Abstract
Introduction:
Aim of this study was identify the prevalence of frailty in patients with idiopathic Parkinson’s disease (PD), to describe the relationship between severity of the disease and frailty, and to evaluate if timed up and go (TUG) is an eligible test for determination of frailty in idiopathic PD patients.
Methods:
We conducted a cross-sectional study which included 66 patients, aged 60 and over in a tertiary hospital. Frailty was assessed by the Fried Frailty Index (FFI). Severity of the idiopathic PD was detected by the Hoehn and Yahr (H&Y) scale. Mobility was measured by the TUG test. Demographic characteristics and comprehensive geriatric assessments were evaluated. Descriptive statistics and logistic regression were used in analyses. Receiver operating characteristic (ROC) curves were used to identify the discriminative effect of TUG test on frailty.
Results:
The numbers of frail, prefrail, and robust subjects were 34 (51.5%), 24 (36.4%), and 8 (12.1%), respectively. Dependency in instrumental activities of daily living (IADL) was significantly associated with frailty (Odds ratio (OR): 36.00, Confidence interval (CI): 8.43–153.80). Multivariate logistic regression analysis results yielded, depression (OR: 10.37, CI: 2.82–38.12) and higher levodopa doses (OR: 6.28, CI: 1.77–22.24) were independently associated with frailty. TUG test performance was strongly associated with frailty with high sensitivity (0.806) and specificity (0.826) (Area under the curve (AUC): 0.831).
Conclusions:
Frailty is highly prevalent in idiopathic PD and is strongly associated with disabilities as well as specific risk factors of the disease. The TUG may be a reliable test for prediction of frailty in patients with idiopathic PD.
Keywords: Disability, fried frailty index, idiopathic Parkinson’s disease, timed up and go test
INTRODUCTION
Parkinson’s disease is an age related neurodegenerative disease that affects as many as 1–2% of persons aged 60 years and older (1). Besides motor signs, non-motor findings of Parkinson’s disease (PD) necessitates the comprehensive evaluation of patients, particularly in terms of associated geriatric syndromes like frailty (2). Frailty is a geriatric syndrome associated with a burden of worse outcomes, such as functional decline, disability, increased hospitalization, and mortality. To date, few studies have explored the prevalence of frailty in PD (3–5). It is known that prevalence of frailty is high in PD but little is known about the relationship between frailty and clinical situations in PD. In fact, PD patients have been excluded from frailty studies due to similarity of the clinical symptoms between PD and frailty (4, 6). Different instruments are available for assessment of frailty but the Fried Frailty Index (FFI) is one of the most used instruments and includes objective measurements (7).
Timed up and go (TUG) is a well-known functional mobility test in PD and community-dwelling older adults (8, 9). TUG is associated with global health decline, disability in activities of daily living, falls in community dwelling older adults (10, 11), and is also useful to identify patients at risk of falls and hospitalization in PD (12). It is an objective measurement and can be applied in all settings without specialization. It has been shown that TUG is closely associated with frailty in community-dwelling older adults (8). The relationship between TUG and frailty in PD patients has not been examined so far.
The aim of this study was to identify the prevalence of frailty in patients with idiopathic PD by using the FFI, to describe the relationship between severity of the disease and frailty and to evaluate if TUG is an eligible test for prediction of frailty in idiopathic PD patients.
METHODS
This was a cross-sectional study conducted between June 2017 and August 2017 in a tertiary hospital. A total of 66 patients with the diagnosis of idiopathic PD, aged 60 years and older, were included in the study. Individuals diagnosed with idiopathic PD were confirmed by a neurologist according to the UK Brain Bank criteria (13). Patients with a history of malignancy, advanced stage congestive heart failure, chronic renal disease, and bedridden were not included in the study to avoid the high probability of association of these clinical situations with frailty.
Anthropometric measurements of the patients were taken. The Body mass index (BMI) was calculated by dividing weight in kilograms by square of height in meters. A structured questionnaire was administered to the patients for demographic information and for comprehensive geriatric assessment. The Mini-Mental State Examination (MMSE) was administered for evaluation of cognitive status (14). A score of <24 (total of 30 points) points was regarded as cognitive impairment. The Geriatric Depression Scale (GDS), validated in Turkish population, was administered for identification of depression (15). A score of ≥14 points (total of 30 points) was regarded as depression. The instance of at least one fall within the past 12 months was recorded. Recurrent falls were described as the presence of two or more falls in the previous year. Fear of falling as self-reported was also recorded. Regular physical exercise was assessed by self-report and defined as a minimum of 30 minutes of exercise at least three days per week. Medications and comorbidities of the patients and stages of idiopathic PD were recorded. Polypharmacy was defined as five or more different medications in chronic use. The Charlson comorbidity index (CCI) was calculated according to accompanying comorbidities of the patients (16). Patients were grouped according to total daily levodopa requirements as ≥400 and <400 mg based on risk of development of motor complications with higher doses (17). The stage of PD and severity was assessed by the Hoehn and Yahr (H&Y) scale that is the most common and widely used scale to describe severity of PD (18). It reflects the severity of motor disability in PD. Stage 3 is defined as the development of postural instability which is an important hallmark of clinical progression. Indeed H&Y is accepted categorical rather than a continuous scale, the study population was divided into early stages (1; 1.5; 2; 2.5) and advanced stages (3; 4; 5) based on the postural instability (19).
Frailty Assessment
The FFI, characterized by five criteria, was used for frailty assessment: 1) Weight loss: Unintentional weight loss of >% 5 or >4.5 kg in past 12 months; 2) Exhaustion: In response to the twenty-first question of the GDS ‘Do you feel full of energy’, the answer ‘no’ was accepted as one score; 3) Weakness: Maximal grip strength in kg in the stronger arm after three consecutive measurements using a Jamar hand-held dynamometer was obtained. Low grip strength cut points were adjusted for BMI and gender specified by Fried and colleagues (7): For men, low grip strength (kg) was determined as ≤29 kg for BMI ≤24 kg/m2, ≤30 kg for BMI 24.1–26 kg/m2, ≤31 kg for BMI 26.1–28 kg/m2 and ≤32 kg for BMI >28 kg/m2. For women low grip strength was determined as ≤17 kg for BMI ≤23 kg/m2, ≤17.3 kg for BMI 23.1–26 kg/m2, ≤18 kg for BMI 26.1–29 kg/m2 and ≤21 kg for BMI >29 kg/m2.4) Slow walking time: Time in seconds to walk a 4-meter distance at a normal pace was evaluated, slow gait speed cut points were adjusted for height and sex specified by Fried and colleagues (7): For men, slow 4-m gait speed was determined as ≥7 s for height ≤1.73 m and ≥6 s for height >1.73 m. For women, slow 4-m gait speed was determined as ≥7 s for height ≤1.59 m and ≥6 s for height >1.59 m. 5) Low physical activity: The short form of the International Physical Activity Questionnaire was administered (20). A metabolic equivalent (MET) minutes per week <600MET considered as low physical activity. Patients who met at least three criteria were considered to be frail, 1 to 2 criteria prefrail and zero as robust.
Disability
The Activities of Daily Living (ADL) (21) and instrumental activities of daily living (IADL) (22) were applied to define the disability. The ADL ranks adequacy of performance in six functions; bathing, dressing, toileting, transferring, continence, and feeding. Each item is evaluated on a three-point scale to determine whether a person functioned alone or required personal assistance partially or completely. (1=unable, 2=needs assistance, 3=independent) (23). After summing the six responses, a total maximum score of 18 points was obtained. Data recorded in this three-point scale was translated into a dependent or independent dichotomy and a score of ≤12 points was considered as dependent and >12 points as independent. There are 8 domains of function measured with the IADL scale; ability to use the telephone, shopping, food preparation, housekeeping, laundry, mode of transportation, responsibility for own medications, and ability to handle finances. Each item is evaluated on a three-point scale (1=unable, 2=needs assistance, 3=independent) (23). After summing the eight responses, a maximum total score of 24 points was obtained; a score of ≤17 points was considered as dependent and >17 points as independent.
Mobility Assessment
The TUG test was applied by using a standard armchair (46 cm high). Patients were seated with their back against the chair. Patients were instructed to stand up from the chair without support, walk three meters marked on the floor, turn around, walk back to the chair, and sit down again. Patients were told that the test must be performed at a usual speed. The stopwatch was started on the word “start” and stopped as the patient sat down. The TUG time was measured in second (s) (24).
Mobility tests and structured questionnaires were applied by a geriatrician, anthropometric measurements were taken by a family physician, and tests for cognitive status and depression were carried out by a psychologist. All patients were examined by the same neurologist. All patients included in the study were evaluated in the morning, 1 to 2 hours after taking their medications for PD.
The present study was approved by the Local Ethics Committee (Ethics Committee decision no: 2016/595). Informed consents were taken from the patients with intact cognitive function and from the closest relatives of patients with cognitive impairment.
Statistical Analysis
We described the frailty status of the study population as frail and nonfrail (prefrail and robust).
A descriptive analysis was performed based on frailty status. Histogram and q-q plots were examined to assess the data normality. A two-sided independent samples t test was conducted to compare the differences between continuous variables; while the Pearson chi-square test or Fisher exact test were used to compare differences between categorical variables. Receiver operating characteristic (ROC) curves were used to identify the discriminative effect the TUG mobility test had on frailty. Area under the ROC curves were calculated with 95% confidence intervals. The Youden index was applied to determine the optimal cut-off value. Sensitivity, specificity, and positive and negative predictive values were calculated with 95% confidence intervals. Univariate and multiple binary logistic regression analysis were used to identify the risk factors of frailty. Odds ratios (OR) were calculated with 95% confidence intervals (CI). Significant variables related with frailty (Gender, regular exercising, polypharmacy, H&Y stage, levodopa dose, and depression) at p<0.150 on univariate analysis were taken in to multiple models and backward stepwise selection was performed using likelihood ratio statistic at p<0.10 stringency level. The calibration of the model was assessed using the Hosmer-Lemeshow goodness of fit test. p<0.05 was considered as statistically significant. Point biserial and Phi correlation coefficients were calculated to check for multicollinearity. Analyses were conducted using TURCOSA (Turcosa Analytics Ltd. Co., Turkey) statistical softwares (https://turcosa.com.tr/) and SPSS version 22.
RESULTS
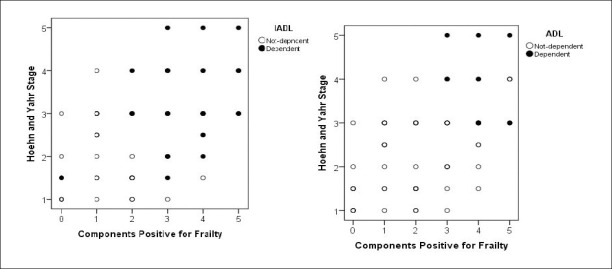
Sixty six patients with idiopathic PD were enrolled in the study. The average age of the study population (mean ± standard deviation) was 67.9±5.9 (43.9% female and 56.1% male). The number of frail, prefrail, and robust were 34 (51.5%), 24 (36.4%), and 8 (12.1%), respectively. All the diagnostic components of the FFI significantly demonstrated positive scores in frail patients when compared to nonfrail (Table 1). Age, gender, BMI, education, marital status, and income did not differ between frail and nonfrail. The median duration of the disease in frail and nonfrail was 7.0 (4–10) and 5.0 (2–10) years, respectively (p=0.158). Two thirds of the frail patients had polypharmacy, experienced falls, had levodopa doses ≥400 mg, and were depressed. There were 10 patients with cognitive impairment and none of them had advanced dementia. All the ADL dependent patients were frail. The number of IADL dependent patients were four times more than the IADL independent patients in the frail group. Three out of four frail patients were in advanced stages of the disease and had fear of falling. A significant relationship of IADL dependency with frailty continued after adjusting for the stage of the disease (OR: 29.6, % 95 CI: 6.4–135.5, p<0.001). Distribution of IADL and ADL dependent patients according to both the stage of the disease and the frailty is shown in Figure 1. A significant relationship between patient characteristics according to frailty status (frail versus nonfrail) are described in Table 2. When gender, regular exercising, polypharmacy, H&Y stage, levodopa, and depression were taken into a model, multivariate logistic regression analysis, results revealed that higher levodopa doses (OR: 6.28, CI: 1.77–22.24, p=0.004) and depression (OR: 10.37 (CI: 2.82–38.12), p<0.001) were independent risk factors for frailty (Table 3). The Hosmer-Lemeshow test result χ2=0.325, p=0.850 revealed the appropriateness of the builted binary logistic regression model in order to predict the independent risk factors of the frailty. Correlation matrices among the independent variables showed significant but low collinearity with ∀ri<0.8, thus, we kept these variables in the model.
Table 1.
Number of positive components of FFI according to frailty status
| Frailty componenets | Frail N=34 (51.5%) | Non-frail N=32 (48.5%) | p | Odds ratio (% 95 CI) |
|---|---|---|---|---|
| Exhaustion | 31 (91.2%) | 13 (40.6%) | <0.001 | 15.103 (3.803–59.981) |
| Weakness | 28 (82.4%) | 5 (15.6%) | <0.001 | 25.200 (6.873–92.395) |
| Low physical activity | 27 (79.4%) | 3 (9.4%) | <0.001 | 37.286 (8.742–159.036) |
| Slow walking time | 25 (75.8%) | 7 (21.9%) | <0.001 | 11.161 (3.513–35.459) |
| Weight loss | 17 (50%) | 8 (25%) | 0.045 | 3.000 (1.055–8.531) |
Figure 1.
Distribution of IADL and ADL dependent patients according to both the stage of the disease and the positive components of frailty.
Table 2.
Patient characteristics and frailty status
| Variables | All (n=66) | Frail (n=34) | Nonfrail (n=32) | p |
|---|---|---|---|---|
| Age | 67.50 (63–72) | 67.50 (60.00–72.25) | 67.00 (62.00–71.00) | 0.378 |
| Gender | ||||
| Female | 27 (40.9) | 17 (50.0) | 10 (31.3) | 0.124 |
| Male | 39 (59.1) | 17 (50.0) | 22 (68.8) | |
| BMI kg/m2 | 30.13±5.04 | 30.52±5.08 | 29.71±5.05 | 0.517 |
| Education | ||||
| Illiterate | 17 (25.8) | 10 (29.4) | 7 (21.9) | 0.246 |
| 5 years | 29 (43.9) | 16 (47.1)) | 13 (40.6) | |
| Over 5years | 20 (30.3) | 8 (23.5) | 12 (37.5) | |
| Marital status | ||||
| Married | 51 (77.3) | 25 (73.5) | 26 (81.3) | 0.561 |
| Widow | 15 (22.7) | 9 (26.5) | 6 (18.8) | |
| Income | ||||
| Low | 49 (74.2) | 24 (70.6) | 25 (78.1) | 0.578 |
| Middle/high | 17 (25.8) | 10 (29.4) | 7 (21.9) | |
| Current smoker | ||||
| Yes | 7 (10.6) | 4 (11.8) | 3 (9.4) | 0.999 |
| No | 59 (89.4) | 30 (88.2) | 29 (90.6) | |
| Regularly exercising | ||||
| Yes | 10 (15.2) | 2 (5.9) | 8 (25.0) | 0.041 |
| No | 56 (84.8) | 32 (94.1) | 24 (75) | |
| CCI | 3.45±1.44 | 3.53±1.40 | 3.38±1.52 | 0.669 |
| Polypharmacy | ||||
| Yes | 38 (57.6) | 23 (67.6) | 15 (46.9) | 0.090 |
| No | 28 (42.4) | 11 (32.4) | 17 (53.1) | |
| H&Y scale | ||||
| Early stages | 30 (45.5) | 9 (26.5) | 21 (65.6) | 0.002 |
| Advanced stages | 36 (54.5) | 25 (73.5) | 11 (34.4) | |
| Levodopa | ||||
| ≥400 mg | 35 (53.0) | 24 (70.6) | 11 (34.4) | 0.003 |
| <400 mg | 31 (47.0) | 10 (29.4) | 21 (65.6) | |
| Urinary incontinance | ||||
| Yes | 39 (59.1) | 21 (61.8) | 18 (56.3) | 0.651 |
| No | 27 (40.9) | 13 (38.2) | 14 (43.8) | |
| Falls | ||||
| Yes | 34 (51.5) | 22 (64.7) | 12 (37.5) | 0.028 |
| No | 32 (48.5) | 12 (35.3) | 20 (62.5) | |
| Recurrent falls | ||||
| Yes | 46 (69.7) | 19 (55.9) | 27 (84.4) | 0.012 |
| No | 20 (30.3) | 15 (44.1) | 5 (15.6) | |
| Fear of falling | ||||
| Yes | 33 (50.0) | 25 (73.5) | 8 (25.0) | <0.001 |
| No | 33 (50.0) | 9 (26.5) | 24 (75.0) | |
| ADL dependency | ||||
| Yes | 8 (12.1) | 8 (23.5) | 0 (0.0) | 0.004 |
| No | 58 (87.9) | 26 (76.5) | 32 (100.0) | |
| IADL dependency | ||||
| Yes | 30 (45.5) | 27 (79.4) | 3 (9.4) | <0.001 |
| No | 36 (54.5) | 7 (20.6) | 29 (90.6) | |
| Depression | ||||
| Yes | 28 (42.4) | 22 (64.7) | 6 (18.8) | <0.001 |
| No | 38 (57.6) | 12 (35.3) | 26 (81.3) | |
| Cognitive impairment | ||||
| Yes | 10 (15.2) | 7 (20.6) | 3 (9.4) | 0.208 |
| No | 56 (84.8) | 27 (79.4) | 29 (90.6) | |
| TUG (sn) | 15.32 (11.47–17.02) | 17.08 (15.68–22.46) | 13.55 (10.40–15.33) | <0.001 |
Values are expressed either as n (%), mean ± SD or median (1st–3rd quartiles). BMI: Body mass index, CCI: Charlson comorbidity index, H&Y: Hoehn and Yahr, ADL: Activities of daily living, IADL: Instrumental activities of daily living, TUG: Timed up and go
Table 3.
Univariate and multivariate logistic regression analysis results in identifying the risk factors of frailty
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p |
| Gender | ||||
| Male | 1.00 | - | - | - |
| Female | 2.20 (0.81–6.01) | 0.124 | - | - |
| Education | ||||
| Over 5years | 1.00 | - | ||
| 5 years | 1.16 (0.35–3.90) | 0.810 | ||
| Illiterate | 2.14 (0.57–7.99) | 0.257 | ||
| Income | ||||
| Middle/high | 1.00 | - | ||
| Low | 1.49 (0.49–4.55) | 0.485 | ||
| Marital status | ||||
| Married | 1.00 | - | ||
| Widow | 1.56 (0.48–5.02) | 0.456 | ||
| Current smoker | ||||
| No | 1.00 | - | ||
| Yes | 1.29 (0.27–6.27) | 0.753 | ||
| Regular exercising | ||||
| Yes | 1.00 | - | - | - |
| No | 5.33 (1.04–27.42) | 0.045 | - | - |
| CCI | 1.07 (0.76–1.51) | 0.663 | ||
| Polypharmacy | ||||
| No | 1.00 | - | - | - |
| Yes | 2.37 (0.87–6.44) | 0.091 | - | - |
| H&Y stage | ||||
| Early stages | 1.00 | - | - | - |
| Advanced stages | 5.30 (1.85–15.23) | 0.002 | - | - |
| Levodopa | ||||
| <400 mg | 1.00 | - | 1.00 | - |
| ≥400 mg | 4.58 (1.62–12.93) | 0.004 | 6.28 (1.77–22.24) | 0.004 |
| Depression | ||||
| No | 1.00 | - | 1.00 | - |
| Yes | 7.94 (2.56–24.66) | <0.001 | 10.37 (2.82–38.12) | <0.001 |
| Cognitive impairment | ||||
| No | 1.00 | - | ||
| Yes | 2.50 (0.59–10.69) | 0.214 | ||
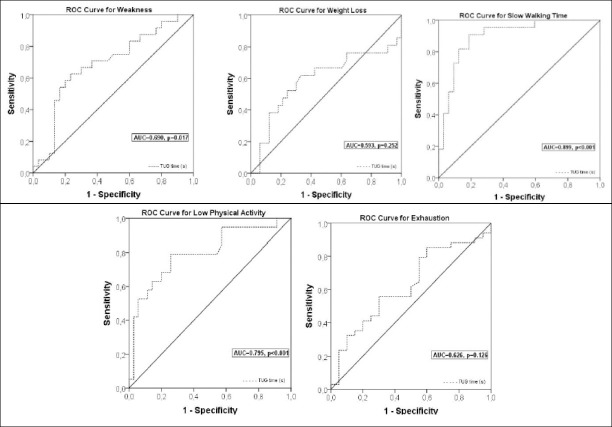
Twelve patients could not perform the TUG test due being unable to get up from the chair without support; one of whom also could not perform the 4-meter test. The median time of the TUG test was 3.53 seconds longer in frail patients than the nonfrail (p<0.001). A long TUG test performance was significantly related with frailty with high sensitivity (0.806, CI: 0.625–0.925) and specificity (0.826, CI: 0.612–0.950) (AUC: 0.831). Positive and negative predictive values were 0.862 (CI: 0.675–0.949) and 0.760 (CI: 0.559–0.928) respectively. The Youden Index determined the cut off value of the TUG as 15.36 seconds for prediction of frailty (Figure 2). Evaluation of the diagnostic components of the FFI revealed that the TUG discriminated the slow walking time, low physical activity, and weakness (AUC: 0.899, AUC: 0.795, AUC: 0.690, respectively) well but did not discriminate the weight loss or exhaustion (Figure 3).
Figure 2.
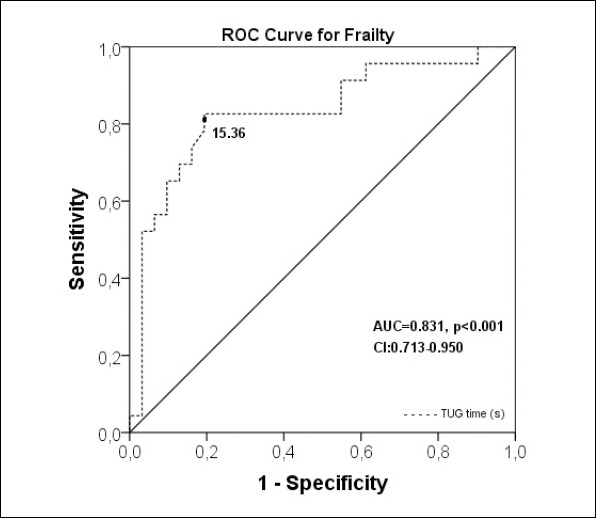
ROC curve to predict the optimal cut off of Timed Up and Go test in seconds for discrimination of frailty with an area under the ROC curve.
Figure 3.
ROC curves of Timed Up and Go test in seconds for discrimination of the each diagnostic components of Fried Frailty Index.
DISCUSSION
A limited number of studies are present in the literature evaluating frailty in idiopathic PD (3–5). We found the prevalence of frailty as 51.5% in idiopathic PD patients by using the FFI. Previously Ahmed et al. studied the prevalence of frailty in PD and reported as 32.6% (3), Roland et al. evaluated the relationship between muscle activity and frailty in 13 females with Parkinson disease; 3 of whom reported as frail (4) and in another study Roland et al assessed the quality of life with frailty in PD and reported one as frail and 19 as prefrail out of 29 PD patients (5). In all three studies the FFI had been used. In our study, exhaustion was the most prevalent component of the FFI in frail patients (91.2%) and the least prevalent component was weight loss (50%). Exhaustion is a disabling feature of PD and it was previously emphasized that exhaustion may be related to the changes within the peripheral or central nervous system, in PD patients (25). It also affects other components of frailty by declining physical capacity and leading to weakness and results in further vulnerability to frailty (5).
Frailty studies conducted with community-dwelling older adults have indicated that frailty is significantly related with older age, low education, low income, living alone, and multimorbidity (26). In our study none of these demographic variables were different between frail and nonfrail patients. However higher levodopa doses and depression were independently associated with frailty in idiopathic PD patients. Higher doses of levodopa is associated with an increased risk of motor complications like dyskinesia, which results in functional limitation and disability, and inevitably predisposes to frailty (17).
Depression is one of the most frequently reported neuropsychiatric disturbances in PD (27). Depression results in an earlier initiation of dopaminergic therapy, more functional decline, greater physical and cognitive deterioration, and increased mortality in PD patients (27). In our study, nearly half of the patients (42.4%) were depressed and depression was significantly related with frailty. Exhaustion as measured by the Fried criterion, is very common in depressed individuals and it has been showed that the risk of frailty increases if depressive symptoms are present while depression interferes with functional status and can facilitate further progression to frailty. In addition, it has been showed that severity of each frailty criterion was worse in depressed individuals (28). In our study exhaustion was found in 27 out of 28 (96.4%) depressed patients.
Advanced stages of idiopathic PD is associated with postural instability and severe disability that results in increased dependency. Both the ADL and IADL are good indicators of dependency in PD (29). In our study, the number of both ADL-dependent and IADL-dependent patients were significantly higher in advance stages. Also, frail patients were significantly more dependent in the ADL and IADL than the nonfrail and this relationship continued after adjustment with the stage of the disease. In this instance, not only the severity of the idiopathic PD, but also the co-existence of frailty may induce the worsening of disabilities and dependency in advanced stages of the disease (30).
The TUG is one of the most used tests to evaluate functional mobility in PD (9). A prolonged TUG test performance discloses a strong relationship with frailty and has been shown to be a strong predictor of frailty in community-dwelling older adults (8). We performed ROC analysis and the Youden index for prediction of frailty by applying the TUG test in idiopathic PD patients. A TUG test performance >15.36 seconds was strongly associated with frailty with high sensitivity and specificity. The TUG also discriminated weakness, low physical activity, and slow walking time well but could not discriminate the exhaustion and weight loss components of FFI. Similar findings have been mentioned in The Irish Longitudinal Study on aging in community-dwelling older adults (8). They concluded that the TUG discriminated components of frailty that become more common with age but did not discriminate components that do not, like unintended weight loss or exhaustion. In the context of these results the TUG test may be a reliable mobility test for awareness and prediction of frailty in patients with idiopathic PD and facilitating early interventions for frailty. While the FFI is time consuming and not a familiar tool for neurologist, the TUG is a well-known mobility test used by neurologists.
The main strengths of our study are that frailty was assessed with the FFI, which comprises objective measurements, and including frailty a comprehensive geriatric assessment was performed in idiopathic PD patients. Some limitations of this study are present. First is the small sample size may introduce an inference error, reduce power of analysis, and limit generalization of the results. Secondly, the cross-sectional design of the study limited interpretation of the direct cause-effect relationship between frailty and disability.
In conclusion, while frailty is highly prevalent in PD and strongly associated with disabilities, it is important to develop effective and applicable frailty interventions to prevent disability and improve physical functions and quality of life in PD patients (30). Due to the diversity of the PD findings and high prevalence of accompanied geriatric syndromes in PD patients, a multidisciplinary approach is needed in these patients, including geriatricians.
Acknowledgment:
The authors thank Merve Çalişkan for taking anthropometric measurements of the patients.
Footnotes
Ethics Committee Approval: The present study was approved by the Local Ethics Committee (Ethics Committee decision no: 2016/595).
Informed Consent: Informed consents were taken from the patients with intact cognitive function and from the closest relatives of patients with cognitive impairment.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - FFÖ, SA, MG; Design - FFÖ, SA, MG, GEZ, AES; Supervision - FFÖ, SA, MG; Resource - FFÖ, SA, MG; Materials - FFÖ, SA, MG; Data Collection and/ or Processing - FFÖ, SA, MG, GEZ, AES; Analysis and/or Interpretation - FFÖ, SA, MG, GEZ; Literature Search - FFÖ, SA, MG; Writing - FFÖ, SA, MG; Critical Reviews - FFÖ, SA, MG, GEZ, AES.
Conflicts of interest: None.
Financial Disclosure: This research did not receive any specific grant from funding agencies in the public.
REFERENCES
- 1.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology. 2009;72:S1–S136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- 2.Lauretani F, Maggio M, Silvestrini C, Nardelli A, Saccavini M, Ceda GP. Parkinson's disease (PD) in the elderly:an example of geriatric syndrome (GS)? Arch Gerontol Geriatr. 2012;54:242–246. doi: 10.1016/j.archger.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed NN, Sherman SJ, VanWyck D. Frailty in Parkinson's disease and its clinical implications. Parkinsonism Relat Disord. 2008;14:334–337. doi: 10.1016/j.parkreldis.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Roland KP, Jones GR, Jakobi JM. Daily electromyography in females with Parkinson's disease:a potential indicator of frailty. Arch Gerontol Geriatr. 2014;58:80–87. doi: 10.1016/j.archger.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Roland KP, Jakobi JM, Powell C, Jones GR. Quality of life as a determinant of frailty phenotype in community dwelling persons with Parkinson's disease. J Am Geriatr Soc. 2012;60:590–592. doi: 10.1111/j.1532-5415.2011.03862.x. [DOI] [PubMed] [Google Scholar]
- 6.Powell C. Frailty and Parkinson's disease:Theories and clinical implications. Parkinsonism Relat Disord. 2008;14:271–272. doi: 10.1016/j.parkreldis.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults:evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.Savva GM, Donoghue OA, Horgan F, O'Regan C, Cronin H, Kenny RA. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci. 2013;68:441–446. doi: 10.1093/gerona/gls190. [DOI] [PubMed] [Google Scholar]
- 9.Bloem BR, Marinus J, Almeida Q, Dibble L, Nieuwboer A, Post B, Ruzicka E, Goetz C, Stebbins G, Martinez-Martin P, Schrag A Movement Disorders Society Rating Scales Committee. Measurement instruments to assess posture, gait, and balance in Parkinson's disease:critique and recommendations. Mov Disord. 2016;31:1342–1355. doi: 10.1002/mds.26572. [DOI] [PubMed] [Google Scholar]
- 10.Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59:887–892. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wennie Huang WN, Perera S, Van Swearingen J, Studenski S. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc. 2010;58:844–852. doi: 10.1111/j.1532-5415.2010.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi-Izquierdo M, Basta D, Rubio-Rodríguez JP, Santos-Pérez S, Ernst A, Sesar-Ignacio Á, Alberte-Woodward M, Guijarro-Del Amo M, Estany-Gestal A, San Román-Rodríguez E, Faraldo-García A, Zubizarreta-Gutiérrez A, Soto-Varela A. Is posturography able to identify fallers in patients with Parkinson's disease? Gait Posture. 2014;40:53–57. doi: 10.1016/j.gaitpost.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Hughes AJ, Daniel SE, Kilfor L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease:a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Ertan T, Eker E. Reliability, validity, and factor structure of the geriatric depression scale in Turkish elderly:are there different factor structures for different cultures? Int Psychogeriatr. 2000;12:163–172. doi: 10.1017/s1041610200006293. [DOI] [PubMed] [Google Scholar]
- 16.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 17.Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, Nissinen H, Leinonen M, Stocchi F Stalevo Reduction in Dyskinesia Evaluation in Parkinson's Disease (STRIDE-PD) investigators. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson's disease. Mov Disord. 2013;28:1064–1071. doi: 10.1002/mds.25364. [DOI] [PubMed] [Google Scholar]
- 18.Hoehn MM, Yahr MD. Parkinsonism:onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 19.Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. Mov Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 20.Saglam M, Arikan H, Savci S, Inal-Ince D, Bosnak-Guclu M, Karabulut E, Tokgozoglu L. International physical activity questionnaire:reliability and validity of the Turkish version. Percept Mot Skills. 2010;111:278–284. doi: 10.2466/06.08.PMS.111.4.278-284. [DOI] [PubMed] [Google Scholar]
- 21.Katz S, Akpom CA. 12. Index of ADL. Med Care. 1976;14:116–118. doi: 10.1097/00005650-197605001-00018. [DOI] [PubMed] [Google Scholar]
- 22.Lawton MP, Brody EM. Assessment of older people:self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 23.Cho C-Y, Alessi CA, Cho M, Aronow HU, Stuck AE, Rubenstein LZ, Beck JC. The association between chronic illness and functional change among participants in Comprehensive Geriatric Assessment Program. J Am Geriatr Soc. 1998;46:677–682. doi: 10.1111/j.1532-5415.1998.tb03800.x. [DOI] [PubMed] [Google Scholar]
- 24.Podsiadlo D, Richardson S. The timed “Up &Go”:a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Grace J, Mendelsohn A, Friedman JH. A comparison of fatigue measures in Parkinson's disease. Parkinsonism Rel Disord. 2007;13:443–445. doi: 10.1016/j.parkreldis.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Gray WK, Richardson J, McGuire J, Dewhurst F, Elder V, Weeks J, Walker RW, Dotchin CL. Frailty Screening in Low- and Middle-Income Countries:A Systematic Review. J Am Geriatr Soc. 2016;64:806–823. doi: 10.1111/jgs.14069. [DOI] [PubMed] [Google Scholar]
- 27.Marsh L. Depression and Parkinson's disease:current knowledge. Curr Neurol Neurosci Rep. 2013;13:409. doi: 10.1007/s11910-013-0409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buigues C, Padilla-Sánchez C, Garrido JF, Navarro-Martínez R, Ruiz-Ros V, Cauli O. The relationship between depression and frailty syndrome:a systematic review. Aging Ment Health. 2015;19:762–772. doi: 10.1080/13607863.2014.967174. [DOI] [PubMed] [Google Scholar]
- 29.Shulman LM. Understanding disability in Parkinson's disease. Mov Disord. 2010;25(Suppl 1):S131–135. doi: 10.1002/mds.22789. [DOI] [PubMed] [Google Scholar]
- 30.Kojima G. Frailty as a predictor of disabilities among community-dwelling older people:a systematic review and meta-analysis. Disabil Rehabil. 2017;39:1897–1908. doi: 10.1080/09638288.2016.1212282. [DOI] [PubMed] [Google Scholar]