Abstract
Background. Prader-Willi syndrome (PWS) is a rare neurodevelopmental disorder causing quality of life impairments such as insatiable hunger (hyperphagia) and obesity. We explored caregivers’ willingness to assume treatment risk in exchange for reduced hyperphagia according to a PWS-validated observer-reported outcome measure. Methods. We partnered with PWS patient organizations to develop a discrete-choice experiment exploring caregivers’ benefit-risk tradeoffs for emerging PWS treatments. The treatment benefit was a reduction in hyperphagia (as measured by a 0-, 5-, or 10-point change on the Hyperphagia Questionnaire for Clinical Trials [HQ-CT]). Treatment risks included weight gain (none, 5%, 10%), added risk of skin rash (none, 10%, 20%), and risk of liver damage (none, 1 in 1000, 10 in 1000). Preference models were estimated using mixed logistic regression and maximum acceptable risk. We explored differences in preferences across familial caregivers of patients with and without hyperphagia. Results. Four hundred sixty-eight caregivers completed the online survey. The majority of caregivers reported that patients experienced hyperphagia (68%) and half of patients experienced obesity (52%). Caregivers of patients without hyperphagia were willing to accept greater weight gain (16.4% v. 8.1%, P = 0.004) and a higher risk of skin rash (11.7% v. 6.2% P = 0.008) as compared to caregivers of patients with hyperphagia. Caregivers of patients with hyperphagia would accept a higher risk of liver damage as compared to caregivers of patients without hyperphagia (11.9 out of 1000 v. 6.4 out of 1000, P = 0.04). Conclusions. This research demonstrates that caregivers are willing to accept risk in exchange for a five-point improvement on the HQ-CT, a smaller marginal improvement than had been previously classified as meaningful. Patient experience with hyperphagia is a modifier in how much risk caregivers will accept.
Keywords: hyperphagia, patient preferences, patient-focused drug development, patient-reported outcome, Prader-Willi syndrome, rare diseases
Introduction
Prader-Willi syndrome (PWS) is a complex neurodevelopmental disorder, initially presenting with hypotonia, poor feeding, hypogonadism, and developmental delay.1–3 PWS results from lack of expression of paternally inherited imprinted genes on chromosome 15q11-q13, most commonly due to an interstitial deletion. It is usually sporadic and occurs in approximately 1 in 15,000 live births.4,5 The characteristic PWS behavioral phenotype includes cognitive rigidity, obsessive-compulsive behaviors, skin-picking, anxiety, temper outbursts, and other mental health issues.1–3 Patients with PWS have annual direct medical costs 8.8 times higher than patients without PWS,6 and the condition affects the quality of life of both the patient and their caregivers.7
Many individuals with PWS experience hyperphagia, which is an intense, incessant sensation of hunger. Hyperphagia induces an inability to control food intake, which represents a major impediment to full functioning for individuals with PWS. Hyperphagia in PWS is characterized by impaired satiety, food seeking behavior, preoccupation with food, and binge eating.8–10 Complications stemming from uncontrolled hyperphagia are the leading cause of death in PWS,8 often as a result of binge episodes that can lead to life-threatening gastric dilation, perforation, and necrosis.11,12 While hyperphagia is associated with obesity, it has an impact on patient and caregiver well-being that extends far beyond the effect of weight gain alone. Addressing hyperphagia was ranked as the highest priority in a study to identify priorities for PWS treatment endpoints among caregivers, above and beyond obesity.13 Despite the significant impact of hyperphagia on individuals with PWS, there are currently no approved treatment options targeting this aspect of the syndrome.14,15 At present, the only approach to manage the negative health outcomes associated with hyperphagia are through strict supervision and environmental controls to restrict access to food. Over the lifetime, these restrictions can reduce independence and quality of life.10 Growth hormone is the only US Food and Drug Administration (FDA)–approved treatment for PWS and it does not alter hyperphagia.7,16 As treatments with potential hyperphagia and weight loss benefit are in the pipeline,17 there is regulatory interest on the impacts of such treatments on not only biomedical health outcomes but also quality of life factors.
Numerous approaches have been used to measure health impairment associated with hyperphagia, including visual analog scales, behavioral observations, self- and caregiver-administered questionnaires, and an eye-tracking device.8,18,19 The Hyperphagia Questionnaire for Clinical Trials (HQ-CT) is considered the gold standard to measure hyperphagia by PWS-specialized clinicians.20 This caregiver-reported instrument was initially developed by Dykens and colleagues8 and has since been adapted for clinical trials with input from the FDA. It is suitable for reporting on PWS patients who have impaired cognitive functions.13 The HQ-CT generates a score ranging from 0 to 36 based on the caregiver’s assessment of the patient on nine food-related behaviors over a 2-week period.9
We sought to quantify familial caregivers’ preferences for PWS treatments and to assess if changes in hyperphagia would present a meaningful benefit by comparing equivalent changes in obesity and willingness to tolerate risk. Our work supports calls by patient advocacy groups for PWS who have advocated for hyperphagia as meaningful endpoint. A number of pharmacological agents are currently in clinical development for hyperphagia in PWS.14,17 Concerns persist regarding the meaningfulness of hyperphagia as a clinical endpoint and the potential risk associated with past therapies targeting hyperphagia.14,20 Given the uncertainty and risks in emergent treatment options for targeting hyperphagia, there is an urgent need to investigate the benefit-risk tolerance of the PWS community.
Methods
Community Engagement
A community advisory board (CAB) including patients (n = 2), caregivers (n = 6), and clinicians specializing in PWS (n = 2) oversaw the study. The CAB provided continuous guidance on the design, analysis, and dissemination of research.21,22 CAB members were engaged bimonthly via teleconferences and via email. We also engaged the PWS community more widely through presentations and other communications regarding methods, results, and publications.
Survey Design
A discrete-choice experiment (DCE) was used to examine the benefit-risk profile of the PWS caregivers. DCEs are an established method used by the FDA to investigate preferences for regulatory decision making.23 The preference elicitation was framed in the context of a clinical vignette where the respondents were told that the information presented in the choice tasks were 12-month clinical trial results from a hypothetical drug. The caregivers were given two hypothetical profiles and asked to choose which drug was better for their PWS family member. Twelve choice tasks were designed using a D-efficient design with zero priors.
Paired drug profiles in each task were constructed in consultation with the CAB to include relevant treatment benefits and risks. The survey described each attribute and its levels of risk prior to initiation to ensure that the caregivers fully understood the effects of the hypothetical drug. Benefits were described as improvement in hyperphagia (no improvement, 5-point improvement, 10-point improvement on the HQ-CT scale) and improvement in obesity (no improvement, 10% improvement, 20% improvement in body weight). Risks were defined by increased risk of skin rash (no additional risk, 10% higher risk, 20% higher risk) and risk of liver damage (no risk, 1 in 1000 risk, 10 in 1000 risk). Following good practices,24,25 a risk grid was used to present the effects of the benefits and risks. An example of the choice task is shown in Figure 1. Skin-picking is one of the self-mutilation behaviors in PWS and can result in life-threatening infections, severe scarring, as well as stress and shame in social conditions.26 Liver damage was described in the survey as a severe side effect, which can lead to irreversible liver damage and may result in the discontinuation of the drug. However, liver damage was not explicitly described as being associated with mortality.
Figure 1.
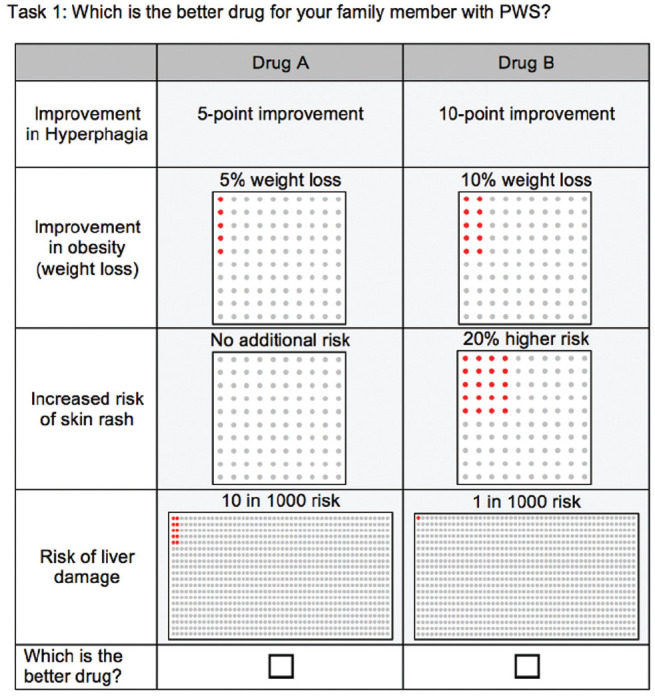
Choice task example in DCE.
Demographic and clinical questions for both the patient and the caregivers were included in the survey. A Likert-type scale was also used to evaluate five dimensions of the caregiver’s personality, including optimistic, health-conscious, risk-taker, numbers-savvy, and self-control. Caregivers also completed standard debriefing questions regarding the DCE,27 evaluating their experience with the DCE by indicating their agreement with three statements along a Likert-type scale, including the following: 1) I found it easy to understand the questions; 2) I found it easy to answer the questions; 3) My answers showed my real preferences.
PWS caregivers were recruited through PWS-CTC’s network to pretest and pilot the survey. The results of the pretest changed the wording, risk grid presentation, and design of the survey. The pilot ensured that the survey language was acceptable, that contents were relevant to the PWS community, and that PWS caregivers were able to follow the instructions and complete the survey.
Participants
We recruited familial, adult caregivers to participate in the survey as we sought to engage caregivers who were active participants in medical decision making. Individuals were eligible if they 1) were either a parent, grandparent, sibling, or legal guardian of a family member with PWS; 2) were involved in the decision-making process for the care and treatment of the person with PWS; and 3) had a family member with PWS who was 4 years or older. We limited the sample to caregivers of individuals 4 years or older since the main symptoms of PWS such as hyperphagia are not fully developed in younger children with PWS.28 Genetic subtype information was reported by the caregiver and was not independently confirmed by genetic testing.
The survey was disseminated through the digital platform on the PWS-CTC’s website, PWS Global patient registry, e-mail lists, blogs, and newsletters of the Foundation for Prader-Willi research (FPWR) and Prader-Willi syndrome Association (PWSA USA) and private PWS-associated Facebook groups. No compensation or incentives were provided to participate in the survey.
Statistical Analysis
Descriptive analysis was used to report characteristics of the caregiver respondents and their patient with PWS. For the primary analysis of the DCE, the dependent variable was the respondents’ choice within each choice task (i.e., drug profile a or b). The independent variables were the attribute levels offered in the profiles within each choice task. Separate mixed-logistic regression preference models were built for those with and without hyperphagia. Differences in scale between groups was explored using a heteroskedastic conditional logit to estimate a scale parameter, and differences in overall models and preference for individual attributes were compared using Wald and Chow tests, respectively. Attribute levels were anchored at zero to improve the data visualization of models.
We estimated maximum acceptable risk (MAR) models for each group to compare tolerance for changes in weight, liver damage, and skin picking in exchange for a 5-point improvement on the HQ-CT. MAR is an approach for identifying how much risk the respondent is willing to accept in exchange for a given treatment benefit and is routinely used in regulatory contexts to infer hypothetical tradeoffs between potential therapeutic benefits and risks.23,29
MAR compares the preference weights for risk attributes in comparison to one referent benefit attribute.30 In the current study, the benefit attribute was as a 5-point reduction in hyperphagia given the particular interest in this attribute to regulatory decision makers. In order to identify an equivalency between the benefit of hyperphagia reduction and changes in weight, which was presented in the DCE as weight loss, the weight attribute was reverse coded to reflect a risk rather than a benefit, in effect demonstrating weight gain. This reverse coding approach is consistent with standard practice.27 Differences in MAR across groups were compared using t tests.
Debriefing questions used to evaluate the DCE were compared across hyperphagia groups. Responses to debriefing questions were also compared to an a priori 75% threshold using a two-tailed Z-test, wherein a significant P value could indicate if the evaluation of the item was either significantly above or below the threshold.
Analyses were conducted in Stata version 16 (StataCorp, College Station, TX). Community engagement was deemed non–human subjects research (No. 7285), and the survey was deemed exempt from further human subject review (No. 7769) by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
Demographics
A total of 468 completed caregiver responses were included in the final analysis. Demographics and personality traits are documented in Table 1. Caregivers had a mean age of 49 years, with an age range of 22 to 83 years. The caregivers were predominantly parents (97%), female (84%), and Caucasian (87%). Seventy-one percent of caregivers had at least a bachelor’s degree, and 53% had an annual household income of $100,000 or more. Forty-seven percent of caregivers were at least somewhat familiar with the drug development process and the FDA’s regulatory approval process. The majority identified as optimistic (72%), health-conscious (84%), numbers-savvy (69%), and having self-control (71%). A third of caregivers were risk-taking (29%).
Table 1.
Caregiver and Patient Characteristics
| Total, N = 468 | Hyperphagia, n = 319 | No Hyperphagia, n = 149 | P Valuea | |
|---|---|---|---|---|
| Caregiver | ||||
| Caregiver age, mean (range) | 49.2 (22–83) | 51.6 (22–83) | 43.9 (25–74) | <0.001 |
| Parent | 97.4% | 96.6% | 99.3% | 0.077 |
| Gender: Woman | 83.5% | 83.3% | 83.9% | 0.88 |
| Race: White | 87.4% | 86.8% | 88.6% | 0.59 |
| Education: Bachelor’s degree or higher | 70.7% | 68.6% | 75.2% | 0.14 |
| Income: Above $100,000 annually | 59.3% | 54.2% | 69.6% | 0.006 |
| Personality features | ||||
| Optimistic | 72.3% | 70.3% | 76.5% | 0.16 |
| Health-seeking | 83.7% | 85.4% | 79.9% | 0.13 |
| Risk-taking | 28.7% | 29.2% | 27.5% | 0.71 |
| Good with numbers | 69.2% | 68.0% | 71.8% | 0.41 |
| Control-seeking | 71.2% | 71.3% | 70.9% | 0.93 |
| Familiar with regulatory process | 46.7% | 45.6% | 49.0% | 0.49 |
| Patient | ||||
| Patient age, mean (range) | 15.6 (4–54) | 18.0 (4–54) | 10.2 (4–47) | <0.001 |
| Genetic subtype | 0.036 | |||
| Deletion | 48.9% | 50.2% | 46.3% | |
| Uniparental disomy | 38.5% | 34.8% | 46.3% | |
| Privately insured | 68.8% | 65.2% | 76.5% | 0.014 |
| Symptoms | ||||
| Skin picking | 69.9% | 80.3% | 47.7% | <0.001 |
| Overweight/obesity | 51.5% | 64.9% | 22.8% | <0.001 |
Comparing hyperphagia versus no hyperphagia.
Caregivers reported that the mean age of PWS patients was 16 years, with an age range of 4 to 54 years. Patients were largely diagnosed through blood or DNA (99%), and most of their genetic subtypes were deletion (49%) or uniparental disomy (38%). Most of the patients (69%) used private insurance to pay for their PWS treatments.
The majority of caregivers (68%) reported that the individual with PWS experienced hyperphagia. Those caregivers reporting on patients with hyperphagia were older (P < 0.001), had a lower income (P = 0.006), and were less likely to be privately insured (P = 0.014) than those without hyperphagia. Patients with hyperphagia were older than those without hyperphagia (P < 0.001), consistent with a natural history study showing an average age of onset of “Phase 3” hyperphagia of 8 years old.31 Patients experiencing hyperphagia were also more likely to have symptoms such as skin picking (P < 0.001) and overweight/obesity (P < 0.001). They were also more likely to have a deletion genetic subtype and less likely to have uniparental disomy (P = 0.036).
Preference Models
Preference weights for the hyperphagia-stratified models are presented in Table 2 and visualized in Figure 2. When stratified by hyperphagia status, preference models estimated using mixed logistic were significantly different (P < 0.001). Differences in preference for attribute levels across the two groups were observed for the following: no improvement in hyperphagia (P = 0.03), no additional risk of skin rash (P = 0.01), 20% additional risk of skin rash (P = 0.01), 1 in 1000 risk of liver damage (P = 0.049), and 10 in 1000 risk of liver damage (P < 0.001). A scale difference was observed between the hyperphagia/no hyperphagia models, with those in the hyperphagia group having less consistency in their preferences (scale coefficient = −0.120, P = 0.047). Adjusting for differences in scale did not alone account for differences in preferences (P = 0.002).
Table 2.
Preference Weights by Patient’s Hyperphagia Statusa
| Hyperphagia, n = 319 | No Hyperphagia, n = 149 | P Value | |||
|---|---|---|---|---|---|
| Coeff. | SE | Coeff. | SE | ||
| Hyperphagia | |||||
| No improvement | −1.583** | 0.14 | −2.399** | 0.36 | 0.03 |
| 5 point improvement | 0.185** | 0.07 | 0.348* | 0.14 | 0.23 |
| 10 point improvement | 1.399** | 0.12 | 2.051** | 0.30 | 0.07 |
| Weight loss | |||||
| None | −1.273** | 0.12 | −1.088** | 0.17 | 0.85 |
| 10% | 0.357** | 0.05 | 0.332** | 0.09 | 0.73 |
| 20% | 0.915** | 0.09 | 0.756** | 0.14 | 0.99 |
| Risk of skin rash | |||||
| No additional | 1.088** | 0.11 | 1.697** | 0.27 | 0.01 |
| 10% additional | 0.341** | 0.06 | 0.227* | 0.11 | 0.65 |
| 20% additional | −1.429** | 0.11 | −1.925** | 0.26 | 0.01 |
| Risk of liver damage | |||||
| None | 0.926** | 0.11 | 1.327** | 0.27 | 0.12 |
| 1 in 1000 | 0.579** | 0.07 | 0.836** | 0.14 | 0.049 |
| 10 in 1000 | −1.505** | 0.14 | −2.163** | 0.36 | <0.001 |
Wald test assessing overall model difference: P < 0.001. Scale parameter significance: P = 0.047.
P < 0.001; *P < 0.05.
Figure 2.
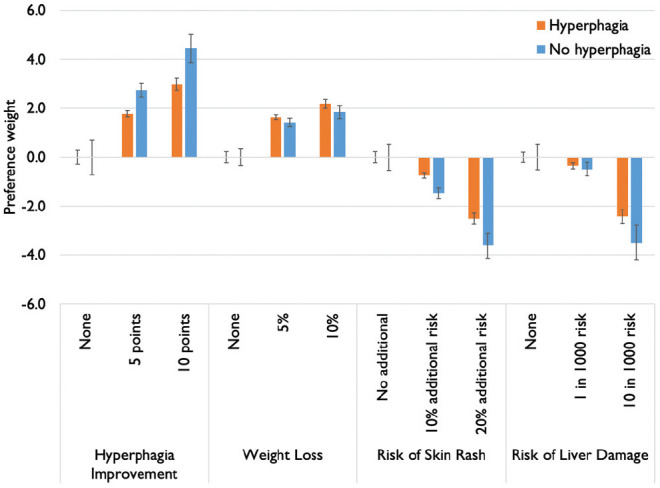
Preference weights, generated from mixed logistic regression stratified by experience of hyperphagia (n = 319 hyperphagia, n = 149 no hyperphagia).
Results of the maximal acceptable risk stratified by the hyperphagia groups are displayed in Figure 3. Caregivers of patients without hyperphagia would accept greater weight gain (16.4% v. 8.1%, P = 0.004) and a higher risk of skin rash (11.7% v. 6.2%, P = 0.008) as compared to caregivers of patients with hyperphagia. Caregivers of patients with hyperphagia would accept a higher risk of liver damage as compared to caregivers of patients without hyperphagia (11.9 out of 1000 v. 6.4 out of 1000, P = 0.04).
Figure 3.
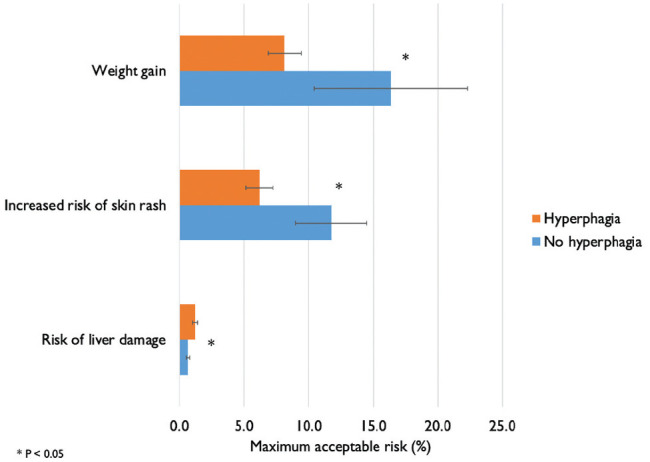
Maximal acceptable risk in exchange for a 5-point improvement in hyperphagia (bars are 95% confidence intervals).
Hyperphagia and no hyperphagia groups were equally likely to endorse the DCE as being easy to understand, answer, and consistent with preferences (Table 3). The DCE met the a priori 75% endorsement threshold for both groups across all evaluation criteria (P > 0.05 in two-tailed Z-test).
Table 3.
DCE Evaluation
| Hyperphagia, n = 315, % | No Hyperphagia, n = 148, % | P Valuea | |
|---|---|---|---|
| Easy to understand | 81.7b | 83.8b | 0.57 |
| Easy to answer | 67.3b | 66.9b | 0.93 |
| Consistent with my preferences | 80.4b | 82.3b | 0.62 |
Chi-squared, P value is between groups.
Item evaluation meets a priori 75% threshold, calculated using two-tailed Z-test.
Discussion
A national sample of caregivers of PWS patients expressed their willingness to accept risks in exchange for smaller degrees of improvement in hyperphagia than previously thought to be meaningful in this choice experiment. Although two-thirds of caregivers did not identify as risk-taking, preference findings indicated that caregivers would accept a higher risk in side effects of skin-picking and liver damage in exchange for improvements in hyperphagia.
Given the context and severity of the side effects, caregivers of patients with and without hyperphagia were still willing to accept risks of body weight increase, skin rash, and liver damage in exchange for a 5-point improvement in HQ-CT. This demonstrates that a 5-point improvement in HQ-CT may be a meaningful change in the caregivers’ perspective. A clinical trial evaluating an experimental drug in individuals with PWS indicated a 7.7-point improvement on the HQ-CT was determined to represent a meaningful change as identified using an anchor-based method that incorporated Caregiver Global Impression of Change.20
That preferences varied across caregivers reporting on patients with and without hyperphagia suggests that the lived experience with the condition influences the importance of the hyperphagia endpoint. Those without hyperphagia were willing to accept greater risk of weight gain and skin rash in exchange for treatments that reduced hyperphagia suggesting at first glance that those without hyperphagia may be more intent on reducing hyperphagia than those with the symptom itself. Because those without hyperphagia were younger families overall, most of whom expect to experience hyperphagia in the future, caregiver anticipation of the impact of hyperphagia and concern about management may contribute to the difference in risk tolerance. In general, people with hyperphagia were also more likely than those without hyperphagia to experience risks presented in the DCE such as having skin issues and obesity. These experiences might therefore have reduced the amount of risk that the group was willing to take on, ultimately suggesting that while hyperphagia is an important endpoint, it is not the only outcome that matters in PWS.
It is notable that parents would be willing to accept increased weight gain in exchange for hyperphagia improvement as based on MAR results. Obesity can result from hyperphagia and is associated with poorer health. This may reflect the perception of hyperphagia in the PWS community as encompassing a number of associated behaviors, including food seeking, stealing food, eating inappropriate food (e.g., uncooked, spoiled), temper outbursts when denied food, and repetitively asking for food. Caregivers prioritize the development of treatments to reduce hyperphagia and potentially mitigate these behaviors because they interfere with socialization in the community, job opportunities, independence, and family dynamics. These results demonstrate the importance of hyperphagia to parents irrespective of its impact on weight.
It is possible that preference differences may also be attributable to other differences observed across the hyperphagia and non-hyperphagia groups, such as patient age, having skin issues, and/or obesity. A limitation of the modeling approach of DCE is that it cannot be adjusted for covariates or account for baseline difference between groups, making it difficult to disentangle all possible sources of preference heterogeneity. The current study focused on differences in preferences based on hyperphagia given specific interest in this outcome from the PWS patient groups and the FDA. The effect of multilevel patient and caregiver factors on preferences for treatments that alleviate hyperphagia was recently explored by Lavelle and colleagues using a time tradeoff approach.32 This research demonstrated that even adjusting for numerous patient and clinical characteristics such as obesity, hyperphagia was an important endpoint for which caregivers would be willing to trade years of life to alleviate.
Scale differences were observed across hyperphagia and non-hyperphagia groups. Disaggregating scale and preference differences is not entirely possible as scale is confounded by utility.33 Because of this, we opted to use a mixed-logit model when estimating preferences, as this modeling approach allows for preference heterogeneity across individuals.34 Despite observed differences in scale, there was not a difference in how respondents evaluated their ability to answer in a way consistent with their preferences. This is notable given that scale heterogeneity is intended to reflect consistency across respondents and suggests that scale may be composed not only of within-person consistency (as the debriefing item reflected) but also within-group heterogeneity.
The current study mathematically conceptualized weight gain as the opposite of weight loss in order to conduct MAR modeling. Although this approach of reverse-coding attributes to calculate MAR is consistent with standard practice, it is possible that weight gain and weight loss have different utility to respondents rather than having nearly opposite utility as the analysis assumes. Given that the current study explored hypothetical treatments by asking respondents to make hypothetical tradeoffs more generally, this research is not meant to inform routine medical decision making.
Caregivers in the PWS community are uniquely qualified to provide critical insight to help advise the FDA on patient-focused drug development and patient-centered regulatory processes given that patients have cognitive issues that may prevent them from consenting to research, irrespective of age, let alone complete a DCE.35–37 This study is unique in the DCE literature as it focuses on an efficacy measure, as measured by an observer-reported outcome (ObsROs; the HQ-CT), and shedding light on what might be a minimal meaningful delta on a clinical trial endpoint. The use of the HQ-CT outcome measure in our DCE complements the importance of the psychometric properties of the measure. Just because a change is observable does not mean it is meaningful.38 Conversely, just because a concept is meaningful does not mean that a scale is well positioned to measure it. Using DCEs and other preference elicitation approaches may have a role in identifying the meaningfulness of measures/domains of measures. For the HQ-CT specifically, work is ongoing to refine the scale and quantify a meaningful change. This demonstrates the potential value of DCEs in designing and interpreting clinical trial data.
Patient-reported outcomes and ObsROs have been used to determine the endpoints in clinical trials. Patient preference information (PPI) is defined by the FDA as “qualitative or quantitative assessments of the relative desirability or acceptability to patients of specified alternatives or choices among outcomes or other attributes that differ among health interventions,”39 Included in these assessments may be the perspectives of patient partners (e.g., spouses, parents) and health care professionals who are involved in the care of the patient. In contrast, patient-reported outcomes are defined by the FDA as “any report of the status of the patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.” While patient-reported outcome instruments are designed to measure personal perceptions of health status, patient preference studies measure the tradeoffs that patients would be willing to make. The HQ-CT is an ObsRO that has been developed to evaluate the clinical effectiveness of hyperphagia in clinical trials.
The current study reflects the preferences of caregivers rather than patients. Individuals with PWS typically present with cognitive impairment including rigidity and social cognition deficits, which could affect their ability to understand the hypothetical scenarios of a stated-preference question. Furthermore, individuals with PWS are often unable to live independently and mostly live at home even as they age into adults.7 Caregivers in pediatric rare diseases have often been used as surrogates since they are often involved in the decision-process of the care and treatment approaches of their family member40 and the FDA acknowledges the role of patient partners, such as caregivers, in reporting on patient experiences. There is currently a paucity of experience in understanding patient perspectives in intellectually disabled populations, reflecting the challenges in collecting data from a population with cognitive deficits and expressive language difficulties. However, the perspective of the individuals who will participate in clinical trials and will ultimately be the consumers of new treatments for PWS is critical to elicit and understand, and represents an important area for future investigation.
We recruited PWS caregivers through online mediums such as PWS-CTC’s platform, patient registries, and Facebook groups. We recognize this online sampling approach may result in selection bias, as we may be more likley to recruit caregivers connected to the PWS community who are more tech-savvy and perhaps more resourceful. The large number of respondents from this patient community is a strength given the challenge of recruiting from rare disease populations.
Conclusion
We quantified PWS caregivers’ benefit-risk profile and demonstrated that PWS caregivers have a significant risk tolerance in exchange for improvements in hyperphagia. Through the choice experiment presented in this large, community-engaged, sample, caregivers of people with PWS expressed their willingness to accept risks in exchange for smaller degrees of improvement in hyperphagia than previously thought to be meaningful—namely, that a 5-point change in HQ-CT constituted meaningful benefit.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided entirely by International Consortium to Advance Clinical Trials for PWS. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
ORCID iD: Norah L Crossnohere  https://orcid.org/0000-0002-2811-1330
https://orcid.org/0000-0002-2811-1330
Contributor Information
Jui-Hua Tsai, Pharmerit International, Bethesda, Maryland.
Norah L. Crossnohere, Department of Biomedical Informatics, The Ohio State University College of Medicine, Columbus, Ohio.
Theresa Strong, Foundation for Prader-Willi Research, Walnut, California.
John F. P. Bridges, Department of Biomedical Informatics, The Ohio State University College of Medicine, Columbus, Ohio
References
- 1.Angulo MA, Butler M, Cataletto ME.Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest. 2015;38(12):1249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griggs JL, Sinnayah P, Mathai ML. Prader–Willi syndrome: from genetics to behaviour, with special focus on appetite treatments. Neurosci Biobehav Rev. 2015;59:155–72. [DOI] [PubMed] [Google Scholar]
- 3.Cohen M, Hamilton J, Narang I.Clinically important age-related differences in sleep related disordered breathing in infants and children with Prader-Willi syndrome. PLoS One. 2014;9(6):e101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstone AP.Prader-Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab. 2004;15(1):12–20. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy SB, Driscoll DJ.Prader-Willi syndrome. Eur J Hum Genet. 2009;17(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoffstall AJ, Gaebler JA, Kreher NC, et al. The high direct medical costs of Prader-Willi syndrome. J Pediatr. 2016;175:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayadjanian N, Schwartz L, Farrar E, Comtois KA, Strong TV.High levels of caregiver burden in Prader-Willi syndrome. PloS One. 2018;13(3):e0194655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dykens EM, Maxwell MA, Pantino E, Kossler R, Roof E.Assessment of hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring). 2007;15(7):1816–26. [DOI] [PubMed] [Google Scholar]
- 9.Goldstone A, Holland AJ, Butler JV, Whittington JE.Appetite hormones and the transition to hyperphagia in children with Prader-Willi syndrome. Int J Obes (Lond). 2012;36(12):1564–70. [DOI] [PubMed] [Google Scholar]
- 10.Heymsfield SB, Avena NM, Baier L, et al. Hyperphagia: current concepts and future directions proceedings of the 2nd International Conference on Hyperphagia. Obesity (Silver Spring). 2014;22(suppl 1):S1–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson DA, Heinemann J, Angulo M, et al. Gastric rupture and necrosis in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr. 2007;45(2):272–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J.Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet Med. 2017;19:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai JH, Scheimann AO, McCandless SE, Strong TV, Bridges JFP. Caregiver priorities for endpoints to evaluate treatments for Prader-Willi syndrome: a best-worst scaling. J Med Econ. 2018;21(12):1230–7. [DOI] [PubMed] [Google Scholar]
- 14.Miller JL, Strong TV, Heinemann J.Medication trials for hyperphagia and food-related behaviors in Prader-Willi syndrome. Diseases. 2015;3(2):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salehi P, Hsu I, Azen CG, Mittelman SD, Geffner ME, Jeandron D.Effects of exenatide on weight and appetite in overweight adolescents and young adults with Prader-Willi syndrome. Pediatr Obes. 2017;12(3):221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan Q, Orsso CE, Deehan EC, et al. Current and emerging therapies for managing hyperphagia and obesity in Prader-Willi syndrome: a narrative review. Obes Rev. 2020;21(5):e12992. [DOI] [PubMed] [Google Scholar]
- 17.US National Library of Medicine. Co-administration of tesofensine/metoprolol in subjects with Prader-Willi Syndrome (PWS) (2016-003694-18) [cited August 4, 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT03149445
- 18.Key AP, Dykens EM.Eye tracking as a marker of hyperphagia in Prader-Willi syndrome. Dev Neuropsychol. 2018;43(2):152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dykens EM, Roof E.Behavior in Prader-Willi syndrome: relationship to genetic subtypes and age. J Child Psychol Psychiatry. 2008;49(9):1001–8. [DOI] [PubMed] [Google Scholar]
- 20.McCandless SE, Yanovski JA, Miller J, et al. Effects of MetAP2 inhibition on hyperphagia and body weight in Prader-Willi syndrome: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2017;19(12):1751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollin IL, Young C, Hanson C, Bridges JFP, Peay H.Developing a patient-centered benefit-risk survey: a community-engaged process. Value Health. 2016;19(6):751–7. [DOI] [PubMed] [Google Scholar]
- 22.Janssen EM, Segal JB, Bridges JF.A framework for instrument development of a choice experiment: an application to type 2 diabetes. Patient. 2016;9(5):465–79. [DOI] [PubMed] [Google Scholar]
- 23.Ho MP, Gonzalez JM, Lerner HP, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29(10):2984–93. [DOI] [PubMed] [Google Scholar]
- 24.Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A.The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73(3):448-55. [DOI] [PubMed] [Google Scholar]
- 25.Tait AR, Voepel-Lewis T, Zikmund-Fisher BJ, Fagerlin A.The effect of format on parents’ understanding of the risks and benefits of clinical research: a comparison between text, tables, and graphics. J Health Commun. 2010;15(5):487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller JL, Angulo M.An open-label pilot study of N-acetylcysteine for skin-picking in Prader-Willi syndrome. Am J Med Genet A. 2014;164(2):421–4. [DOI] [PubMed] [Google Scholar]
- 27.Bridges JFP, Tsai JH, Janssen E, Crossnohere NL, Fischer R, Peay H. How do members of the Duchenne and Becker muscular dystrophy community perceive a discrete-choice experiment incorporating uncertain treatment benefit? An application of research as an event. Patient. 2019;12:247–57. [DOI] [PubMed] [Google Scholar]
- 28.Goldstone A, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M; Speakers Contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93(11):4183–97. [DOI] [PubMed] [Google Scholar]
- 29.Hauber AB, Fairchild AO, Johnson FR.Quantifying benefit–risk preferences for medical interventions: an overview of a growing empirical literature. Appl Health Econ Health Policy. 2013;11(4):319–29. [DOI] [PubMed] [Google Scholar]
- 30.Najafzadeh M, Schneeweiss S, Choudhry N, et al. A unified framework for classification of methods for benefit-risk assessment. Value Health. 2015;18(2):250–9. [DOI] [PubMed] [Google Scholar]
- 31.Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A. 2011;155A(5):1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavelle T, Crossnohere N, Bridges J.Quantifying the burden of hyperphagia in Prader-Willi syndrome using quality-adjusted life years (QALYs). Clin Ther. Published online June27, 2021. doi: 10.1016/j.clinthera.2021.05.013 [DOI] [PubMed] [Google Scholar]
- 33.Swait J, Louviere J.The role of the scale parameter in the estimation and comparison of multinomial logit models. J Market Res. 1993;30:305–14. [Google Scholar]
- 34.Crossnohere NL, Janse S, Janssen E, Bridges JF.Comparing the preferences of patients and the general public for treatment outcomes in type 2 diabetes mellitus. Patient. 2021;14(1):89–100. [DOI] [PubMed] [Google Scholar]
- 35.Peay H, Hollin I, Fischer R, Bridges JFP. A community-engaged approach to quantifying caregiver preferences for the benefits and risks of emerging therapies for Duchenne muscular dystrophy. Clin Ther. 2014;36(5):624–37. [DOI] [PubMed] [Google Scholar]
- 36.Mühlbacher AC, Juhnke C, Beyer AR, Garner S.Patient-focused benefit-risk analysis to inform regulatory decisions: the European Union perspective. Value Health. 2016;19(6):734–40. [DOI] [PubMed] [Google Scholar]
- 37.Crossnohere NL, Fischer R, Crossley E, Vroom E, Bridges JFP. The evolution of patient-focused drug development and Duchenne muscular dystrophy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:57–68. [DOI] [PubMed] [Google Scholar]
- 38.Weinfurt KP.Clarifying the meaning of clinically meaningful benefit in clinical research: noticeable change vs valuable change. JAMA. 2019;322(24):2381–2. [DOI] [PubMed] [Google Scholar]
- 39.Center for Disease and Radiological Health. Patient preference information—voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and de novo requests, and inclusion in decision summaries and device labeling. Guidance for Food and Drug Administration Staff, and Other Stakeholders [cited August 4, 2021]. https://www.fda.gov/media/92593/download
- 40.Hollin I, Peay H, Bridges J.Caregiver preferences for emerging Duchenne muscular dystrophy treatments: a comparison of best-worst scaling and conjoint analysis. Patient. 2015;8(1):19–27. [DOI] [PubMed] [Google Scholar]


