Abstract
Objective
Cerebellar neurodegenerative disorders (CDs) are a heterogeneous group of disorders. It is known that the cerebellum plays a role not only in motor, but also in cognitive and social cognitive functions. The aim of this study was to investigate social cognition in patients with different CDs.
Materials and Methods
Social cognition was examined in 34 patients, 12 with spinocerebellar ataxia type 1 (SCA1), 6 with spinocerebellar ataxia type 2 (SCA2), and 16 with idiopathic late onset cerebellar ataxia (ILOCA). All patients were clinically evaluated using the Scale for the Rating and Assessment of Ataxia. In addition, 34 age, sex, and education-matched healthy control (HC) subjects were similarly analyzed. Social cognition was studied using two tests: the Faux Pas Recognition Test and the Reading the Mind in the Eyes Test (RMET). An appropriate array of neuropsychological tests was used to assess the global cognitive status as well as the frontal functions and mood.
Results
CD patients achieved significantly worse results on both tests of social cognition compared to the HCs. The SCA1 + 2 group achieved the poorest results on the Faux Pas Recognition Test and exhibited poor performance on all cognitive tests, but was only significantly worse compared to the ILOCA group on the Free and Cued Selective Reminding Test (FCSRT) – recognition. The patients in the SCA1 + 2 and ILOCA groups obtained similar scores on RMET. In the SCA1 + 2 group the findings significantly correlated with clinical parameters of disease severity and duration and executive functions (EFs), and with mood and executive functions in the ILOCA group. In the SCA group EFs appeared as the only significant predictor of RMET achievement. The Boston Naming Test (BTN) was a significant predictor of the CD patients’ achievement on RMET, while the BTN, the Trail Making Test Part A and FCSRT – Delayed free recall predicted their performance on the Faux Pas Recognition Test.
Conclusion
Patients with CD have social cognitive impairments as demonstrated by the Faux Pas Test and the RMET test results. The SCA1 and 2 patients exhibited a more pronounced impairment compared with the ILOCA patients. The independent cognitive predictors of social cognition impairment were EFs and language.
Keywords: cerebellum, neurodegenerative disorder, spinocerebellar ataxia, idiopathic late-onset cerebellar ataxia, theory of mind, social cognition
Introduction
Cerebellar neurodegenerative disorders (CD) encompass a group of diverse disorders affecting the cerebellum and its pathways. Some of these disorders, such as autosomal dominant cerebellar ataxias or spinocerebellar ataxias (SCAs) are characterized by cerebellar signs, as well as additional non-cerebellar signs, including extrapyramidal features, pyramidal signs, peripheral neuropathy, and in some types of SCA, cognitive deficits (Shakkottai and Fogel, 2013; Sullivan et al., 2019). SCA1 and SCA2, the two types of cerebellar ataxias which are caused by trinucleotide expansion, represent the most common types in the Serbian population (Dragasević et al., 2006). Another group of ataxias is idiopathic late-onset cerebellar ataxias (ILOCA) or sporadic adult-onset ataxias, whose etiology still remains unknown. These disorders are non-hereditary and degenerative ataxias characterized by a slowly progressive cerebellar syndrome (Barbosa et al., 2016; Klockgether, 2018).
It is a well-known and widely reported fact that the cerebellum plays a vital role in motor functions (Glickstein, 1992; Manto et al., 2012). It is equally well-known that this role also extends to cognitive and behavioral functioning (Schmahmann, 1991, 1996; Middleton and Strick, 1994; Schmahmann and Sherman, 1997; Riva and Giorgi, 2000; Ravizza et al., 2006; Frings et al., 2007; Baillieux et al., 2008; Stoodley and Schmahmann, 2009; Thompson and Steinmetz, 2009; Stoodley, 2012; Koziol et al., 2014; Hoche et al., 2018). However, scientists still disagree as to the exact role of the cerebellum in cognition (Cooper et al., 2010). Some sources have suggested that different cognitive functions are localized to specific regions of the cerebellum (Cooper et al., 2010). Patients with cerebellar disturbances have significant and relevant deficits in the working memory, visuospatial, language, and executive function (EF) domains (Ahmadian et al., 2019; Silveri, 2020). Patients with different types of hereditary ataxias, especially SCA1 and SCA2, show impairments in EF, verbal memory, and attention (Bürk et al., 2001; Le Pira et al., 2002), while ILOCA patients show impairments in visuospatial function, verbal fluency, and mental flexibility (Rentiya et al., 2018). In addition, patients with degenerative ataxias exhibit changes in emotions (Adamaszek et al., 2017) and social cognition (SC) (Sokolovsky et al., 2010).
The ability to estimate mental states of other people is a fundamental aspect of social cognition (Van Overwalle et al., 2014). Social cognition primarily implies a basic, automatic level of recognizing and attributing emotions to others by immediate perception, which can be assessed by Reading the Mind in the Eyes Test (RMET) (Baron-Cohen et al., 2001; Serafin and Surian, 2004; Clausi et al., 2018). In addition to this, the theory of mind (ToM) is considered as a higher level of social interference, defined as the ability to attribute mental states to others and adopting the perspectives of other persons to understand and predict behavior (Premack and Woodruff, 1978; Van Overwalle et al., 2014). According to Baron Cohen, having “theory of mind” means being able to infer a full range of mental states (beliefs, desires, intentions, imagination, emotions), or the ability to reflect on the content of one’s own and others’ minds (Baron-Cohen et al., 1999). One of the tools commonly used to assess ToM is the Faux-Pas Recognition Test (Stone et al., 1998; Liverta Sempio et al., 2005; Clausi et al., 2018).
The cerebellum is incorporated in associative and paralimbic circuits which are involved in social cognition (Van Overwalle et al., 2014; Heleven and Van Overwalle, 2018). Our knowledge of how exactly the neuronal circuitry supports complex human behaviors remains insufficient; however, it is known that it includes fronto-limbic connections, the medial precuneus and medial prefrontal cortex, temporoparietal junction, superior temporal sulcus (Saxe and Kanwisher, 2003; Aichhorn et al., 2009; Van Overwalle and Vandekerckhove, 2013), orbitofrontal regions, dorsal and lateral prefrontal cortex (Abu-Akel and Shamay-Tsoory, 2011), the amygdala (Adolphs, 2004), and the insula (Kipps et al., 2007). Other important participants in social cognition are mirror neurons in the ventral premotor and rostral posterior parietal cortices (Rizzolatti et al., 2006) and middle temporal gyrus (Johnstone et al., 2006).
The cerebellum and its connections have recently been added to the neural network of social cognition. It has previously been shown that not only the patients with complex cerebello-cerebral degeneration, but also those with isolated cerebellar lesions, have specific impairments in social cognition abilities (Abel et al., 2007; Sokolovsky et al., 2010; D’Agata et al., 2011; Hoche et al., 2016). Moreover, imaging studied have shown evidence of cerebellar activation during ToM tasks (Brunet et al., 2000; Calarge et al., 2003; Stoodley, 2012; Van Overwalle et al., 2014; Clausi et al., 2018; Guell et al., 2018).
The aim of this study was to assess social cognition in patients with different hereditary and non-hereditary degenerative ataxias using social cognition tests. Our hypotheses were the following: (1) Patients with hereditary and non-hereditary cerebellar disorders would exhibit impairments on the Faux Pas Recognition Test and RMET compared with healthy controls. (2) Patients with SCA1 and SCA2 would exhibit a more pronounced impairment on the Faux Pas Recognition Test and RMET compared with ILOCA patients. (3) Patients with SCA1/SCA2 would exhibit different neuropsychological profiles to those with ILOCA. (4) Executive dysfunctions would be correlated with social cognition in both groups. (5) Executive dysfunctions would be a significant predictor of social cognition in both groups of patients.
Materials and Methods
Participants
Social cognition was studied in 34 patients [mean age/SD: 48.9/11.8 (years); mean education/SD: 12.7/2.1 (years); M/F: 20/14]: 18 with spinocerebellar ataxias (12 SCA1 and 6 SCA2 patients) and 16 ILOCA patients. All patients presented with diffuse cerebellar atrophy on MRI, while SCA1 and SCA2 patients further exhibited brainstem atrophy and generalized cortical atrophy, and genetic disorders were confirmed by genetic analyses. SCA1 and SCA2 patients carried a heterozygous CAG triplet expansion with 53.5 ± 5.2 repeats (range 48 to 63) in the coding ATXN1 region and CAG triplet expansion with 37.6 ± 1.5 repeats (range 36 to 40) in the coding ATXN2 region, respectively. The subjects were diagnosed and tested at the Neurology Clinic of the Clinical Center of Serbia (CCS) in 2017–2020. The patients were taking dietary supplements (vitamin E and selenium, Coenzyme Q10). None of the patients showed any major psychiatric disorders, anxiety or depressive traits, nor were they taking antidepressants or any other psychoactive drugs. In addition to the previously described body of patients with ataxias, 34 age, sex, and education-matched healthy control (HC) subjects were enrolled in the study. An appropriate battery of tests was used to assess global cognitive status, frontal neuropsychological functions, social cognition, and mood. All participants were informed of the purpose, procedures, and scope of all the examination and were included in the screening only after providing their written informed consent for participation.
Clinical Assessment
The patients were clinically evaluated using SARA (Scale for the Rating and Assessment of Ataxia), which assesses a range of different impairments in cerebellar ataxia (Schmitz-Hübsch et al., 2006). This Scale assesses the following aspects: truncal ataxia, speech disturbance and limb ataxia. It has eight categories with an accumulative score ranging from 0 (no ataxia) to 40 (most severe ataxia).
Social Cognition
Faux Pas Recognition Test
A social faux pas occurs when a speaker says something without considering that the listener might not want to hear it or might be hurt by what has been said, implying a false or mistaken belief (Baron-Cohen et al., 1999). In normal circumstances, people are usually able to recognize the faux pas and understand that the person who said something inappropriate did not know that that the listener would be upset by the faux pas. To understand faux pas the subject must identify wrong behavior in the context of predicted social interactions. The events that constitute a faux pas are unpredicted, and the subject needs to compare events and social expectations constantly while in no-faux pas stories the events are expected and require a low level of prediction.
We used a Serbian adaptation of the revised version of the faux pas stories (Baron-Cohen et al., 1999; orđević, 2013) to evaluate the ability to recognize a social faux pas. The detection of a faux pas requires both a cognitive understanding of false or mistaken beliefs and an appreciation of the emotional impact of a statement on the listener. Twenty stories were read to the participants, who were provided with copies of the stories to read along as the stories were read and check back over their contents. Ten of the stories involved a social faux pas (Faux Pas stories), and 10 were control stories in which no faux pas occurred (No-Faux Pas stories). After listening to each story, the subject was asked whether anyone had said anything they should not have said. When a faux pas was identified, specific clarifying questions were asked to verify the participant’s understanding of the mental and emotional state of the agent involved in the stories. Samples of Faux Pas and No-Faux Pas stories with respective questions are reported. Separate reports were made for the: (a) faux pas-related questions about the stories containing a faux pas, (b) control questions on the faux pas stories, (c) faux-pas-related questions on the control stories, and (d) control questions on the control stories. Separate reporting helped keep track of the type of errors the participants had made i.e., whether they had more faux-pas-related errors (ToM errors) or errors on the factual control questions. If a control question was answered incorrectly, other errors made in connection with that particular story were interpreted with caution. Each correctly answered question related to a Faux Pas story was scored as 1, yielding a maximum score of 6 for each correctly identified Faux Pas story and altogether 60 for the total of 10 Faux Pas stories. For each No-Faux Pas story a score of 2 was given if the subject correctly identified the absence of a faux pas. Two questions assessing the comprehension of the story material were asked after each story. The results were converted into percentages.
Emotion Recognition Test
To examine the participants’ ability to infer the mental states of others, we used a Serbian adaptation of the revised version of the Reading the Mind in the Eyes Test (RMET) (Baron-Cohen et al., 2001; orđević et al., 2017). The test evaluates the first stage (automatic) mentalizing and consists of 36 photographs of the actors’ eye region, each printed on a separate sheet of paper. Four adjectives corresponding to complex internal mental state descriptors were given.
The participants were required to identify the sex of the people in the pictures. They were also asked to decide which of the four words best described what the individuals in the photographs were thinking or feeling. Only one of the four words (the target word) correctly described the mental state of the person in the photograph. Although more than one word might have seemed applicable, the participants were instructed to choose only one, making sure to carefully read all four before making a decision. The answers were not timed. However, the participants were asked to complete the task as quickly as possible. The use of a dictionary was allowed in case of an unfamiliar word. For each correct answer, the participants received 1 point. The maximum number of points was 36. The test results were then divided by the maximum number of points (36) and converted into percentages.
Neuropsychological Screening
Cognitive functions were assessed using selected tests from a neuropsychological battery that included the following:
Global cognitive functioning was measured with the Addenbrooke’s Cognitive Examination –
Revised (ACE-R) (Mioshi et al., 2006). The test consists of 5 subscales designed to assess attention and orientation, fluency, language, visuospatial skills, and verbal memory. For the assessment of global functioning we used the total score. To test this aspect we also used the standard Mini-Mental State Examination (MMSE) (Folstein et al., 1975).
Learning and episodic verbal memory were assessed with the Free and Cued Selective Reminding Test (FCSRT) (Buschke, 1984; Grober et al., 1988) using the immediate total free recall (total number of items retrieved over 3 learning trials), delayed free recall (number of words freely recalled after a 30-min delay), and recognition (number of recognized words after delayed free and cued recall).
Orientation and attention were tested with the help of several subtests: the Orientation and attention subscale from the ACE-R; the Digit Span-subtest from the VITI (Vekslerov Individualni Test Inteligencije) (Pavlović, 2003), a Serbian adaptation of Wechsler’s Adult Intelligence Scale – Revised. The subjects were asked to repeat auditorily presented sequences of digits forward (up to 9 digits) and backward (up to 8 digits), and trials were administered until two failures of each span length. The total number of correct answers after the transformation into a scaled score was then used. To assess attention and psychomotor speed, we used the Trail Making Test Part A (TMT-A), a timed measure of selective attention to visually presented information (Lezak, 1995). The TMT-A consists of 25 circles distributed over a sheet of paper. The patients were asked to draw lines to connect them in ascending order as fast as possible.
Calculation was assessed using the Arithmetics-subtest from VITI (Pavlović, 2003), which consists of a series of verbally presented arithmetic questions of increasing difficulty. Transformed total scores were used.
Confrontation naming was assessed with the Boston Naming Test (BNT) using a raw score that represents the total of correctly named objects (spontaneously or after giving a semantic cue) (Kaplan et al., 1983).
Visuospatial processing was tested using the Hooper Visual Organization Test (HVOT) (Western Psychological Services, 1983). The total number of correct answers was recorded.
Executive functions were evaluated using several subtests: the Stroop Color and Word Test (SCWT) (Lezak, 1995). This test involves an interference task, requiring the naming of color ink, preventing the reading of words for which the color and meaning of the words are incongruent (for example, the word “red” written in green). The analysis includes the time (in seconds) required to complete the task. Verbal working memory was assessed using the Digit Ordering Test (DOT) (Cooper et al., 1991): a series of seven digits that has to be memorized and immediately recalled in ascending order. The total number of correct answers and the maximum forward range were used in the analysis. Letter fluency (number of words beginning with the letters S, K, and L produced in 60 s) and category fluency (number of animals produced in 60 s) (Lezak, 1995) were used as measures of verbal divergent thinking. The analysis included the total raw score for all three letters, the score for category fluency, and the corrected values for both of these measures. All tests were conducted by a qualified neuropsychologist in a standardized manner consistent across subjects.
Mood Assessment
Mood was evaluated with the help of two tests:
Depression Assessment
The Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960), which consists of 17 questions, was used to assess the patients’ levels of depression. The assessment, which was in the form of a structured interview, was conducted by a trained physician. Total scores vary from 0 to 53. For the HDRS, a score of 0–7 is generally accepted as being within the normal range, while a score of 20 or higher indicates at least moderate severity.
Anxiety Assessment
The Hamilton Anxiety Rating Scale (HARS) (Hamilton, 1959) was used to determine the presence of anxiety in our patients. The Scale is completed by a trained physician acting as the examiner, based on interviews held with patients. The Scale consists of 14 items which are individually rated from 0 (not present) to 4 (severe). Total scores vary from 0 to 56. Patients with score ≥13 were considered anxious.
Data Analysis
The assumption of normality of dependent variables (Faux Pas Recognition Test, RMET, neuropsychological tests, the duration of the disease, SARA, mood assessment and EF, and other neuropsychological functions tests) was determined based on the results of the Kolmogorov-Smirnov and Shapiro-Wilk tests, which indicate the application of non-parametric techniques (p < 0.05). The non-parametric Kruskal Wallis Test, with post hoc pairwise comparisons, was used to compare the three groups (SCA, ILOCA, and HC) in terms of socio-demographic variables, mood assessment tests, Faux Pas Recognition Test, RMET and neuropsychological tests. The Mann-Whitney U Test for independent samples was used to detect differences in the scores between the two CD groups, on variables assessed only on these groups, such as duration of the disease, clinical characteristics/SARA. The Spearman rank-order correlation coefficient was used to correlate the Faux Pas Recognition Test and RMET results with the duration of the disease, clinical characteristics/SARA, psychiatric tests and EF tests in all three groups (SCA1 + 2, ILOCA, HC). Multiple regression analysis was used to determine whether EFs qualify as significant predictors on the Faux Pas Recognition Test and RMET in SCA and ILOCA groups. Same analysis was used to determine whether all neuropsychological tests could significantly predict scores on the Faux Pas Recognition Test and RMET in both CD groups. The statistical analyses were performed using IBM SPSS software 21.
Results
Demographic details of CD patients and HC are displayed in Table 1. The Kruskal Wallis Test showed a significant difference in the mean age (χ2 = 6,961; p = 0.031) between the three groups. The test showed no significant difference in the mean level of education (χ2 = 1,702; p = 0.427) between the three groups. The mood assessment test results seem to be within the normal expected range for each of the three groups, and the Kruskal Wallis Test showed a significant difference in the mean HDRS score (χ2 = 29,414; p = 0.0001) and HARS score (χ2 = 20,825; p = 0.0001). Post hoc pairwise comparisons with Bonferroni correction indicated differences between:
TABLE 1.
Socio-demographic characteristics of groups, clinical characteristics and mood scales – descriptive statistics.
|
SCA1 + 2 (n = 18, 10M + 8F)
|
ILOCA (n = 16, 10M + 6F)
|
HC (n = 34, 16M + 18F)
|
||||
| Mean/Median (sd) | Range | Mean/Median (sd) | Range | Mean/Median (sd) | Range | |
| Age (yrs) | 43.78/42.5 (12.4) | 22–68 | 54.8/55.5 (7.9) | 38–68 | 49.1/44 (11.8) | 22–68 |
| Education (yrs) | 12.2/12 (2.3) | 8–16 | 13.1/12 (1.7) | 11–16 | 12.7/12 (2.1) | 8–16 |
| Onset of disease (yrs) | 32.7/30.5 (10.6) | 16–53 | 47.6/48.5 (8.9) | 28–62 | – | – |
| Disease duration (yrs) | 10.9/8.5 (9.1) | 2–38 | 7.1/7.5 (4.2) | 2–15 | – | – |
| Truncal ataxia | 7.4/7 (2.1) | 4–12 | 4.4/4 (1.9) | 2–8 | – | – |
| Dysarthria | 2.5/3 (0.6) | 1–3 | 1.7/1.5 (1.1) | 0–4 | – | – |
| Limb ataxia | 5.3/5 (2.2) | 3–12 | 2.9/2.5 (1.6) | 0–6 | – | – |
| SARA1 | 15.3/15 (3.8) | 10–22 | 9.0/8.5 (3.7) | 3–18 | – | – |
| HDRS2 | 6.7/7.5 (4.1) | 0–17 | 7.1/7 (3.9) | 1–15 | 1.7/0 (2.7) | 0–13 |
| HARS3 | 9.6/10.5 (5.9) | 0–17 | 7.3/6 (6.2) | 0–17 | 2.1/1 (3.6) | 0–19 |
1Scale for the Rating and Assessment of Ataxia (SARA), 2Hamilton Depression Rating Scale, 3Hamilton Anxiety Rating Scale. Source: Author’s calculation.
-
-
SCA1 + 2 and ILOCA groups in age (t = −26,781; p = 0.025),
-
-
HC and SCA1 + 2 groups (t = −24,346; p = 0.0001) and HC and ILOCA groups (t = −26,735; p = 0.0001) in HDRS score
-
-
HC and SCA1 + 2 groups (t = −17,173; p = 0.011) and HC and ILOCA groups (t = −24,346; p = 0.0001) in HARS score.
For variables applicable only to the CD groups (SCA1 + 2 and ILOCA), Mann-Whitney U Tests indicated significant differences in onset of disease (U = 42.5; z = −3.505; p = 0.0001), truncal ataxia (U = 39.5; z = −3.632; p = 0.0001), dysarthria (U = 78; z = −2.398; p = 0.017), limb ataxia (U = 47; z = −3.372 p = 0.001), and SARA (U = 34.5; z = −3.783; p = 0.0001) between patients in the SCA1 + 2 and ILOCA groups.
Mean values, standard deviations and range are shown in Table 1.
Social Cognition Profile in CD Patients vs. HC
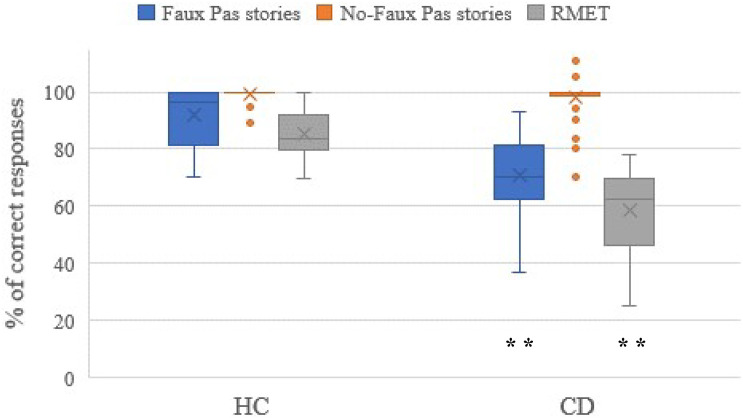
We identified significant differences in the performance on the Faux Pas Recognition Test and RMET between the CD patients and HCs. Box plots illustrating the differences in the average scores (in%) obtained from CD and HC subjects in each social cognition task (Faux Pas stories, No-Faux Pas stories and RMET) are shown in Figure 1. In the Faux Pas Recognition Test the patients with cerebellar hereditary and non-hereditary disorders showed impaired scores compared to HCs [U = 129.5, z = −5.544, p = 0.0001, r = 0.67 (high effect size, 95%CI: 0.523 to 0.788)]. Moreover, the patients with cerebellar hereditary and non-hereditary disorders obtained significantly lower scores than HCs in the RMET [U = 17.0, z = −6.895, p = 0.0001, r = 0.84 (high effect size, 95%CI: 0.756 to 0.899)].
FIGURE 1.
The scores of the social cognition battery. Data are presented as the percentage of the total number of correct responses for the Faux Pas stories (max = 60) and the No-Faux Pas stories (max = 20), and for the RMET (max = 36). The mean scores of the accuracy percentage, where 0% is totally wrong and 100% is totally correct, are reported for the CD and HC groups, and significant differences of p < 0.01 are indicated by ∗∗.
Differences in the Faux Pas Recognition Test and RMET Outcomes Among All Studied Groups
We further investigated the differences in the performance on the Faux Pas Recognition Test and RMET tests between groups. The average score (standard deviation) on the Faux Pas Recognition Test was 63.8 (11.6) for SCA1 + 2, 78.8 (13.9) for ILOCA and 91.9 (10.2) for HC. The average score (standard deviation) on RMET was 52.8 (14.4) for SCA1 + 2, 64.4 (10.4) for ILOCA and 85.3 (8.3) for HC. Kruskal Wallis Test showed a significant difference in the mean Faux Pas Recognition Test score (χ2 = 36,661; p = 0.0001) and RMET score (χ2 = 49,316; p = 0.0001). Post hoc pairwise comparisons with Bonferroni correction indicated differences between:
-
-
The scores for all three groups on the Faux Pas Recognition Test; SCA1 + 2 and ILOCA (t = −16,417; p = 0.045), SCA1 + 2 and HC (t = 34,108; p = 0.0001), ILOCA and HC (t = 17,691; p = 0.009). Based on medians, the patients in the SCA1 + 2 group obtained lower scores than the patients in the ILOCA group, while both of these groups obtained lower scores than the HC group participants.
-
-
CD groups in comparison to HC group on RMET scores, SCA1 + 2 and HC (t = 37,250; p = 0.0001), ILOCA and HC (t = 28,219; p = 0.0001). Based on median values, the patients in the SCA1 + 2 and ILOCA group obtained similar scores, which were lower than HC group participants.
Neuropsychological Findings
We investigated intergroup comparisons (SCA1 + 2, ILOCA, and HC) in scores on the neuropsychological tests. Median and significance for intergroup comparisons can be seen in Table 2.
TABLE 2.
Neuropsychological screening – descriptive statistics and group comparison.
|
SCA1+2 (n = 18)
|
ILOCA (n = 16)
|
HC (n = 34)
|
SCA1+2 - HC
|
ILOCA - HC
|
||||
| Mean/Median (sd) | Range | Mean/Median (sd) | Range | Mean/Median (sd) | Range | t | t | |
| Global cognitive functioning | ||||||||
| MMSE1 | 27.2/27.5 (2.8) | 19–30 | 27.8/27.5 (1.7) | 25–30 | 29.1/30 (1.2) | 27–30 | 16.428* | 15.22* |
| ACE-R-total2 | 82.6/85.5 (12.0) | 56–99 | 89.9/89 (4.9) | 80–98 | 95.9/97(4.2) | 86–100 | 29.601** | 19.41** |
| Learning and episodic verbal memory3 | ||||||||
| FCSRT-total free recall | 20.6/20.5 (6.8) | 5–33 | 22.1/22.5 (4.9) | 15–32 | 29.2/34 (6.9) | 8–40 | 23.662** | 19.756** |
| FCSRT-delayed free recall | 8.4/8.5 (2.7) | 5–13 | 10.4/10 (2.5) | 5–15 | 11.3/12 (2.6) | 4–15 | 19.941** | |
| FCSRT-recognition | 14.4/14.5 (1.7) | 12–16 | 15.5/16 (1.5) | 10–16 | 15.9/16 (0.2) | 15–16 | 17.905** | |
| ACE-R-verbal memory | 21.7/23 (4.9) | 6–26 | 23.3/23 (2.2) | 19–26 | 25.3/26 (1.4) | 21–26 | 22.26** | 17.774** |
| Orientation and attention | ||||||||
| ACE-R-orientation and attention | 16.6/17 (1.8) | 12–18 | 17.3/17.5 (0.9) | 16–18 | 17.6/18 (0.7) | 16–18 | ||
| TMT-A | 83.6/74 (36.6) | 30–157 | 49.3/42.5 (17.0) | 22–82 | 33.4/30 (14.7) | 18–78 | 31.183** | 17.169* |
| Digit-span | 7.7/8 (2.1) | 4–11 | 8.3/8 (2.9) | 4–13 | 11.3/11.5 (2.1) | 7–15 | 24.977** | 20.088** |
| Calculation | ||||||||
| Arithmetic-VITI4 | 9.1/9 (3.2) | 3–14 | 11.3/11 (1.6) | 9–14 | 13.8/14 (0.5) | 12–14 | 32.647** | 24.014** |
| Language | ||||||||
| ACE-R-language | 23.2/25 (3.2) | 15–26 | 24.8/25 (0.8) | 24–26 | 25.4/26 (1.2) | 22–26 | 17.188** | |
| BNT-Total | 50.3/52 (6.4) | 40–58 | 53.3/54 (3.1) | 45–57 | 54.4/55 (3.9) | 40–59 | ||
| Visuospatial processing | ||||||||
| HVOT5 | 17.2/17 (6.3) | 8–25 | 21.3/21.5 (3.1) | 16–25 | 24.6/25 (2.9) | 17–29 | 26.103** | 18.39** |
| ACE-R-visuospatial skills | 13.2/14 (3.1) | 8–16 | 15.3/16 (1.0) | 13–16 | 15.3/16 (1.4) | 11–16 | 14.634** | |
| Executive functions | ||||||||
| SCWT6 | 91.6/90 (37.6) | 45–154 | 97.3/98 (41.2) | 36–180 | 57.9/53.5 (21.5) | 10–98 | 18.088** | 19.488** |
| DOT Total7 | 5.3/5 (1.9) | 2–9 | 6.0/6 (1.3) | 4–9 | 7.9/8 (2.1) | 4–12 | 23.5** | 18.188** |
| ACE-R-phonemic fluency | 3.8/4 (1.6) | 1–7 | 4.4/4 (1.5) | 2–7 | 5.7/6 (0.9) | 4–7 | 23.034** | 17.086* |
1Mini Mental State Examination, 2Addenbrooke’s Cognitive Examination – Revised, 3Free and Cued Selective Reminding Test, 4Serbian adaptation of the Wechsler Adult Intelligence Scale – Revised, 5Hooper Visual Organization Test, 6Stroop Color and Word Test, 7Digit Ordering Test. Source: Author’s calculation (** indicate p < 0.01, while * indicates p < 0.05).
The Kruskal Wallis Test showed a significant difference in the mean MMSE score (χ2 = 11,299; p = 0.004), ACE-R – total (χ2 = 27,801; p = 0.0001), FCSRT – total free recall (χ2 = 21,097; p = 0.0001), FCSRT – delayed free recall (χ2 = 12,177; p = 0.002), FCSRT – recognition (χ2 = 18,528; p = 0.0001), ACE-R – verbal memory (χ2 = 19,121; p = 0.0001), TMT-A (χ2 = 30,569; p = 0.0001), Digit Span (χ2 = 23,136; p = 0.0001), Arithmetic-VITI (χ2 = 41,979; p = 0.0001), ACE-R – phonemic fluency (χ2 = 19,495; p = 0.0001), HVOT (χ2 = 23,541; p = 0.0001), SCWT (χ2 = 15,779; p = 0.0001), ACE-R-Language (χ2 = 11,356; p = 0.003), ACE-R-visuospatial skills (χ2 = 8,273; p = 0.016), DOT Total (χ2 = 20,185; p = 0.0001).
Post hoc pairwise comparisons with Bonferroni correction indicated differences between both CD groups in comparison to the HC group on:
-
-
MMSE score, ACE-R – total, FCSRT – total free recall, ACE-R – verbal memory, TMT-A, Digit Span, Arithmetic-VITI, ACE-R – phonemic fluency, HVOT, SCWT, DOT Total. Based on median values, the patients in SCA1 + 2 and ILOCA group obtained similar scores, and worse than HC group participants.
On some scales, post hoc pairwise comparisons with Bonferroni correction indicated differences only between SCA1 + 2 and HC group:
-
-
FCSRT – delayed free recall, ACE-R-Language, ACE-R-visuospatial skills. Based on medians, the patients in SCA1 + 2 group obtained worse scores than HC group participants, while patients in the ILOCA group obtained scores between the other two.
And lastly, on FCSRT – recognition scale, post hoc pairwise comparisons with Bonferroni correction indicated differences between the SCA1 + 2 and HC groups (t = 17,905; p = 0.0001), and between the SCA1 + 2 and ILOCA groups (t = −13,424; p = 0.019). Median values indicate that the patients in SCA1 + 2 group obtained lower scores than the other two groups, patients in the ILOCA group and HC participants.
Associations of the Social Cognitive Profile With Sociodemographic, Clinical, and Genetic Features of CD Patients With Their Neuropsychological Findings and Mood State
The duration of the disease (r = −0.555, 95%CI: −0.119 to −0.811, p = 0.017), truncal ataxia (r = −0.557, 95%CI: −0.122 to −0.812, p = 0.016) and SARA score (r = −0.561, 95%CI: −0.128 to −0.814, p = 0.015) showed a significant inverse (negative) correlation with the Faux Pas Recognition Test in the SCA1 and SCA2 groups of patients, while the SCWT (r = −0.482, 95%CI: −0.002 to −0.781, p = 0.050) correlated inversely with RMET. In the ILOCA group of patients, RMET correlated with HDRS (r = 0.498, 95%CI: 0.004 to 0.796, p = 0.050) and HARS (r = 0.541, 95%CI: 0.062 to 0.817, p = 0.030) and ACE-Phonemic Fluency (r = 0.545, 95%CI: 0.068 to 0.819, p = 0.029). There was no significant correlation in the SCA1 and SCA2 groups of patients between the number of repeats in the expanded allele and the achievement on Faux Pas Recognition Test and RMET.
Predictors of Social Cognition Impairment in CD Patients
The final goal of this study was to determine whether EFs are significant predictors of performance in social cognition tests in SCA1 + 2 and ILOCA patients. The predictor variables (SCWT, DOT Total, ACE-R-Phonemic Fluency) did not show significant prediction of the Faux Pas Recognition Test scores in the SCA1 + 2 group (F = 1.802; df = 3.13; p > 0.05), nor in the ILOCA group (F = 1.269; df = 3.11; p > 0.05). In addition, in the ILOCA group, EFs (SCWT, DOT Total, ACE-R-Phonemic Fluency) did not show significant prediction of RMET (F = 1.95; df = 3.11; p > 0.05). On the other hand, in the SCA1 + 2 group, EFs appeared as significant predictors of the Emotion Recognition Test (F = 4.05; df = 3.13; p = 0.031), explaining 48.3% of the variance. The only significant predictor was SCWT (β = −0.631, 95% CI from −1.04 to −0.22, t = −3.006, p < 0.05). If the SCWT score were to increase by one standard deviation, the achievement scores on the RMET would likely decrease by 0.63 units of standard deviation in the patients of the SCA1 + 2 group. Since only one EF test could predict social cognition impairment, we wanted to determine which other domains of neuropsychological tests might be significant predictors of failure in social cognition in both groups of CD patients.
First, we entered all neuropsychological tests in the regression model, but some of them showed suppression effects that needed to be removed from the model. The final list of neuropsychological predictors (MMSE score, FCSRT – Delayed free recall, TMT-A, BNT – Total, SCWT and DOT Total) showed significant prediction of Faux Pas Recognition Test (F = 7.458; df = 6.23; p = 0.0001) in CD patients, explaining 66% of the variance. Standardized beta coefficients show that BNT – Total (β = 0.47, 95%CI from 0.16 to 0.77, t = 2.97, p = 0.007) has the greatest effect, followed by TMT-A (β = −0.37, 95%CI from −0.09 to −0.65, t = −2.64, p = 0.014) and FCSRT – Delayed free recall (β = 0.26, 95%CI from 0.01 to 0.51, t = 2.07, p = 0.049), while MMSE score, SCWT and DOT Total did not appear as significant predictors.
Before predicting the Emotion Recognition Test (RMET) findings, after removing suppressors, the final list of predictors contained FCSRT-Total free recall, FCSRT – Delayed free recall, ACE-R Verbal Memory, TMT-A, BNT, and ACE-R-Language. Regression model was significant (F = 11.088; df = 6.24; p = 0.0001) in CD patients, explaining 73.5% of the variance. Standardized beta coefficients show that only BNT – Total (β = 0.612, 95%CI from 0.34 to 0.88, t = 4.406, p = 0.0001) represents a significant predictor of achievement on RMET in CD patients.
Discussion
Our study shows that patients with CD (SCA1, SCA2, and ILOCA) exhibit impaired performance on both the Faux Pas Recognition Test and the RMET compared with HC. The patients have difficulty understanding the mental states of others in everyday interactions and from their facial expressions.
Our study examined two groups of patients: a group of SCA1 and SCA2 patients with severe cerebellar and extra-cerebellar signs and a group of ILOCA patients with primarily isolated cerebellar degeneration. The poor performance of both groups possibly suggests that a cerebellar pathology alone produces social cognition impairment, as previously shown by Hoche (Hoche et al., 2016). SCA patients achieved the worst results on the social cognition tests used in our study, which may suggest that social cognition also involves extra-cerebellar structures (Van Overwalle et al., 2015).
Cerebellar disorders are a group of subentities with origins in various pathophysiological mechanisms; they are also characterized by different levels of social cognitive impairment. Some studies suggest that patients with a left lateral cerebellar tumor (Sokolov et al., 2010) and patients with ponto-cerebellar ischemia (Roldan Gerschcovich et al., 2011) can also exhibit social cognition impairment. Schizophrenia and autism spectrum disorders are further characterized by dysfunctions of cerebellar-cortical networks and an impairment of the “mentalizing” process (Andreasen and Pierson, 2008; Penn et al., 2008; Green et al., 2015).
The duration of the disease was similar in all the patients who took part in our study. The SCA group consisted of younger patients with a more severe clinical presentation and disability (SARA), exhibiting a more serious truncal and limbic ataxia and dysarthria before the commencement of the study. The deleterious effect of the disease in SCA patients, corroborated by the neuropshychological tests, was also evident in other domains of cognition. Unlike our ILOCA patients, the SCA group consistently achieved poorer results compared with the HCs in all examined domains. Routine clinical practice may not have detected these deficits if only MMSE tests had been used, as the SCA patients’ group scores were within the prescribed normal values. However, a detailed neuropsychological evaluation and screening tests such as ACE-R can detect a more serious cognitive deficit. The severity of ataxia in all SCA patients positively correlated with the patients’ cognitive deficit (Ma et al., 2014), although some sources suggest that cognitive functions are affected first and have a slower progression than motor dysfunctions (Fancellu et al., 2013).
Compared to healthy subjects, our SCA patients exhibited consistently poorer results on tests measuring attention and verbal memory than ILOCA patients, which other authors have also previously recorded (Bürk et al., 2001; Le Pira et al., 2002; Fancellu et al., 2013). The ILOCA patients exhibited worse results than the HC in most cognitive domains, but verbal memory, speech and visuospatial deficits were less frequent. Finally, the executive functioning of both the ILOCA and the SCA patients was equally impaired, which previous studies have also found (Bürk et al., 1999, 2001; Rentiya et al., 2018), while the differences in visuospatial tasks proved less consistent (Wallesch and Horn, 1990; Schmahmann and Sherman, 1998; Rentiya et al., 2018). The extent of cognitive impairment, especially that of attention and executive functioning, verbal and semantic memory, language and speech competence, may explain the patients’ poor performance on social cognition tests (Haxby et al., 2000; Siegal and Varley, 2002).
The duration of the disease, functional disability and truncal ataxia significantly correlated with the SCA patients’ performance on the Faux Pas Recognition Test. SCWT and Phonemic Fluency Test, and significantly correlated with the RMET in the SCA and ILOCA group, respectively, but no correlation was determined with verbally presented social situations in the Faux Pas stories.
Although prior studies have shown that social cognition impairments are often associated with executive dysfunction (Aboulafia-Brakha et al., 2011), the literature offers conflicting data (Fine et al., 2001; Clausi et al., 2021). Analysis of connectivity suggests that the cerebellar modules active during social cognition are more likely to be involved in socio-cognitive and not executive networks (Van Overwalle et al., 2015).
Depression and depressive symptoms were the most common non-cognitive symptoms in several studies of patients with cerebellar disorders (Leroi et al., 2002; Liszewski et al., 2004). Although the mean scores in both our groups of patients were within the range for the healthy population, significant difference was observed between the CD patients and the HC. Further, several patients in the SCA1 and 2 groups had a score above 14. The ILOCA group’s anxiety and depression scores correlated with the patients’ performance on the RMET. A larger sample of CD patients is necessary to determine the impact of depression on social cognition. It is possible that the patient’s impaired perception hampers the understanding of other people’s mental states.
The predictive value of EFs in the assessment of social cognition was ambiguous. The EF tests we used did not significantly predict the Faux Pas test performance in our SCA patients. SCWT showed significant predictive value for the RMET performance in the SCA group, but not in the ILOCA patients. The most significant predictor in all groups and for both tests was the BNT language and speech test. Language and speech, skills inherent to humans, are primarily an instrument for interpersonal communication (Schmahmann, 2019) and involve the comprehension of words, sentences and stories arising from and serving social interaction. It is thus natural that speech proved to be the best predictor of social cognition among our CD patients (Mariën and Beaton, 2014; Mariën et al., 2014). The social cognition impairment in our CD patients involved diminished semantic lexicon. Errors of this segment inevitably cause difficulties in conducting original cognitive functions and normal social functioning.
Our patients with primary cerebellar disease exhibited impairments in the Faux Pas Recognition Test and the RMET, which are traditionally considered indicators of social cognition. As mentioned above, the deficit was primarily caused by semantic processing impairment, but the extent of damage in other cognitive domains determined the severity of social deficit. The literature describes the role of the cerebellum in the cognitive deficit of SCA1 and 2 pathologies, which is commonly attributed to extra-cerebellar degeneration and damaged ties primarily between the basal ganglia and the thalamocortical circles (Gilman et al., 1996; Kawai et al., 2009). Other studies agree with our results, which associate social cognition with specific regions of the brain: mesial and orbitofrontal cortex (Amodio and Frith, 2006; Frith and Frith, 2006; Völlm et al., 2006) and mesial temporal cortex (Gallagher and Frith, 2003; Völlm et al., 2006). Furthermore, (Charlton et al., 2009) it has been shown that poor performance on social cognition tests can be associated with changes in the white matter. We support the assertion that mentalizing processes depend on a complex network which connect different regions and involves ties in the brain’s white matter.
It is unclear whether the obtained data give insight into the patients’ overall social abilities or only into routine components necessary for opinion forming. The metric characteristics of the tests, ecological as well as criterion validity, are of special importance in assessing social cognition. Cognition in real social settings is a dynamic, changeable, and context-dependent process. We concur with (Byom and Mutlu, 2013) that the assessment of social cognition in experimental settings may lead to the under- or overestimation of this complex function, resulting in inconsistent results.
In summary, the findings from this study is that social cognition impairment is present in both the SCA 1 + 2 and the ILOCA patients and that the predictors of this impairment are Efs and language. The conclusions of this study are limited due to the small sample of patients and the fact that they were in the advanced stage of the disease, with great physical and cognitive deterioration. The SCA group was in itself heterogeneous and included two somewhat different entities, where the SCA2 group consisted of patients with severe cognitive impairments, even dementia (Bürk et al., 1999). Because of this, the implication that some brain regions contribute to social cognition must be considered with caution. Future studies must avoid the current study’s limitations.
The body of evidence suggesting the existence of cognitive and mentalizing deficits in CD is growing. We hope our study will encourage theories which embrace the possibility of a completely different profile of the deficit within this heterogeneous disorder. Socialization is the core of all human relationships, which is why the implications of impaired social cognition need to be taken seriously when considering treatment options.
Future research should branch into two directions: researching the cognitive changes in CD and determining the profile of some entities of this heterogeneous disorder. Special attention should be dedicated to operationalizing the mentalizing process, determining the main neuropsychological processes and neural correlates involved, and defining adequate assessment instruments.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Board of Neurology Clinic, Medical Faculty University of Belgrade. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
OT and ND-M contributed to the conception and design of the study. OT wrote the first draft of the manuscript. DS performed the statistical analysis. MK, AK, ES, B, AM, M, and NG contributed to the organization and data collection. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abel C. G., Stein G., Galarregui M., Garretto N., Mangone C., Genovese O., et al. (2007). Social cognition and theory of mind assessment in non-demented patients with isolated cerebellar degeneration. Arq. Neuropsiquiatr. 65 304–312. 10.1590/s0004-282x2007000200022 [DOI] [PubMed] [Google Scholar]
- Aboulafia-Brakha T., Christe B., Martory M.-D., Annoni J.-M. (2011). Theory of mind tasks and executive functions: a systematic review of group studies in neurology. J. Neuropsychol. 5 39–55. 10.1348/174866410X533660 [DOI] [PubMed] [Google Scholar]
- Abu-Akel A., Shamay-Tsoory S. (2011). Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia 49 2971–2984. 10.1016/j.neuropsychologia.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Adamaszek M., D’Agata F., Ferrucci R., Habas C., Keulen S., Kirkby K. C., et al. (2017). Consensus paper: cerebellum and emotion. Cerebellum 16 552–576. 10.1007/s12311-016-0815-8 [DOI] [PubMed] [Google Scholar]
- Adolphs R. (2004). Emotional vision. Nat. Neurosci. 7 1167–1168. 10.1038/nn1104-1167 [DOI] [PubMed] [Google Scholar]
- Ahmadian N., van Baarsen K., van Zandvoort M., Robe P. A. (2019). The cerebellar cognitive affective syndrome-a meta-analysis. Cerebellum 18 941–950. 10.1007/s12311-019-01060-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aichhorn M., Perner J., Weiss B., Kronbichler M., Staffen W., Ladurner G. (2009). Temporo-parietal junction activity in theory-of-mind tasks: falseness, beliefs, or attention. J. Cogn. Neurosci. 21 1179–1192. 10.1162/jocn.2009.21082 [DOI] [PubMed] [Google Scholar]
- Amodio D. M., Frith C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7 268–277. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Andreasen N. C., Pierson R. (2008). The role of the cerebellum in schizophrenia. Biol. Psychiatry 64 81–88. 10.1016/j.biopsych.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillieux H., De Smet H. J., Paquier P. F., De Deyn P. P., Mariën P. (2008). Cerebellar neurocognition: insights into the bottom of the brain. Clin. Neurol. Neurosurg. 110 763–773. 10.1016/j.clineuro.2008.05.013 [DOI] [PubMed] [Google Scholar]
- Barbosa R., Lampreia T., Bugalho P. (2016). The aetiology of idiopathic late onset cerebellar ataxia (ILOCA): clinical and imaging clues for a definitive diagnosis. J. Neurol. Sci. 365 156–157. 10.1016/j.jns.2016.04.029 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., O’Riordan M., Stone V., Jones R., Plaisted K. (1999). Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. J. Autism Dev. Disord. 29 407–418. 10.1023/a:1023035012436 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. (2001). The “reading the mind in the eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry 42 241–251. 10.1111/1469-7610.00715 [DOI] [PubMed] [Google Scholar]
- Brunet E., Sarfati Y., Hardy-Baylé M. C., Decety J. (2000). A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage 11 157–166. 10.1006/nimg.1999.0525 [DOI] [PubMed] [Google Scholar]
- Bürk K., Bösch S., Globas C., Zühlke C., Daum I., Klockgether T., et al. (2001). Executive dysfunction in spinocerebellar ataxia type 1. Eur. Neurol. 46 43–48. 10.1159/000050755 [DOI] [PubMed] [Google Scholar]
- Bürk K., Globas C., Bösch S., Gräber S., Abele M., Brice A., et al. (1999). Cognitive deficits in spinocerebellar ataxia 2. Brain J. Neurol. 122(Pt 4) 769–777. 10.1093/brain/122.4.769 [DOI] [PubMed] [Google Scholar]
- Buschke H. (1984). Cued recall in amnesia. J. Clin. Neuropsychol. 6 433–440. 10.1080/01688638408401233 [DOI] [PubMed] [Google Scholar]
- Byom L. J., Mutlu B. (2013). Theory of mind: mechanisms, methods, and new directions. Front. Hum. Neurosci. 7:413. 10.3389/fnhum.2013.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge C., Andreasen N. C., O’Leary D. S. (2003). Visualizing how one brain understands another: a PET study of theory of mind. Am. J. Psychiatry 160 1954–1964. 10.1176/appi.ajp.160.11.1954 [DOI] [PubMed] [Google Scholar]
- Charlton R. A., Barrick T. R., Markus H. S., Morris R. G. (2009). Theory of mind associations with other cognitive functions and brain imaging in normal aging. Psychol. Aging 24 338–348. 10.1037/a0015225 [DOI] [PubMed] [Google Scholar]
- Clausi S., Olivito G., Lupo M., Siciliano L., Bozzali M., Leggio M. (2018). The cerebellar predictions for social interactions: theory of mind abilities in patients with degenerative cerebellar atrophy. Front. Cell. Neurosci. 12:510. 10.3389/fncel.2018.00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausi S., Olivito G., Siciliano L., Lupo M., Bozzali M., Masciullo M., et al. (2021). The neurobiological underpinning of the social cognition impairments in patients with spinocerebellar ataxia type 2. Cortex 138 101–112. 10.1016/j.cortex.2020.12.027 [DOI] [PubMed] [Google Scholar]
- Cooper F. E., Grube M., Elsegood K. J., Welch J. L., Kelly T. P., Chinnery P. F., et al. (2010). The contribution of the cerebellum to cognition in Spinocerebellar ataxia Type 6. Behav. Neurol. 23:724861. 10.3233/BEN-2010-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sagar H. J., Jordan N., Harvey N. S., Sullivan E. V. (1991). Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain J. Neurol. 114(Pt 5) 2095–2122. 10.1093/brain/114.5.2095 [DOI] [PubMed] [Google Scholar]
- D’Agata F., Caroppo P., Baudino B., Caglio M., Croce M., Bergui M., et al. (2011). The recognition of facial emotions in spinocerebellar ataxia patients. Cerebellum 10 600–610. 10.1007/s12311-011-0276-z [DOI] [PubMed] [Google Scholar]
- Ðorđević J., Živanović M., Pavlović A., Mihajlović G., Stašević Karlièić I., Pavlović D. (2017). Psychometric evaluation and validation of the Serbian version of “reading the mind in the eyes” test. Psihologija 50 483–502. 10.2298/PSI170504010D [DOI] [Google Scholar]
- Ðorđević J. (2013). Procena Socijalne Kognicije i Neurokognicije Kod Bolesnika sa Šizofrenijom i Bipolarnim Afektivnim Poremećajem. Available online at: https://nardus.mpn.gov.rs/handle/123456789/10524. [Google Scholar]
- Dragasević N. T., Culjković B., Klein C., Ristić A., Keckarević M., Topisirović I., et al. (2006). Frequency analysis and clinical characterization of different types of spinocerebellar ataxia in Serbian patients. Mov. Disord. 21 187–191. 10.1002/mds.20687 [DOI] [PubMed] [Google Scholar]
- Fancellu R., Paridi D., Tomasello C., Panzeri M., Castaldo A., Genitrini S., et al. (2013). Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. J. Neurol. 260 3134–3143. 10.1007/s00415-013-7138-1 [DOI] [PubMed] [Google Scholar]
- Fine C., Lumsden J., Blair R. J. (2001). Dissociation between “theory of mind” and executive functions in a patient with early left amygdala damage. Brain J. Neurol. 124 287–298. 10.1093/brain/124.2.287 [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). Mini-mental state”. a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Frings M., Maschke M., Timmann D. (2007). Cerebellum and cognition: viewed from philosophy of mind. Cerebellum 6 328–334. 10.1080/14734220701200063 [DOI] [PubMed] [Google Scholar]
- Frith C. D., Frith U. (2006). The neural basis of mentalizing. Neuron 50 531–534. 10.1016/j.neuron.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Gallagher H. L., Frith C. D. (2003). Functional imaging of “theory of mind”. Trends Cogn. Sci. 7 77–83. 10.1016/s1364-6613(02)00025-6 [DOI] [PubMed] [Google Scholar]
- Gilman S., Sima A. A., Junck L., Kluin K. J., Koeppe R. A., Lohman M. E., et al. (1996). Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions. Ann. Neurol. 39 241–255. 10.1002/ana.410390214 [DOI] [PubMed] [Google Scholar]
- Glickstein M. (1992). The cerebellum and motor learning. Curr. Opin. Neurobiol. 2 802–806. 10.1016/0959-4388(92)90137-a [DOI] [PubMed] [Google Scholar]
- Green M. F., Horan W. P., Lee J. (2015). Social cognition in schizophrenia. Nat. Rev. Neurosci. 16 620–631. 10.1038/nrn4005 [DOI] [PubMed] [Google Scholar]
- Grober E., Buschke H., Crystal H., Bang S., Dresner R. (1988). Screening for dementia by memory testing. Neurology 38 900–903. 10.1212/wnl.38.6.900 [DOI] [PubMed] [Google Scholar]
- Guell X., Gabrieli J. D. E., Schmahmann J. D. (2018). Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage 172 437–449. 10.1016/j.neuroimage.2018.01.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1959). The assessment of anxiety states by rating. Br. J. Med. Psychol. 32 50–55. 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J. V., Hoffman E. A., Gobbini M. I. (2000). The distributed human neural system for face perception. Trends Cogn. Sci. 4 223–233. 10.1016/s1364-6613(00)01482-0 [DOI] [PubMed] [Google Scholar]
- Heleven E., Van Overwalle F. (2018). The neural basis of representing others’ inner states. Curr. Opin. Psychol. 23 98–103. 10.1016/j.copsyc.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Hoche F., Guell X., Sherman J. C., Vangel M. G., Schmahmann J. D. (2016). Cerebellar contribution to social cognition. Cerebellum 15 732–743. 10.1007/s12311-015-0746-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoche F., Guell X., Vangel M. G., Sherman J. C., Schmahmann J. D. (2018). The cerebellar cognitive affective/Schmahmann syndrome scale. Brain J. Neurol. 141 248–270. 10.1093/brain/awx317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C. M., Oakes T. R., Davidson R. J. (2006). The voice of emotion: an FMRI study of neural responses to angry and happy vocal expressions. Soc. Cogn. Affect. Neurosci. 1 242–249. 10.1093/scan/nsl027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., Weintrab S. (1983). The Boston Naming Test. Philadelphia, PA: Lea and Febiger. [Google Scholar]
- Western Psychological Services (1983). Hooper Visual Organization Test (VOT). Manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Kawai Y., Suenaga M., Watanabe H., Sobue G. (2009). Cognitive impairment in spinocerebellar degeneration. Eur. Neurol. 61 257–268. 10.1159/000206850 [DOI] [PubMed] [Google Scholar]
- Kipps C. M., Duggins A. J., McCusker E. A., Calder A. J. (2007). Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington’s disease. J. Cogn. Neurosci. 19 1206–1217. 10.1162/jocn.2007.19.7.1206 [DOI] [PubMed] [Google Scholar]
- Klockgether T. (2018). Sporadic adult-onset ataxia. Handb. Clin. Neurol. 155 217–225. 10.1016/B978-0-444-64189-2.00014-7 [DOI] [PubMed] [Google Scholar]
- Koziol L. F., Budding D., Andreasen N., D’Arrigo S., Bulgheroni S., Imamizu H., et al. (2014). Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum 13 151–177. 10.1007/s12311-013-0511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pira F., Zappalà G., Saponara R., Domina E., Restivo D., Reggio E., et al. (2002). Cognitive findings in spinocerebellar ataxia type 2: relationship to genetic and clinical variables. J. Neurol. Sci. 201 53–57. 10.1016/s0022-510x(02)00194-6 [DOI] [PubMed] [Google Scholar]
- Leroi I., O’Hearn E., Marsh L., Lyketsos C. G., Rosenblatt A., Ross C. A., et al. (2002). Psychopathology in patients with degenerative cerebellar diseases: a comparison to Huntington’s disease. Am. J. Psychiatry 159, 1306–1314. 10.1176/appi.ajp.159.8.1306 [DOI] [PubMed] [Google Scholar]
- Lezak M. D. (1995). Neuropsychological Assessment. New York, NY: Oxford University Press. [Google Scholar]
- Liszewski C. M., O’Hearn E., Leroi I., Gourley L., Ross C. A., Margolis R. L. (2004). Cognitive impairment and psychiatric symptoms in 133 patients with diseases associated with cerebellar degeneration. J. Neuropsychiatry Clin. Neurosci. 16, 109–112. 10.1176/jnp.16.1.109 [DOI] [PubMed] [Google Scholar]
- Liverta Sempio O., Marchetti A., Lecciso F. (2005). Faux Pas: Traduzione Italiana. Milan: Theory of Mind Research Unit. Milan: Catholic University of the Sacred Heart. [Google Scholar]
- Ma J., Wu C., Lei J., Zhang X. (2014). Cognitive impairments in patients with spinocerebellar ataxia types 1, 2 and 3 are positively correlated to the clinical severity of ataxia symptoms. Int. J. Clin. Exp. Med. 7 5765–5771. [PMC free article] [PubMed] [Google Scholar]
- Manto M., Bower J. M., Conforto A. B., Delgado-García J. M., da Guarda S. N. F., Gerwig M., et al. (2012). Consensus paper: roles of the cerebellum in motor control–the diversity of ideas on cerebellar involvement in movement. Cerebellum 11 457–487. 10.1007/s12311-011-0331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P., Ackermann H., Adamaszek M., Barwood C. H. S., Beaton A., Desmond J., et al. (2014). Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum 13 386–410. 10.1007/s12311-013-0540-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P., Beaton A. (2014). The enigmatic linguistic cerebellum: clinical relevance and unanswered questions on nonmotor speech and language deficits in cerebellar disorders. Cerebellum Ataxias 1:12. 10.1186/2053-8871-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F. A., Strick P. L. (1994). Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 266 458–461. 10.1126/science.7939688 [DOI] [PubMed] [Google Scholar]
- Mioshi E., Dawson K., Mitchell J., Arnold R., Hodges J. R. (2006). The Addenbrooke’s cognitive examination revised (ACE-R): a brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry 21 1078–1085. 10.1002/gps.1610 [DOI] [PubMed] [Google Scholar]
- Pavlović M. D. (2003). Dijagnostièki Testovi u Neuropsihologiji [Diagnostic Tests in Neuropsychology]. Beograd: Pavlovicì. [Google Scholar]
- Penn D. L., Sanna L. J., Roberts D. L. (2008). Social cognition in schizophrenia: an overview. Schizophr. Bull. 34 408–411. 10.1093/schbul/sbn014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza S. M., McCormick C. A., Schlerf J. E., Justus T., Ivry R. B., Fiez J. A. (2006). Cerebellar damage produces selective deficits in verbal working memory. Brain J. Neurol. 129 306–320. 10.1093/brain/awh685 [DOI] [PubMed] [Google Scholar]
- Premack D., Woodruff G. (1978). Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1 515–526. 10.1017/s0140525x00076512 [DOI] [Google Scholar]
- Rentiya Z. S., Jung B. C., Bae J., Liszewski C. M., Fishman A., Du A. X., et al. (2018). Selective patterns of cognitive impairment in spinocerebellar ataxia type 6 and idiopathic late-onset cerebellar ataxia. Arch. Clin. Neuropsychol. 33 427–436. 10.1093/arclin/acx077 [DOI] [PubMed] [Google Scholar]
- Riva D., Giorgi C. (2000). The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain J. Neurol. 123(Pt 5) 1051–1061. 10.1093/brain/123.5.1051 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Ferrari P. F., Rozzi S., Fogassi L. (2006). The inferior parietal lobule: where action becomes perception. Novartis Found. Symp. 270 129–140. 10.1002/9780470034989.ch11 [DOI] [PubMed] [Google Scholar]
- Roldan Gerschcovich E., Cerquetti D., Tenca E., Leiguarda R. (2011). The impact of bilateral cerebellar damage on theory of mind, empathy and decision making. Neurocase 17 270–275. 10.1080/13554791003730618 [DOI] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people. the role of the temporo-parietal junction in “theory of mind”. Neuroimage 19 1835–1842. 10.1016/s1053-8119(03)00230-1 [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D. (1991). An emerging concept. the cerebellar contribution to higher function. Arch. Neurol. 48 1178–1187. 10.1001/archneur.1991.00530230086029 [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D. (1996). From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum. Brain Mapp. 4 174–198. [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D. (2019). The cerebellum and cognition. Neurosci. Lett. 688 62–75. 10.1016/j.neulet.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D., Sherman J. C. (1997). Cerebellar cognitive affective syndrome. Int. Rev. Neurobiol. 41 433–440. 10.1016/s0074-7742(08)60363-3 [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D., Sherman J. C. (1998). The cerebellar cognitive affective syndrome. Brain J. Neurol. 121(Pt 4) 561–579. 10.1093/brain/121.4.561 [DOI] [PubMed] [Google Scholar]
- Schmitz-Hübsch T., du Montcel S. T., Baliko L., Berciano J., Boesch S., Depondt C., et al. (2006). Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66 1717–1720. 10.1212/01.wnl.0000219042.60538.92 [DOI] [PubMed] [Google Scholar]
- Serafin M., Surian L. (2004). Il test degli occhi: uno strumento per valutare la‘teoria della mente‘. G. Ital. Psicol. 31 839–862. [Google Scholar]
- Shakkottai V. G., Fogel B. L. (2013). Clinical neurogenetics: autosomal dominant spinocerebellar ataxia. Neurol. Clin. 31 987–1007. 10.1016/j.ncl.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal M., Varley R. (2002). Neural systems involved in “theory of mind”. Nat. Rev. Neurosci. 3 463–471. 10.1038/nrn844 [DOI] [PubMed] [Google Scholar]
- Silveri M. C. (2020). Contribution of the cerebellum and the basal ganglia to language production: speech, word fluency, and sentence construction-evidence from pathology. Cerebellum 20 282–294. 10.1007/s12311-020-01207-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov A. A., Gharabaghi A., Tatagiba M. S., Pavlova M. (2010). Cerebellar engagement in an action observation network. Cereb. Cortex 1991 486–491. 10.1093/cercor/bhp117 [DOI] [PubMed] [Google Scholar]
- Sokolovsky N., Cook A., Hunt H., Giunti P., Cipolotti L. (2010). A preliminary characterisation of cognition and social cognition in spinocerebellar ataxia types 2, 1, and 7. Behav. Neurol. 23 17–29. 10.3233/BEN-2010-0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone V. E., Baron-Cohen S., Knight R. T. (1998). Frontal lobe contributions to theory of mind. J. Cogn. Neurosci. 10 640–656. 10.1162/089892998562942 [DOI] [PubMed] [Google Scholar]
- Stoodley C. J. (2012). The cerebellum and cognition: evidence from functional imaging studies. Cerebellum 11 352–365. 10.1007/s12311-011-0260-7 [DOI] [PubMed] [Google Scholar]
- Stoodley C. J., Schmahmann J. D. (2009). The cerebellum and language: evidence from patients with cerebellar degeneration. Brain Lang. 110 149–153. 10.1016/j.bandl.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Sullivan R., Yau W. Y., O’Connor E., Houlden H. (2019). Spinocerebellar ataxia: an update. J. Neurol. 266 533–544. 10.1007/s00415-018-9076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. F., Steinmetz J. E. (2009). The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience 162 732–755. 10.1016/j.neuroscience.2009.01.041 [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K., Mariën P., Vandekerckhove M. (2014). Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage 86 554–572. 10.1016/j.neuroimage.2013.09.033 [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K., Mariën P., Vandekerckhove M. (2015). Cerebellar areas dedicated to social cognition? A comparison of meta-analytic and connectivity results. Soc. Neurosci. 10 337–344. 10.1080/17470919.2015.1005666 [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Vandekerckhove M. (2013). Implicit and explicit social mentalizing: dual processes driven by a shared neural network. Front. Hum. Neurosci. 7:560. 10.3389/fnhum.2013.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völlm B. A., Taylor A. N. W., Richardson P., Corcoran R., Stirling J., McKie S., et al. (2006). Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage 29 90–98. 10.1016/j.neuroimage.2005.07.022 [DOI] [PubMed] [Google Scholar]
- Wallesch C. W., Horn A. (1990). Long-term effects of cerebellar pathology on cognitive functions. Brain Cogn. 14 19–25. 10.1016/0278-2626(90)90057-u [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.