Abstract
Growth modulation (GM) with tension-band plates (TBPs) by tethering part of the growth plate is an established technique for the correction of angular deformities in children, and it has increasingly supplanted more invasive osteotomies.
Growth modulation with TBPs is a safe and effective method to correct a variety of deformities in skeletally immature patients with idiopathic and pathological physes. The most common indication is a persistent deformity in the coronal plane of the knee exceeding 10°, with anterior and/or lateral joint pain, patellofemoral instability, gait disturbance, or cosmetic concerns. GM has also shown good results in patients with fixed flexion deformity of the knee and ankle valgus.
This paper reviews the history of the procedure, current indications, and recent advances underlying physeal manipulation with TBPs.
Cite this article: EFORT Open Rev 2021;6:658-668. DOI: 10.1302/2058-5241.6.200098
Keywords: hemiepiphysiodesis, lower limb angular deformity, tension-band plates
Introduction
Growth modulation (GM) by tethering part of the growth plate is a well-established and widely accepted technique for the correction of angular deformities in children. Gradual correction by temporary hemiepiphysiodesis with tension-band plates (TBPs) has gained popularity worldwide in the last decade. This minimally invasive method is predictable, reversible and well tolerated. The simplicity and lower complication risk compared to osteotomies make GM an attractive option for paediatric orthopaedic surgeons. This paper reviews the history of the procedure, current indications, and recent advances underlying surgical physeal manipulation.
History of the procedure
In the 19th century, Hueter, Volkmann, and Delpech described the effect of physeal pressure on bone growth.1 Phemister was the first surgeon to describe an open epiphysiodesis technique in 1933.2 This procedure consisted of the removal of a rectangular bone block from the medial and lateral physes, which included part of the adjacent metaphysis and epiphysis. This block would then be replaced in reverse position, producing ultimately a bar across the growth plate. Haas first used instrumentation to guide growth in 1945.3 He demonstrated that metal wire loops affixed to a skeletally immature physis cause tethering and decrease of the growth potential. Subsequently, he concluded that growth resumes once the device is removed.4 Blount et5 described stapling for epiphysiodesis to achieve correction of limb length discrepancy and then6 described stapling for temporary hemiepiphysiodesis to achieve correction of angular deformities. For more than 50 years, the Blount staple (Zimmer, Warsaw, IN, USA) has been widely used. However, downsides related to implant failure, migration or breakage have led to the search for improved techniques.
In 1998, Métaizeau and colleagues introduced a percutaneous technique using transphyseal screws (PETS).7 The technique involved placing threaded screws across the physis to inhibit growth. The potential advantages of PETS were percutaneous insertion with minimal blood loss, and immediate postoperative weight bearing. However, problems with hardware bending, migration, and retrieval, and the theoretical disadvantage of violating the physis with a rigid implant in a temporary procedure, have prevented its popularity.
First described by Stevens in 2007,8 tension-band plates (TBPs) are currently the most commonly used implants for growth modulation. In this procedure, an extra-periosteal plate with two non-locking screws, one inserted in the metaphysis and the other in the epiphysis, serves as a focal hinge at the perimeter of the physis. TBPs have become a safe and effective method to correct a variety of deformities and diagnoses in skeletally immature patients.
Surgical technique
With the patient supine on the operating table and after the administration of general or spinal anaesthesia, the entire leg is prepped and draped. The leg is elevated, and a tourniquet is applied and inflated. A 2 to 3 cm incision is made centred at the level of the physis. Care is taken not to damage the periosteum. Under cross-table fluoroscopic guidance, a Keith needle is inserted into the physis. The 8-plate, which has a centre hole, is slipped over the needle and two 1.6-mm guide wires are inserted. The cortex is drilled, and the self-tapping cannulated screws are inserted. The length of the screw is selected at the discretion of the surgeon.
Masquijo et al9 compared the Stevens technique with a modified technique for TBP insertion around the knee. The modified technique differs in that the guidewires are inserted into the epiphysis and metaphysis before the skin incision. In this study, the modified technique was found to reduce operative time, radiation exposure, and incision size.
Surgical considerations and preoperative planning
During the process of decision making for treating angular deformities, different aspects have to be evaluated. One great advantage of tension-band plating is that it can be used in patients with almost any underlying condition, although incomplete correction is more common in patients with severe deformities (greater physeal compression on the concave side of the deformity/greater inhibition of physeal growth) and skeletal dysplasias. Another advantage is the wide range of ages at which this method can be used. In our personal experience we have treated patients as young as three years old. Implant size is critical when deciding the time of surgery in younger patients, especially when the deformity to be treated is around smaller joints such as the ankle. Available implants on the market are as small as 12 mm plates and 16 mm length 4.5 mm diameter cannulated screws or 12 mm length 3.5 mm diameter solid screws.
For older patients, you should make sure that there is at least one year of bone growth remaining, which is an acceptable time frame to allow the tension band to achieve the desired correction. We find it very useful at this point to assess bone age. Chronologic age only corresponds to skeletal age within a six-month range in 49% of boys and 51% of girls10 and as many as 26% of individuals have a skeletal age that varies more than one year from their chronologic age.10,11 Assessment can be easily achieved through a left hand radiograph and the analysis of any of the validated methods such as the Greulich and Pyle atlas12 or the Shorthand age assessment.13
Before making any surgical decision, it is important to investigate the existence of pain, difficulties with everyday activities or sports, and even whether there are any aesthetic concerns. Besides a thorough physical examination including gait pattern, strength testing, torsional profile, joint motion and stability, and neuromuscular function, a complete deformity analysis should be made. The mechanical axis, joint orientation lines and angles should be carefully outlined and measured.
Determining the time of surgery is another important issue. Various growth-predicting methods have been described: White-Menelaus,14 Green-Anderson,15 Moseley’s straight-line method16, and the Multiplier, popularized by Paley et al.17 that has been validated for growth calculations and epiphysiodesis timing.18
Correction of angular deformities
Coronal plane deformities
Knee
Idiopathic coronal plane angular deformities around the knee with the consequent mechanical axis deviation are the most common lower limb deformities.8,19 It is important to keep in mind that most of these deformities are part of the normal development of lower extremities in a child and they will eventually resolve spontaneously without further treatment. Surgical correction is indicated for persistent deformities of > 10°, with anterior and/orlateral joint pain, patellofemoral instability, gait disturbance, or cosmetic concerns. This usually represents a deviation of the mechanical axis (MAD) beyond the central two quadrants. Evaluation of the individual segment angles is useful to detect the levels and determine where the hardware is needed (femoral vs. tibial). TBPs has been widely used with satisfactory and reproducible results since it was first reported by Stevens in 2007.8 This technique has demonstrated advantages, effectiveness, and to be a safe procedure when compared to corrective osteotomies (Fig. 1).20,21
Fig. 1.
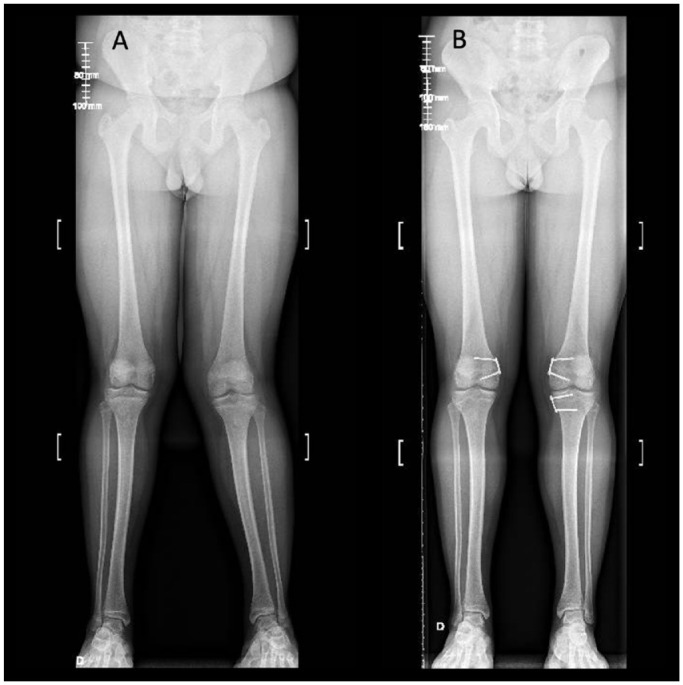
14-year-old boy with bilateral idiopathic genu valgum and patellofemoral instability. (A) Preoperative long leg film. (B) Postoperative radiograph 18 months after growth modulation.
An important number of publications have reviewed the efficacy of TBPs for correcting coronal plane deformities around the knee (Table 1). We would like to highlight the multicentre study published by Danino et al.23 A total of 206 patients with 362 physes were analysed. Average age at the time of surgery was 12.5 years with an average follow-up of 16 months. They achieved 93% standard alignment (mechanical lateral distal femoral angle (mLDFA) between 85° and 89°) in the femoral physis group, and 92% correction to standard alignment parameters (mechanical medial proximal tibial angle (mMPTA) between 85° and 89°) in the tibial physis group. Furthermore, in this study they calculated a rate of correction (ROC) expressed in degrees/month and the result was used as a constant which was multiplied by the number of months from initial surgery. By doing this they were able to predict the amount of correction at several time points throughout the follow-up period. The constant derived is the first tool which enables predicting and monitoring the amount of correction in hemiepiphysiodesis when correcting angular deformities around the knee. Factors significantly influencing success and ROC were age, direction and magnitude of deformity. Valgus correction in the distal femur and proximal tibia and varus correction in the proximal tibia are highly predictable. They concluded through their data analysis that femur corrects faster than tibia. Last but not least, this study gives the first evidence for higher ROC of valgus compared with varus femoral deformity.
Table 1.
Correction with tension-band plates in idiopathic coronal knee deformities
| Author | N | Mean age (years) | No of deformities | Successful correction (%) | Follow-up (months) | Complications |
|---|---|---|---|---|---|---|
| Stevens8 | 34 | 10.0 | 65 | 100 | 12 | N/A |
| Burghardt20 | 43 | 9.7 | 54 | 92.5 | 26 | Screw loosening |
| Ballal22 | 25 | 11.6 | 51 | 100 | 12.4 | Plate and screw migration, deep infection, rebound |
| Danino23 | 206 | 12.5 | 362 | 93% femur 92% tibia |
16 | Infection, limited range motion knee |
| Jelinek24 | 17 | 11.6 | 33 | 100 | N/A | Overcorrection Limited range motion knee |
| Aslani25 | 21 | 10.3 | 42 | 86 | 17 | Screw breakage |
| Boero26 | 30 | 12.5 | N/A | 100 | 21 | None |
Note. N/A, not available.
A common scenario in idiopathic genu valgum is to have an asymmetric deformity. In that case there are two available alternatives:
Perform a staged removal of the implants.
Use a combined TBP for distal femur + proximal tibia in the more severe affected leg, and only distal femur in the contralateral one to perform a single hardware removal.
Another common question that arises regarding the use of TBPs is whether the screw insertion angle will influence the rate of correction. Stevens recommends divergent screws at the outset to reduce the lag time for correction.27 Eltayeby et al28 showed that divergence angle ranging from 0° to 30° results in similar rates of angular correction, and their final recommendation was that adequate screw placement should be the priority rather than favouring any particular divergence angle.
Ankle
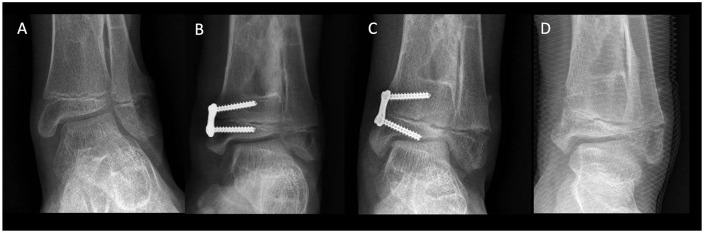
Coronal plane deformities around the ankle joint have been treated successfully by guided growth with TBPs. There is a growing body of evidence that shows good results for ankle valgus. On the other hand, literature is scarce regarding ankle varus and TBPs. Ankle valgus deformity may occur in children with various disorders. This deformity will eventually progress and cause malalignment and abnormal loading of the ankle joint.29 Valgus deformity is most often seen in children with neuromuscular disorders such as myelomeningocele, poliomyelitis, and cerebral palsy. It also occurs in children with postaxial hypoplasia, clubfoot, congenital pseudarthrosis of the tibia, or hereditary multiple exostoses (HME) (Fig. 2).29 Hemiepiphysiodesis with a medial malleolar screw is a well know technique to correct ankle valgus. It was first described by Belle and Stevens in 199230 and Stevens and Belle in 1997.31 More recently, Stevens et al32 published the first cohort of patients treated with TBPs for ankle valgus. This series included 33 patients (57 ankles) with an average age of 10.4 years at the time of surgery and an average follow-up of 27 months. The mechanical lateral distal tibial angle (mLDTA) improved from an average of 78.7 to 90.0 degrees at implant removal and measured 88.2 degrees at final follow-up. The authors calculated the rate of correction of 0.6 degrees per month. This rate of correction is similar to the one achieved with a medial malleolar screw.33,34
Fig. 2.
12-year-old boy with multiple hereditary exostoses (MHE) and ankle valgus. (A) Preoperative anteroposterior ankle radiograph. (B) Six months post growth modulation of the distal tibia. (C) 24 months postoperative. (D) After hardware removal.
Driscoll et all35 compared TBPs with medial malleolar transphyseal screw (MMS) epiphysiodesis for ankle valgus with particular emphasis on the rate of deformity correction and the frequency of complications. Forty-two patients (25 ankles with TBPs and 35 with MMS) with different underlying conditions with a mean follow-up of 34 months were included. Both techniques resulted in successful correction of ankle valgus. The mean rate of correction was faster in ankles treated with MMS than TBPs, but differences did not reach statistical significance (0.55 vs. 0.36 degrees/month, respectively; P = 0.057). Tension-band plate technique was associated with fewer hardware-related complications (4.0% vs. 17.1%).
As mentioned before, the information available for ankle varus correction with TBPs is limited. Probably because ankle varus in children is a much less frequent deformity than ankle valgus. Fu et al36 presented a series of 45 patients, mean age nine years, with post-traumatic physeal arrest and varus deformity with significant growth remaining. They performed a bar resection associated with lateral tension-band plating hemiepiphysiodesis. Bar resection was successfully achieved in 31 patients. In this group, statistically significant mLDFA varus correction was obtained with a mean correction of 15°.
Hip
Growth modulation within the hip with TBPs has been used mainly in Legg-Calve-Perthes disease (LCPD) to prevent trochanteric overgrowth.37 There are also a few reports addressing coxa vara.38
The proximal femoral epiphysis is the primary affected anatomical region in LCPD.39,40 The medial two-thirds of the proximal femoral chondroepiphysis have an intra-capsular blood supply, thus ischemia may cause a residual deformity such as an aspherical femoral head and a short, broad neck.37 On the other hand, the greater trochanter has a separate extra-capsular blood supply, not being affected by the ischemic process, becoming relatively overgrown and prominent, reducing the effective abductor lever arm (functional coxa vara).37 Also, this relative overgrowth can cause extra-articular impingement between the greater trochanter and the ilium.41–43 At the same time, and because of the development of a coxa magna, the centre of rotation of the femoral head is displaced distally, exacerbating the abductor lever arm problem.37
To prevent or minimize deformity associated with greater trochanter overgrowth is the rationale to try to control the growth of the greater trochanter apophysis.43 Stevens et al37 reported a retrospective review of 12 patients, with an average age 7.3 years, who underwent non-osteotomy surgery for LCPD. A TBP was applied to the greater trochanteric apophysis at the time of an arthrogram, open adductor and iliopsoas tenotomy, and Petrie cast application with an average follow-up of 49 months. Eleven of 12 patients experienced improvement in pain, and alleviation of limp and Trendelenburg sign. Neck-shaft angles, Shenton’s line, extrusion index, centre edge angles and trochanteric height did not change significantly. Furthermore, Stevens and Novais38 reported a series of three consecutive patients who underwent guided growth with TBPs in the proximal femur (along with distal femur and proximal tibia) for the treatment of coxa vara and genu varum secondary to Schmid-type metaphyseal chondrodysplasia with an average follow-up of four years. All the radiographic parameters of coxa vara improved in all three patients along with complete correction of genu varum and neutralization of the mechanical axis.
There are several case series about eccentrical transphyseal screws for coxa valga in patients with developmental dysplasia of the hip and/or cerebral palsy.44–46 To our knowledge there are no reports of the use of TBPs in these patients. Recently d’Heurle et al47 compared screws, TBPs and drilling techniques for coxa valga in an experimental model with lambs. Their purpose was to determine whether screw, plate, or drilling techniques decreased the femoral neck-shaft angle (NSA) and articular trochanteric disease (ATD). The plate was a modified 1/3 of a semitubular stainless steel plate, and care was taken to ensure the plate was placed intra-articularly, but not within the weight-bearing area of the acetabulum. Their results suggest that implantation of a screw is likely to be more effective than a plate or drilling procedure in decreasing the NSA in skeletally immature hips.
Pathological physes
There is a subset of patients who have different conditions: skeletal dysplasias, genetic syndromes and endocrinopathies where bone development is affected by an abnormal growth of the physis. Mankin48 coined the term ‘sick physes’ or ‘pathological physes’ to describe these patients. Several previous authors have explored the use of growth modulation for the treatment of lower extremity deformities in patients with pathological physes. In general, pathological physes have a slower rate of correction,26 more complications,49 and a lower success rate than idiopathic deformities.26,49
A comparative study by Boero et al26 included 58 patients with angular deformities around the knee treated with TBP, 28 of them with pathological physes (different aetiologies). In this last group they reported complete correction in 22 patients (78.5%), partial correction in five (17.9%), and no correction in one patient (3.6%). This group showed a significantly slower significant correction rate (P = 0.003) that could be explained by a significant difference in growth speeds. They suggested starting treatment at a very young age, when the deformities are not severe and there is plenty of time for the desired correction to be achieved.
Yilmaz et al50 reported 29 patients (50 limbs) with different skeletal dysplasias that were treated with TBPs to correct varus or valgus deformity with an average follow-up of 25 months. Thirty-four of 38 valgus deformities (89%) and 7 of 12 varus deformities (58%) were successfully corrected with significant change in the final LDFA (lateral distal femoral angle) and MPTA (proximal medial tibial angle) (P < 0.001). The MAD (mechanical axis deviation), LDFA and MPTA improved in all except six patients: Morquio syndrome (N = 1), spondylo-metaphyseal dysplasia (N = 3), pseudoachondroplasia (N = 2).
Rickets has also been successfully treated using TBP. Stevens and Klatt51 reviewed 14 children with rickets, four of them treated with TBPs. These four patients achieved complete correction of their mechanical axis and no complications were reported. It is interesting to mention that they observed improvement in the appearance and width not only of the physis around the knee, but also of remote physes at the hip and ankle while gradually correcting the mechanical axis. The average follow-up reported at the time of publication was 12 months, so no conclusions could be made on rebound effect. Horn et al52 evaluated 24 patients with X-linked hypophosphataemic rickets treated at a mean age of 10.3 years. Neutral mechanical axis was restored in 70% of the limbs. The peri-articular deformity corrected at a rate of 0.3° and 0.7° per month for the mMPTA and mLDFA, respectively. Interestingly, femoral and tibial diaphyseal bowing also improved, particularly in the younger children. The authors observed that patients with ≥ three years of growth remaining responded significantly better than older patients, and treatment was more successful in correcting valgus than varus deformity.
Several reports of smaller series of patients with different conditions have been published. McClure et al53 reported four patients with achondroplasia and genu varus with bilateral distal femoral and proximal tibial growth modulation in three of them and only tibial correction in the remaining. All limbs had some improvement of alignment; however, one patient went on to bilateral osteotomies. Welborn et al54 published a small retrospective series of three patients with focal fibrocartilaginous dysplasia with progressive angular deformities who had undergone GM around the knee. Average follow-up was 56 months, and none of the patients required further surgical intervention for their angular deformity, nor had they shown any evidence of recurrence. Complete deformity correction with growth modulation has also been reported in Albers-Schönberg disease,55 and Schmid-type metaphyseal dysplasia.56
Regarding infantile and adolescent tibia vara, TBPs have been effective for its treatment. Tibia vara, or Blount’s disease, affects the posteromedial aspect of the proximal tibial epiphysis, physis and metaphysis, generating the progressive characteristic deformity described by Blount in 1937.57 The fundamental requirements for effective physeal tethering with TBPs are sufficient growth potential in the patient (at least two years of remaining growth) and an open and functioning medial part of the physis.58 Recently, Griswold et al.59 presented a series of 11 patients (17 extremities) with infantile Blount’s disease who underwent treatment with TBPs, subdivided into cohorts based upon preoperative Langenskiöld classification: ≤ 2 or ≥ 3. They reported a 100% rate of angular correction without the need for corrective osteotomy in patients with Langenskiöld stage ≤ 2 at the beginning of treatment. The treatment course can expect a 33% rate of recurrent deformity, which can be treated successfully with repeat growth modulation. On the other hand, they suggested caution when considering guided growth for children presenting with Langenskiöld stage ≥ 3, who achieved only 40% of angular correction.
McIntosh et al60 reported on 49 adolescents (54 limbs) with adolescent Blount’s disease with an average age of 13.4 years and an average follow-up of 3.3 years. Based on multivariate analysis, age at surgery greater than 14 years (p = 0.0009), body mass index (BMI) greater than or equal to 45 kg/m2 (p = 0.01), and greater preoperative varus deformity (p = 0.03) predicted treatment failure. This is consistent with results demonstrated in previous studies.
Heflin et al, with the exception of those patients with a defined medial tibial physeal bar, have taken the approach of initially managing all infantile, juvenile, and adolescent patients presenting with pathologic tibia vara without specific consideration for age or BMI as long as there is growth remaining, with TBPs.61 The authors presented a series of 17 patients (27 limbs) affected by either infantile or adolescent Blount’s disease, aged from 1.8 years to 15.1 years, who were managed by means of guided growth of the proximal tibia. Twenty-one (78%) limbs had complete normalization of their mechanical axis (middle 50% of knee). Time to correction averaged 13.5 months (8–19 months). There were no peri-operative complications. The authors reported hardware failure in three patients: two with screw breakage and one patient with hardware migration. Also two patients had rebound varus, one was being observed and the other had undergone a repeat procedure.61 As shown in the articles reviewed, TBPs can be effectively used in the treatment of infantile and adolescent Blount’s disease. If this technique fails there will always be time for more complex procedures such as corrective osteotomies.
Finally, we would like to mention a special subset of physeal pathology. Physeal insults such as trauma, infections or tumours, may result in complete or partial growth arrest, generating progressive deformities and functional disability. Recently, Masquijo et al62 published a small case series with partial distal femoral growth arrest that underwent distal femoral physeal bar resection, fat graft interposition, and growth modulation with tension-band plates. Four of the five patients had complete correction and one patient required corrective osteotomy and external fixation. Two patients had rebound valgus: one is being observed and another has undergone a repeat procedure. They concluded that physeal bar resection combined with tension-band hemiepiphysiodesis provides a viable option for the correction of angular deformities associated physeal arrest.
Growth modulation with TBPs is an effective procedure for deformity correction in patients with sick physis (Fig. 3). When elaborating the preoperative planning, the surgeon must be aware that TBPs should be applied earlier than in typical idiopathic patients, as the response to treatment of this physis and the calculations done might not be accurate. As suggested by several authors cited in this section, growth modulation should be the first-line treatment in these patients. Osteotomy may still be required after growth modulation for incomplete correction or very severe deformities.
Fig. 3.
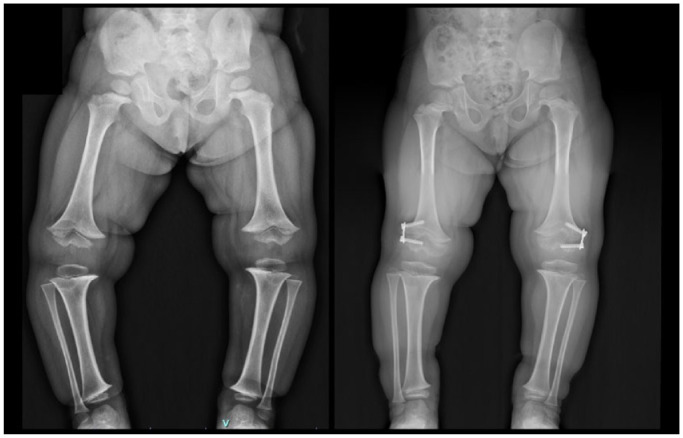
Four-year-old girl with diagnosis of achondroplasia. Preoperative and 20 months after progressive correction with growth modulation.
Sagittal plane deformities
The success of growth modulation for the correction of coronal plane angular deformity in the knee has stimulated surgeons in applying related methods to correct deformities in the sagittal plane. Fixed knee flexion deformity (FKFD) is a common problem in patients with spina bifida, cerebral palsy, and arthrogryposis.63–65 Deformities of less than 10 degrees may be treated conservatively with physical therapy and the use of orthoses. FKFD exceeding 10 degrees may lead to anterior knee pain, decreased endurance, and progressive crouch gait in ambulatory patients, and with respect to wheelchair users, this deformity may impair standing, transfers, and activities of daily living.63,66 Anterior femoral growth modulation (AFGM) is a viable option to treat FKFD to avoid extensive procedures (Fig. 4). Multiple studies examine simultaneous implantation of anteromedial/anterolateral tension-band plates for children with cerebral palsy and other neuromuscular disorders67–70 and agree that the use of TBPs is reasonable in children with mild to moderate flexion deformities, and at least two years of residual growth. The use of AFGM with TBPs in the knee was first described by Klatt and Stevens.67 The authors reported on 18 patients (29 deformities with various aetiologies) with an average preoperative fixed flexion deformity of 23.4° (range, 10° to 50°). At final follow-up, the average fixed flexion deformity was 8° (range, 0° to 30°). No broken screws, migration, or other complications were observed. One patient (one knee) had a superficial wound infection, and one patient (one knee) had recurrence 18 months after removal. One patient who was treated too close to skeletal maturity (16 years old) did not achieve correction. Recently, Wang et al70 reported the outcomes of a case–control study in children with neuromuscular problems. The authors included 42 knees (26 patients) who underwent AFGM compared with a non-surgical control group of 49 knees (43 patients). Average preoperative knee flexion deformity in the AFGM group was 13 ± 8 degrees. Following AFGM, deformity improved by 8 ± 7 degrees (P < 0.001) as measured radiographically and by 7 ± 7 degrees (P < 0.001) as measured on physical examination. The average rate of correction was 0.7 ± 0.6 degrees per month or 8 ± 8 degrees per year. Instrumented three-dimensional gait analyses were also significantly improved following AFGM. Two knees (two patients) experienced wound infection requiring operative irrigation and debridement. Three knees (two patients) had arthrofibrosis limiting maximum flexion, suspected to be secondary to intra-articular irritation by the implants in deep flexion. A significant portion of knees (29%) experience failure of correction. Older age, lower functional status, and greater degree of preoperative deformity appear to be risk factors. Careful patient selection and meticulous surgical technique are key in order to achieve the desired results with AFGM.
Fig. 4.
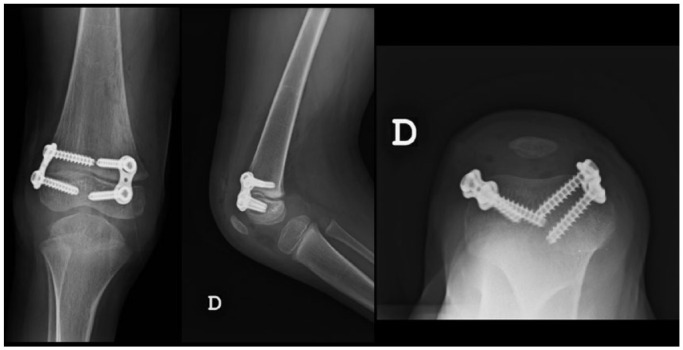
Anteroposterior, lateral and axial views demonstrating the positioning of the tension band plate (TBP). One TBP is placed on each side of the patellofemoral sulcus for knee flexion deformity correction.
There are emerging reports of using TBPs for correction of ankle deformities in the sagittal plane. Al-Aubaidi et al,71 evaluated 25 children (31 feet) with recurrent equinus deformity after surgical treatment of clubfoot. Mean follow-up was 22 months. Mean improvement of dorsiflexion was 2 degrees, with a mean of dorsiflexion of 4.5 degrees, and mean radiological change in the anterior distal tibia angle (ADTA) was 13 degrees. The authors found no correlation between the radiographic changes and the clinically measured dorsiflexion. In addition to the low efficacy demonstrated in this study, a hypothetical drawback of this procedure is that the correction is performed at a site far away from the CORA (centre of rotation of angulation), and the ankle joint is moved relatively forward in regard to the distal tibia. The long-term clinical effect of a change of alignment is uncertain. Sinha et al72 evaluated the radiographic outcomes of nine patients (11 ankles) with calcaneus deformity, treated with extra-periosteal application of a TBP on the posterior aspect of the distal tibial. The indications for treatment were residual clubfoot deformity (N = 9), post-traumatic (N = 1), and neurologic (N = 1). The average age of the patients was 10 years (range, 4 to 13 years). The ADTA showed a modest mean correction of 8° (range, 3.1° to 16.6°), and 55% of the patients required revision. The authors reported no evidence of anterior ankle impingement; however, clinical data (range of motion, pain, quality of life, and patient satisfaction) were not reported. Future studies with more rigorous research designs are required before growth modulation could be considered as a standard treatment in the ankle.
Rotational deformities
Osteotomies are the gold standard for the treatment of rotational deformities. Guided growth has been successfully used in animal studies.73–76 However, human clinical studies are sparse. Arami et al73 showed that oblique placement of two plates on the medial and the lateral side of the distal femur of a six-week-old rabbits can affect the rotational profile. Similarly, Cobanoglu et al75 reported a statistically significant difference in torsion with guided-growth constructs on a rabbit proximal tibia. Lazarus et al,76 performing a 3D analysis by micro-CT scans of the femur, confirmed that the torsional effect of oblique plating seems to correlate with the amount of initial plate angle. More recently, Metaizeau et al77 described a method that converts part of the axial growth into rotation by placing a system composed of two screws and a cable around the distal femoral physis. Eleven patients (20 knees) were reviewed. Mean age at surgery was 10.1 years (range, 8.6–12.7 years). Mean time to screw removal was 21.5 months. The preliminary outcomes indicate good efficacy with about 1.2° of derotation per month and a total mean derotation of 25° over 22 months. Main postoperative complications were knee arthrofibrosis and secondary deformity. Knee stiffness occurred in all the cases. Physical therapy was required to restore full range of motion in 14 knees, and gentle manipulation under general anaesthesia in six knees.
A common problem encountered in the experimental studies was shortening and additional coronal plane deformity when the plates reached alignment with the longitudinal axis of the treated bone. Previous animal studies73,76 showed an average of 4.2% to 7.0% decrease in the length of femurs receiving plates. In Metaizeau et al’s cohort,77 40% of the knees developed secondary mild recurvatum deformity. Future research is required to design a solution that addresses these limitations, and to demonstrate its safety before a widespread use in children.
Risk of coronal plane rebound after TBP removal
Patients with pathological physes, Cozen’s deformity, and patients aged < 10 years have a higher rebound recurrence.26,78,79 Increased frequency of rebound has also been described in patients with initial deformity > 20 degrees.26 In patients with higher risk, Stevens80 recommends removal of the metaphyseal screw leaving the TBP with the epiphyseal screw in situ once the desired correction is achieved (sleeper plate). If rebound occurs, this would allow the re-insertion of the metaphyseal screw, avoiding the need for a new plate insertion. The potential benefits of this strategy must be considered against the possible undesired effects generated by its application. A recent multicentre study including three centres from Argentina and Chile81 reviewed 28 sleeper plate surgeries. Only 22% of the limbs required re-insertion of the metaphyseal screw. Two patients presented complications from the procedure that required unplanned surgery: soft tissue irritation (N = 1) and angular deformity (N = 1). Keshet et al82 reported a similar experience in 55 segments of which only 12 (22%) required re-insertion of the metaphyseal screw. Unfortunately, in only three of these cases, the plate and the epiphyseal screw were in an adequate position to only re-insert the metaphyseal screw. In the remaining nine cases, the authors had to change the plate and both screws. Also, there were two cases where leaving the plate with an epiphyseal screw caused a radiographic bone bar and undesired clinical growth arrest.
Overcorrection has also been proposed to mitigate rebound.21,79 However, as rebound effect is unpredictable, a marked overcorrection of every patient treated with GM may create an opposite deformity in those patients who do not go on to rebound. The authors of this review recommend a mild overcorrection (about 3 degrees) of leg alignment before hardware removal in patients with higher risk and more than a year of expected growth. Recurrent deformity in a skeletally immature patient can be corrected by repeating the process and does not preclude osteotomy if eventually needed.
Complications related to growth modulation
Reported complications of GM with TBP are screw migration, infection, over or undercorrection, permanent physeal arrest, and broken screws/plates. Prevention and treatment of complications are described in Table 2.
Table 2.
Prevention and treatment of complications related to growth modulation
| Complication | Predisposing factor | Prevention | Treatment |
|---|---|---|---|
| Postoperative pain | > 11 years old, four or more plates, femoral plates, bilateral operations83 | Physical therapy84 | NSAIDs, physical therapy |
| Screw migration | < 10 years old, rickets, neurofibromatosis, or other conditions that produce osteopenia85 | Accurate epiphyseal screw placement Sufficient screw length Close monitoring85 |
Hardware exchange |
| Superficial surgical site infection | Obesity, diabetes mellitus | Optimization of patient status, proper asepsis, and surgical site preparation | Antibiotics ± debridement |
| Overcorrection | Poor follow-up86 | Regular follow-up appointments (every 3 months) |
Mild: Observation Moderate/severe: Growth modulation vs. osteotomy |
| Undercorrection | Blount’s disease, obesity, advanced skeletal age, pseudoachondroplasia, or severe deformities21 | Proper patient selection | Osteotomy |
| Broken screws | Poor surgical technique Blount’s disease, obesity87,88 |
Metaphyseal end of the plate coapted to bone89
Solid screws/Double plate or quad plates21 |
Hardware exchange |
| Physeal arrest | Poor surgical technique | Avoid iatrogenic injury of the perichondral ring | Physeal bar resection ± deformity correction |
Notes. NSAIDs, non-steroidal anti-inflammatory drugs.
Conclusions
Growth modulation with tension-band plates has proven to be a successful method of angular deformity correction in growing children. This technique of temporary, asymmetric tethering has matured, and continues to evolve as ongoing research offers new insights into new applications.
Compared to osteotomies, TBP surgery is quicker, safer, and much less invasive. Recovery during the postoperative period is fast and patients can bear weight from the same day of surgery. The complication rate is much lower and with less morbidity.
TBPs can be used in coronal or sagittal plane deformities, in idiopathic patients and also in those with pathologic physis. Careful patient selection and preoperatory planning are mandatory, including chronological and bone age, implant size, and timing of the correction. Finally, parents must be informed about potential complications, failure risk, and possible rebound after TBP removal. Future studies with follow-up till skeletal maturity will help in better understanding for timing of growth modulation and implant removal.
Footnotes
ICMJE Conflict of interest statement: The authors declare no conflict of interest relevant to this work.
Social media: Twitter @javimasq
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.Arkin AM, Katz JF. The effects of pressure on epiphyseal growth: the mechanism of plasticity of growing bone. J Bone Joint Surg [Am] 1956;38-A:1056–1076. [PubMed] [Google Scholar]
- 2.Phemister D. Operative arrestment of longitudinal growth of bones in the treatment of deformities. J Bone Joint Surg [Am] 1933;15-A:1–15. [Google Scholar]
- 3.Haas SL. Retardation of bone growth by a wire loop. J Bone Joint Surg Am 1945;27:25–36. [Google Scholar]
- 4.Haas SL. Mechanical retardation of bone growth. J Bone Joint Surg [Am] 1948;30-A:506–512. [PubMed] [Google Scholar]
- 5.Blount WP, Clarke GR. Control of bone growth by epiphyseal stapling: a preliminary report. J Bone Joint Surg [Am] 1949;31-A:464–478. [PubMed] [Google Scholar]
- 6.Zuege RC, Kempken TG, Blount WP. Epiphyseal stapling for angular deformity at the knee. J Bone Joint Surg [Am] 1979;61-A:320–329. [PubMed] [Google Scholar]
- 7.Métaizeau JP, Wong-Chung J, Bertrand H, Pasquier P. Percutaneous epiphysiodesis using transphyseal screws (PETS). J Pediatr Orthop 1998;18:363–369. [PubMed] [Google Scholar]
- 8.Stevens PM. Guided growth for angular correction: a preliminary series using a tension band plate. J Pediatr Orthop 2007;27:253–259. [DOI] [PubMed] [Google Scholar]
- 9.Masquijo JJ, Lanfranchi L, Torres-Gomez A, Allende V. Guided growth with the tension band plate construct: a prospective comparison of 2 methods of implant placement. J Pediatr Orthop 2015;35:e20–e25. [DOI] [PubMed] [Google Scholar]
- 10.Dimeglio A. Growth in pediatric orthopaedics. In: Lovell WW, Winter RB, Morrissy RT, Weinstein SL, eds. Lovell and Winter’s pediatric orthopaedics. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2006:35–63. [Google Scholar]
- 11.Makarov MR, Jackson TJ, Smith CM, Jo CH, Birch JG. Timing of epiphysiodesis to correct leg-length discrepancy: a comparison of prediction methods. J Bone Joint Surg Am 2018;100:1217–1222. [DOI] [PubMed] [Google Scholar]
- 12.Greulich WW, Pyle SI, Todd TW. Radiographic atlas of skeletal development of the hand and wrist: based on the Brush Foundation Study of Human Growth and Development initiated by T. Wingate Todd. Stanford: Stanford University Press, 1950:xiii. [Google Scholar]
- 13.Heyworth BE, Osei DA, Fabricant PD, et al. The shorthand bone age assessment: a simpler alternative to current methods. J Pediatr Orthop 2013;33:569–574. [DOI] [PubMed] [Google Scholar]
- 14.Menelaus MB. Correction of leg length discrepancy by epiphysial arrest. J Bone Joint Surg Br 1966;48:336–339. [PubMed] [Google Scholar]
- 15.Anderson M, Green WT, Messner MB. Growth and predictions of growth in the lower extremities. J Bone Joint Surg [Am] 1963;45-A:1–14. [PubMed] [Google Scholar]
- 16.Moseley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg [Am] 1977;59-A:174–179. [PubMed] [Google Scholar]
- 17.Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg [Am] 2000;82-A:1432–1446. [DOI] [PubMed] [Google Scholar]
- 18.Wagner P, Standard SC, Herzenberg JE. Evaluation of a mobile application for multiplier method growth and epiphysiodesis timing predictions. J Pediatr Orthop 2017;37:e188–e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White GR, Mencio GA. Genu valgum in children: diagnostic and therapeutic alternatives. J Am Acad Orthop Surg 1995;3:275–283. [DOI] [PubMed] [Google Scholar]
- 20.Burghardt RD, Herzenberg JE. Temporary hemiepiphysiodesis with the eight-Plate for angular deformities: mid-term results. J Orthop Sci 2010;15:699–704. [DOI] [PubMed] [Google Scholar]
- 21.Shabtai L, Herzenberg JE. Limits of growth modulation using tension band plates in the lower extremities. J Am Acad Orthop Surg 2016;24:691–701. [DOI] [PubMed] [Google Scholar]
- 22.Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg [Br] 2010;92-B:273–276. [DOI] [PubMed] [Google Scholar]
- 23.Danino B, Rödl R, Herzenberg JE, et al. Growth modulation in idiopathic angular knee deformities: is it predictable? J Child Orthop 2019;13:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jelinek EM, Bittersohl B, Martiny F, Scharfstädt A, Krauspe R, Westhoff B. The 8-plate versus physeal stapling for temporary hemiepiphyseodesis correcting genu valgum and genu varum: a retrospective analysis of thirty five patients. Int Orthop 2012;36:599–605. SICOT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aslani H, Panjavy B, Bashy RH, Tabrizi A, Nazari B. The efficacy and complications of 2-hole 3.5 mm reconstruction plates and 4 mm noncanulated cancellous screws for temporary hemiepiphysiodesis around the knee. J Pediatr Orthop 2014;34:462–466. [DOI] [PubMed] [Google Scholar]
- 26.Boero S, Michelis MB, Riganti S. Use of the eight-Plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop 2011;5:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens PM. Invalid comparison between methods of epiphysiodesis. J Pediatr Orthop 2018;38:e29–e30. [DOI] [PubMed] [Google Scholar]
- 28.Eltayeby HH, Iobst CA, Herzenberg JE. Hemiepiphysiodesis using tension band plates: does the initial screw angle influence the rate of correction? J Child Orthop 2019;13:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rupprecht M, Spiro AS, Breyer S, Vettorazzi E, Ridderbusch K, Stücker R. Growth modulation with a medial malleolar screw for ankle valgus deformity: 79 children with 125 affected ankles followed until correction or physeal closure. Acta Orthop 2015;86:611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belle RM, Stevens PM. Medial malleolar screw epiphysiodesis for ankle valgus. Orthop Trans 1992;16:655. [PubMed] [Google Scholar]
- 31.Stevens PM, Belle RM. Screw epiphysiodesis for ankle valgus. J Pediatr Orthop 1997;17:9–12. [PubMed] [Google Scholar]
- 32.Stevens PM, Kennedy JM, Hung M. Guided growth for ankle valgus. J Pediatr Orthop 2011;31:878–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang FM, Ma J, Pan Z, Hoversten L, Novais EN. Rate of correction and recurrence of ankle valgus in children using a transphyseal medial malleolar screw. J Pediatr Orthop 2015;35:589–592. [DOI] [PubMed] [Google Scholar]
- 34.Davids JR, Valadie AL, Ferguson RL, Bray EW, III, Allen BL, Jr. Surgical management of ankle valgus in children: use of a transphyseal medial malleolar screw. J Pediatr Orthop 1997;17:3–8. [PubMed] [Google Scholar]
- 35.Driscoll MD, Linton J, Sullivan E, Scott A. Medial malleolar screw versus tension-band plate hemiepiphysiodesis for ankle valgus in the skeletally immature. J Pediatr Orthop 2014;34:441–446. [DOI] [PubMed] [Google Scholar]
- 36.Fu G, Wang W, Dong YF, Lv XM, Yang Z. Treatment of post-traumatic pediatric ankle Varus Deformity with Physeal Bar Resection and Hemi-Epiphysiodesis. Curr Med Sci 2019;39:604–608. [DOI] [PubMed] [Google Scholar]
- 37.Stevens PM, Anderson LA, Gililland JM, Novais E. Guided growth of the trochanteric apophysis combined with soft tissue release for Legg-Calve-Perthes disease. Strateg Trauma Limb Reconstr 2014;9:37–43. PMCID: PMC3951627. PubMed: 24563149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens PM, Novais EN. Multilevel guided growth for hip and knee varus secondary to chondrodysplasia. J Pediatr Orthop 2012;32:626–630. [DOI] [PubMed] [Google Scholar]
- 39.Sponseller PD, Desai SS, Millis MB. Abnormalities of proximal femoral growth after severe Perthes’ disease. J Bone Joint Surg [Br] 1989;71-B:610–614. PubMed: 2768308. [DOI] [PubMed] [Google Scholar]
- 40.Park KW, Rejuso CA, Cho WT, Song HR. Timing of premature physeal closure in Legg-Calve-Perthes disease. Int Orthop 2014;38:2137–2142. PubMed: 24916137. [DOI] [PubMed] [Google Scholar]
- 41.Albers CE, Steppacher SD, Schwab JM, Tannast M, Siebenrock KA. Relative femoral neck lengthening improves pain and hip function in proximal femoral deformities with a high-riding trochanter. Clin Orthop Relat Res 2015;473:1378–1387. PMCID: PMC4353530. PubMed: 25373936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneidmueller D, Carstens C, Thomsen M. Surgical treatment of overgrowth of the greater trochanter in children and adolescents. J Pediatr Orthop 2006;26:486–490. PubMed: 16791067. [DOI] [PubMed] [Google Scholar]
- 43.Bouchard M. Guided growth: novel applications in the hip, knee, and ankle. J Pediatr Orthop 2017;37:S32–S36. [DOI] [PubMed] [Google Scholar]
- 44.Lee WC, Kao HK, Yang WE, Ho PC, Chang CH. Guided growth of the proximal femur for hip displacement in children with cerebral palsy. J Pediatr Orthop 2016;36:511–515. [DOI] [PubMed] [Google Scholar]
- 45.Portinaro N, Turati M, Cometto M, Bigoni M, Davids JR, Panou A. Guided growth of the proximal femur for the management of hip dysplasia in children with cerebral palsy. J Pediatr Orthop 2019;39:e622–e628. [DOI] [PubMed] [Google Scholar]
- 46.Peng SH, Lee WC, Kao HK, Yang WE, Chang CH. Guided growth for caput valgum in developmental dysplasia of the hip. J Pediatr Orthop B 2018;27:485–490. [DOI] [PubMed] [Google Scholar]
- 47.d’Heurle A, McCarthy J, Klimaski D, Stringer K. Proximal femoral growth modification: effect of screw, plate, and drill on asymmetric growth of the hip. J Pediatr Orthop 2018;38:100–104. [DOI] [PubMed] [Google Scholar]
- 48.Mankin H. Rickets, osteomalacia, and renal osteodystrophy Part II. J Bone Joint Surg (Am) 1974;56-A:352–386. [PubMed] [Google Scholar]
- 49.Wiemann JM, IV, Tryon C, Szalay EA. Physeal stapling versus 8-plate hemiepiphysiodesis for guided correction of angular deformity about the knee. J Pediatr Orthop 2009;29:481–485. [DOI] [PubMed] [Google Scholar]
- 50.Yilmaz G, Oto M, Thabet AM, et al. Correction of lower extremity angular deformities in skeletal dysplasia with hemiepiphysiodesis: a preliminary report. J Pediatr Orthop 2014;34:336–345. [DOI] [PubMed] [Google Scholar]
- 51.Stevens PM, Klatt JB. Guided growth for pathological physes: radiographic improvement during realignment. J Pediatr Orthop 2008;28:632–639. [DOI] [PubMed] [Google Scholar]
- 52.Horn A, Wright J, Bockenhauer D, Van’t Hoff W, Eastwood DM. The orthopaedic management of lower limb deformity in hypophosphataemic rickets. J Child Orthop 2017;11:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McClure PK, Kilinc E, Birch JG. Growth modulation in achondroplasia. J Pediatr Orthop 2017;37:e384–e387. [DOI] [PubMed] [Google Scholar]
- 54.Welborn MC, Stevens P. Correction of angular deformities due to focal fibrocartilaginous dysplasia using guided growth: a preliminary report. J Pediatr Orthop 2017;37:e183–e187. [DOI] [PubMed] [Google Scholar]
- 55.Popkov D. Guided growth for valgus deformity correction of knees in a girl with osteopetrosis: a case report. Strateg Trauma Limb Reconstr 2017;12:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens PM, Novais EN. Multilevel guided growth for hip and knee varus secondary to chondrodysplasia. J Pediatr Orthop 2012;32:626–630. [DOI] [PubMed] [Google Scholar]
- 57.Blount WP. Tibia vara: osteochondrosis deformans tibiae. J Bone Joint Surg [Am] 1937;19-A:1–29. [PubMed] [Google Scholar]
- 58.de Pablos J, Arbeloa-Gutierrez L, Arenas-Miquelez A. Update on treatment of adolescent Blount disease. Curr Opin Pediatr 2018;30:71–77. [DOI] [PubMed] [Google Scholar]
- 59.Griswold BG, Shaw KA, Houston H, Bertrand S, Cearley D. Guided growth for the Treatment of infantile Blount’s disease: is it a viable option? J Orthop 2020;20:41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McIntosh AL, Hanson CM, Rathjen KE. Treatment of adolescent tibia vara with hemiepiphysiodesis: risk factors for failure. J Bone Joint Surg [Am] 2009;91-A:2873–2879. [DOI] [PubMed] [Google Scholar]
- 61.Heflin JA, Ford S, Stevens P. Guided growth for tibia vara (Blount’s disease). Medicine (Baltimore) 2016;95:e4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masquijo JJ, Allende V, Ferreyra A, Hernández Bueno JC. Distal femoral physeal bar resection combined with guided growth for the treatment of angular limb deformity associated with growth arrest: a preliminary report. J Pediatr Orthop 2020;40:e958–e962. [DOI] [PubMed] [Google Scholar]
- 63.Williams JJ, Graham GP, Dunne KB, Menelaus MB. Late knee problems in myelomeningocele. J Pediatr Orthop 1993;13:701–703. [DOI] [PubMed] [Google Scholar]
- 64.van Bosse HJ, Feldman DS, Anavian J, Sala DA. Treatment of knee flexion contractures in patients with arthrogryposis. J Pediatr Orthop 2007;27:930–937. [DOI] [PubMed] [Google Scholar]
- 65.Wren TA, Rethlefsen S, Kay RM. Prevalence of specific gait abnormalities in children with cerebral palsy: influence of cerebral palsy subtype, age, and previous surgery. J Pediatr Orthop 2005;25:79–83. [DOI] [PubMed] [Google Scholar]
- 66.Rueter K, Pierre M. Energy cost and gait characteristics of flexed knee ambulation. In: Bunch WE, Keagy R, Knitter AE, et al., eds. Atlas of orthotics. St Louis, MO: CV Mosby, 1985:154–155. [Google Scholar]
- 67.Klatt J, Stevens PM. Guided growth for fixed knee flexion deformity. J Pediatr Orthop 2008;28:626–631. [DOI] [PubMed] [Google Scholar]
- 68.Macwilliams BA, Harjinder B, Stevens PM. Guided growth for correction of knee flexion deformity: a series of four cases. Strateg Trauma Limb Reconstr 2011;6:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Aubaidi Z, Lundgaard B, Pedersen NW. Anterior distal femoral hemiepiphysiodesis in the treatment of fixed knee flexion contracture in neuromuscular patients. J Child Orthop 2012;6:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang KK, Novacheck TF, Rozumalski A, Georgiadis AG. Anterior guided growth of the distal femur for knee flexion contracture: clinical, radiographic, and motion analysis results. J Pediatr Orthop 2019;39:e360–e365. [DOI] [PubMed] [Google Scholar]
- 71.Al-Aubaidi Z, Lundgaard B, Pedersen NW. Anterior distal tibial epiphysiodesis for the treatment of recurrent equinus deformity after surgical treatment of clubfeet. J Pediatr Orthop 2011;31:716–720. [DOI] [PubMed] [Google Scholar]
- 72.Sinha A, Selvan D, Sinha A, James LA. Guided growth of the distal posterior tibial physis and short term results: a potential treatment option for children with calcaneus deformity. J Pediatr Orthop 2016;36:84–88. [DOI] [PubMed] [Google Scholar]
- 73.Arami A, Bar-On E, Herman A, Velkes S, Heller S. Guiding femoral rotational growth in an animal model. J Bone Joint Surg [Am] 2013;95-A:2022–2027. [DOI] [PubMed] [Google Scholar]
- 74.Cullu E, Cobanoglu M, Peker MK. The effect of guided growth on rotational deformities of the long bones: a biomechanical study on Sawbone. Int J Pediatr Orthop 2015;1:44–47. [Google Scholar]
- 75.Cobanoglu M, Cullu E, Kilimci FS, Ocal MK, Yaygingul R. Rotational deformities of the long bones can be corrected with rotationally guided growth during the growth phase. Acta Orthop 2016;87:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lazarus DE, Farnsworth CL, Jeffords ME, Marino N, Hallare J, Edmonds EW. Torsional growth modulation of long bones by oblique plating in a rabbit model. J Pediatr Orthop 2018;38:e97–e103. [DOI] [PubMed] [Google Scholar]
- 77.Metaizeau JD, Denis D, Louis D. New femoral derotation technique based on guided growth in children. Orthop Traumatol Surg Res 2019;105:1175–1179. [DOI] [PubMed] [Google Scholar]
- 78.Morin M, Klatt J, Stevens PM. Cozen’s deformity: resolved by guided growth. Strateg Trauma Limb Reconstr 2018;13:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leveille LA, Razi O, Johnston CE. Rebound deformity after growth modulation in patients with coronal plane angular deformities about the knee: who gets it and how much? J Pediatr Orthop 2019;39:353–358. [DOI] [PubMed] [Google Scholar]
- 80.Stevens PM. The role of guided growth as it relates to limb lengthening. J Child Orthop 2016;10:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Masquijo JJ, Allende V, Artigas C, Hernández Bueno JC, Morovic M, Sepúlveda M. Partial hardware removal in guided growth surgery: A convenient strategy? Rev Esp Cir Ortop Traumatol.2021;65:195-200. [DOI] [PubMed] [Google Scholar]
- 82.Keshet D, Katzman A, Zaidman M, Eidelman M. Removal of metaphyseal screw only after hemiepiphysiodesis correction of coronal plane deformities around the knee joint: is this a safe and advisable strategy? J Pediatr Orthop 2019;39:e236–e239. [DOI] [PubMed] [Google Scholar]
- 83.Fillingham YA, Kroin E, Frank RM, Erickson B, Hellman M, Kogan M. Post-operative delay in return of function following guided growth tension plating and use of corrective physical therapy. J Child Orthop 2014;8:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fillingham YA, Luthringer T, Erickson BJ, Kogan M. Does physical therapy prevent post-operative delay in return of function following tension-band plating? J Child Orthop 2015;9:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masquijo JJ, Firth GB, Sepúlveda D. Failure of tension band plating: a case series. J Pediatr Orthop B 2017;26:449–453. [DOI] [PubMed] [Google Scholar]
- 86.Kemppainen JW, Hood KA, Roocroft JH, Schlechter JA, Edmonds EW. Incomplete follow-up after growth modulation surgery: incidence and associated complications. J Pediatr Orthop 2016;36:516–520. [DOI] [PubMed] [Google Scholar]
- 87.Schroerlucke S, Bertrand S, Clapp J, Bundy J, Gregg FO. Failure of Orthofix eight-Plate for the treatment of Blount disease. J Pediatr Orthop 2009;29:57–60. [DOI] [PubMed] [Google Scholar]
- 88.Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plateguided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop 2010;30:594–597. [DOI] [PubMed] [Google Scholar]
- 89.Stevens PM. The broken screw dilemma. J Pediatr Orthop 2014;34:e5. [DOI] [PubMed] [Google Scholar]