Abstract
Oral immunotherapy (OIT) has emerged to build sustained unresponsiveness or tolerance in patients with egg allergy. However, it is important to increase compliance and ensure safety because OIT requires an extended period of time and has a risk of side effects like anaphylaxis. We aimed to show the feasibility and safety of OIT during the build-up phase using a home-based, up-dosing method in children with egg allergy. Sixteen patients aged 4 to 12 years with egg allergy were enrolled. Patients increased the dose of boiled egg white (EW) by 5% per day at home and 25% per month at the hospital, with a target dose of 40 g of boiled EW (4.0 g of EW proteins). A historical control group (n = 16) was matched for age, sex, and clinical characteristics for comparisons with the OIT group. Oral food challenge (OFC) tests were performed after completing the build-up phase. In the OIT group, 93.8% (15/16) of patients achieved desensitization, with only 1 patient discontinuing OIT before the maintenance phase due to repeated allergic reactions. Mild allergic reactions and anaphylaxis occurred in 12 (75.0%) and 2 patients (12.5%), respectively. However, there were no significant adverse reactions such as serious anxiety or life-threatening events that required discontinuation of treatment. On the contrary, only 1 patient (6.3%) in the control group passed an OFC of 40 g of boiled EW during the same period (P < 0.001). Our results suggest that home-based up-dosing protocols using boiled eggs may be safe and feasible for the build-up phase of OIT in children with egg allergy.
Keywords: Food allergy, egg hypersensitivity, anaphylaxis, immunotherapy, immune tolerance, desensitization, allergens, safety
INTRODUCTION
Egg allergy occurs in about 1.2% of children under the age of 6 years in the United States and in 0.75% in Korea.1,2,3 Of importance, 17.5% and 17.3% of children in the United States and Korea, respectively, experience anaphylaxis that could lead to death.1,3 Although the most important management option of food allergy (FA) is the strict avoidance of all causative foods, dietary restriction is challenging for patients and their caregivers as eggs are ubiquitous in many foods.4
Therefore, oral immunotherapy (OIT) has emerged as a method to induce sustained unresponsiveness (SU) or tolerance for patients with long-lasting FAs.5 OITs begins with an initial escalation and a build-up phase in which food antigen intake is gradually increased.5 Once the target dose is reached, the maintenance phase of oral intake of food antigens is maintained for years.5 However, some patients experience psychological problems, such as severe anxiety, due to repeated adverse reactions during OIT.6 Recent studies have reported that up to 20% of patients undergoing OIT discontinue treatment, which supports the need for an OIT strategy to reduce side effects and improve compliance.7,8 Therefore, we aimed to demonstrate the feasibility and safety of a modified OIT for egg allergies in real-world practice.
MATERIALS AND METHODS
Study population
We enrolled 16 patients aged 4 to 12 years who were diagnosed with egg allergy and whose parents agreed to OIT. A diagnosis of FA was based on a positive oral food challenge (OFC) test or a convincing history (reproducible symptoms within 2 hours or anaphylaxis) within a year plus positive serum specific IgE (≥ 0.35 kU/L). We classified the allergic reactions reported according to affected organs: 1) skin or mucosal symptoms 2) gastrointestinal symptoms 3) respiratory symptoms and 4) anaphylaxis.9,10 We collected patients' clinical and demographic information, including symptoms after eating eggs, age at which OIT was initiated, duration of the build-up phase, and symptoms reported by patients or parents during OIT.
For the historical control group, we selected 16 patients who were diagnosed with egg allergy at our hospital between 2009 and 2017 and were matched for age, sex, levels of sIgE to egg white (EW), symptoms after eating eggs, and duration of follow-up similar to the build-up phase in the OIT group.
This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center, Seoul, Korea (IRB No. 2012-09-026, 2020-02-163, and 2020-05-135).
OIT protocol
At the hospital, ingestion of EW began with 0.1 g, followed by 0.5 g and 1 g at 20-minute intervals. Daily intake of boiled EW at home began with half of the maximum tolerated dose, increased by 0.1 g each week for the first 4 weeks and then by 0.1 g every 3 days to 1.0 g of boiled EW. Thereafter, ingestion of boiled EW increased by 0.1 g each day at home to 2.0 g of boiled EW. Dose was increased in 5% daily increments from 2.0 g of boiled EW at home and in 25% increments per month in the hospital to 40 g of boiled EW (4 g of EW protein). For preparation, the eggs were heated in cold water, and cooked for another 15 minutes after the water boiled. The amount of egg to be ingested was measured using an electronic scale that measured up to 2 decimal places. When allergic symptoms were experienced at home, the patients were given antihistamine or an epinephrine auto-injectors.
The severity of allergic reactions was classified according to the Consortium of Food Allergy Research (CoFAR) grading system.6 Desensitization was defined as no reaction during egg OFC with 40 g of boiled EW (4 g of EW protein). OFCs were performed to a total dose of 4 g of EW protein under the supervision of pediatric allergists.
Laboratory test
Before the start of OIT (T1) and after the build-up phase (T2), the children underwent clinical evaluations and blood tests. Levels of specific IgE (sIgE) and specific IgG4 (sIgG4) against EW and ovomucoid (OM) were measured using immunoCAP (ThermoFisher Scientific Inc., Waltham, MA, USA). Serum sIgG4 levels were measured in 10 and 9 patients in the OIT and control groups, respectively. The cutoff values of sIgE and IgG4 were 0.35 kU/L and 0.01 mg/L, respectively.
Statistical analysis
Statistical analysis was performed using SPSS version 25.0 (IBM Corp, Armonk, NY, USA). Data are expressed as median with range. Fisher's exact test and the Mann-Whitney U test were used to determine differences in baseline characteristics and laboratory data at T1 between the OIT and control groups. The desensitization rate was compared between the 2 groups using Fisher's exact test. The Wilcoxon signed rank test was performed to compare immunologic profiles between T1 and T2. The percent change of immunological profiles between T1 and T2 was calculated as and compared between the groups by the Mann-Whitney U test. Levels of sIgE antibodies higher than 100 kU/L and sIgG4 levels higher than 30 mg/L were assigned as 101 kU/L and 31 mg/L, respectively, for analyses. A P value less than 0.05 was considered significant.
RESULTS
Baseline characteristics of the subjects
For egg allergy, 75.0% (12/16) and 68.8% (11/16) of patients in the OIT group and the control group, respectively, were confirmed by positive OFC test (P = 0.780). There were no differences in age, sex, personal and family history of allergic diseases, combined FAs, symptoms after eating eggs, total IgE levels, or levels of specific IgE against EW between the OIT and control groups (all P > 0.05) (Table). In addition, no differences were found in personal history of allergic diseases, combined FAs, symptoms after eating eggs, total IgE levels, or levels of specific IgE against EW between patients confirmed by positive OFC and those with a convincing history in the OIT group (all P > 0.05).
Table. Baseline clinical characteristics of the subjects.
| Characteristics | OIT group (n = 16) | Control group (n = 16) | P value | |
|---|---|---|---|---|
| Male | 9 (56.3) | 9 (56.3) | 1.000 | |
| Age (yr) | 5 (4–11) | 5 (3–10) | 0.491 | |
| Comorbid conditions | ||||
| Atopic dermatitis | 11 (68.8) | 11 (68.8) | 1.000 | |
| Allergic rhinitis | 11 (68.8) | 12 (75.0) | 0.780 | |
| Asthma | 6 (37.5) | 7 (43.8) | 0.780 | |
| Family history of allergic diseases | 8 (50.0) | 8 (50.0) | 1.000 | |
| SCORAD | 12.0 (0–49.8) | 14.2 (6–33.7) | 0.300 | |
| Combined FAs | 15 (93.8) | 14 (87.5) | 0.780 | |
| Cow's milk | 13 (81.3) | 10 (62.5) | ||
| Wheat | 7 (43.8) | 6 (37.5) | ||
| Peanut | 1 (6.3) | 1 (6.3) | ||
| Tree nuts | 4 (25.0) | 7 (43.8) | ||
| Symptoms of egg allergy | 0.897 | |||
| Skin & mucosa | 2 (12.5) | 3 (18.8) | ||
| Gastrointestinal | 2 (12.5) | 2 (12.5) | ||
| Respiratory | 1 (6.3) | 0 (0.0) | ||
| Anaphylaxis | 11 (68.8) | 11 (68.8) | ||
| Total IgE (kU/L) | 618.0 (135.0–2,677.0) | 715.0 (180.0–5,001.0) | 0.599 | |
| sIgE to EW (kU/L) | 24.8 (2.8–101.0) | 25.6 (3.1–101.0) | 0.867 | |
| sIgG4 to EW (mg/L)* | 0.63 (0.05–6.36) | 0.77 (0.10–5.61) | 0.661 | |
Data are represented as number (%) or median (range).
OIT, oral immunotherapy; SCORAD, scoring of atopic dermatitis; FA, food allergy; sIgE, specific IgE; sIgG4, specific IgG4; EW, egg white; OM, ovomucoid.
*Serum sIgG4 levels were measured in 10 and 9 patients in the OIT and control groups, respectively.
Clinical responses and adverse reactions
In the OIT group, the median build-up phase was 7 (5–13) months and the median number of visits to the hospital was 6 (4–10). After completion of the build-up phase, 93.8% (15/16) of the patients obtained desensitization, with only 1 patient discontinuing the OIT before the maintenance phase due to repeated allergic reactions. During the build-up phase, allergic reactions of grade 1 and grade 2 (anaphylaxis) of the CoFAR occurred in 10 (62.5%) and 2 patients (12.5%), respectively, in the OIT group, but no patients required psychiatric intervention for anxiety or experienced severe adverse events (≥ grade 3 of the CoFAR). In the OIT group, 4 patients were able to consume eggs ad libitum 10 months after the maintenance period, and 11 subjects have ingested 40 g of boiled EW at least 4 times a week for 1–9 months of maintenance period without adverse events.
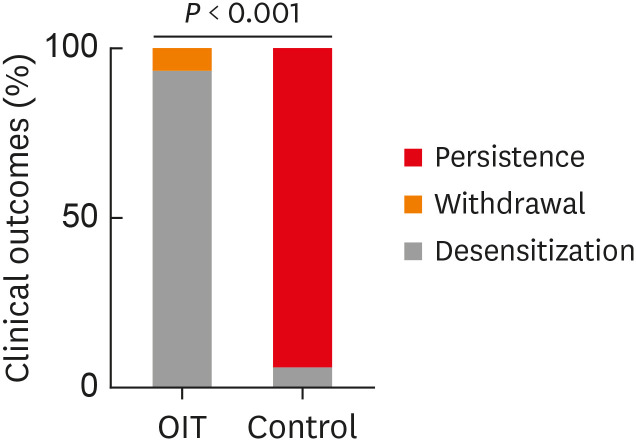
On the contrary, only 1 patient (6.2%) passed the OFC of 40 g of boiled EW in the control group (P < 0.001) (Fig. 1). In the control group, during the follow-up period (median 12 months, range 9–24 months), 12 patients (75.0%) showed allergic reactions to eggs including 4 cases of anaphylaxis, while 3 patients (18.8%) continued to avoid eggs without receiving OFCs due to fear of possible allergic reactions.
Fig. 1. Clinical outcomes after the build-up period in oral immunotherapy and control groups.
OIT, oral immunotherapy group; Control, control group; Persistence, persistence of egg allergy; Withdrawal, withdrawal of oral immunotherapy; Desensitization, egg allergy desensitization.
Immunological profiles
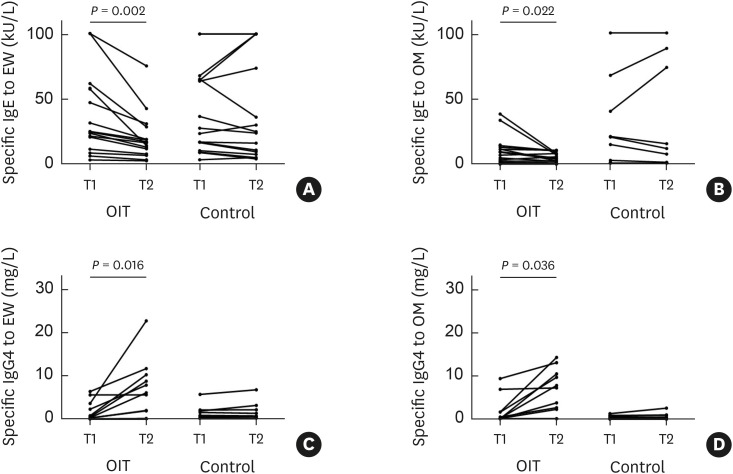
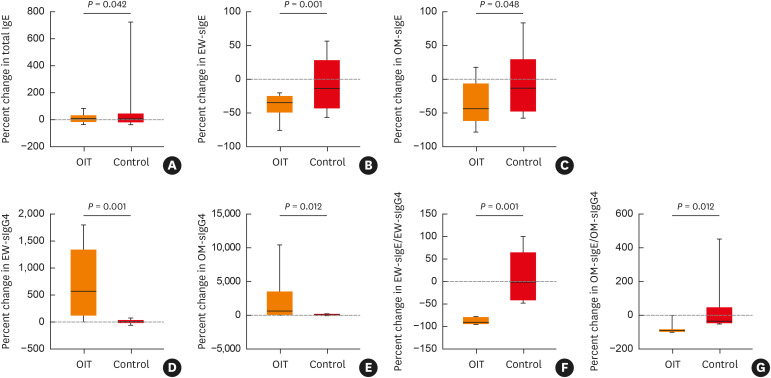
In the OIT group, levels of sIgE to EW and OM were significantly lower at T2 than at T1 (P = 0.002 and 0.022, respectively), while levels of sIgG4 to EW and OM were higher at T2 (P = 0.016 and 0.036, respectively) (Fig. 2). However, there were no differences in levels of EW-sIgE, OM-sIgE, EW-sIgG4, or OM-sIgG4 between T1 and T2 in the control group (all P > 0.05). No relevant changes in total IgE levels were found at T2 compared to baseline in either group (all P > 0.05). The percent changes in levels of total IgE, EW-sIgE, OM-sIgE, EW-sIgG4, OM-sIgG4, the ratio of EW-sIgE to EW-sIgG4, and the ratio of OM-sIgE to OM-sIgG4 were higher in the OIT group than in the control group (P = 0.042, 0.001, 0.048, 0.001, 0.012, 0.001, and 0.012, respectively) (Fig. 3).
Fig. 2. Levels of EW- and OM-specific IgE (A and B) and EW- and OM-specific IgG4 (C and D) at T1 and T2 in the OIT and control groups. T1 and T2 in the OIT group are at baseline and after the build-up phase, respectively, whereas the time between T1 and T2 in the control group refers to the elapsed period from diagnosis matched with the OIT group. Serum specific IgG4 levels were measured in 10 and 9 patients in the OIT and control groups, respectively.
EW, egg white; OM, ovomucoid; Ig, immunoglobulin; OIT, oral immunotherapy.
Fig. 3. Percent changes at the end of the build-up phase/follow-up from baseline in total IgE (A), EW-sIgE (B), OM-sIgE (C), EW-sIgG4 (D), OM-sIgG4 (E), the ratio of EW-sIgE to EW-sIgG4 (F) and the ratio of EW-sIgE to EW-sIgG4 (G) in the OIT and control groups. Serum sIgG4 levels were measured in 10 and 9 patients in the OIT and control groups, respectively.
Ig, immunoglobulin; sIg, specific immunoglobulin; OIT, oral immunotherapy group; Control, control group; EW, egg white; OM, ovomucoid.
DISCUSSION
Although OIT induces desensitization or SU in food-allergic patients, 30%–100% of patients experience adverse reactions during the build-up phase.10,11,12,13 In our preliminary study, we conducted conventional OIT using EW powder in 4 patients with egg allergy based on the protocol suggested by Burks et al.,6 and 3 of these cases have previously been reported.14 Unfortunately, 2 of 4 patients received psychiatric counseling due to anxiety, and one finally withdrew from OIT due to severe anxiety and repeated allergic symptoms. The median build-up phase was 14 (11–18) months, and the median number of hospital visits was 23 (16–25), which was longer and greater than those in the present study (all P = 0.002). In our current study using the modified protocol, we demonstrated safe increase in daily intake of boiled EWs at home and reduced number of hospital visits, leading to improved compliance during the build-up phase. Notably, no patients exhibited psychological problems, which was one of the biggest obstacles in maintaining OIT in our previous study.
The desensitization rate higher than 90% after the build-up phase in our study was not inferior to those from previous studies, which ranged from 55% to 94%.6,11,15,16,17 Immunological profiles in the present study showed patterns similar to those in other studies.11,12 These findings suggest that home-based up-dosing in the build-up phase of OIT can be safely applied in real-world practice while exhibiting a similar efficacy to the conventional method for treatment of egg allergy.
In a recent trial in Spain, children in an OIT group increased the amount of pasteurized EWs by 5% increase per day at home and 30% increase per week at hospitals with a target dose of 3.3 g of pasteurized EW proteins.11 After 1 year of follow‐up, 84.2% of patients in the OIT group showed desensitization, while only 16.0% of patients in the control group passed OFC. No serious allergic reactions of Sampson’s grade 5 were reported in that study. In a Japanese study, egg-allergic patients increased the amount of dried egg powder at home every 3–4 days for OIT, with 0.1 mg of dried egg powder as the starting dose.17 Out of 18 patients, 94.44% had allergic symptoms, but most of them were mild reactions. The cumulative tolerated dose of egg powder in the OIT group was increased up to 1.4–4.0 g except for 1 patient who rejected OFC 5–8 months after enrollment. In real-world practice, most caregivers do not want to evaluate SUs due to fear of a recurrence of egg allergy. For this reason, previous studies have also extended the maintenance period for up to 4–7 years without interruption of egg ingestion.16,18 Together with our findings, these results suggest that OIT methods should be further improved to reduce health care costs and to promote convenience leading to greater compliance. A key advantage of our protocol is the usage of boiled eggs, which led to greater compliance due to the ease of preparation and consumption at home compared to previous studies. In addition, our study showed high desensitization rate and low incidence of serious adverse reactions. Our results are clinically meaningful in that an alternative method has been proposed to overcome issues of OIT, such as adverse reactions and inconvenience. However, boiled eggs may affect the starting and target doses of OIT as well as the induction of SU due to their lower allergenicity compared to dry powder or raw eggs.19,20,21 In addition, 25% of the initial diagnoses of egg allergy in the current study were not based on OFC.
In conclusion, the home-based up-dosing protocol using boiled eggs can be used safely and effectively during the build-up phase of OIT for the treatment of egg allergy in real-world practice.
ACKNOWLEDGMENTS
This study was supported by SMC Research and Development Grant.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Park M, Kim D, Ahn K, Kim J, Han Y. Prevalence of immediate-type food allergy in early childhood in Seoul. Allergy Asthma Immunol Res. 2014;6:131–136. doi: 10.4168/aair.2014.6.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol. 2010;126:1105–1118. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141:41–58. doi: 10.1016/j.jaci.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowak-Węgrzyn A, Wood RA, Nadeau KC, Pongracic JA, Henning AK, Lindblad RW, et al. Multicenter, randomized, double-blind, placebo-controlled clinical trial of vital wheat gluten oral immunotherapy. J Allergy Clin Immunol. 2019;143:651–661.e9. doi: 10.1016/j.jaci.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Bird JA, Spergel JM, Jones SM, Rachid R, Assa'ad AH, Wang J, et al. Efficacy and safety of AR101 in oral immunotherapy for peanut allergy: results of ARC001, a randomized, double-blind, placebo-controlled phase 2 clinical trial. J Allergy Clin Immunol Pract. 2018;6:476–485.e3. doi: 10.1016/j.jaip.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, Chang HY, Kim SH, Yang MS, Koh YI, Kang HR, et al. A prospective observation of psychological distress in patients with anaphylaxis. Allergy Asthma Immunol Res. 2020;12:496–506. doi: 10.4168/aair.2020.12.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín-Muñoz MF, Belver MT, Alonso Lebrero E, Zapatero Remón L, Fuentes Aparicio V, Piquer Gibert M, et al. Egg oral immunotherapy in children (SEICAP I): Daily or weekly desensitization pattern. Pediatr Allergy Immunol. 2019;30:81–92. doi: 10.1111/pai.12974. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Rangel I, Rodríguez Del Río P, Escudero C, Sánchez-García S, Sánchez-Hernández JJ, Ibáñez MD. Efficacy and safety of high-dose rush oral immunotherapy in persistent egg allergic children: a randomized clinical trial. Ann Allergy Asthma Immunol. 2017;118:356–364.e3. doi: 10.1016/j.anai.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Dello Iacono I, Tripodi S, Calvani M, Panetta V, Verga MC, Miceli Sopo S. Specific oral tolerance induction with raw hen's egg in children with very severe egg allergy: a randomized controlled trial. Pediatr Allergy Immunol. 2013;24:66–74. doi: 10.1111/j.1399-3038.2012.01349.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim I, Lee BR, Kyung Y, Jung M, Yang HK, Kim M, et al. Three cases of oral immunotherapy for IgE-mediated egg allergy. Allergy Asthma Respir Dis. 2020;8:161–164. [Google Scholar]
- 15.Caminiti L, Pajno GB, Crisafulli G, Chiera F, Collura M, Panasci G, et al. Oral immunotherapy for egg allergy: a double-blind placebo-controlled study, with postdesensitization follow-up. J Allergy Clin Immunol Pract. 2015;3:532–539. doi: 10.1016/j.jaip.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Meglio P, Giampietro PG, Carello R, Galli E. Oral immunotherapy in children with IgE-mediated hen's egg allergy: follow-ups at 2.5 and 7 years. Allergy Rhinol (Providence) 2017;8:157–169. doi: 10.2500/ar.2017.8.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akashi M, Yasudo H, Narita M, Nomura I, Akasawa A, Ebisawa M, et al. Randomized controlled trial of oral immunotherapy for egg allergy in Japanese patients. Pediatr Int. 2017;59:534–539. doi: 10.1111/ped.13210. [DOI] [PubMed] [Google Scholar]
- 18.Jones SM, Burks AW, Keet C, Vickery BP, Scurlock AM, Wood RA, et al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol. 2016;137:1117–1127.e10. doi: 10.1016/j.jaci.2015.12.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim EH, Perry TT, Wood RA, Leung DY, Berin MC, Burks AW, et al. Induction of sustained unresponsiveness after egg oral immunotherapy compared to baked egg therapy in children with egg allergy. J Allergy Clin Immunol. 2020;146:851–862.e10. doi: 10.1016/j.jaci.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin M, Han Y, Ahn K. The influence of the time and temperature of heat treatment on the allergenicity of egg white proteins. Allergy Asthma Immunol Res. 2013;5:96–101. doi: 10.4168/aair.2013.5.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horino S, Kitazawa H, Satou T, Miura K. Hyperresponsiveness to boiled egg yolk in early life leads to prolonged egg allergy. Allergy Asthma Immunol Res. 2019;11:433–437. doi: 10.4168/aair.2019.11.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]