Abstract
Recurring episodes of acute pain, also referred to as vaso-occlusive crises (VOC), are characteristic of sickle cell disease (SCD), during which pro-inflammatory cytokines, chemokines, adhesion markers and white cell count, some already elevated at steady state, increase further. Hydroxyurea (HU) is licensed by the FDA for reducing frequency of VOCs in SCD; increased fetal hemoglobin (HbF) together with reduction of the neutrophil count and circulating inflammatory markers, contribute to its clinical efficacy.
Here, using paired plasma samples from HbSS patients (in steady-state and VOC) we determined that despite HU treatment, the SCD environment remained highly inflammatory and particularly at VOC, triggered neutrophil activity. While neutrophil extracellular traps (NETs) induction by the steady state plasmas were comparable to that of plasma from healthy donors, the NETs response triggered by crisis plasmas was significantly increased over that of the steady state (P = 0.0124*). Levels of IL-6 and IL-1α, IL-1ra/IL1F3 and adhesion molecule P-selectin were significantly increased in the VOC plasma when compared with steady state plasma. Higher levels of IL-6 and IL-1ra were also found in the crises samples that yielded an increased NETs response suggesting that increased NETs production associated with increased levels of the inflammatory products of the IL-6 family and regulators of IL-1 family of cytokines during sickle VOCs.
Keywords: Sickle cell disease, Neutrophils, NETs, Pro-inflammatory environment
1. Introduction
Sickle cell disease (SCD) is caused by the presence of hemoglobin S (HbS) due to a point mutation in the β-hemoglobin (Hb) gene. Polymerization of deoxygenated-HbS leads to “sickling” of red blood cells, chronic hemolytic anemia and recurrent episodes of acute vaso-occlusive crises (VOC). All patients have progressive end-organ damage and a reduced life expectancy caused by a complex pathophysiology. Increased circulating levels of pro-inflammatory, chemotactic and adhesion products (e.g. TNF-α, IL-6, IL-18, IL-17α, IL-1β, ICAM, P-selectin) are present at both steady state and VOC [1,2]. Early clinical epidemiological studies have pointed to neutrophils having a major role in promoting SCD pathophysiology. High neutrophil count and their overactivation correlate with poor disease outcome and early death [3,4]. Activated neutrophils can also produce Neutrophil Extracellular Traps (NETs), the extracellular DNA-scaffolded structures that anchors granules components with potent anti-pathogen properties that allow for a more effective antimicrobial activity. NETs formation has also been described in sterile pathologies with a major inflammatory component, including SCD [5,6]. Hydroxyurea (HU) administration reduces the frequency and severity, but does not abolish the occurrence of the sickle VOCs, and reduction of neutrophil count due to the HU myelosuppressive effect is thought to contribute to its efficacy [7].
We asked whether under HU therapy, the SCD environment remained pro-inflammatory enough to cause neutrophils to produce harmful NETs, and whether increased NETs formation associated with high levels of known pro-inflammatory products.
2. Patients and methods
2.1. Patients
All SCD patients had HbSS genotype; paired plasma samples were obtained from 14 patients, age 22–57 (34.3 ± 9.4) years, all undergoing HU treatment, in crises and then again at steady state. Due to limitations of sample size, only 13 of the 14 pairs were used for both Luminex and indirect NETs assays. A “pain crisis” was defined as an episode of acute pain with no evident cause other than SCD, resulting in hospitalization and treatment with parenteral opioids. The “steady state” was when the patient was in the usual state of health. Patients were excluded when they were < 18 or > 80 years of age, pregnant or had a blood transfusion within 8 weeks prior to the blood draw. Plasma from 6 ethnic-matched healthy volunteers, age 28–47 (34.8 ± 7.3) years, was pooled and used as control in the NETs formation assays. Plasma from 28 ethnic-matched healthy donors (HbAA) were used as control for the Luminex assays.
All donors provided written informed consent under their respective IRB-approved protocols (NCT00081523 for SCD plasma donors and NCT00047996 and NCT01441141 for the healthy volunteers).
2.2. ELISA and laboratory parameter measurements
Peripheral blood samples were collected in EDTA and processed within 60 min of collection. Platelet-poor plasma (PPP) was prepared by centrifugation of whole blood at 1500g (15 min, 4 °C) and the plasma removed was further centrifuged for 10 min at 15,000g (10 min, 4 °C). The PPP was stored at −80 °C until analysis. A custom made human premixed multi-analyte kit (Magnetic Luminex Screening Assay) from R&D Systems, MN, was used to screen 35 analytes, in a 10-plex plate (IL-18BPa, IFN-γ, IL-31, IL-13, ICAM1/CD54, IL-27, IL-22, IL-9, IL-5, GRO1/CXCL1), and a 25-plex plate (TNF-α, IL8/CXCL8, IP-10/CXCL10, MCP-1/CCL2, IL-7, MIP-1α/CCL3, MIP-1β/CCL4, IL-4, IL-2, GM-CSF, IL-12p70, IL-23, IL-18, IL-6, SDF-1α/CXCL12, IL-10, IL-1β, IL-1ra/IL-1F3, RANTES/CCL5, IL-1α, IL-17a, E-selectin, P-selectin, IL-15, Eotaxin/CCL11). Not all the 13 patient and 28 healthy samples met sensitivity of the different assays. Eleven of the analytes (IL-31, IFN-γ, IL-5, IL-27, IL-22, IL-9, IL-2, GRO1/CXCL1, IL-12p70, IL-17a, RANTES/CCL5) were not detected in any of the samples from patients. Several analytes were measured in fewer than the 13 paired patients’ samples and fewer than the 28 healthy controls (Supplemental Table 1).
2.3. Neutrophil extracellular traps (NETs) assay
Neutrophils were purified from whole blood collected in EDTA from healthy donors using Polymorphprep gradient medium (Cosmo Bio USA, cat # AXS-1114683). Neutrophils (0.5x105 in 100 μl) were plated in poly-L-Lysine coated 8-well glass chambers (Lab Tek II, cat# 154534) and allowed to rest in the incubator (37 °C, 5% CO2) for 1 h with the lids on and then further incubated for 4 h with plasma from patients or healthy controls diluted to 10% in RPMI. Reactions were stopped with freshly diluted paraformaldehyde (PFA) at final concentration 4% for 30 min at room temperature. NETs were visualized with elastase-Alexa Fluor 488, myeloperoxidase (MPO)-PE and DAPI nuclear staining with a BZ-X710 All-in-One Fluorescence Microscope (Keyence, Osaka, Japan). For statistical purposes 10 to 15 fields at 20x magnification per conditions were acquired in the Multi-color Image Capturing Mode. The number of overlaid DAPI and elastase positive strands (i.e. NETs) were counted in all acquired fields. Average number of NETs per field ± S.D. is shown in Fig. 2. NETotic cells were counted and shown as percentage of total number of counted DAPI-stained nuclei.
Fig. 2.
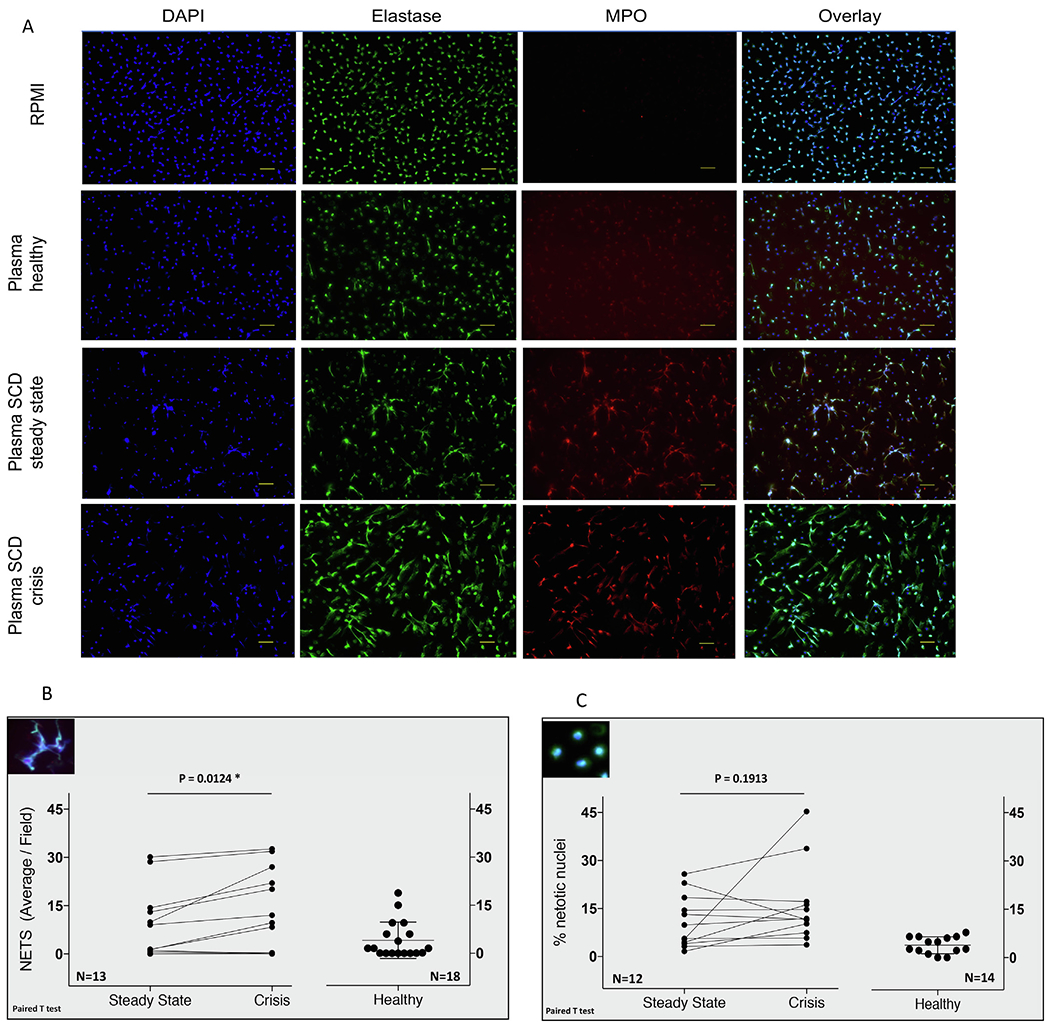
Plasma from SCD patients in crisis induced higher NETosis response in healthy neutrophils than the steady state or control healthy plasma samples. (A) NETs in healthy neutrophils treated for 4 h with paired plasma samples from a SCD patient. Bar is 50 μm. (B) NETs counts for plasma sample from 13 patients and the healthy plasma. (C) NETotic cells were counted and are shown as percentage of total number of DAPI-stained nuclei counted in all acquired fields.
2.4. Statistical analysis
Paired T test was used to asses NETs formation induced with the paired steady state/crisis plasma. Otherwise, Wilcoxon signed-rank test and Mann-Whitney tests were used for two group comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Statistical analyses were performed with PRISM7 software (Graph Pad Software, CA).
3. Results and discussion
Due to assay sensitivity giving rise to variability in the number of analytes that could be detected, only 24 of the 35 analytes could be assessed. Across the study cohort, 13 (P-selectin, IL-18, IL-18PBa, IL8/CXCL8, MIP-1β/CCL4, SDF1α/CXCL12, Eotaxin/CCL11, TNF-α, IL-4, IL-7, IL-15, IL-1β, and E-selectin) of the total 24 analytes had statistically significant higher plasma levels in the patients’ samples at both steady state and crisis compared to the healthy controls (Supplemental Table 1). Within the paired samples, 3 analytes (IL-1ra/IL1F3, P-selectin and IL-6) were significantly higher in the crises samples as compared to steady state (Fig. 1 and Supplemental Table 1). Leukocytes recruitment to the vessel walls are initiated by P-selectin, an adhesive factor that contributes to SCD VOC pathogenesis [8]. In our cohort, P-selectin levels in SCD patients were significantly increased when compared with healthy controls, at both steady state and crisis (P = 0.0022**), increases at crises vs steady state were also significant (P = 0.0215*), supporting P-selectin as a therapy target, for reducing frequency of VOC even in SCD patients while on HU therapy [9]. High levels of IL-6, a multifunctional cytokine with both pro- and anti-inflammatory properties, have been previously measured in non-paired samples from SCD patients at steady state and crisis [10]. Although we did not find a significant increase in IL-6 at steady state as compared to healthy levels in the 12 analyzable samples, we found IL-6 to be significantly increased in the paired crises samples (P = 0.0068**). IL-6 which is involved in the regulation of acute inflammation and consistently found to be increased in SCD, presents a strong potential candidate for further research regarding its association and/or contribution to VOC pathophysiology. In our paired cohort, plasma levels of three analytes (IL-1ra/IL-1F3, P-selectin and IL-6) were significantly increased at crisis compared to steady state (Fig. 1 and Supplemental Table 1). In our study, IL-1α levels were decreased at crises (P = 0.0327*) although notably, IL-1α together with IP-10/CXCL10, were previously found to be elevated in SCD patients with a history of acute splenic sequestration [11]. The other member of the IL-1 family tested in our study, IL-1β, showed similar levels at steady state and crises, but elevated in comparison with the healthy controls (P = 0.0266* and P = 0.0298*, respectively).
Fig. 1.

Analytes with increased plasma expression at SCD crisis included members of IL-6 family and regulators of inflammasome products. Plasma samples from SCD steady state and crisis (N = 13) and healthy race matched donors were used for Luminex assay according to the company’s instructions. Significance between steady state and crisis data was calculated with a Wilcoxon matched-pairs signed rank test. Significance between healthy data and either the steady state or the crisis ones, was calculated with Mann-Whitney test.
Our data showed that the sickle environment remained highly pro-inflammatory even during hydroxyurea treatment and likely still contributed to specific cellular inflammatory responses.
Neutrophilia is a feature of SCD, reduction of neutrophil count as part of HU treatment is thought to contribute to its efficacy. Hydroxyurea also diminishes some of neutrophils activation features, such as increased adhesivity, that can contribute to SCD pathophysiology [12]. Activated neutrophils can also produce NETs, extracellular DNA-scaffolded structures that anchor granules components with potent anti-pathogen properties that allow for a more effective antimicrobial activity. NETs can induce coagulation and even trigger vascular injury [13] and hence can contribute to the vasculopathy in SCD, further supported in a SCD mouse model [6]. These studies suggest that NETS formation is an important contributor to SCD pathophysiology, and delineating its occurrence and pro-thrombotic properties under heightened pro-inflammatory conditions can assist in development of targeted therapies.
We treated healthy neutrophils with the paired SCD plasmas used in the Luminex assays and used immunofluorescence microscopy to quantify the number of mature DNA-elastase-MPO-containing strings and the percentage of NETotic cells following 4 h treatment (Fig. 2). Healthy plasma induced a minor basal NETs response (4 ± 5.70). Increased NETs formation was induced with both plasma from SCD steady state (7.80 ± 9.48) and crises (11.70 ± 12.64) (Fig. 2B) compared to plasma from healthy controls. Amongst the 13 paired plasma samples tested, NETs were induced by plasma collected in steady state and in crisis, but the crises plasma, generally induced higher number of NETs (statistically significant at P = 0.0124*); these crises plasma also corresponded with higher circulating levels of IL-6 and IL-1ra (Supplemental Fig. 1). Three plasma pairs did not produce NETs at steady state or at crisis. Steady state and crises plasma induced comparable NETotic phenotype in the healthy neutrophils (NETotic cells were defined as round, enlarged cells, with co-localized spot-like DAPI and elastase staining) (Fig. 2C).
Our study shows that higher NETs production under SCD crisis conditions associates with increased plasma levels of the inflammatory products of the IL-6 family, a regulator of IL-1 cytokines and adhesion molecule. Interestingly, IL-1ra has been reported as an inhibitor of NETosis [14] and its relevance for NETs formation in SCD is yet to be determined. Although a previous study showed that plasma obtained at sickle crisis was more efficient in triggering NETs production compared to steady state plasma [6], these plasmas were not paired samples from the same individuals, and it is well known that clinical severity and laboratory variables differ greatly between patients. Using paired samples from the same subject, we confirmed that healthy plasma and steady state plasma induced comparable NETs production in healthy neutrophils, but crisis plasmas were more potent compared to their steady state counterpart.
Numerous mediators with known inflammatory potential have been shown to be increased in SCD, either at steady state, or during painful crisis, or at both. Here, using an extended Luminex panel, we provide a comprehensive view on the inflammatory milieu that maintains the SCD basal inflammatory state outside the painful crises. We identified 13 mediators with elevated levels compared to the healthy controls at steady state that were further increased in crises (Supplemental Table 1). It is likely that such a complex pro-inflammatory environment would result in complex responses from the target cells (including neutrophils), that can explain the persistence of the recurrent inflammatory processes in SCD even during a seemingly successful ongoing HU treatment. It is notable that 3 paired samples did not induce NETs at steady-state or crisis, a feature that could account for the variability in clinical response to HU therapy in SCD patients.
One interesting finding in our study refers to the increased expression of IL-1ra/IL1F3 at sickle cell crises. IL-1ra/IL1F3 (IL-1 receptor antagonist) binds with no signaling to cell surface IL-1 receptor (IL-1R) and inhibits cellular response from both IL-1α and IL-1β. Increased circulating levels IL-1β as well as the tissue-produced IL-1α have been previously reported in steady state SCD [11]. In our study we showed that at crises, IL-1β plasma levels did not change, but IL-1α decreased, while their negative regulator IL-1ra/IL1F3 presented significantly increased levels (P = 0.0215*). Whether cytokines of the IL-1 family might be specifically downregulated during SCD crises is yet to be determined.
We showed here that plasma from SCD patients in VOC whilst on hydroxyurea therapy, still caused increased NETs formation, and the increased NETs production correlated with high plasma levels of cytokines of the IL-6 family and IL-1ra. We also confirmed significantly high P-selectin levels at SCD crises, supporting increased adhesivity as a viable therapeutic target for mitigating vaso-occlusive crises [15].
Further tests are required to determine whether a causality relationship exists between increased IL-6, IL-18BP and IL-1ra and increased NETs formation and to determine whether any of these products is a major NETs trigger on its own.
4. Capsule summary
Hydroxyurea therapy reduces frequency but does not abolish sickle cell vaso-occlusive crises (VOC). Plasma from SCD patients in VOC whilst on hydroxyurea treatment, can still trigger NETosis, and degree of NETosis appears to correlate with increased levels of IL-6 and IL-1ra, a regulator of the IL-1 family of cytokines, suggesting that the latter might serve as biomarkers of sickle cell crisis episodes.
5. Authorship
EAB designed and executed experiments, analyzed data and wrote the manuscript. LS and LM executed experimental work, contributed to data analysis and reviewed the manuscript. SLT supervised the project, wrote and reviewed the manuscript.
Supplementary Material
Acknowledgements
Authors thank Jim Nichols and Darlene Allen for their help with the donors’ recruitment process and all patients and healthy volunteers who participated in our study.
Funding
This work was supported by NHLBI intramural support to SLT.
Abbreviations
- SCD
sickle cell disease
- NETs
neutrophil extracellular traps
- VOC
vaso-occlusive crisis
- HbF
fetal hemoglobin
- HbS
hemoglobin S
- MPO
myeloperoxidase
Footnotes
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cyto.2019.154933.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Keikhaei B, Mohseni AR, Norouzirad R, Alinejadi M, Ghanbari S, Shiravi F, Solgi G, Altered levels of pro-inflammatory cytokines in sickle cell disease patients during vaso-occlusive crises and the steady state condition, Eur. Cytokine Network 24 (1) (2013) 45–52. [DOI] [PubMed] [Google Scholar]
- [2].Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N, Costa FF, Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy, J. Leukoc. Biol 85 (2) (2009) 235–242. [DOI] [PubMed] [Google Scholar]
- [3].Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP, Mortality in sickle cell disease. Life expectancy and risk factors for early death, N. Engl. J. Med 330 (23) (1994) 1639–1644. [DOI] [PubMed] [Google Scholar]
- [4].Anyaegbu CC, Okpala IE, Akren’Ova YA, Salimonu LS, Peripheral blood neutrophil count and candidacidal activity correlate with the clinical severity of sickle cell anaemia (SCA), Eur. J. Haematol 60 (4) (1998) 267–268. [DOI] [PubMed] [Google Scholar]
- [5].Castanheira FVS, Kubes P, Neutrophils and NETs in modulating acute and chronic inflammation, Blood 133 (20) (2019) 2178–2185. [DOI] [PubMed] [Google Scholar]
- [6].Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD, Frenette PS, Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease, Blood 123 (24) (2014) 3818–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Charache S, Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults, Semin. Hematol 34 (3 Suppl 3) (1997) 15–21. [PubMed] [Google Scholar]
- [8].Matsui NM, Borsig L, Rosen SD, Yaghmai M, Varki A, Embury SH, P-selectin mediates the adhesion of sickle erythrocytes to the endothelium, Blood 98 (6) (2001) 1955–1962. [DOI] [PubMed] [Google Scholar]
- [9].Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, Guthrie TH, Knight-Madden J, Alvarez OA, Gordeuk VR, Gualandro S, Colella MP, Smith WR, Rollins SA, Stocker JW, Rother RP, Crizanlizumab for the prevention of pain crises in sickle cell disease, N. Engl. J. Med 376 (5) (2017) 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taylor SC, Shacks SJ, Mitchell RA, Banks A, Serum interleukin-6 levels in the steady state of sickle cell disease, J. Interferon Cytokine Res 15 (12) (1995) 1061–1064. [DOI] [PubMed] [Google Scholar]
- [11].Driss A, Wilson NO, Mason K, Hyacinth HI, Hibbert JM, Serjeant GR, Stiles JK, Elevated IL-1alpha and CXCL10 serum levels occur in patients with homozygous sickle cell disease and a history of acute splenic sequestration, Dis. Markers 32 (5) (2012) 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Charache S, Barton FB, Moore RD, Terrin ML, Steinberg MH, Dover GJ, Ballas SK, McMahon RP, Castro O, Orringer EP, Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia, Medicine 75 (6) (1996) 300–326. [DOI] [PubMed] [Google Scholar]
- [13].Moschonas IC, Tselepis AD, The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis, Atherosclerosis 288 (2019) 9–16. [DOI] [PubMed] [Google Scholar]
- [14].Fernandez-Ruiz I, Inflammation: NETs are involved in AAA, Nat. Rev. Cardiol 15 (5) (2018) 257. [DOI] [PubMed] [Google Scholar]
- [15].Ataga KI, Kutlar A, Kanter J, Crizanlizumab in sickle cell disease, N. Engl. J. Med 376 (18) (2017) 1796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


