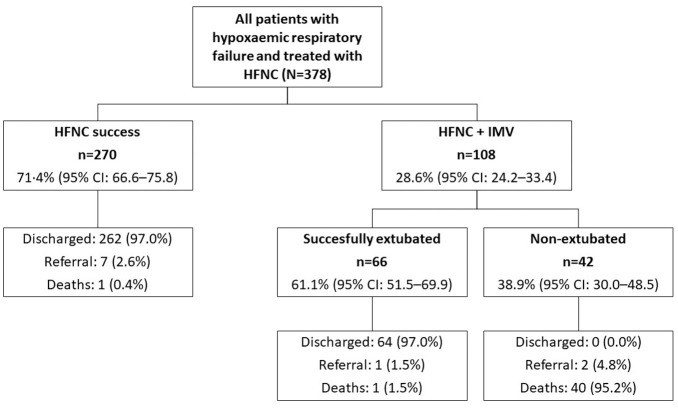
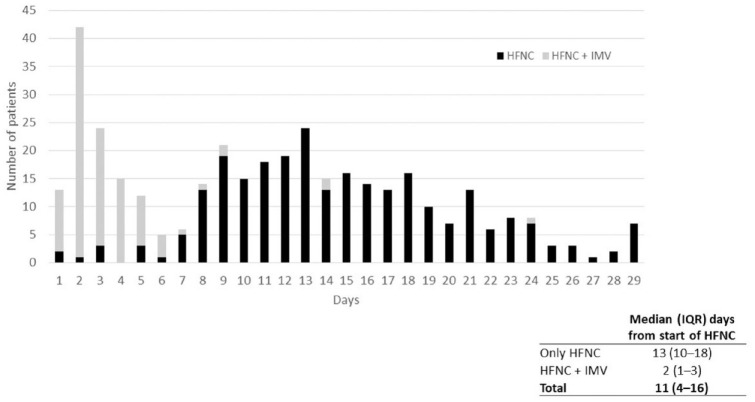
A flow chart of the outcomes of 378 patients with hypoxemic respiratory failure who were treated with HFNC is shown in Figure 2. The HFNC success rate, defined as patients who did not require IMV, was 71.4% (n = 270; 95% CI 66.6–75.8) compared with 28.6% (n = 108; 95% CI 24.2–33.4) of patients who required IMV. Of the 270 patients who were successfully treated with HFNC, 262 patients (97.0%) were discharged, seven patients (2.6%) were referred, and one patient (0.4%) died. Among those 108 patients who required IMV, 61.1% (n = 66; 95% CI 51.5–69.9) were successfully extubated. Of those 66 patients who required IMV and who were successfully extubated, 64 patients (97.0%) were discharged, and one patient (1.5%) died. In addition, the proportion of patients with HFNC success increased over time from when HFNC treatment was started (Figure 3). The median (IQR) number of days of HFNC administration was 13 (10–18) days in patients who had success compared with 2 (1–3) days in patients who then also required IMV.
Figure 2.
Flow chart of outcomes of patients treated with HFNC.
HFNC success was defined as the non-progression from HFNC to IMV.
HFNC, high-flow nasal cannula; IMV, invasive mechanical ventilation.
Figure 3.
Proportion of patients with HFNC success and failure over time.
*p-value < 0.0001.
HFNC, high-flow nasal cannula; IMV, invasive mechanical ventilation; IQR, interquartile range.
When comparing patients who required IMV and those who did not require IMV, age and the presence of hypertension were significantly different (p < 0.0001 and p = 0.03, respectively). The Charlson comorbidity index categories also differed significantly between these two groups (p < 0.0001) (Table 1). Patients who only received HNFC were treated for a significantly longer period of time versus those who eventually required IMV (13 versus 2 days, respectively; p < 0.0001). Patients with HFNC success rarely required admission to the ICU and had shorter lengths of hospital stay [19/270 (7.0%) and 15.0 days, respectively] than those who required IMV [104/108 (96.3%) and 26.5 days, respectively].
Laboratory results at admission and when HFNC was commenced are summarized in Table 2 and Supplemental Table 2 online. Patients who required IMV had less favorable laboratory parameters versus those who did not require IMV. It should be noted that there were significant differences at admission and when commencing HFNC in absolute lymphocyte counts (p < 0.01 and p < 0.0001, respectively). Although markers of inflammation (median D-dimer and ferritin levels) were not significantly different between patients who only required HFNC and those who also required IMV at admission (p = 0.32 and p = 0.69, respectively), they were significantly different when commencing HFNC (p < 0.01 and p = 0.03, respectively).
Table 2.
Laboratory results at admission and when HFNC was started.
| Total |
Only HFNC |
HFNC + IMV |
p-value | |
|---|---|---|---|---|
| N = 378 | n = 270 | n = 108 | ||
| Creatinine (mg/dl), n = 353 | ||||
| At admission | 0.9 (0.7–1.0) | 0.8 (0.7–1.0) | 0.9 (0.8–1.2) | 0.002 |
| At HFNC start | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) | 0.8 (0.6–1.2) | 0.85 |
| FiO2 (mmHg), n = 348 | ||||
| At admission | 35 (24–56) | 35 (24–53) | 32 (24–60) | 0.82 |
| At HFNC start | 45 (32–75) | 40 (30–60) | 63 (34–94) | <0.0001 |
| Lymphocyte (%) n = 362 | ||||
| At admission | 11.7 (6.9–18.6) | 12.4 (7.7–18.6) | 10.6 (6.3–17.4) | 0.06 |
| At HFNC start | 11.5 (7.4–19.1) | 12.5 (8.0–20.7) | 8.8 (5.5–16.1) | <0.0001 |
| D-dimer (ng/ml), n = 357 | ||||
| At admission | 550 (380–890) | 555 (390–940) | 550 (360–790) | 0.32 |
| At HFNC start | 580 (370–970) | 540 (350–860) | 735 (490–1145) | <0.0001 |
| Ferritin (µg/l), n = 279 | ||||
| At admission | 466.6 (227.3–836.8) | 466.6 (229.2–770.6) | 467.8 (219.8–961.7) | 0.69 |
| At HFNC start | 457.4 (249.9–780.7) | 438.4 (230.5–714.1) | 556.4 (305.2–971.7) | 0.03 |
Data are presented as median (interquartile range).
FiO2, fraction of inspired oxygen; HFNC, high-flow nasal cannula; IMV, invasive mechanical ventilation.
Rox index values were significantly higher at each time point in patients who did not require IMV versus those who required IMV (Figure 4). In patients with HFNC success, Rox index values increased from 5.98 at baseline to 6.41, 6.83, 7.02, 7.37, 7.87, and 8.20 after 1, 2, 4, 6, 12, and 16 hours, respectively. In contrast, in patients with HFNC failure, Rox index values remained low from 5.40 at baseline to 5.70, 5.88, 5.76, 5.93, 5.74, and 5.62 after 1, 2, 4, 6, 12, and 16 hours, respectively. When comparing the Rox index values between 2 hours and 16 hours, the difference was significant in the HFNC only group, but not in the HFNC and IMV group (p < 0.0001 and p = 0.91, respectively).
Figure 4.
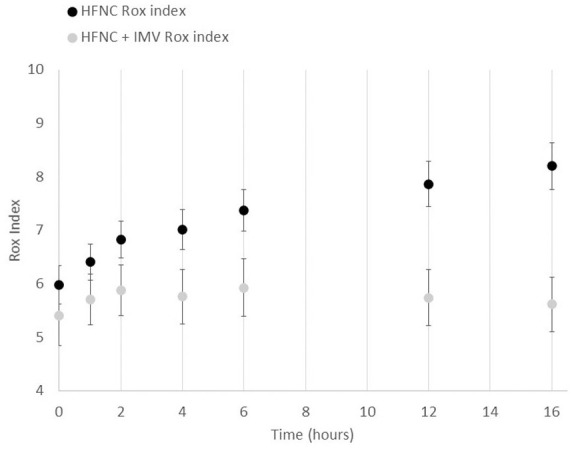
Rox index in patients with HFNC success and failure.
Rox index values at different time points in patients who did not require IMV (success of HFNC; black dots) versus those who required IMV (failure of HFNC; grey dots) among patients with hypoxemic respiratory failure who underwent treatment with HFNC.
HFNC, high-flow nasal cannula; IMV, invasive mechanical ventilation.
The changes in arterial blood gas parameters from 2 hours after HFNC to 16 hours after HFNC are summarized in Supplemental Table 3 online. In patients with HFNC success, the median (IQR) ratio of SpO2 over FiO2 (SPFI) increased from 135.7 (115.3, 160.0) after 2 hours to 158.4 (127.2, 192.2) after 16 hours of HFNC. In patients with HFNC failure, the median (IQR) SPFI ratio decreased from 115.0 (98.0, 140.0) after 2 hours to 110.4 (96.5, 134.9) after 16 hours of HFNC. When comparing SPFI ratios between 2 hours and 16 hours, the difference was only significant in the HFNC only group and not in the HFNC and IMV group (p < 0.0001 and p = 0.34, respectively).
Finally, we show that the CALL score at admission (adjusted HR 1.27; 95% CI 1.09–1.47; p < 0.01), Rox index at 1 hour (adjusted HR 0.82; 95% CI 0.70–0.96; p = 0.02), and absence of treatment with steroids (adjusted HR 0.34; 95% CI 0.19–0.62; p < 0.0001) were all significant predictors of HFNC failure (Table 3).
Table 3.
Predictors of HFNC failure (n = 238) (adjusted Cox regression model).
| Variable | Adjusted HR (95% CI) | p-value |
|---|---|---|
| Age (per year increase) | 1.003 (0.99–1.01) | 0.53 |
| Male versus female | 0.73 (0.41–1.32) | 0.28 |
| CALL score (per 1-point increase) | 1.27 (1.09–1.47) | <0.01 |
| No diabetes (versus uncontrolled) | 0.81 (0.43–1.51) | 0.50 |
| Diabetes (versus uncontrolled) | 0.46 (0.16–1.29) | 0.14 |
| Hypertension (versus no hypertension) | 1.08 (0.47–2.50) | 0.85 |
| Overweight (versus normal weight) | 1.05 (0.38–2.93) | 0.92 |
| Obesity (versus normal weight) | 1.49 (0.55–4.01) | 0.43 |
| Rox index 1 hour (per 1-point increase) | 0.82 (0.70–0.96) | 0.02 |
| Treatment with steroids (versus no treatment) | 0.34 (0.19–0.62) | <0.0001 |
| Absolute lymphocytes (per unit increase) | 0.99 (0.99–1.00) | 0.24 |
| D-dimer 550–1000 (versus normal levels) | 1.23 (0.66–2.27) | 0.51 |
| D-dimer > 1000–1500 (versus normal levels) | 1.52 (0.61–3.76) | 0.37 |
| D-dimer > 1500 (versus normal levels) | 0.31 (0.09–1.09) | 0.07 |
| PAFI (per unit increase) | 0.99 (0.99–1.00) | 0.94 |
| Lactate–mmol/l (per unit increase) | 1.25 (0.80–1.97) | 0.33 |
| PaCO2 (per unit increase) | 1.04 (0.98–1.11) | 0.18 |
CALL, comorbidity–age–lymphocyte–and LDH; CI, confidence interval; HFNC, high-flow nasal cannula; HR, hazard ratio; PAFI, ratio of PaO2 over FiO2
Discussion
The present study is one of the largest observational prospective studies to evaluate the efficacy of HFNC use in patients with severe SARS-CoV-2 pneumonia. Our results showed that the HFNC success rate of 71.4% significantly prevented escalation to IMV in patients with hypoxemic respiratory failure due to COVID-19. This finding is important, because if HFNC was not available at our temporary COVID-19 hospital, the majority of these patients would have required IMV, which is supported in the literature across several studies.13–17
CALL score at admission predicted a linear increase in the risk of IMV, with an HR of 1.27 for every point increase. The Rox index at 1 hour after starting HFNC predicted an 18% decrease in the risk of IMV for every point increase; in contrast, steroid treatment predicted a 66% decrease in the risk of IMV compared with the absence of steroid treatment.
In a multicenter prospective observational study of 293 consecutive patients with severe COVID-19-related hypoxemic respiratory failure in South Africa, the HFNC success rate (defined as the proportion of patients successfully weaned from HFNC) was 47% (137/293 patients), which is substantially lower than the 71.4% we report in the present study.13 It was also reported that the median duration of HFNC was significantly higher in those with HFNC success versus those with HFNC failure (p < 0.001). In a study of 28 consecutive patients with hypoxemic acute respiratory failure due to SARS-CoV-2 infection in Italy, the HFNC success rate (defined as a reversal of hypoxemia or SpO2 ⩾ 92%), was 67.8% (19/28 patients).4 However, it was reported that only 17.8% of patients subsequently required IMV (versus 28.6% in the present study), although the small number of patients (n = 28) in that study is likely to be a confounding factor. In a larger multicenter, retrospective cohort study conducted in Wuhan, HFNC failure (defined as upgrading respiratory support to positive pressure ventilation or death) was reported in 46.5% of patients. Among these patients, 13 (30.2%) subsequently required IMV, with failure of HFNC associated with a higher mortality rate.18 Finally, in a small study of eight patients with severe and critical COVID-19 in China, it was reported that after 2 hours of HFNC use, the Rox index was ⩾4.88 in 100% of patients and this remained above this cut-off for 12 hours.19 It was concluded that even in severe and critical patients who were experiencing hypoxemic respiratory failure, HFNC was successful. In a previous retrospective analysis of patients with moderate-to-severe hypoxemia due to highly suspected or proven COVID-19 infection who were treated with HFNC, 67 of 104 patients (64.4%) did not require IMV,20 which is similar to the HFNC success rate (71.4%) in the present study. Overall, the present study findings are consistent with those reported in these previous studies in terms of HFNC success.4,13,18–20 However, direct comparisons cannot be made because of differences in the study design and definitions of HFNC success between the studies.
When investigating factors associated with HFNC failure, the present study found that the CALL score at admission was a significant predictor of HFNC failure. In contrast, the Rox index at 1 hour (per 1-point increase) and prior treatment with steroids were significant predictors of HFNC success. Our findings are consistent with those of a previous study, which reported that a Rox index of 6 after HFNC commencement and the use of steroids were associated with HFNC success.13 The results of the RECOVERY trial on corticosteroid use in patients hospitalized with COVID-19 showed that early corticosteroid use (within ⩽7 days of admission) reduced mortality and ICU admissions in these patients; however, no significant difference was shown in IMV rates between early and later corticosteroid use.21 Similar to the present study, the severity of hypoxemia and lower oxygen saturation at admission were reported to be factors associated with HFNC failure in previous studies.4,18 Other factors, including C reactive protein level4 and male sex,18 have also been previously associated with HFNC failure.
There are many potential advantages of HFNC use, including efficacy, less training needed for health-care personnel, and lower cost compared with IMV. Furthermore, in hospitals with saturated critical care capacities, the early use of HFNC is likely to have a positive effect. In contrast, some of the potential barriers of HFNC use in low resource settings include the fact that the training of health-care personnel is needed, HFNC requires the cooperation of the patient for adequate use, and, as with IMV, there are limitations in the chain of supply.
The present study has some limitations, including those inherent to the observational, single-center study design and the retrospective analysis of patients according to the management/treatment algorithms, which may have introduced bias. In addition, we did not collect data on radiological implications.
Conclusions
In conclusion, the present study showed that 71.4% of patients with SARS-CoV-2 pneumonia and hypoxemic respiratory failure did not require IMV when treated with HFNC; this reinforces the benefits of the timely use of HFNC. HFNC led to improvement in respiratory parameters in many patients with COVID-19 and might reduce the length of stay in the hospital and the ICU. CALL score, the Rox index at 1 hour after starting HFNC, and the presence/absence of steroid treatment were identified as predictors of HFNC outcome. These results should be validated in prospectively registered, randomized controlled trials.
Supplemental Material
Supplemental material, sj-docx-2-tai-10.1177_20499361211042959 for High-flow nasal cannula therapy for hypoxemic respiratory failure in patients with COVID-19 by Adrian Palacios Chavarria, Erika Salinas Lezama, Mauricio Gonzalez Navarro, Rafael Ricardo Valdez Vazquez, Héctor Herrera Bello, Julieta Lomelín Gascon, Linda Morales Juárez, Mónica Arboleya Avendaño, Luis Esteban Ramirez Gonzalez, Rodrigo Ville Benavides, Renate Victoria Álvarez Wyssmann, Brenda Sandoval Ortiz, Mariana Lizbeth Rodríguez de la Cerda, Lidia Moreno Castañeda, Luis Alberto Martinez-Juarez, Héctor Gallardo-Rincón and Roberto Tapia-Conyer in Therapeutic Advances in Infectious Disease
Supplemental material, sj-jpg-1-tai-10.1177_20499361211042959 for High-flow nasal cannula therapy for hypoxemic respiratory failure in patients with COVID-19 by Adrian Palacios Chavarria, Erika Salinas Lezama, Mauricio Gonzalez Navarro, Rafael Ricardo Valdez Vazquez, Héctor Herrera Bello, Julieta Lomelín Gascon, Linda Morales Juárez, Mónica Arboleya Avendaño, Luis Esteban Ramirez Gonzalez, Rodrigo Ville Benavides, Renate Victoria Álvarez Wyssmann, Brenda Sandoval Ortiz, Mariana Lizbeth Rodríguez de la Cerda, Lidia Moreno Castañeda, Luis Alberto Martinez-Juarez, Héctor Gallardo-Rincón and Roberto Tapia-Conyer in Therapeutic Advances in Infectious Disease
Footnotes
Authors’ contributions: APC, ESL, MGN, RRVV, HHB, MAA, LERG, RVB, RVAW, BSO, MLRC, and LMC conceived and designed the study. HGR, JLG, LMJ, LAMJ, and RTC conceived and designed the study; drafted the manuscript; acquired, analyzed, and interpreted the data; and revised the manuscript for important intellectual content. All authors read and approved of the final version to be published and agree to be accountable for all aspects of the work.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Carlos Slim Foundation. We thank Michelle Belanger, MD and James Graham, PhD of Edanz Pharma, for providing medical writing support, which was funded by the Carlos Slim Foundation.
Conflict of interest statement: The Carlos Slim Foundation funded this study. APC, ESL, MGN, RRVV, HHB, LMJ, MAA, LERG, LMC, RVB, RVAW, BSO, and MLRC are full-time employees of the Temporary COVID-19 Hospital. HGR, JLG, LAMJ, and RTC are full-time employees of the Carlos Slim Foundation in Mexico. The authors declare no other conflicts of interest or outside funding from any other organizations.
ORCID iDs: Mauricio Gonzalez Navarro  https://orcid.org/0000-0001-5247-2083
https://orcid.org/0000-0001-5247-2083
Rodrigo Ville Benavides  https://orcid.org/0000-0003-2158-831X
https://orcid.org/0000-0003-2158-831X
Luis Alberto Martinez-Juarez  https://orcid.org/0000-0001-7550-7867
https://orcid.org/0000-0001-7550-7867
Hector Gallardo-Rincón  https://orcid.org/0000-0002-0811-4606
https://orcid.org/0000-0002-0811-4606
Availability of supporting data: Data will be made available upon reasonable request to the corresponding author.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Adrian Palacios Chavarria, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Erika Salinas Lezama, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Mauricio Gonzalez Navarro, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Rafael Ricardo Valdez Vazquez, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Héctor Herrera Bello, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Julieta Lomelín Gascon, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico; Fundación Carlos Slim, Miguel Hidalgo, Mexico City, Mexico.
Linda Morales Juárez, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Mónica Arboleya Avendaño, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Luis Esteban Ramirez Gonzalez, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Rodrigo Ville Benavides, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Renate Victoria Álvarez Wyssmann, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Brenda Sandoval Ortiz, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Mariana Lizbeth Rodríguez de la Cerda, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Lidia Moreno Castañeda, Temporary COVID-19 Hospital, Hipódromo de las Américas, Miguel Hidalgo, Mexico City, Mexico.
Luis Alberto Martinez-Juarez, Fundación Carlos Slim, Miguel Hidalgo, Mexico City, Mexico; London School of Hygiene and Tropical Medicine, London, UK.
Héctor Gallardo-Rincón, Fundación Carlos Slim, Lago Zurich 245, Presa Falcon Building (Floor 20), Col. Ampliacion Granada, Miguel Hidalgo, Mexico City, 11529, Mexico.
Roberto Tapia-Conyer, Fundación Carlos Slim, Miguel Hidalgo, Mexico City, Mexico; National Autonomous University of Mexico, Coyoacán, Mexico City, Mexico.
References
- 1.Cinesi Gómez C, Peñuelas Rodríguez Ó, Luján Torné M, et al. Clinical consensus recommendations regarding non-invasive respiratory support in the adult patient with acute respiratory failure secondary to SARS-CoV-2 infection. Arch Bronconeumol 2020; 56: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med 2020; 8: 433–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricard JD, Roca O, Lemiale V, et al. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Med 2020; 46: 2238–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vianello A, Arcaro G, Molena B, et al. High-flow nasal cannula oxygen therapy to treat patients with hypoxemic acute respiratory failure consequent to SARS-CoV-2 infection. Thorax 2020; 75: 998–1000. [DOI] [PubMed] [Google Scholar]
- 5.Whittle JS, Pavlov I, Sacchetti AD, et al. Respiratory support for adult patients with COVID-19. J Am Coll Emerg Physicians Open 2020; 1: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J 2020; 55: 2000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton F, Arnold D, Bzdek BR, et al. Aerosol generating procedures: are they of relevance for transmission of SARS-CoV-2? Lancet Respir Med 2021; 9: 687–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med 2015; 41: 623–632. [DOI] [PubMed] [Google Scholar]
- 9.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007; 132: 410–417. [DOI] [PubMed] [Google Scholar]
- 10.Roca O, Messika J, Caralt B, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care 2016; 35: 200–205. [DOI] [PubMed] [Google Scholar]
- 11.Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med 2019; 199: 1368–1376. [DOI] [PubMed] [Google Scholar]
- 12.Ji D, Zhang D, Xu J, et al. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis 2020; 71: 1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calligaro GL, Lalla U, Audley G, et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: a multi-centre prospective observational study. EClinicalMedicine 2020; 28: 100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically III patients with severe COVID-19. Am J Respir Crit Care Med 2020; 202: 1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin SM, Liu KX, Lin ZH, et al. Does high-flow nasal cannula oxygen improve outcome in acute hypoxemic respiratory failure? A systematic review and meta-analysis. Respir Med 2017; 131: 58–64. [DOI] [PubMed] [Google Scholar]
- 16.Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med 2019; 45: 563–572. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth 2020; 67: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia J, Zhang Y, Ni L, et al. High-flow nasal oxygen in coronavirus disease 2019 patients with acute hpoxemic respiratory failure: a multicenter, retrospective cohort study. Crit Care Med 2020; 48: e1079–e1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung 2020; 49: 444–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel M, Gangemi A, Marron R, et al. Retrospective analysis of high flow nasal therapy in COVID-19-related moderate-to-severe hypoxaemic respiratory failure. BMJ Open Respir Res 2020; 7: e000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho KS, Narasimhan B, Difabrizio L, et al. Impact of corticosteroids in hospitalised COVID-19 patients. BMJ Open Respir Res 2021; 8: e000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-2-tai-10.1177_20499361211042959 for High-flow nasal cannula therapy for hypoxemic respiratory failure in patients with COVID-19 by Adrian Palacios Chavarria, Erika Salinas Lezama, Mauricio Gonzalez Navarro, Rafael Ricardo Valdez Vazquez, Héctor Herrera Bello, Julieta Lomelín Gascon, Linda Morales Juárez, Mónica Arboleya Avendaño, Luis Esteban Ramirez Gonzalez, Rodrigo Ville Benavides, Renate Victoria Álvarez Wyssmann, Brenda Sandoval Ortiz, Mariana Lizbeth Rodríguez de la Cerda, Lidia Moreno Castañeda, Luis Alberto Martinez-Juarez, Héctor Gallardo-Rincón and Roberto Tapia-Conyer in Therapeutic Advances in Infectious Disease
Supplemental material, sj-jpg-1-tai-10.1177_20499361211042959 for High-flow nasal cannula therapy for hypoxemic respiratory failure in patients with COVID-19 by Adrian Palacios Chavarria, Erika Salinas Lezama, Mauricio Gonzalez Navarro, Rafael Ricardo Valdez Vazquez, Héctor Herrera Bello, Julieta Lomelín Gascon, Linda Morales Juárez, Mónica Arboleya Avendaño, Luis Esteban Ramirez Gonzalez, Rodrigo Ville Benavides, Renate Victoria Álvarez Wyssmann, Brenda Sandoval Ortiz, Mariana Lizbeth Rodríguez de la Cerda, Lidia Moreno Castañeda, Luis Alberto Martinez-Juarez, Héctor Gallardo-Rincón and Roberto Tapia-Conyer in Therapeutic Advances in Infectious Disease