Abstract
Objective:
Older patients with Alzheimer’s disease (AD) are challenged with adhering to complex medication regimens. We examined effects of Comprehensive Medication Review (CMR), a required Medicare Part D Medication Therapy Management (MTM) program component, on medication adherence among AD patients.
Methods:
This retrospective study analyzed 100% of 2016–2017 Medicare claims covering the entire United States, linked to Area Health Resources Files. Medicare beneficiaries aged ≥65 years were included. Propensity score matching identified comparable intervention and comparison groups with the intervention defined as receiving a CMR in 2017. A difference-in-differences analysis included in multivariate logistic regressions an interaction term between CMR receipt and year 2017. The outcome measured was the nonadherence to diabetes, hypertension, and hyperlipidemia medications, with nonadherence defined as proportion of days covered <80% for study medications.
Results:
Unadjusted comparisons indicated the proportion of nonadherence for intervention group members decreased from 2016 to 2017 but increased for the comparison group. In adjusted analyses, reduction in medication nonadherence among the intervention group remained higher: odds ratios for the interaction term were 0.62 (95% confidence interval [CI]=0.54–0.71), 0.54 (95% CI=0.50–0.58), and 0.50 (95% CI=0.47–0.53) respectively for diabetes, hypertension, and hyperlipidemia medications. This suggests that the likelihood of nonadherence in the intervention group was respectively reduced by 38%, 46%, and 50% more than the comparison group.
Conclusions:
CMR was found to reduce nonadherence to diabetes, hypertension, and hyperlipidemia medications among older Medicare beneficiaries with AD. This provides evidence that the MTM program is effective for a population with unique medication compliance challenges.
Keywords: Medicare, Medication Therapy Management, comprehensive medication review, adherence, Alzheimer’s
Introduction
One in ten Americans age 65 years or above has dementia caused by Alzheimer’s disease (AD) [1], the most common neurological disorder among older adults [2]. As the U.S. population continues to age, the older segment is projected to grow from 16% in 2020 to over 20% in 2030 [3]. Accordingly, the number of those having Alzheimer’s dementia is estimated to increase from the current 5.8 million to 8.4 million over the next decade, reaching 13.8 million by 2050 [4].
While the older population is generally susceptible to medication nonadherence as a result of age-related decline in hearing, vision, and physical movement [5], having AD exacerbates the problem. The disease is characterized by progressive cognitive impairment affecting the mental capacity crucial for proper medication management, such as understanding and following instructions [2,5]. In addition to AD-related behavioral and psychiatric symptoms, older patients typically have age-related comorbid chronic conditions such as diabetes, hypertension, and dyslipidemia [1,2]. The complex medication regimens present particular adherence challenges to cognitively impaired AD patients, resulting in an increased risk of adverse drug events [2]. Furthermore, the challenges of managing AD have significant financial implications. The total cost of care for older Americans with Alzheimer’s or other dementias in 2020 is estimated at $305 billion, over half of which is covered by Medicare [1]. Medicare is a U.S. federal health insurance program for older individuals as well as people with qualified disabilities or end-stage renal disease [6]. Among Medicare beneficiaries age 65 years and above, the average annual per-capita cost incurred for those with Alzheimer’s or other dementias is more than three times as much as the cost for those without [1].
The Centers for Medicare & Medicaid Services (CMS) has long recognized the necessity of reducing costs and improving health outcomes for its beneficiaries who are most at risk of medication therapy problems, including AD patients. In 2006, CMS adopted medication therapy management (MTM) as a required component for Medicare Part D plans, which provide prescription drug benefit for eligible Medicare beneficiaries [6, 7]. A strategy to optimize medication use, MTM services typically are provided by pharmacists and composed of elements such as medication therapy review, medication-related action plan, and intervention and/or referral [8]. The Medicare Part D MTM program only targets beneficiaries meeting eligibility criteria based on multiple chronic conditions, taking multiple Part D medications, and having high medication expenditures [6]. While CMS establishes eligibility guidelines, Part D plans determine their own eligibility criteria, which vary across plans. Although the CMS’s eligibility guidelines have evolved since the inception of the program, AD has been frequently listed as one of the core chronic conditions that Part D plans are required to select from when establishing their MTM eligibility criteria [9].
Despite its great promise to improve pharmacotherapy outcomes, the MTM program has been suffering from low enrollment rates that average only around 10% of Medicare Part D population [10]. To increase MTM enrollment, CMS lowered the thresholds for MTM eligibility criteria based on the number of chronic conditions and the number of prescription medications, but these measures resulted in limited success in improving MTM enrollment [7,11]. A key obstacle in realizing the program’s full potential lies in the paucity of evidence on its effectiveness. The only national study of the Medicare Part D MTM effects reported improved medication adherence for beneficiaries with chronic conditions including diabetes, congestive heart failure, and chronic obstructive pulmonary disease [12]. Findings from studies of non-Medicare MTM interventions likewise suggested a generally positive effect on medication adherence; however, most studies suffered from insufficient statistical power [13]. Among previous literature on MTM effects for patients with chronic conditions, there was a lack of sufficient evidence for most of the assessed outcomes due to wide latitude in program interventions and study populations [14]. No known studies have been conducted of the effects of the Medicare Part D MTM program on medication adherence among older AD patients. We sought to address the research gap by utilizing the newly available Medicare Part D MTM data, with comprehensive medication review (CMR) as our focus.
A required component of the Part D MTM program, CMR entails a consultation provided annually either in person or over the phone mostly by a pharmacist to the beneficiary or an authorized individual such as the beneficiary’s caregiver [15]. The consultation results in a written summary in CMS’s standardized format [15]. The objective of our study was to examine the effects of CMR on adherence to medications for diabetes, hypertension, and hyperlipidemia among older beneficiaries with AD. Developed by the Pharmacy Quality Alliance (PQA), medication adherence measures for the three diseases are core measures in the Star Ratings, a Medicare health plans quality evaluation system established by CMS to assist beneficiaries in making more informed plan enrollment decisions [16].
Methods
Data Sources and Sample Selection
We used 2016 and 2017 Medicare data covering the entire United States linked to the Area Health Resources Files (AHRF). The Medicare data were obtained from the Master Beneficiary Summary File (MBSF), Parts A/B claims, Part D Event (PDE) File, and the Part D MTM Data File. We derived from these Medicare data files beneficiary-level demographic, prescription claims, MTM enrollment, and CMR receipt information [17]. For county-level characteristics, we obtained from AHRF data that provided information such as income, education, and health service supply [18].
To be included in the study, beneficiaries had to meet the following criteria: (1) had AD; (2) aged 65 years or older at the beginning of 2016; (3) were alive at the end of 2017; and (4) had continuous coverage for Medicare Parts A, B, and D in both years. AD diagnosis was determined using codes listed in the CMS Chronic Conditions Data Warehouse [19].
MTM Eligibility Criteria
Part D plans have the flexibility of determining their own eligibility criteria within the parameters of CMS guidelines. The 2016 guidelines required plans to enroll beneficiaries who had at least two to three chronic conditions, took at least two to eight Part D covered prescription medications, and were likely to incur a minimum annual medication cost of $3,507 [20]. The 2017 guidelines were identical except the minimum annual medication cost was $3,919 [15]. Because the eligibility thresholds utilized by Part D plans for the numbers of chronic conditions and covered medications varied, we examined the minimum, median, maximum, and mode values of those thresholds in both years. Given that the majority of the plans used three chronic conditions and eight covered medications [21,22], we decided to use these mode values, which were also the ceiling levels in the guidelines, along with the corresponding annual medication costs as the eligibility criteria to identify comparison group members for our main analysis. Additionally, we explored other possible combinations of threshold values in sensitivity analyses. A beneficiary’s number of chronic conditions was determined from among the 25 MTM-targeted chronic diseases [23] by utilizing ICD-10 codes from Medicare Parts A and B claims, while information on the number of covered medications and annual medication cost was obtained from the PDE file [24].
Identifying Intervention and Comparison Groups
We defined the intervention as receiving a CMR in 2017. To identify the most comparable intervention and comparison groups, we first divided the study population that met the inclusion criteria into two mutually exclusive pools, with one consisting of beneficiaries who were enrolled in MTM for at least one of the two years and the other of those who were not enrolled in both years. We then identified from the first pool those who were new MTM enrollees in 2017 and received a CMR as our potential intervention group members. From the second pool, we identified potential comparison group members as those who met the MTM eligibility criteria adopted for our main analysis, namely a minimum of three chronic conditions and eight Part D drugs plus a minimum annual drug cost of $3,507 in 2016 and $3,919 in 2017.
In the final step of identification, we used propensity score matching to ensure that beneficiary characteristics were balanced between the two groups. A propensity score for each potential group member was obtained by predicting the probability of CMR receipt using logistic regression that included all covariates used in the main analysis. Based on the propensity score, we used nearest neighbor matching without replacement to match comparison to intervention group members in a 3:1 ratio [25,26]. A ratio greater than 3:1 was not used because of the limit in the number of potential comparison group members. Further, efficiency gain was poor beyond this level [27].
Outcome Variables
The outcome variables included three dummies with each representing nonadherence to medications for one of the three diseases of interest, namely diabetes, hypertension, and hyperlipidemia. Medication adherence was determined by the proportion of days covered (PDC), calculated as the number of days covered by at least one prescription medication in the class over the total number of days in the measurement period [28], which started from the first prescription claim date for the medications of interest to the end of the year. For intervention group members, the measurement period started from the first prescription claim date after their CMR receipt. A beneficiary was coded as nonadherent if his or her PDC was under 80% [28].
Conceptual Framework
We used Gelberg-Andersen’s Behavioral Model for Vulnerable Populations as the conceptual framework [29]. Widely used in health care research, the model theorized that health care use is influenced by individual- and community-level predisposing, enabling, and need factors. We selected covariates based on these factors for the analyses. Specifically, the predisposing factors are demographic and social characteristics, measured in this study by age, sex, race/ethnicity, and county-level variables including per capita income, the percentages of married-couple families, population having high school or above education level, and population without health insurance. As resources that facilitate medication utilization, enabling factors were measured by county-level characteristics including metropolitan statistical area, health professional shortage area, and census regions. Need factors represent perceived and evaluated health, measured by a risk adjustment summary score calculated based on a beneficiary’s demographic and diagnostic information. A higher score indicated a higher chance for a beneficiary to incur high healthcare costs. The score served as a proxy of health status.
Statistical Analysis
All statistical analyses were performed using SAS®9.4 (SAS Institute, Inc., Cary, NC) with the Medicare data accessed through the CMS Virtual Research Data Center. The Institutional Review Board at the corresponding author’s institution approved the study (approval number: #20–07197-XM).
In descriptive analyses, we compared the baseline characteristics of the intervention and comparison groups in 2016 by using t-tests for continuous covariates and Chi-square tests for categorical covariates. We also conducted Chi-square tests to examine the unadjusted within-group differences in the proportions of beneficiaries nonadherent to medications between the two years across all three disease states of interest, diabetes, hypertension, and hyperlipidemia.
For multivariate analyses, we conducted logistic regression using a difference-in-differences approach with an interaction term between CMR receipt and year 2017 dummies incorporated. The coefficient estimate of the interaction term represented the effect of CMR on the likelihood of medication nonadherence. If the estimate, an odds ratio (OR), was significantly smaller than 1 (p < .05), it would indicate that CMR receipt was associated with a reduced likelihood of medication nonadherence. Considering the fact that the analyses included county-level covariates and therefore the outcomes of beneficiaries within the same county were likely to correlate with each other, standard errors were clustered within a county to account for potential correlation. While the main analysis examined the immediate effect of CMR, we also performed sensitivity analyses using 3- and 6-month lags to investigate likely long-term effects.
Results
The final study sample consisted of 129,820 Medicare beneficiaries with AD, with 25% in the intervention group (N = 32,455) and 75% in the comparison group (N = 97,365). Baseline characteristics for both groups in 2016 are reported in Table 1. All characteristics were not statistically different across the two groups, except beneficiaries in the intervention group were more likely to have lower risk adjustment summary scores compared to those in the comparison group (2.40 vs 2.55; p < .001).
Table 1.
Baseline Characteristics for Intervention and Comparison Groups in 2016.
| Characteristics | Intervention (n = 32,455, 25%) |
Comparison (n = 97,365, 75%) |
||
|---|---|---|---|---|
| Number | % | Number | % | |
| Predisposing Factors | ||||
| Age, mean (SD) | 79.04 (7.37) | 78.93 (7.86) | ||
| Male | 11,094 | 34.18 | 33,282 | 34.18 |
| Race/Ethnicity | ||||
| Non-Hispanic Whites | 22,775 | 70.17 | 68,325 | 70.17 |
| Blacks (African Americans) | 4,377 | 13.49 | 13,131 | 13.49 |
| Hispanics | 3,647 | 11.24 | 10,941 | 11.24 |
| Asians/Pacific Islanders | 1,199 | 3.69 | 3,597 | 3.69 |
| Other | 457 | 1.41 | 1,371 | 1.41 |
| % Married-couple Families, mean (SD)* | 0.72 (0.07) | 0.72 (0.07) | ||
| % Education >= High School, mean (SD)* | 0.86 (0.06) | 0.86 (0.06) | ||
| Per Capita Income (in $1,000), mean (SD)* | 48.39 (17.13) | 48.77 (16.23) | ||
| % No Insurance, mean (SD)* | 0.10 (0.05) | 0.10 (0.05) | ||
| Enabling Factors | ||||
| Metropolitan Statistical Area* | 26,367 | 81.24 | 79,262 | 81.41 |
| Health Professional Shortage Area* | 29,727 | 91.59 | 89,350 | 91.77 |
| Census Regions* | ||||
| Northeast | 7,844 | 24.17 | 24,051 | 24.70 |
| Midwest | 6,731 | 20.74 | 20,260 | 20.81 |
| South | 12,900 | 39.75 | 38,024 | 39.05 |
| West | 4,980 | 15.34 | 15,030 | 15.44 |
| Need Factor | ||||
| Risk Adjustment Summary Score, mean (SD) | 2.40 (1.60) | 2.55 (1.41) | ||
Note:
indicates a county-level characteristic.
All characteristics were similar between the intervention and comparison groups, except for risk adjustment summary score (p < .001).
Abbreviation: SD=standard deviation.
Figure 1 presents results from unadjusted within-group comparisons of the proportions of nonadherent beneficiaries in 2016 and 2017. For each medication category, the proportions of nonadherent beneficiaries in the intervention group decreased after receiving a CMR whereas the proportions in the comparison group increased over time. For example, among the intervention group, the proportion of beneficiaries nonadherent to diabetes medications dropped from 2016 (13.1%) to 2017 (9.8%) by 3.3 percentage points. In contrast, the proportion grew by 1.2 percentage points (10.8% in 2016 vs. 12.1% in 2017) in the comparison group. We observed similar patterns for hypertension and hyperlipidemia medications. The within-group differences across years were significant at p < .001.
Figure 1.
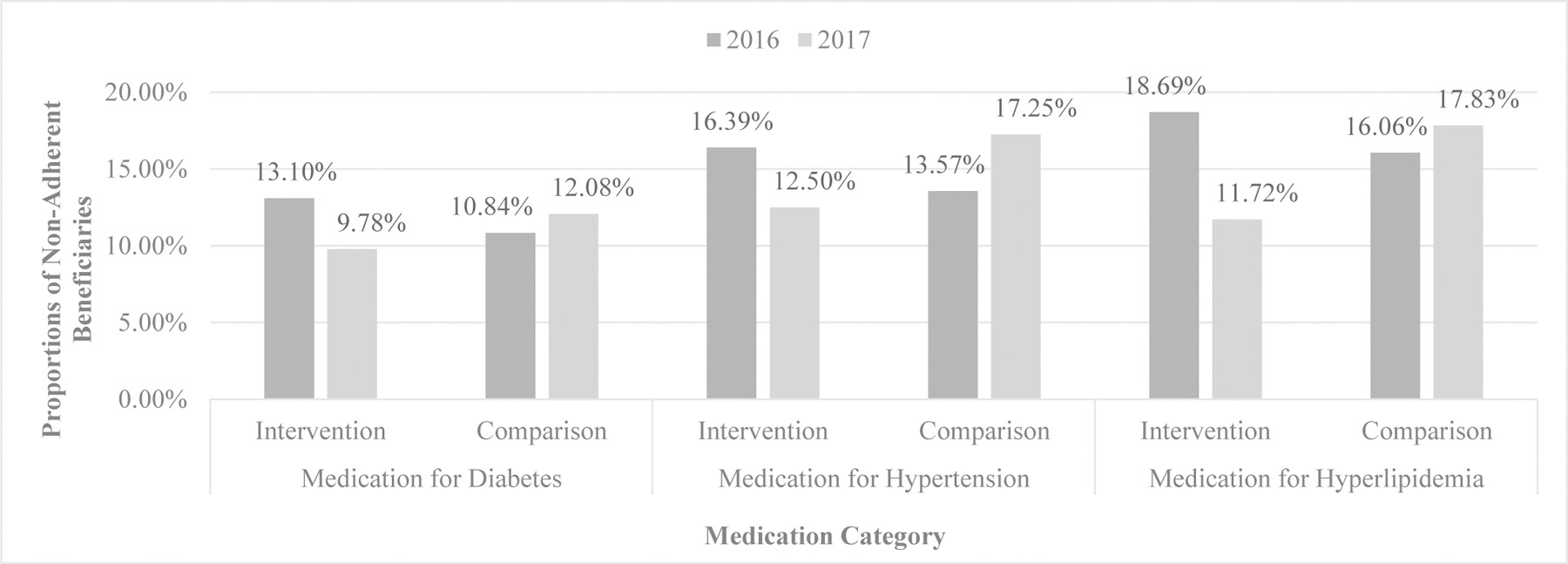
Proportions of Nonadherent Beneficiaries in Intervention and Comparison Groups by Medication Category in 2016 and 2017. For each medication category, the proportions of nonadherent beneficiaries in the intervention group decreased after receiving a comprehensive medication review whereas the proportions in the comparison group increased over time. The within-group differences across years were significant at p < .001.
Results from adjusted difference-in-differences analyses showed that receiving a CMR had a significant negative impact on the likelihood of nonadherence to diabetes, hypertension, and hyperlipidemia medications (Table 2). For diabetes, hypertension, and hyperlipidemia medications, the ORs for the interaction term (CMR × year 2017) were 0.62 (95% confidence interval [CI]=0.54–0.71), 0.54 (95% CI=0.50–0.58), and 0.50 (95% CI=0.47–0.53), respectively. This suggests that the likelihood of nonadherence to diabetes, hypertension, and hyperlipidemia medications in the intervention group was respectively reduced by 38%, 46%, and 50% over time more than the comparison group. Table 2 also indicates that several covariates were significantly associated with the likelihood of nonadherence to each category of medications. Those that had a positive association included Black individuals, the percentage of people without health insurance, individuals living in the South region, and those who had a higher risk adjustment summary score. For example, Black individuals were respectively 1.26 (OR=1.26, 95% CI=1.12–1.43), 1.18 (OR=1.18, 95% CI=1.12–1.25), and 1.29 (OR=1.29, 95% CI=1.22–1.36) times more likely than White individuals to be nonadherent to diabetes, hypertension, and hyperlipidemia medications.
Table 2.
Adjusted Difference-in-Differences Estimates of the Effects of CMR on Nonadherence to Diabetes, Hypertension, and Hyperlipidemia Medications among Beneficiaries with Alzheimer’s Disease.
| Characteristics | Diabetes (n = 27,296) |
Hypertension (n = 100,046) |
Hyperlipidemia (n = 121,644) |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Predisposing Factors | ||||||
| Age | 1.00 | 0.99 – 1.00 | 0.99 | 0.99 – 0.99 | 0.99 | 0.99 – 0.99 |
| Male | 0.87 | 0.80 – 0.96 | 0.98 | 0.94 – 1.02 | 0.88 | 0.85 – 0.91 |
| Race/Ethnicity | ||||||
| Blacks (African Americans) | 1.26 | 1.12 – 1.43 | 1.18 | 1.12 – 1.25 | 1.29 | 1.22 – 1.36 |
| Hispanics | 1.09 | 0.96 – 1.25 | 1.16 | 1.09 – 1.24 | 1.30 | 1.23 – 1.38 |
| Asians/Pacific Islanders | 0.73 | 0.59 – 0.90 | 0.97 | 0.88 – 1.07 | 1.07 | 0.98 – 1.17 |
| Other | 1.09 | 0.81 – 1.49 | 1.11 | 0.96 – 1.29 | 1.06 | 0.92 – 1.21 |
| Year 2017 | 1.11 | 1.02 – 1.21 | 1.31 | 1.26 – 1.36 | 1.13 | 1.09 – 1.16 |
| CMR receipt | 1.28 | 1.15 – 1.42 | 1.25 | 1.19 – 1.32 | 1.21 | 1.16 – 1.27 |
| CMR receipt × Year 2017: CMR Effect | 0.62 | 0.54 – 0.71 | 0.54 | 0.50 – 0.58 | 0.50 | 0.47 – 0.53 |
| % Married-couple Families* | 0.67 | 0.33 – 1.37 | 0.71 | 0.51 – 0.98 | 0.52 | 0.39 – 0.69 |
| % Education >= High School* | 4.70 | 1.59 – 13.89 | 1.62 | 0.99 – 2.65 | 1.81 | 1.17 – 2.79 |
| Per Capita Income (in $1,000)* | 1.00 | 1.00 – 1.00 | 1.00 | 1.00 – 1.00 | 1.00 | 1.00 – 1.00 |
| % No Insurance* | 11.66 | 3.12 – 43.54 | 3.02 | 1.63 – 5.60 | 5.02 | 2.94 – 8.58 |
| Enabling Factors | ||||||
| Metropolitan Statistical Area* | 0.95 | 0.84 – 1.08 | 1.03 | 0.97 – 1.08 | 1.07 | 1.02 – 1.12 |
| Health Professional Shortage Area* | 1.05 | 0.89 – 1.23 | 0.99 | 0.92 – 1.07 | 1.03 | 0.96 – 1.10 |
| Census Regions* | ||||||
| Midwest | 0.99 | 0.86 – 1.14 | 0.98 | 0.92 – 1.04 | 0.94 | 0.89 – 0.99 |
| South | 1.17 | 1.02 – 1.35 | 1.14 | 1.07 – 1.22 | 1.11 | 1.05 – 1.18 |
| West | 1.06 | 0.92 – 1.23 | 1.04 | 0.98 – 1.12 | 1.03 | 0.97 – 1.09 |
| Need Factor | ||||||
| Risk Adjustment Summary Score | 1.20 | 1.17 – 1.23 | 1.27 | 1.25 – 1.28 | 1.18 | 1.17 – 1.19 |
Note:
indicates a county-level characteristic.
Reference groups: female, non-Hispanic Whites, year 2016, comparison group (CMR non-recipients), non-metropolitan statistical area, non-health professional shortage area, and Northeast region.
Abbreviations: CMR=comprehensive medication review; OR=odds ratio; CI=confidence interval.
Sensitivity analyses using combinations of alternative MTM eligibility threshold values as well as 3- and 6-month lags yielded consistent results, which are not presented in this article.
Discussion
Our study utilized the latest Medicare data to evaluate the effects of CMR on medication adherence among older Medicare beneficiaries with AD in years 2016 and 2017. Relative to the CMR non-recipients, CMR recipients were found to have experienced significantly lower nonadherence to diabetes, hypertension, and hyperlipidemia medications. Our findings provided critical evidence of MTM effects on medication compliance that is particularly challenging for the older AD population.
In discussing the significance of any pharmacologic intervention targeting AD, it is important to note that currently no medications cure or slow down the disease progression [1]. All five medications approved by the U.S. Food and Drug Administration (FDA) for AD treatment only serve to temporarily improve patients’ cognitive function [1], with three of them classified as acetylcholinesterase inhibitors (AChEIs) [30]. As for behavioral and psychiatric symptoms that are commonly found among AD patients, there are no FDA-approved medications [1,2]. It is solely at the healthcare provider’s discretion to prescribe medications deemed appropriate, while some of the antipsychotics are known to have a high risk of interaction with other medications, including AChEIs [2]. Although some researchers observed that AChEIs also address comorbidities such as cardiovascular conditions, therefore advocated using one medication for multiple conditions to reduce polypharmacy and improve medication adherence among AD patients [30], others cautioned that AChEIs are associated with high rates of adverse drug events and unknown long-term effect, hence effective MTM strategies are essential for AD treatments [2,31,32].
Results from our study demonstrated the value of Medicare MTM program for improving medication compliance; however, low enrollment has hindered the program from fully realizing its potential. Due to overall low enrollment, it is reasonable to expect that the enrollment rate among the older AD population would not be higher than that for the general Medicare beneficiary population. While CMS sets eligibility guidelines, Part D plans have considerable freedom in determining their own criteria, resulting in a wide range of enrollment rates from under 0.2% to over 57% [10]. The majority of plans aligned their criteria with the CMS threshold ceiling levels, and those with the lowest enrollment tended to cluster diseases that do not normally occur together [10]. Research suggested that plans lack incentives to increase enrollment because the associated costs are paid out of administrative funds as part of the plans’ annual bids for a contract and thus those costs are not reimbursable from CMS [33,34]. To address this potential cause of low enrollment, CMS launched a five-year pilot program in 2017 known as the Medicare Part D Enhanced MTM Model, which provides more regulatory flexibility and financial incentives to the 22 participating plans [34]. As indicated in the interim evaluation in 2019, the plans capitalized on the incentives and enrolled a substantially larger number of beneficiaries using revised eligibility criteria. The average MTM enrollment rate among the participating plans reached 73.5% [34]. While still midway through its course, the pilot program has shown promising directions for future MTM program reforms.
Similar to MTM enrollment, CMR receipt suffers from a low rate. Until recently, the percentage of Medicare MTM enrollees who received a CMR had been under 17% [35, 36]. Whereas both MTM enrollment and CMR receipt have low-rate issues, their plausible causes differ. Unlike MTM enrollment, Part D plans are motivated to increase their CMR completion rate because it is a Star Ratings quality measure [16]. However, most of them struggled and until recently scored only two stars or less on a scale of one to five with five stars representing the highest quality [16, 36]. Research revealed that the standardized format that plans are required to use for their written CMR summaries was not perceived by many beneficiaries as a helpful medication management tool [37]. Additionally, the CMR delivery was suboptimal due to some of the elements in the standardized format [38]. Thus far, the driver of CMR receipt has mainly come from regulatory requirements focusing on the provider side of the MTM program. Given that pharmacists are typically the ones performing CMRs, additional incentives could be considered for these frontline providers so that the Star Ratings measure could be translated into a more effective delivery. Moreover, future reforms should devote more attention to the consumer side by making CMR better serve beneficiaries’ medication management needs and thereby winning more buy-in.
Our study has the following limitations. First, the analysis was limited by the availability of variables in Medicare data. As administrative data, they were not collected for research purposes; beneficiary characteristics such as education and income that are potentially associated with medication adherence were unattainable. Even though corresponding community-level variables had been controlled for, they only represented aggregated characteristics. Second, the outcome measures were based on PDC, which essentially only indicated whether refills were made but did not reflect whether the patient actually took the medications or took them according to prescribed dosage and frequency. The PDC therefore did not measure the actual medication adherence. Nonetheless, given that there is no validated measure of actual medication adherence, PDC is the closest proxy available that has been widely used in research and adopted by CMS as a core Star Ratings measure. Third, we applied a universal set of eligibility criteria to identify a comparable comparison group, thus the results might not generalize to individual patients in different plans because, as previously discussed, there is considerable variation in eligibility criteria across plans. However, we did examine different combinations of thresholds in sensitivity analyses in addition to using the ceiling levels in our main analyses. The results were robust and presented a relatively comprehensive picture of the study outcomes.
Despite the limitations, our study was among the first that utilized 100% of the latest Medicare data to examine the effects of Part D MTM on older AD patients. By analyzing a national population, it overcame the issue of insufficient statistical power that plagues many previous studies. Further, it improved the rigor of research in this area by employing a quasi-experimental method characterized by the inclusion of a comparison group identified through propensity score matching. With more data becoming available, future studies can explore subgroup differences such as gender and racial/ethnic disparities. Given that the older AD population is a high-risk group for adverse drug events due to polypharmacy, the assessment of Part D MTM effects can be extended to other medication utilization measures such as high-risk medication.
Conclusions
In conclusion, CMR was found to have reduced nonadherence to medications for diabetes, hypertension, and hyperlipidemia among older Medicare beneficiaries with AD. The results therefore provided critical evidence of the effectiveness of the MTM program for a vulnerable population having unique challenges in medication compliance. The program will further realize its potential when both MTM enrollment and CMR receipt rate have increased. The enhanced MTM pilot program has shown promises for ways to expand the MTM enrollment, while a more consumer-oriented approach might be desired to increase the CMR receipt among Medicare beneficiaries.
Declaration of funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG040146. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of financial/other relationships
Xiaobei Dong: None. Chi Chun Steve Tsang: None. Shirong Zhao: None. Jamie Browning: None. Jim Y. Wan: None. Marie A. Chisholm-Burns: Received funding from Carlos and Marguerite Mason Trust. Christopher K. Finch: None. Jack W. Tsao: None. Lisa Hines: None. Junling Wang: Received funding from AbbVie, Curo, Bristol Myers Squibb, Pfizer, and Pharmaceutical Research and Manufacturers of America (PhRMA), and serves on Heath Outcomes Research Advisor Committee of the PhRMA Foundation.
Data availability statement
The Medicare data were accessed through virtual access to the CMS Virtual Research Data Center.
References
- [1].2020 Alzheimer’s Disease Facts and Figures [Internet]. Chicago (IL): Alzheimer’s Association; c2020. [cited 2020 Dec 18]. Available from: https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf [Google Scholar]
- [2].Pasqualetti G, Tognini S, Calsolaro V, et al. Potential drug–drug interactions in Alzheimer patients with behavioral symptoms. Clin Interv Aging 2015;10:1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].2017 National Population Projections Tables: Main Series [Internet]. The United States Census Bureau. [cited 2020 Dec 18]. Available from: https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html [Google Scholar]
- [4].Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013;80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smith D, Lovell J, Weller C, et al. A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLOS ONE 2017;12(2):e0170651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Centers for Medicare & Medicaid Services. HHS. Medicare program; Medicare prescription drug benefit. Final rule. Fed Regist 2005;70:4193–4585. [PubMed] [Google Scholar]
- [7].Medicare Program; Contract Year 2015 Policy and Technical Changes to the Medicare Advantage and the Medicare Prescription Drug Benefit Programs; Proposed Rule [Internet]. Department of Health and Human Services; 2014. [cited 2020 Dec 18]. Available from: https://www.govinfo.gov/content/pkg/FR-2014-01-10/pdf/2013-31497.pdf [Google Scholar]
- [8].American Pharmacists Association, National Association of Chain Drug Stores Foundation. Medication therapy management in pharmacy practice: Core elements of an MTM service model (version 2.0). J Am Pharm Assoc 2008;48(3):341–353. [DOI] [PubMed] [Google Scholar]
- [9].Medication Therapy Management [Internet]. Baltimore (MD): Centers for Medicare & Medicaid Services; 2020. May 27 [cited 2020 Dec 18]. Available from: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/MTM [Google Scholar]
- [10].Stuart B, Hendrick FB, Shen X, et al. Eligibility for and enrollment in Medicare Part D Medication Therapy Management Programs varies by plan sponsor. Health Aff (Millwood) 2016;35(9):1572–1580. [DOI] [PubMed] [Google Scholar]
- [11].Gray C, Cooke CE, Brandt N. Evolution of the Medicare Part D Medication Therapy Management Program from inception in 2006 to the present. Am Health Drug Benefits 2019;12(5):243–249. [PMC free article] [PubMed] [Google Scholar]
- [12].Perlroth D, Marrufo G, Montesinos A, et al. Medication Therapy Management in Chronically Ill Populations: Final Report [Internet]. Burlingame (CA): Acumen, LLC; 2013. [cited 2020 Dec 18]. Available from: https://innovation.cms.gov/files/reports/mtm_final_report.pdf [Google Scholar]
- [13].Ai AL, Carretta H, Beitsch LM, et al. Medication therapy management programs: Promises and pitfalls. J Manag Care Spec Pharm 2014;20(12):1162–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Viswanathan M, Kahwati LC, Golin CE, et al. Medication therapy management interventions in outpatient settings: A systematic review and meta-analysis. JAMA Intern Med 2015;175(1):76–87. [DOI] [PubMed] [Google Scholar]
- [15].CY 2017 Medication Therapy Management Program Guidance and Submission Instructions [Internet]. Baltimore (MD): Centers for Medicare & Medicaid Services; 2016. April 8 [cited 2020 Dec 18]. Available from: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Memo-Contract-Year-2017-Medication-Therapy-Management-MTM-Program-Submission-v-040816.pdf [Google Scholar]
- [16].Part C and D Performance Data [Internet]. Baltimore (MD): Centers for Medicare & Medicaid Services; 2020. December 30 [cited 2020 Dec 18]. Available from: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/PerformanceData [Google Scholar]
- [17].Data File Search [Internet]. Minneapolis (MN): Research Data Assistance Center; [cited 2020 Dec 18]. Available from: https://www.resdac.org/cms-data?tid_1%5B%5D=1 [Google Scholar]
- [18].Area Health Resources Files [Internet]. Health Resources & Services Administration; 2020. July 31 [cited 2020 Dec 18]. Available from: https://data.hrsa.gov/topics/health-workforce/ahrf [Google Scholar]
- [19].Condition Categories [Internet]. Baltimore (MD): Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse; 2020. Alzheimer’s Disease and Related Disorders or Senile Dementia; [cited 18 Dec 2020]. Available from: https://www2.ccwdata.org/web/guest/condition-categories [Google Scholar]
- [20].CY 2016 Medication Therapy Management Program Guidance and Submission Instructions [Internet]. Baltimore (MD): Centers for Medicare & Medicaid Services; 2015. April 7 [cited 18 Dec 2020]. Available from: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Memo-Contract-Year-2016-Medication-Therapy-Management-MTM-Program-Submission-v-040715.pdf [Google Scholar]
- [21].2016 Medicare Part D Medication Therapy Management (MTM) Programs [Internet]. Baltimore (MD): Centers for Medicare & Medicaid Services; 2016. May 4 [cited 18 Dec 2020]. Available from: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/CY2016-MTM-Fact-Sheet.pdf [Google Scholar]
- [22].2017 Medicare Part D Medication Therapy Management (MTM) Programs [Internet]. Baltimore (MD): Centers for Medicare & Medicaid Services; 2017. August 16 [cited 18 Dec 2020]. Available from: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/CY2017-MTM-Fact-sheet.pdf [Google Scholar]
- [23].Daniel GW, Malone DC. Characteristics of older adults who meet the annual prescription drug expenditure threshold for Medicare Medication Therapy Management Programs. J Manag Care Spec Pharm 2007;13(2):142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Part D Event File [Internet]. Minneapolis (MN): Research Data Assistance Center; [cited 2020 Dec 18]. Available from: https://www.resdac.org/cms-data/files/pde [Google Scholar]
- [25].Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70(1):41–55. [Google Scholar]
- [26].Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014;33(6):1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Strom BL. Pharmacoepidemiology, 4th edition. Hoboken, NJ: John Wiley & Sons; 2005. [Google Scholar]
- [28].Measures Overview [Internet]. Alexandria (VA): Pharmacy Quality Alliance; c2018. [cited 2020 Dec 18]. Available from: https://www.pqaalliance.org/measures-overview [Google Scholar]
- [29].Gelberg L, Andersen RM, Leake BD. The Behavioral Model for Vulnerable Populations: Application to medical care use and outcomes for homeless people. Health Serv Res 2000;34(6):1273–1302. [PMC free article] [PubMed] [Google Scholar]
- [30].Kaushik V, Smith ST, Mikobi E, et al. Acetylcholinesterase inhibitors: Beneficial effects on comorbidities in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2018;33(2):73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Campbell NL, Perkins AJ, Gao S, et al. Adherence and tolerability of Alzheimer’s disease medications: A pragmatic randomized trial. J Am Geriatr Soc 2017;65(7):1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tan EC, Hilmer SN, Garcia-Ptacek S, et al. Current approaches to the pharmacological treatment of Alzheimer’s disease. Aust J Gen Pract 2018;47(9):586–592. [DOI] [PubMed] [Google Scholar]
- [33].Giberson S, Yoder S, Lee MP. Improving Patient and Health System Outcomes through Advanced Pharmacy Practice. A Report to the U. S. Surgeon General. Office of the Chief Pharmacist. U. S. Public Health Service; 2011 Available at: https://www.accp.com/docs/positions/misc/improving_patient_and_health_system_outcomes.pdf. AccessedDecember 18, 2020.
- [34].Fan B, Ghimire E, Habibulla A, et al. Evaluation of the Part D Enhanced Medication Therapy Management (MTM) Model: First Evaluation Report Burlingame, CA. Acumen, LLC; 2019. Available at: https://downloads.cms.gov/files/mtm-firstevalrpt.pdf. AccessedDecember 18, 2020. [Google Scholar]
- [35].Pestka DL, Zillich AJ, Coe AB, et al. Nationwide estimates of Medication Therapy Management delivery under the Medicare prescription drug benefit. J Am Pharm Assoc 2020;60(3):456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Medicare 2021 Part C & D Star Ratings Technical Notes [Internet]. Baltimore (MD): Centers for Medicare & Medicaid Services; 2020. October 1 [cited 18 Dec 2020]. Available from: https://www.cms.gov/files/document/2021technotes20201001.pdf-0 [Google Scholar]
- [37].Brandt NJ, Cooke CE, Sharma K, et al. Findings from a national survey of Medicare beneficiary perspectives on the Medicare Part D Medication Therapy Management Standardized Format. J Manag Care Spec Pharm 2019;25(3):366–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sharma K, Cooke CE, Howard AK, et al. Beneficiary, caregiver, and case manager perspectives on the Medication Therapy Management Program Standardized Format. J Gerontol Nurs 2019;45(4):7–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Medicare data were accessed through virtual access to the CMS Virtual Research Data Center.


